Abstract
Background
Propofol is a common intravenous anesthetic used to induce and maintain anesthesia. Numerous studies have reported that propofol plays an anti-tumor role in diverse human cancers, including glioma. In this research, we explored the roles of propofol and its related molecular mechanisms in glioma.
Material/Methods
U251 and A172 cells were exposed to different doses of propofol for 24 h. Cell proliferation, migration, and invasion in glioma were evaluated using MTT assay and Transwell assay, respectively. The levels of microRNA-410-3p (miR-410-3p) and transforming growth factor-β receptor type 2 (TGFBR2) were detected by quantitative real-time polymerase chain reaction (qRT-PCR) assay and Western blot assay, respectively. The association between miR-410-3p and TGFBR2 was predicted by TargetScan and confirmed by dual-luciferase reporter assay.
Results
Propofol inhibited the proliferation, migration, and invasion of glioma cells in a concentration-dependent way. miR-410-3p was induced and TGFBR2 was inhibited by different concentrations of propofol treatment. Moreover, TGFBR2 was confirmed to be a target gene of miR-410-3p and TGFBR2 was inversely modulated by miR-410-3p in glioma cells. Depletion of miR-410-3p reversed the inhibition of propofol treatment on U251 and A172 cell growth and metastasis, but the effects were further abolished by knocking down the expression of TGFBR2.
Conclusions
Propofol can suppress cell growth and metastasis by regulating the miR-410-3p/TGFBR2 axis in glioma.
MeSH Keywords: Cell Proliferation, Optic Nerve Glioma, Propofol
Background
Glioma is the most common intracranial malignancy in adults and accounts for 40–50% of brain tumors [1]. According to the classification system established by the World Health Organization (WHO), glioma is classified into grades I–IV [2]. In recent years, with the advancement of imaging diagnostic technology and the application of microsurgical techniques, the diagnosis and treatment of glioma have generally made significant progress, but the survival time of patients with high-grade (grade III–IV) glioma remains very low [3,4]. Hence, it is crucial to elucidate the molecular pathogenesis of glioma and discover new treatment methods for glioma patients.
Propofol is an anesthetic that can be used to induce and maintain general anesthesia. Surgical resection is one of the main treatments of tumors, and the use of anesthesia is very necessary to relieve the pain of patients during this process. Hence, the impact of anesthesia on tumor development needs to be studied. More and more reports have confirmed that propofol can suppress the progression of different human cancers. For example, Zhang et al. indicated that propofol induced cell apoptosis and repressed tumor growth in cervical cancer [5]. Ye et al. reported that propofol inhibited cancer progression by inhibiting cell proliferation and invasion in osteosarcoma [6]. Several glioma studies have reports indicated that propofol plays crucial roles in tumor cell growth, apoptosis, and metastasis [7,8]. However, to the best of our knowledge, there are very few studies about the roles and related regulatory mechanisms of propofol in glioma.
MicroRNAs (miRNAs) can modulate gene expression by recognizing the 3′-untranslated region (3′-UTR) of their target messenger RNAs at the post-transcriptional level [9]. Numerous studies have determined that miRNAs are associated with diverse biological processes, including differentiation, migration, invasion, and apoptosis [9–11]. Recently, it has been confirmed that miRNAs are involved in the occurrence and progression of various human cancers, including glioma. For instance, miR-30b-3p was demonstrated to promote cell viability and metastasis in glioma [12]. Zhou et al. found that miR-132 was abnormally expressed and its overexpression repressed glioma cell viability, migration, and invasion [13]. These data suggest that various miRNAs exert different effects in glioma. miR-410 has been verified by several studies to be associated with the progression of glioma [14,15], but the exact regulatory mechanism remains ill-defined.
Transforming growth factor-β receptor type 2 (TGFBR2), a transmembrane protein with serine (Ser)/threonine (Thr) kinase activity, plays vital roles in the occurrence and development of tumors [16,17]. Wei et al. demonstrated that TGFBR2 was a target for miR-373 and induced cell migration and invasion in glioma [18]. However, whether miR-410-3p can target TGFBR2 in glioma has not been confirmed.
Here, we explored the function of propofol in glioma cells and the impact of propofol on miR-410-3p and TGFBR2 expression. We also explored the exact roles of miR-410-3p and TGFBR2 in the promotion of glioma.
Material and Methods
Cell culture
U251 and A172 glioma cell lines were bought from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). All cells were kept in Dulbecco’s modified Eagle’s medium (DMEM; Solarbio, Beijing, China) including 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA). The medium was placed at 37°C in an incubator with 5% CO2.
Cell transfection and treatment
miR-410-3p mimic and its control (miR-NC), inhibitor of miR-410-3p (anti-miR-410-3p) and corresponding control (anti-NC), small interfering RNA targeting TGFBR2 (si-TGFBR2) and its control (si-NC) were synthesized by GenePharma (Shanghai, China). Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA, USA) was then used for U251 and A172 cell transfection based on the descriptions in the manual.
Propofol was dissolved in DMSO (Solarbio) and diluted in a complete culture medium. Then, U251 and A172 cells, transfected or untransfected, were exposed to different doses of propofol (5 μg/mL or 10 μg/mL) or an equal volume of DMSO (0 μg/mL of propofol) for 24 h.
3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-H-tetrazolium bromide (MTT) assay
Glioma cells were seeded into 96-well plates and kept for 24 h, then cells were untransfected or transfected with synthetic oligonucleotides and untreated or treated with propofol. Next, cells were interacted with MTT solution (Sigma-Aldrich, St. Louis, MO, USA) for 4 h at 37°C. Subsequently, we removed the supernatant and added DMSO (Solarbio) to each well to solubilize the formazan crystals. Finally, the absorbance at 490 nm was examined with a microplate reader (BMG LABTECH, Offenburg, Baden-Württemberg, Germany).
Transwell assay
To examine cell migration ability, glioma cells that were treated with propofol or transfected as above together with propofol treatment were suspended and seeded into the upper chamber of a Transwell device (Corning, Inc., Corning, NY, USA). DMEM (Solarbio) including 10% FBS (HyClone) was added to the lower chamber. After 24 h, we removed the cells that remained on the upper chamber, and cells that migrated were fixed with methanol, stained with crystal violet (Sigma-Aldrich), and counted under a microscope. To determine cell invasion ability, the upper chamber was coated with Matrigel (Solarbio), and the other steps were consistent with the migration assay.
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
After being transfected as above or treated with propofol, total RNA was isolated from glioma cells using an RNeasy Mini kit (Qiagen, Hilden, NRW, Germany). Then, the RNA samples were quantified and reverse-transcribed into cDNAs using a MicroRNA Reverse Transcription kit (Takara, Dalian, China) or a Prime Script RT reagent kit (Takara). The expression of miR-410-3p and TGFBR2 was examined using a SYBR Premix Ex Taq™ kit (Takara) and calculated by the 2−ΔΔCt method. U6 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. The primers were:
miR-410-3p: (F) 5′-AGTTGTTCACCACCTTCTCCAC-3′ and
(R) 5′-TATCGTTGTACTCCAGTCCAAGTC-3′;
TGFBR2: (F) 5′-CTAACCTGCTGCCTGTGTGA-3′ and
(R) 5′-TCTGGAGCCATGTATCTTGC-3′;
U6: (F) 5′-CTCGCTTCGGCAGCACATA-3′ and
(R) 5′-AACGATTCACGAATTTGCGT-3′;
GAPDH: (F) 5′-CACCCACTCCTCCACCTTTG-3′ and
(R) 5′-CCACCACCCTGTTGCTGTAG-3′.
Western blot assay
After appropriate transfection or treatment, U251 and A172 cells were lysed in RIPA buffer (Beyotime, Shanghai, China) to isolate total protein. Concentrations of these protein samples were examined using a BCA assay kit (Beyotime). Then, the proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes (Pall, NY, USA). Next, membranes were blocked in 5% skim milk at room temperature for 1 h and maintained with primary antibodies anti-TGFBR2 (Abcam, Cambridge, MA, USA) and anti-β-actin (Abcam) overnight at 4°C and the indicated secondary antibody at room temperature for 2 h. Finally, protein signaling was visualized by an enhanced chemiluminescence system (Pierce, Rockford, IL, USA).
Dual-luciferase reporter assay
The 3′-UTR region TGFBR2, which contained the complementary sequences of wild-type or mutant miR-410-3p, was introduced into the pGL3 vector (Promega, Madison, WI, USA) to establish luciferase reporter vector TGFBR2 WT and TGFBR2 MUT, respectively. Then, TGFBR2 WT or TGFBR2 MUT in combination with miR-410-3p or miR-NC were transfected into glioma cells. A dual-luciferase reporter assay kit (Promega) was used to evaluate luciferase activity after 48 h of co-transfection.
Statistical analysis
The experiments were carried out 3 times and the values are shown as means±standard deviation (SD). The difference was analyzed via the t test or one-way analysis of variance (ANOVA). Data analysis in this study was conducted using GraphPad Prism 7 software (GraphPad, San Diego, CA, USA). A statistically significant difference was defined as P<0.05.
Results
Propofol suppressed cell proliferation, migration, and invasion in glioma
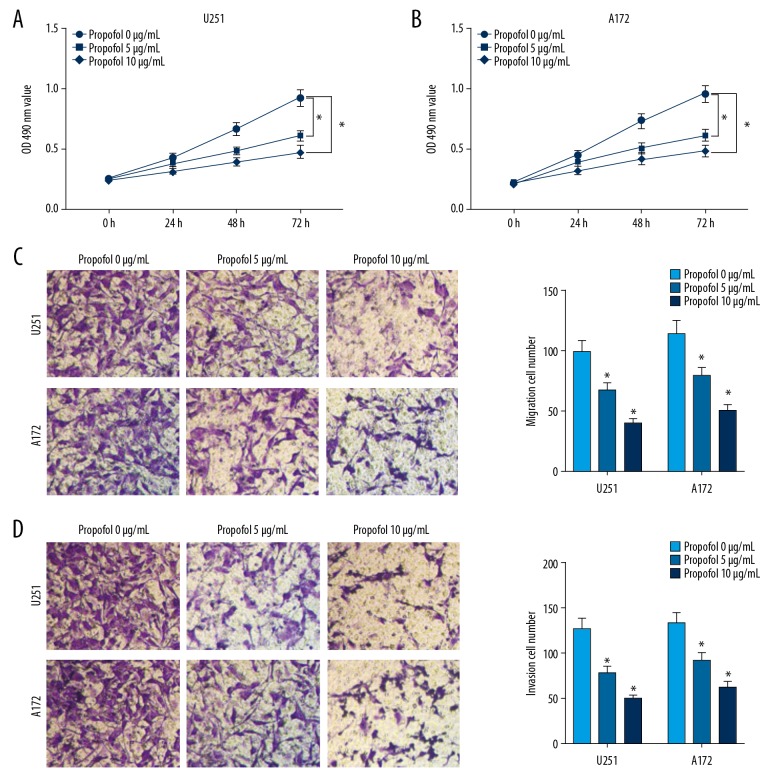
To investigate the functions of propofol in the progression of glioma cells, U251 and A172 cells were exposed to different doses of propofol for 24 h, and an equal volume of DMSO (0 μg/mL of propofol)-treated cells were used as control group. The result of MTT assay revealed that the proliferation of U251 and A172 cells was distinctly repressed by propofol in a concentration-dependent manner (Figure 1A, 1B). Transwell assay demonstrated that different concentrations of propofol treatment led to significant suppression in the migration and invasion of glioma cells compared to the control group (Figure 1C, 1D). These data suggested that propofol treatment suppressed glioma cell growth and metastasis in a concentration-dependent manner.
Figure 1.
Propofol inhibited cell development in glioma. Glioma cells were exposed to 5 μg/mL or 10 μg/mL of propofol or an equal volume of DMSO (0 μg/mL of propofol) for 24 h. (A, B) Proliferation of glioma cells was measured by MTT assay. (C, D) Migration and invasion of glioma cells were assessed through Transwell assay. * P<0.05.
Propofol led to an upregulation of miR-410-3p and a downregulation of TGFBR2 in glioma cells
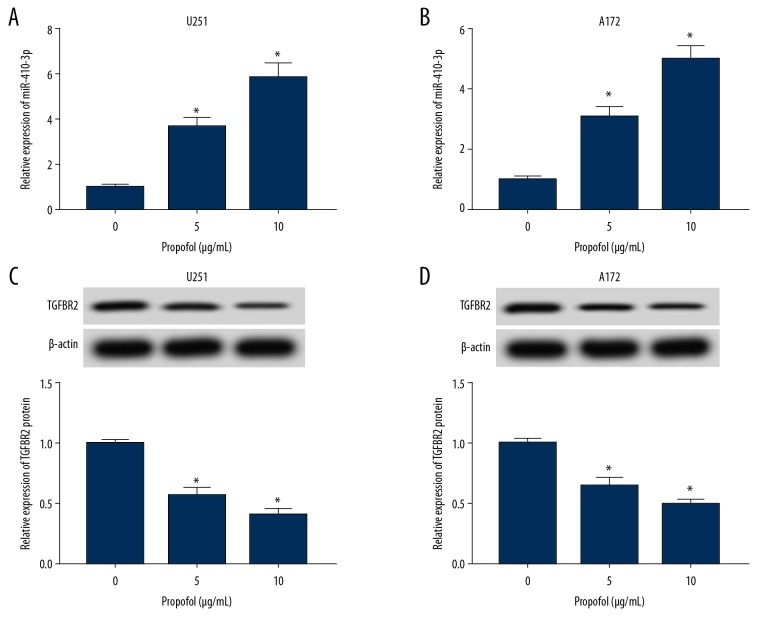
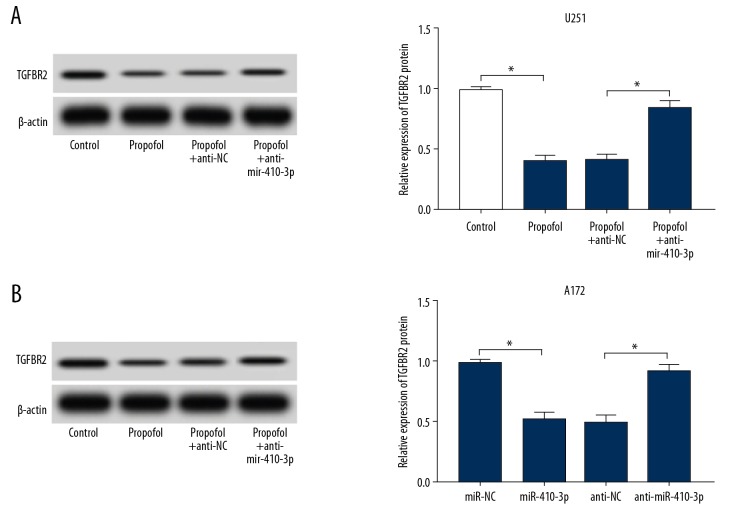
Glioma cells were exposed to propofol for 24 h. Then, the relative expression levels of miR-410-3p and TGFBR2 were analyzed by qRT-PCR and Western blot assay, respectively. We found that miR-410-3p was markedly increased in U251 and A172 cells after treatment with various concentrations of propofol compared to the untreated group, as determined by qRT-PCR assay (Figure 2A, 2B). Western blot analysis displayed that TGFBR2 protein was greatly inhibited by propofol in U251 and A172 cells in a concentration-dependent manner compared to the normal group (Figure 2C, 2D). These results show that propofol treatment promoted miR-410-3p expression and suppressed TGFBR2 expression in glioma cells.
Figure 2.
Propofol activated miR-410-3p expression and inhibited TGFBR2 expression in glioma cells. Glioma cells were exposed to 5 μg/mL or 10 μg/mL of propofol or an equal volume of DMSO (0 μg/mL of propofol) for 24 h. (A, B) The expression of miR-410-3p in glioma cells was assessed by qRT-PCR. (C, D) The expression of TGFBR2 was assessed using Western blot assay. * P<0.05.
MiR-410-3p inhibition restored the inhibition of proliferation, migration, and invasion caused by propofol in glioma cells
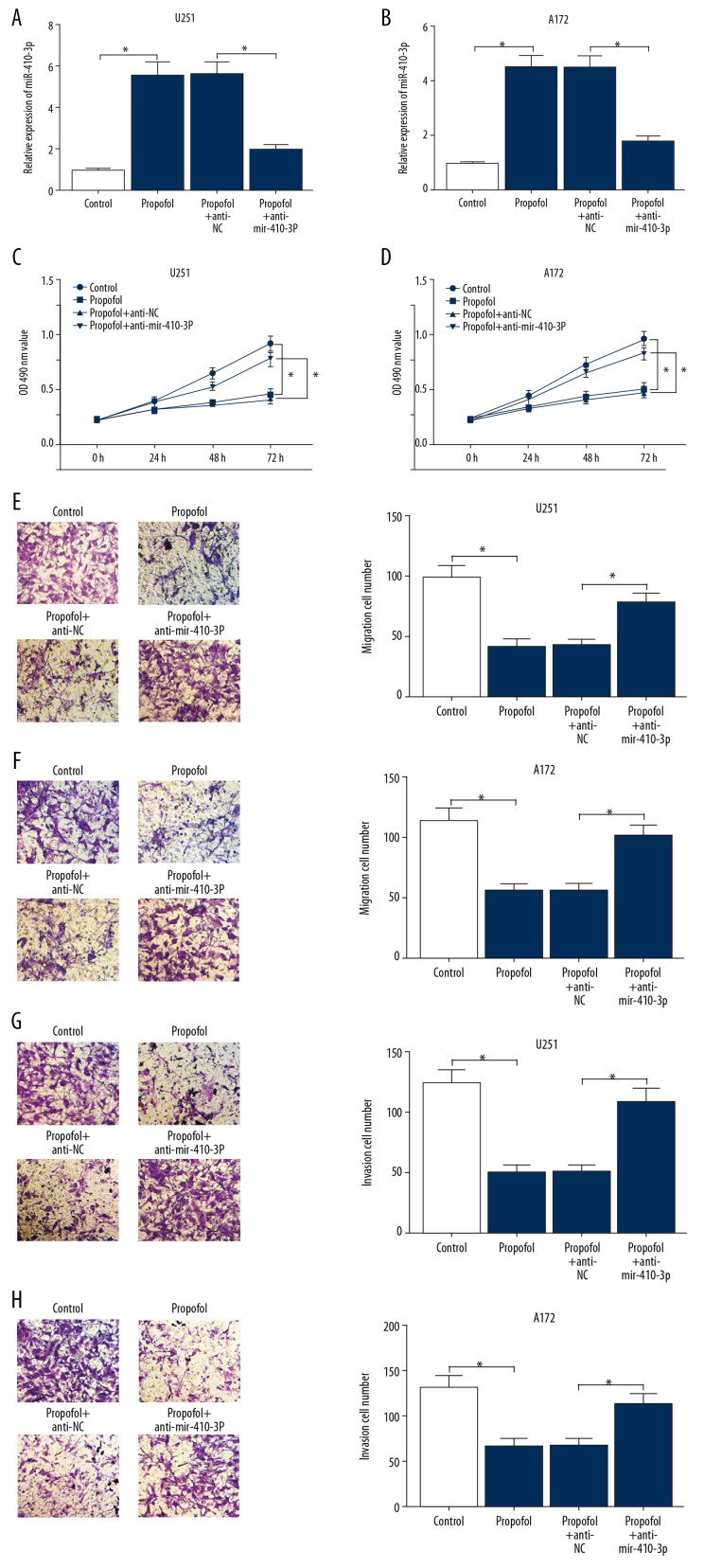
To reveal the potential role of miR-410-3p in the progression of glioma cells, anti-miR-410-3p or anti-NC was transfected into glioma cells, then the cells were exposed to propofol for 24 h. As displayed in Figure 3A and 3B, miR-410-3p expression stimulated by propofol was reduced by anti-miR-410-3p transfection in glioma cells. MTT assay showed that compared to the control group, anti-miR-410-3p restored the inhibition of propofol on cell proliferation in glioma cells (Figure 3C, 3D). The Transwell assay data indicated that miR-410-3p downregulation markedly abolished the inhibitory effects on cell migration and invasion caused by propofol treatment (Figure 3E–3H). All these data demonstrated that miR-410-3p overturned propofol-induced suppression in the progression of glioma cells.
Figure 3.
miR-410-3p restored the inhibition on cell development mediated by propofol in glioma cells. Glioma cells were exposed to DMSO (0 μg/mL of propofol), 10 μg/mL of propofol, or 10 μg/mL of propofol together with anti-miR-410-3p or anti-NC. (A, B) miR-410-3p expression in glioma cells was examined by qRT-PCR. (C, D) Cell proliferation of glioma cells was analyzed by MTT assay. (E–H) glioma cell migration and invasion abilities were evaluated via Transwell assay. * P<0.05.
miR-410-3p directly targeted TGFBR2
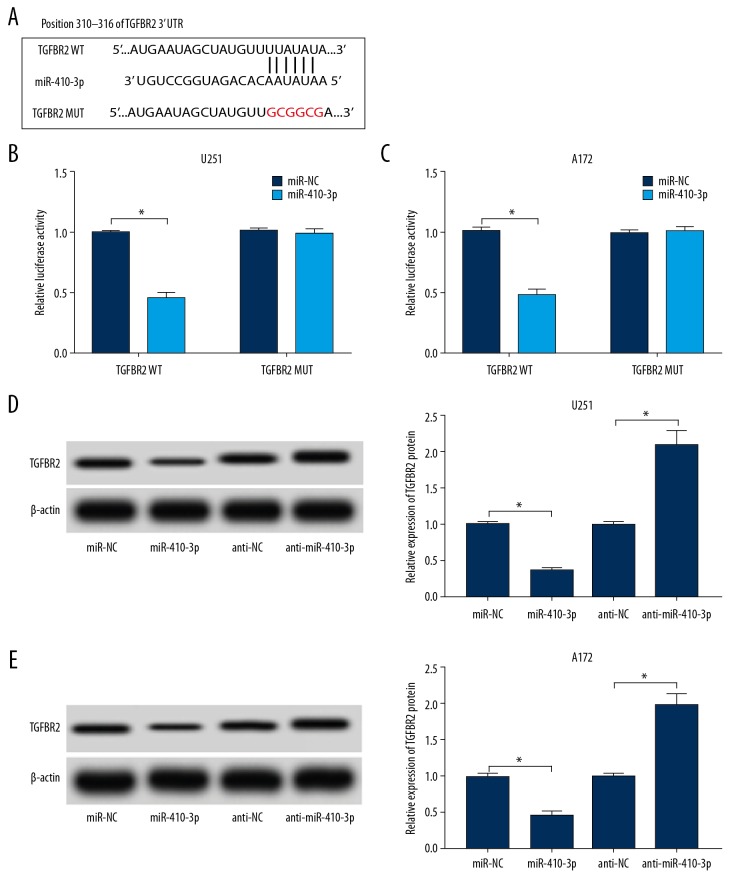
Given that miR-410-3p and TGFBR2 were aberrantly expressed in propofol treated U251 and A172 cells, we hypothesized that TGFBR2 might be a direct target gene of miR-410-3p. Then, online TargetScan software (http://www.targetscan.org/vert_72/) was utilized to predict the potential target of miR-410-3p. The data showed that TGFBR2 was predicted as a target of miR-410-3p, and their potential binding sites are showed in Figure 4A. To further confirm this prediction, dual-luciferase reporter assay was conducted, showing that, compared to the miR-NC and TGFBR2 WT co-transfected group, the luciferase activities in miR-410-3p and TGFBR2 WT co-transfected U251 and A172 cells were notably suppressed; however, there was no change in TGFBR2 MUT and miR-410-3p or miR-NC co-transfected cells (Figure 4B, 4C). Additionally, the protein level of TGFBR2 in glioma cells transfected with miR-NC, miR-410-3p, anti-NC, or anti-miR-410-3p was measured, showing that TGFBR2 protein was clearly decreased by the elevation of miR-410-3p and was drastically increased by the deficiency of miR-410-3p in both U251 and A172 cells (Figure 4D, 4E). These data suggest that miR-410-3p negatively modulated TGFBR2 expression via direct interaction.
Figure 4.
miR-410-3p directly interacted with TGFBR2 and negatively regulated its expression. (A) The complementary sequences between miR-410-3p and TGFBR2 are displayed. (B, C) TGFBR2 WT or TGFBR2 MUT and miR-410-3p or miR-NC were transfected into glioma cells, and luciferase activities were determined. (D, E) The level of TGFBR2 protein in glioma cells treated with miR-NC, miR-410-3p, anti-NC, or anti-miR-410-3p was examined by Western blot assay. * P<0.05.
Silencing of miR-410-3p reversed the downregulation of TGFBR2 caused by propofol in glioma cells
To reveal the association between miR-410-3p and TGFBR2 in propofol-activated U251 and A172 cells, anti-miR-410-3p or anti-NC was transfected into glioma cells, which were then treated with propofol for 24 h. As illustrated by Western blot assay, the downregulation of TGFBR2 caused by propofol was significantly abrogated by the inhibitors of miR-410-3p in U251 and A172 cells (Figure 5A, 5B). The data indicated that miR-410-3p downregulation promoted TGFBR2 expression in propofol-activated glioma cells.
Figure 5.
miR-410-3p knockdown restored the downregulation of TGFBR2 caused by propofol in glioma cells. Glioma cells were exposed to DMSO (0 μg/mL of propofol), 10 μg/mL of propofol, or 10 μg/mL of propofol together with anti-miR-410-3p or anti-NC. (A, B) The protein level of TGFBR2 in glioma cells was assessed by Western blot assay. * P<0.05.
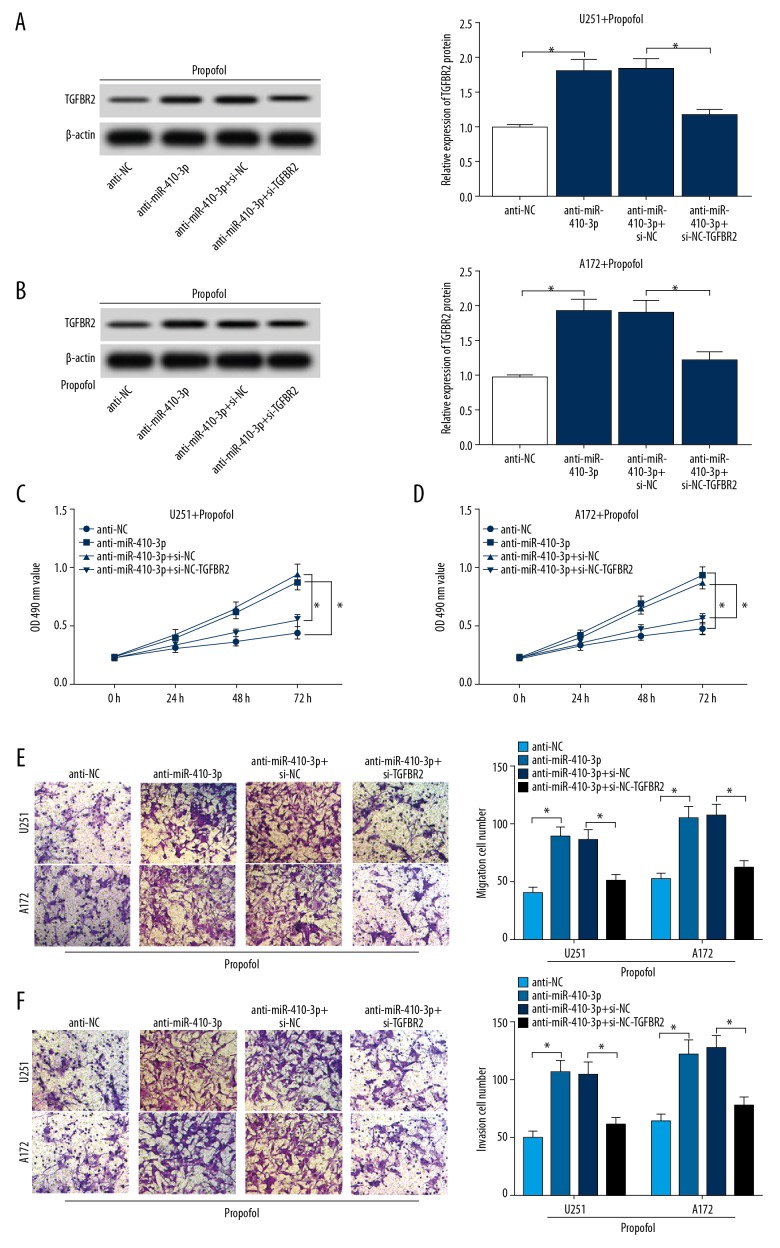
TGFBR2 knockdown abolished the effects of miR-410-3p inhibition on the progression of propofol-activated glioma cells
To further investigate the functional roles of miR-410-3p and TGFBR2 in glioma cell growth and metastasis, anti-NC, anti-miR-410-3p, si-NC, or si-TGFBR2 were transfected into glioma cells and cells were then treated with propofol. Transfection efficiency was examined using Western blot assay. We found that anti-miR-410-3p resulted in the upregulation of TGFBR2, while this effect was reversed by si-TGFBR2 in propofol-treated U251 cells and A172 cells (Figure 6A, 6B). MTT assay proved that miR-410-3p inhibition distinctly promoted cell proliferation, but this promotion was abolished after TGFBR2 knockdown in propofol-stimulated U251 cells and A172 cells (Figure 6C, 6D). The Transwell assay data indicated that cell migration and invasion were induced by anti-miR-410-3p, while these effects were reversed by si-TGFBR2 in propofol-activated U251 cells and A172 cells (Figure 6E, 6F). These results show that miR-410-3p regulated glioma cell development by interacting with TGFBR2.
Figure 6.
TGFBR2 downregulation weakened the promotion of miR-410-3p inhibition on cell progression in propofol-treated glioma cells. Glioma cells were transfected with anti-NC, anti-miR-410-3p, si-NC, or si-TGFBR2 and then exposed to 10 μg/mL of propofol for 24 h. (A, B) The expression of TGFBR2 in glioma cells was determined by Western blot assay. (C, D) Proliferation of glioma cells was evaluated by MTT assay. (E, F) Migration and invasion of glioma cells were evaluated by Transwell assay. * P<0.05.
Discussion
An increasing number of reports have revealed that propofol exerts tumor-suppressive effects in human cancers, such as hepatocellular carcinoma [19], pancreatic cancer [20], and gallbladder cancer [21]. Here, we investigated the functions of propofol in glioma. We used different concentrations of propofol to treat U251 cells and A172 cells, and found that propofol exposure drastically suppressed proliferation and metastasis of glioma cells in a dose-dependent manner. Wang et al. verified that viability and metastasis of glioma cell C6 were markedly depressed by propofol treatment in a concentration-dependent way [22]. A report by Xu et al. also showed that glioma cell growth and invasion were significantly repressed and apoptosis was conspicuously induced by propofol treatment [8]. Our findings in the present study are consistent with results in previous reports, adding evidence to support the tumor-suppressor effect of propofol in glioma.
In addition, we explored the molecular mechanism related to propofol in glioma. We found that miR-410-3p was distinctly increased in propofol-stimulated U251 and A172 cells. Many studies have shown that miRNAs can be induced by propofol in various cancers. For instance, miR-219-5p expression was increased after propofol exposure in hepatocellular carcinoma [19], and miR-486 was stimulated by propofol in lung cancer cells [23]. miR-410-3p was reported to promote or inhibit tumor progression in different cancers, such as prostate cancer [24], rhabdomyosarcoma [25], and breast cancer [26]. In the present study, we transfected anti-miR-410-3p into U251 and A172 cells to knock down miR-410-3p, and found the inhibitory effects on proliferation, migration, and invasion mediated by propofol were abolished after miR-410-3p was knocked down, suggesting that propofol-stimulated miR-410-3p suppresses glioma development. These data are in line with the findings of Chen et al. [14] and Wang et al. [27] in glioma.
TGFBR2 has been verified to take part in diverse biological processes, such as growth, differentiation, and inflammation [17]. In the present research, we revealed for the first time that miR-410-3p can target TGFBR2. Propofol treatment downregulated TGFBR2 expression, and TGFBR2 expression was negatively regulated by miR-410-3p in propofol-activated glioma cells. Further, downregulation of TGFBR2 by si-TGFBR2 restored cell growth and metastasis caused by miR-410-3p inhibition in propofol-stimulated U251 and A172 cells. TGFBR2 has been reported to have dual roles in tumor progression. For example, TGFBR2 suppressed the progression of lung squamous cell carcinoma [28] and colorectal cancer [29] and promoted the progression of colon cancer [30] and breast cancer [31]. Studies by Wei et al. [18] and Hu et al. [32] indicated that TGFBR2 knockdown repressed cell metastasis in glioma, and our results in this study agree with their findings.
Conclusions
Our results show that propofol suppressed cell growth and metastasis by upregulating miR-410-3p and downregulating TGFBR2 in glioma. This study advances our understanding of anesthesia and glioma development, indicating that propofol might be a better anesthetic for use in surgery for glioma patients. Nevertheless, there are still some shortcomings in this research. For example, we did not conduct animal experiments, and more clinical tests need to be performed in the future.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Weller M, Wick W, Aldape K, et al. Glioma. Nat Rev Dis Primers. 2015;1:15017. doi: 10.1038/nrdp.2015.17. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen BK, Hansen S, Laursen RJ, et al. Epidemiology of glioma: clinical characteristics, symptoms, and predictors of glioma patients grade I–IV in the Danish Neuro-Oncology Registry. J Neurooncol. 2017;135:571–79. doi: 10.1007/s11060-017-2607-5. [DOI] [PubMed] [Google Scholar]
- 3.Kao H-W, Chiang S-W, Chung H-W, et al. Advanced MR imaging of gliomas: An update. Biomed Res Int. 2013;2013 doi: 10.1155/2013/970586. 970586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, Van Den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Zhang D, Zhou X-h, Zhang J, et al. Propofol promotes cell apoptosis via inhibiting HOTAIR mediated mTOR pathway in cervical cancer. Biochem Biophys Res Commun. 2015;468:561–67. doi: 10.1016/j.bbrc.2015.10.129. [DOI] [PubMed] [Google Scholar]
- 6.Ye Z, Jingzhong L, Yangbo L, et al. Propofol inhibits proliferation and invasion of osteosarcoma cells by regulation of microRNA-143 expression. Oncol Res. 2014;21:201–7. doi: 10.3727/096504014X13890370410203. [DOI] [PubMed] [Google Scholar]
- 7.Xu W, Zheng J, Bie S, et al. Propofol inhibits Wnt signaling and exerts anticancer activity in glioma cells. Oncol Lett. 2018;16:402–8. doi: 10.3892/ol.2018.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J, Xu W, Zhu J. Propofol suppresses proliferation and invasion of glioma cells by upregulating microRNA-218 expression. Mol Med Rep. 2015;12:4815–20. doi: 10.3892/mmr.2015.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 10.Denli AM, Tops BB, Plasterk RH, et al. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432:231–35. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 11.Shukla GC, Singh J, Barik S. MicroRNAs: Processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. 2011;3:83–92. [PMC free article] [PubMed] [Google Scholar]
- 12.Jian Y, Xu C-H, Li Y-P, et al. Downregulated microRNA-30b-3p inhibits proliferation, invasion and migration of glioma cells via inactivation of the AKT signaling pathway by upregulating RECK. Biosci Rep. 2019;39 doi: 10.1042/BSR20182226. pii: BSR20182226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Zhou K, Zhang C, Yao H, et al. Knockdown of long non-coding RNA NEAT1 inhibits glioma cell migration and invasion via modulation of SOX2 targeted by miR-132. Mol Cancer. 2018;17:105. doi: 10.1186/s12943-018-0849-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Zhang J, Feng Y, et al. MiR-410 regulates MET to influence the proliferation and invasion of glioma. Int J Biochem Cell Biol. 2012;44:1711–17. doi: 10.1016/j.biocel.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 15.Sun W-L, Kang T, Wang Y-Y, et al. Long noncoding RNA OIP5-AS1 targets Wnt-7b to affect glioma progression via modulation of miR-410. Biosci Rep. 2019;39 doi: 10.1042/BSR20180395. pii :BSR20180395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derynck R, Akhurst RJ, Balmain A. TGF-β signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–29. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 17.Seoane J, Gomis RR. TGF-β family signaling in tumor suppression and cancer progression. Cold Spring Harb Perspect Biol. 2017;9:a022277. doi: 10.1101/cshperspect.a022277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei F, Wang Q, Su Q, et al. miR-373 inhibits glioma cell U251 migration and invasion by down-regulating CD44 and TGFBR2. Cell Mol Neurobiol. 2016;36:1389–97. doi: 10.1007/s10571-016-0338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong T, Ning X, Deng Z, et al. Propofol-induced miR-219-5p inhibits growth and invasion of hepatocellular carcinoma through suppression of GPC3-mediated Wnt/β-catenin signalling activation. J Cell Biochem. 2019 doi: 10.1002/jcb.28952. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Zhang J, Hong G, et al. Propofol inhibits growth and invasion of pancreatic cancer cells through regulation of the miR-21/Slug signaling pathway. Am J Transl Res. 2016;8:4120–33. [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Wang N, Zhou S, et al. Propofol induces proliferation and invasion of gallbladder cancer cells through activation of Nrf2. J Exp Clin Cancer Res. 2012;31:66. doi: 10.1186/1756-9966-31-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X-y, Li Y-l, Wang H-y, et al. Propofol inhibits invasion and proliferation of C6 glioma cells by regulating the Ca2+ permeable AMPA receptor-system xc-pathway. Toxicol in Vitro. 2017;44:57–65. doi: 10.1016/j.tiv.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Yang N, Liang Y, Yang P, et al. Propofol inhibits lung cancer cell viability and induces cell apoptosis by upregulating microRNA-486 expression. Braz J Med Biol Res. 2017;50:e5794. doi: 10.1590/1414-431X20165794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Zhang D, Lv J, et al. miR-410–3p promotes prostate cancer progression via regulating PTEN/AKT/mTOR signaling pathway. Biochem Biophys Res Commun. 2018;503:2459–65. doi: 10.1016/j.bbrc.2018.06.176. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Pang Y, Cui X, et al. MicroRNA-410-3p upregulation suppresses proliferation, invasion and migration, and promotes apoptosis in rhabdomyosarcoma cells. Oncol Lett. 2019;18:936–43. doi: 10.3892/ol.2019.10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y-F, Yu Y, Song W-Z, et al. miR-410-3p suppresses breast cancer progression by targeting Snail. Oncol Rep. 2016;36:480–86. doi: 10.3892/or.2016.4828. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z-H, Guo X-Q, Zhang Q-S, et al. Long non-coding RNA CCAT1 promotes glioma cell proliferation via inhibiting microRNA-410. Biochem Biophys Res Commun. 2016;480:715–20. doi: 10.1016/j.bbrc.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Tan X, Tang Y, et al. Dysregulated Tgfbr2/ERK-Smad4/SOX2 signaling promotes lung squamous cell carcinoma formation. Cancer Res. 2019;79(17):4466–79. doi: 10.1158/0008-5472.CAN-19-0161. [DOI] [PubMed] [Google Scholar]
- 29.He H, Zhao X, Zhu Z, et al. MicroRNA-3191 promotes migration and invasion by downregulating TGFBR2 in colorectal cancer. J Biochem Mol Toxicol. 2019 doi: 10.1002/jbt.22308. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Ullmann P, Rodriguez F, Schmitz M, et al. The miR-371~373 cluster represses colon cancer initiation and metastatic colonization by inhibiting the TGFBR2/ID1 signaling axis. Cancer Res. 2018;78:3793–808. doi: 10.1158/0008-5472.CAN-17-3003. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Liang S, Duan X. Molecular mechanism of miR-153 inhibiting migration, invasion and epithelial-mesenchymal transition of breast cancer by regulating transforming growth factor beta (TGF-β) signaling pathway. J Cell Biochem. 2019;120:9539–46. doi: 10.1002/jcb.28230. [DOI] [PubMed] [Google Scholar]
- 32.Hu S, Chen H, Zhang Y, et al. MicroRNA-520c inhibits glioma cell migration and invasion by the suppression of transforming growth factor-β receptor type 2. Oncol Rep. 2017;37:1691–97. doi: 10.3892/or.2017.5421. [DOI] [PubMed] [Google Scholar]