Abstract
Large chondral lesions of the humeral head are often treated with total shoulder arthroplasty, but this may not be an ideal option for young, active patients. Humeral head resurfacing is another option, which better preserves the native biomechanics. This article and the accompanying video present the surgical technique of partial humeral head resurfacing, which further preserves the remaining healthy cartilage. It is described for a chondral lesion due to avascular necrosis, but the method has been successfully used to treat chondral lesions from a broad range of causes.
Large humeral head chondral lesions can result from a number of causes: osteoarthritis, avascular necrosis (AVN), trauma, or idiopathic chondrolysis. Treatment of these injuries is particularly difficult in young, active patients. Traditionally, large chondral lesions have been addressed with total shoulder arthroplasty (TSA), which can be limiting to high-demand patients or which may fail over an extended period of high impact or even normal use. Resurfacing the humeral head is another option. This technique preserves native shoulder biomechanics, maintaining a more anatomic center of rotation with less eccentric loading of the glenoid.1 Resurfacing also preserves bone stock, which can be beneficial if a TSA is needed as a salvage operation. More recently, partial resurfacing, also known as “inlay arthroplasty,” has become a treatment option for younger patients with large chondral lesions. This method preserves not only bone but also any remaining healthy humeral head cartilage.
Although partial humeral head resurfacing has been described for the treatment of Hill-Sachs lesions, this technique has not been described for the treatment of AVN. This article details the steps and considerations necessary for successful partial humeral head resurfacing of a humeral head lesion using an Arthrosurface HemiCAP implant (Franklin, MA), as shown in Video 1.
Surgical Technique
Anesthesia and Positioning
An interscalene nerve block can be performed to decrease postoperative pain, followed by general anesthesia. The patient is then placed in the beach chair position. The operative shoulder is prepared and draped in standard sterile fashion.
Arthroscopic Evaluation
If there is any question of the diagnosis, a diagnostic arthroscopy can be performed. The rotator cuff, labrum, and glenoid surface should be examined, and pathology should be addressed as needed. In this case, synovitis and labral fraying are identified and debrided. Attention is then turned to the humeral head. The cartilage is probed to correlate magnetic resonance imaging and arthroscopic findings. Saline solution can be injected into the cartilage lesion, in this case through a needle placed via the Neviaser portal. The saline solution produces a bulging expansion, similar to that of a rotator cuff bubble sign, which can help determine the extent of the lesion.2
Exposure and Debridement
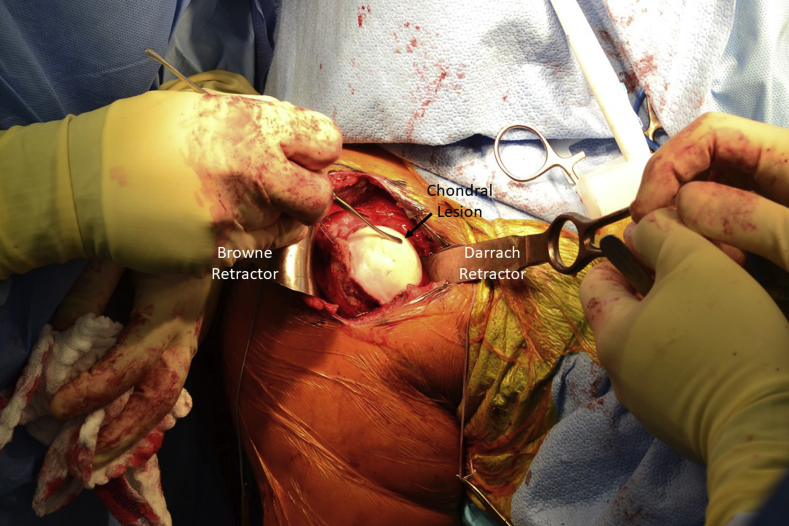
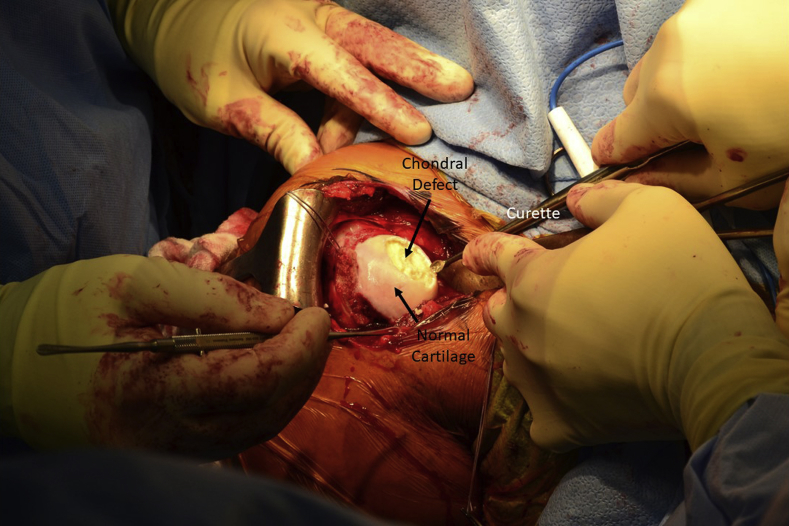
A standard deltopectoral approach is used to expose the glenohumeral joint. Dissection is carried down to the subscapularis, which can be managed per surgeon preference. In this case, a subscapularis tenotomy was performed and later repaired. A Browne deltoid retractor is placed laterally and a Darrach retractor medially to dislocate and expose the humeral head, allowing visualization of the chondral defect (Fig 1). Care should be taken with medial retractors so as to not damage the glenoid cartilage. Saline solution can again be injected into the cartilage lesion to demarcate its margins. The avascular cartilage is sharply excised, leaving distinct borders of normal cartilage (Fig 2).
Fig 1.
The right shoulder, prepared and draped in the standard fashion, is shown. A standard deltopectoral approach is used to dislocate and expose the humeral head. A Browne deltoid retractor is used laterally, and a Darrach retractor is used medially. The chondral lesion can be probed before and after injection of saline solution to define its boundaries, as performed here with a freer elevator.
Fig 2.
The avascular cartilage of the right humeral head is sharply excised using a scalpel and curettes, leaving distinct borders of normal cartilage.
Site Preparation and Implantation
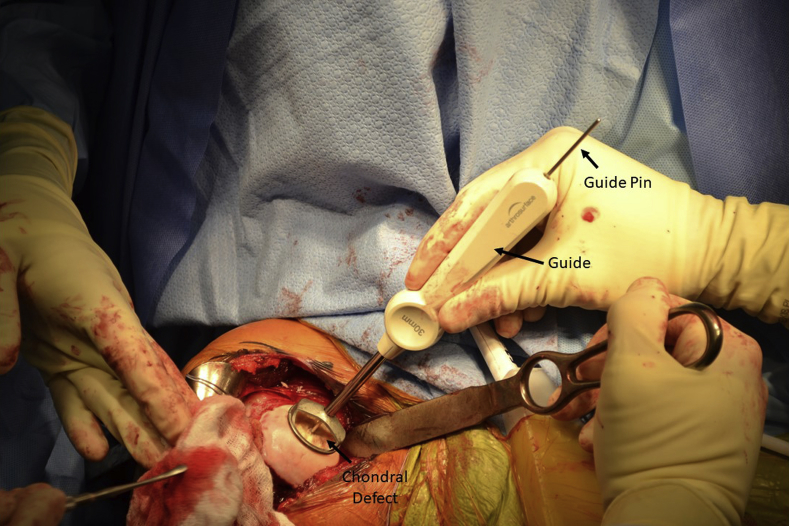
The defect is measured, and a drill guide of matching size is selected. The HemiCAP implant is available in 25-, 30-, 35-, and 40-mm diameters. In this case, a 30-mm implant was selected (Fig 3). A guide pin is inserted through the guide and then drilled into the center of the defect. It is important that the guide pin be perpendicular to the area to be resurfaced; this can be achieved by checking that there are 4 points of contact between the guide and the articular surface. This method ensures that the final implant is symmetrically flush with the surrounding cartilage. The step drill is then placed over the guide pin and drilled down until the proximal shoulder of the drill is flush with the articular surface. The taper post is inserted into the pilot hole that the step drill creates and is advanced until the line on the driver is flush with the articular cartilage.
Fig 3.
A drill guide is selected to match the size of the chondral defect in the right shoulder. The HemiCAP implant is available in 25-, 30-, 35-, and 40-mm diameters; a 30-mm implant was used in this case. A guide pin is then inserted through the guide and drilled into the center of the defect.
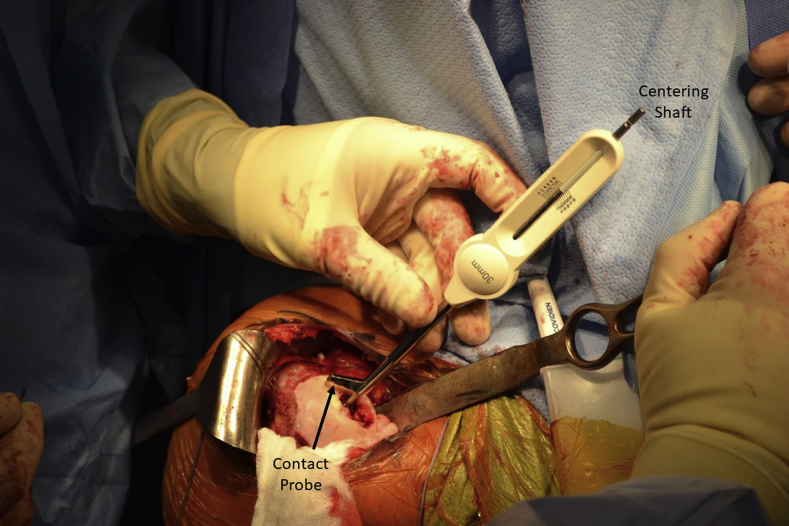
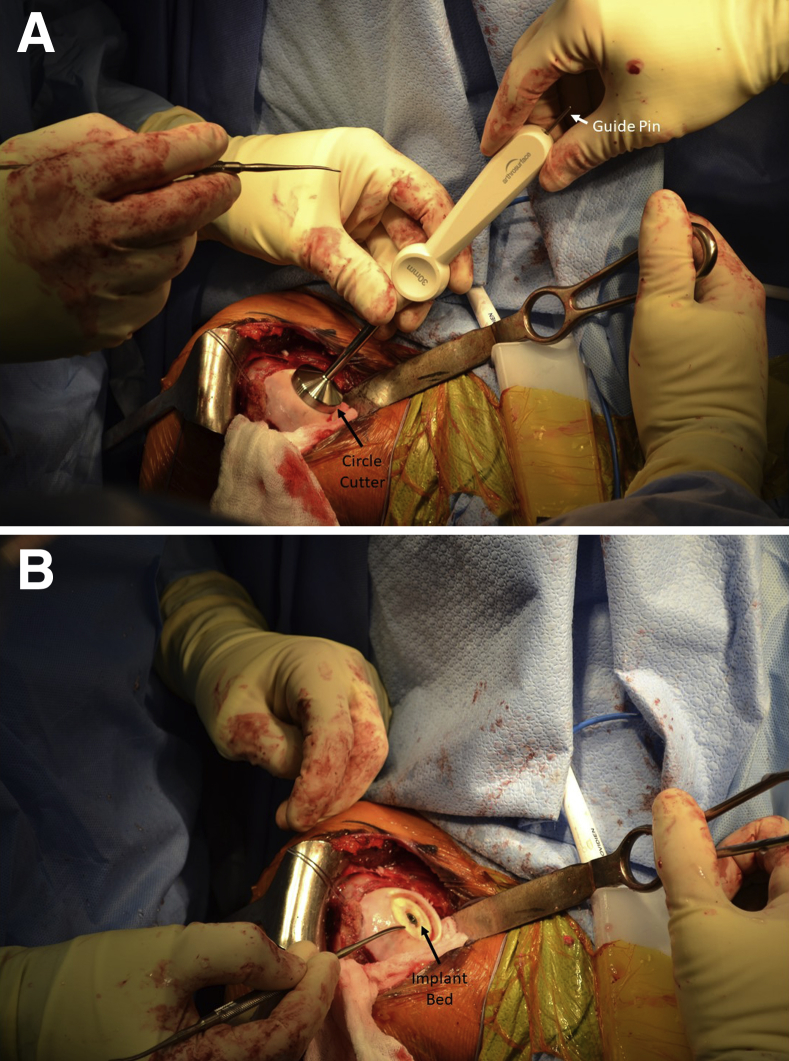
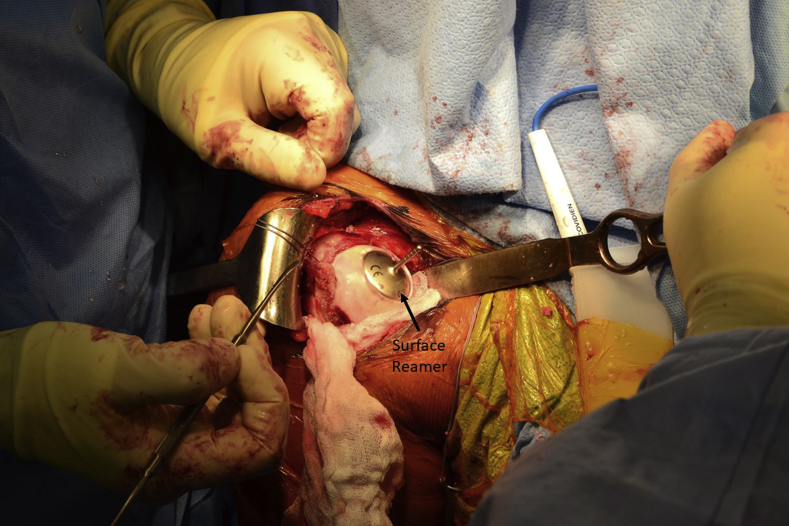
A centering shaft is placed into the taper post, and a contact probe is placed over the centering shaft. Offsets from this centering shaft are then confirmed at 4 index points: superior, lateral, inferior, and medial. A 30-mm implant was confirmed to be appropriate in this case (Fig 4). The centering shaft is replaced with the guide pin. A circle cutter of the appropriate size is advanced over the guide pin, and the articular cartilage is removed down to subchondral bone, by use of a twisting motion to avoid bending the guide pin (Fig 5). The same-sized surface reamer is then placed over the guide pin, and the humeral surface is reamed until the reamer contacts the top of the taper post (Fig 6). It is important to start the reamer prior to contacting bone to prevent chipping the articular rim.
Fig 4.
The contact probe should include the chondral defect and minimal excess healthy cartilage at 4 index points when centered on the centering shaft: superior, lateral, inferior, and medial. A 30-mm contact probe confirms that a 30-mm implant is the best fit for the chondral lesion in the right humeral head in this case.
Fig 5.
A circle cutter of the appropriate size, in this case 30 mm, is advanced over the guide pin, and the articular cartilage is removed down to subchondral bone, by use of a back-and-forth twisting motion to avoid bending the guide pin. The circle cutter is shown in place (A) and the implant bed is shown after its use (B) for a chondral defect in the right shoulder.
Fig 6.
The same-sized surface reamer—in this case 30 mm, used for a chondral defect in the right humeral head—is placed over the guide pin, and the humeral surface is reamed until it contacts the top of the taper post. It is important to start the reamer prior to contacting bone to prevent chipping the articular rim, and care should be taken not to bend the guide pin because this can result in implant malalignment.
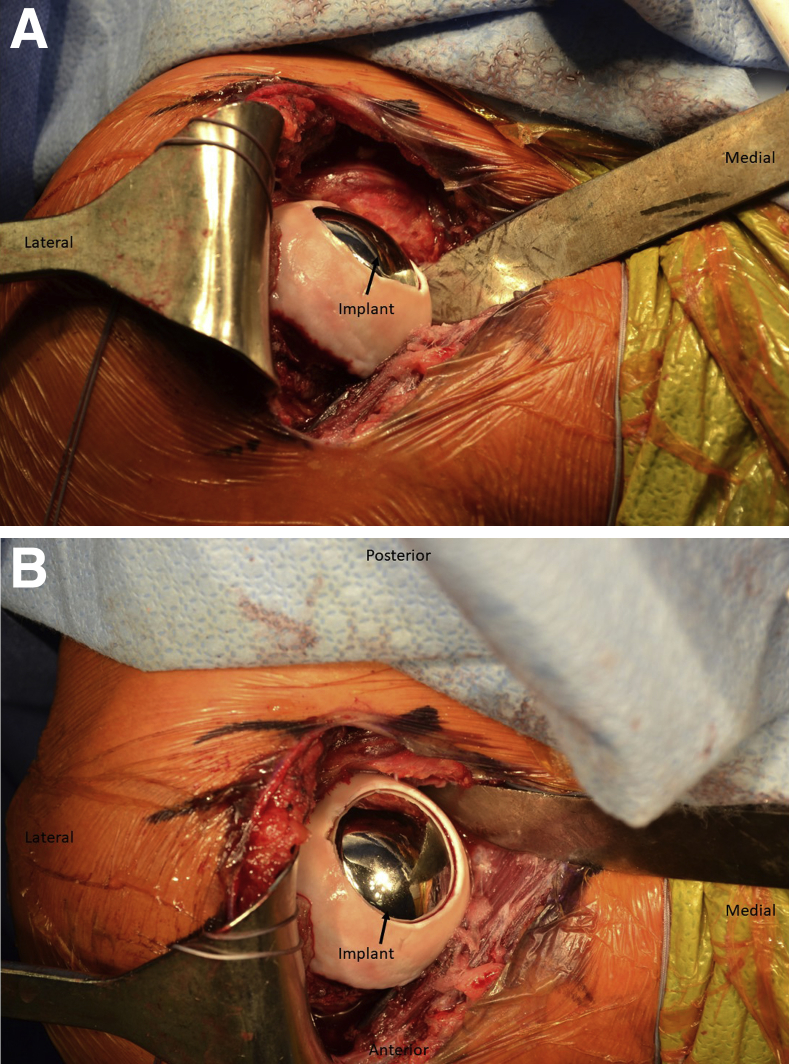
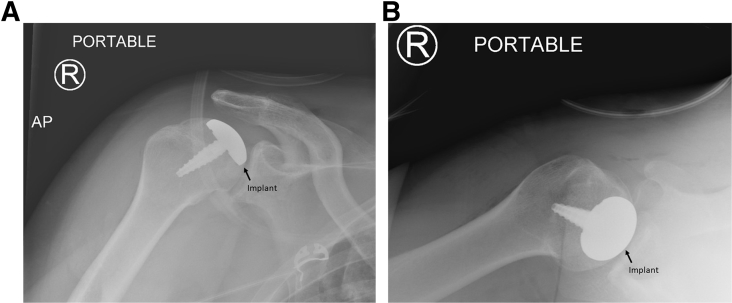
Once reaming is complete, a sizing trial is placed. The sizing trial should be congruent with the surrounding articular surface or slightly recessed. The final implant is set into the taper post and firmly seated (Fig 7). The subscapularis is then repaired per surgeon preference, and the wound is closed in layers. A sterile dressing and a sling are applied. Postoperative radiographs should be obtained, confirming that the implant is flush with or slightly recessed from the articular surface (Fig 8). Pearls and pitfalls of our technique are shown in Table 1, and advantages and disadvantages are presented in Table 2.
Fig 7.
(A, B) After the appropriate size is confirmed with the trial implant, the implant is set into the taper post and lightly tapped into place with a mallet. The implant must be congruent with or slightly recessed from the articular surface, as shown here in the right shoulder.
Fig 8.
Postoperative radiographs confirm that the implant is flush with the articular surface on the anteroposterior (AP) view (A) and axillary view (B) of the right (R) shoulder.
Table 1.
Pearls and Pitfalls
| Pearls | Pitfalls |
|---|---|
| Inject saline solution into the chondral lesion to create a bubble sign and clearly defineits margins. | The glenoid cartilage can be damaged if attention is not paid to careful placement of the medial retractor. |
| Use a contact probe to ensure that all areas of the chondral defect will be prepared and covered by the implant. | A guide pin that is not placed perpendicular to the lesion or one that is bent can lead to an implant that is not flush with the surrounding humeral head on all sides. |
| Start the reamer prior to contacting bone to prevent chipping the articular rim. | The proximal shoulder of the step drill must be flush with the articular surface, and the taper post must be inserted to the line on the driver; otherwise, the implant may sit proud to the articular cartilage. |
| Use the guide and subsequently the trial to confirm that the implant will be flush with the articular surface prior to its implantation. | The guide pin can bend while using the circle cutter. Twisting movements should be used to avoid this pitfall. |
Table 2.
Advantages and Disadvantages
| Advantages |
| Preservation of healthy native cartilage |
| Preservation of native biomechanics including neck-shaft angle, center of rotation, and humeral offset |
| Minimal bone resection |
| Decreased blood loss and operative time compared with stemmed implants |
| Decreased rate of periprosthetic fracture compared with stemmed implants |
| Decreased risk of implant wear and implant loosening compared with implants with glenoid components |
| Low revision rate |
| Easy conversion to another implant design as needed |
| Disadvantages |
| Does not treat concomitant pathology and may have worse outcomes when performed in setting of concomitant pathology |
Postoperative Rehabilitation
The patient is discharged home the day of surgery. The patient can begin passive range of motion (ROM) immediately and can progress to active ROM after 3 weeks. Strengthening exercises can begin at 6 weeks postoperatively.
Discussion
Partial humeral head resurfacing is a viable treatment option for young, active adults with large chondral lesions, including lesions due to AVN. One major advantage of this technique over hemiarthroplasty and TSA is that it preserves the patient's native biomechanics. This is important because changes in biomechanics affect the contact pressure and stress distribution across the glenohumeral joint. The resultant eccentric loading can lead to glenoid loosening in TSAs and can increase the rate of glenoid arthritis when the native glenoid is preserved. In partial resurfacing, the neck-shaft angle is not disturbed nor is the center of rotation changed. Indeed, Hammond et al.3 compared partial resurfacing with hemiarthroplasty and found that partial resurfacing restored the center of rotation significantly closer to normal, resulting in less eccentric loading of the glenoid.
The humeral offset is also maintained in partial hemiarthroplasty, as is the radius of curvature (ROC). Preserving this relation is important given that even a small change in the ROC can change shoulder ROM significantly. Giles et al.1 did not report on ROC but did find ROM after partial resurfacing to be the same as that of intact shoulders in a cadaveric model. Sweet et al.4 reported improved ROM after partial resurfacing, from an average of 100° to 129° of forward elevation and from an average of 23° to 43° of external rotation. In another study, Ranalletta et al.5 showed larger gains in forward elevation, with an average improvement from 101° to 150°, and similar gains in external rotation.
Clinical outcomes after partial resurfacing are also quite good. In the series of 20 partial resurfacings of Sweet et al.,4 statistically significant improvements were noted in American Shoulder and Elbow Surgeons, Simple Shoulder Test, and visual analog scale pain scores from the preoperative visit to final follow-up. Similar trends were seen in smaller studies by Anderl et al.6 and Ranalletta et al.5; the latter study showed that the average American Shoulder and Elbow Surgeons score improved from 31 to 76 (P < .001) and the Constant score improved from 35 to 79 (P < .001). Patient self-assessment ratings in one study also improved from 90% poor preoperatively to 5% poor and 75% good to excellent postoperatively,4 and another study reported good to excellent results in 95% of cases after partial resurfacing.7
Partial resurfacings have an additional theoretical advantage over traditional TSA in that they are stemless. They likely benefit from decreases in blood loss and operative time similar to those that occur with stemless TSAs over TSAs with stems.8 Similarly, partial resurfacings likely have a decreased incidence of humeral shaft and periprosthetic fractures, as seen with stemless total resurfacing.9 This advantage presumably comes from the absence of major stress risers and the minimal amount of bone resected.
The minimal bone resection also allows easy conversion from partial resurfacing to total resurfacing, hemiarthroplasty, or TSA, should the need arise. However, the revision rates of partial resurfacing are quite low. The Australian National Joint Replacement Registry reported a revision rate of 0.6 per 100 observed implant-years, less than half that of primary stemmed hemi-resurfacing, hemiarthroplasty, and TSA.10 It should be noted, though, that concomitant pathology and prior or concomitant surgery have been associated with worse clinical outcomes and higher revision rates.11 Concomitant pathology may worsen postoperatively, or new pathology may develop over time; for example, glenoid wear developed or worsened in a subset of patients in a number of studies, sometimes necessitating conversion to TSA.5,11
Partial resurfacing is not without its perils. The guide pin must be placed perpendicular to the lesion so that the edges of the implant are confluent with the surrounding articular cartilage. Any resultant offset or gap may alter contact forces within the glenohumeral joint or allow the glenoid to catch the edge of the implant and theoretically lever it. Care also must be taken not to bend the guide pin during reaming because similar malalignment can result. Both pitfalls can be avoided with careful attention to technique.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: C.M.J. is a paid consultant and paid presenter or speaker for and receives research support from Acumed; is a paid presenter or speaker for Biomet; is a paid consultant for the Consortium of Focused Orthopedists, DePuy (A Johnson & Johnson Company), Integral Life Sciences, and Integrated Shoulder Collaboration; is a paid consultant and paid presenter or speaker for Wright Medical Technology and Zimmer; is a board or committee member of the American Board of Orthopaedic Surgery and American Shoulder and Elbow Surgeons; and is on the editorial or governing board of the Journal of the American Academy of Orthopaedic Surgeons. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
This video demonstrates partial humeral head resurfacing in the case of a 33-year-old athletic trainer with right humeral head avascular necrosis associated with corticosteroid use. The patient is placed in the beach chair position, and the right shoulder is prepared and draped in the standard fashion. Shoulder arthroscopy can first be used to confirm the location and size of the cartilage lesion, by injecting saline solution into the lesion and observing the devitalized cartilage lift off the subchondral bone. This is seen from the posterior portal as the lesion is probed through the anterior portal. Any other intra-articular pathology can be addressed arthroscopically, before exposure of the glenohumeral joint through a standard deltopectoral approach. The lesion is then demarcated and excised. A drill guide, matching the size of the lesion, is used to place a guide pin. The guide pin is then used with a step drill, creating the pilot hole for the tapered post. Once the post is in place, a centering shaft is used to orient a circle cutter and then a reamer to prepare the implant bed. A trial is used to ensure that all edges of the implant will be confluent with the surrounding articular cartilage; then, the implant is placed. It is imperative that all instruments be used perpendicular to the implant bed so that the implant re-creates a smooth articular surface.
References
- 1.Giles J.W., Elkinson I., Ferreira L.M. Moderate to large engaging Hill-Sachs defects: An in vitro biomechanical comparison of the remplissage procedure, allograft humeral head reconstruction, and partial resurfacing arthroplasty. J Shoulder Elbow Surg. 2012;21:1142–1151. doi: 10.1016/j.jse.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Lo I.K.Y., Gonzalez D.M., Burkhart S.S. The bubble sign: An arthroscopic indicator of an intratendinous rotator cuff tear. Arthroscopy. 2002;18:1029–1033. doi: 10.1053/jars.2002.36486. [DOI] [PubMed] [Google Scholar]
- 3.Hammond G., Tibone J.E., McGarry M.H., Jun B.-J., Lee T.Q. Biomechanical comparison of anatomic humeral head resurfacing and hemiarthroplasty in functional glenohumeral positions. J Bone Joint Surg Am. 2012;94:68–76. doi: 10.2106/JBJS.I.00171. [DOI] [PubMed] [Google Scholar]
- 4.Sweet S.J., Takara T., Ho L., Tibone J.E. Primary partial humeral head resurfacing: Outcomes with the HemiCAP implant. Am J Sports Med. 2015;43:579–587. doi: 10.1177/0363546514562547. [DOI] [PubMed] [Google Scholar]
- 5.Ranalletta M., Bertona A., Tanoira I., Rossi L.A., Bongiovanni S., Maignón G.D. Results of partial resurfacing of humeral head in patients with avascular necrosis. Rev Esp Cir Ortop Traumatol. 2019;63:29–34. doi: 10.1016/j.recot.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Anderl W., Kriegleder B., Neumaier M., Laky B., Heuberer P. Arthroscopic partial shoulder resurfacing. Knee Surg Sports Traumatol Arthrosc. 2015;23:1563–1570. doi: 10.1007/s00167-014-2981-x. [DOI] [PubMed] [Google Scholar]
- 7.Scalise J.J., Miniaci A., Iannotti J.P. Resurfacing arthroplasty of the humerus: Indications, surgical technique, and clinical results. Tech Shoulder Elbow Surg. 2007;8:152–160. [Google Scholar]
- 8.Berth A., Pap G. Stemless shoulder prosthesis versus conventional anatomic shoulder prosthesis in patients with osteoarthritis: A comparison of the functional outcome after a minimum of two years follow-up. J Orthop Traumatol. 2013;14:31–37. doi: 10.1007/s10195-012-0216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy O., Copeland S.A. Cementless surface replacement arthroplasty of the shoulder. 5- to 10-year results with the Copeland mark-2 prosthesis. J Bone Joint Surg Br. 2001;83:213–221. doi: 10.1302/0301-620x.83b2.11238. [DOI] [PubMed] [Google Scholar]
- 10.Australian Orthopaedic Association National Joint Replacement Registry . Australian Orthopaedic Association; Adelaide: 2013. Demographics and outcomes of shoulder arthroplasty: Supplementary report. [Google Scholar]
- 11.Delaney R.A., Freehill M.T., Higgins L.D., Warner J.J.P. Durability of partial humeral head resurfacing. J Shoulder Elbow Surg. 2014;23:e14–e22. doi: 10.1016/j.jse.2013.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video demonstrates partial humeral head resurfacing in the case of a 33-year-old athletic trainer with right humeral head avascular necrosis associated with corticosteroid use. The patient is placed in the beach chair position, and the right shoulder is prepared and draped in the standard fashion. Shoulder arthroscopy can first be used to confirm the location and size of the cartilage lesion, by injecting saline solution into the lesion and observing the devitalized cartilage lift off the subchondral bone. This is seen from the posterior portal as the lesion is probed through the anterior portal. Any other intra-articular pathology can be addressed arthroscopically, before exposure of the glenohumeral joint through a standard deltopectoral approach. The lesion is then demarcated and excised. A drill guide, matching the size of the lesion, is used to place a guide pin. The guide pin is then used with a step drill, creating the pilot hole for the tapered post. Once the post is in place, a centering shaft is used to orient a circle cutter and then a reamer to prepare the implant bed. A trial is used to ensure that all edges of the implant will be confluent with the surrounding articular cartilage; then, the implant is placed. It is imperative that all instruments be used perpendicular to the implant bed so that the implant re-creates a smooth articular surface.