 A new catalytic protocol for the expedient synthesis of oxazolines from oxetanes is developed.
A new catalytic protocol for the expedient synthesis of oxazolines from oxetanes is developed.
Abstract
A new catalytic protocol for the expedient synthesis of oxazolines from oxetanes is disclosed. This mild process complements the conventional oxazoline synthesis based on non-catalytic cyclization of β-hydroxy or unsaturated amides. It is also a new addition to the reactivity profile of oxetanes leading to heterocycles. In the presence of In(OTf)3, various 3-amido oxetanes underwent smooth intramolecular cyclization to form the corresponding 2-oxazolines, including some valuable oxazoline-based bidentate ligands. This protocol also provides rapid access to various natural products and antibacterial molecules.
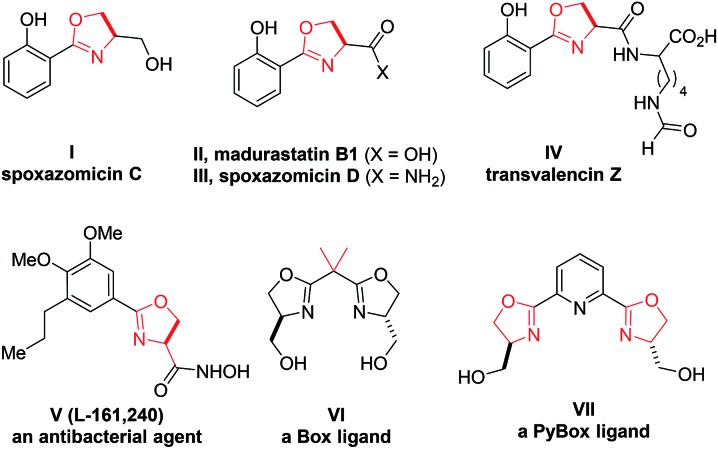
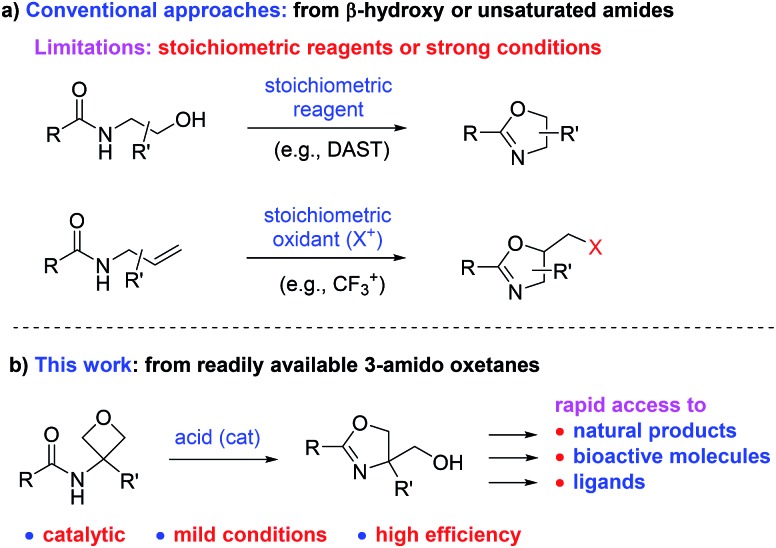
2-Oxazoline is a privileged heterocycle with broad applications.1–3 It is not only an important subunit and pharmacophore of numerous natural products and bioactive molecules (e.g., compounds I–V, Fig. 1),1 but also a versatile functional group in organic synthesis. For example, oxazoline-based ligands, such as Box and PyBox (e.g., VI–VII), have been used as ligands in a wide range of metal-catalyzed reactions.2 Moreover, oxazoline has also been utilized in various other capacities, including protective group, directing group, and synthetic auxiliary.3 Owing to these important applications, the development of efficient approaches for oxazoline synthesis has been a constant pursuit in organic synthesis.4–6 Among the various known methods, the conventional approaches based on cyclization of β-hydroxy or unsaturated amides still remain as the most straightforward and frequently adopted methods (Scheme 1a).4,5 While these approaches have been taken for granted for decades, it is worth noting that they typically suffer from the use of either strong conditions (e.g., heating) or stoichiometric amounts of corrosive reagents (such as DAST and oxidants), which inevitably lead to extra operational cost or stoichiometric waste generation. In this context, the development of new mild catalytic approaches remains in high demand.
Fig. 1. Selected useful molecules containing a 2-oxazoline motif.
Scheme 1. Synthesis of 2-oxazolines: conventional approaches and our design.
Oxetanes have played an important role in medicinal chemistry and organic synthesis.7,8 As a family of readily available synthetic building blocks, they are known precursors to various heterocycles, even in catalytic asymmetric fashion.7,8 However, compared with their three-membered ring homologues, oxiranes (epoxides), their synthetic capability has not been fully explored. As our continued interest in oxetane chemistry,8e–i herein we describe a new mild catalytic protocol for the efficient synthesis of oxazolines from oxetanes enabling rapid access to a diverse family of natural products.
We envisioned that, upon suitable activation by acid, oxetanes bearing a 3-amido group would be expected to undergo intramolecular oxetane ring-opening and concomitantly cyclize to form an oxazoline ring (Scheme 1b). If successful, the oxazolines generated in this process are naturally decorated with a hydroxymethyl group at the 4-position. The presence of this substituent perfectly matches those important molecules and ligands (shown in Fig. 1 and Scheme 2, vide infra), thereby providing expedient access to them (either directly or within a couple of steps). Moreover, such a process can be catalytic in principle, thus complementing the conventional approaches.
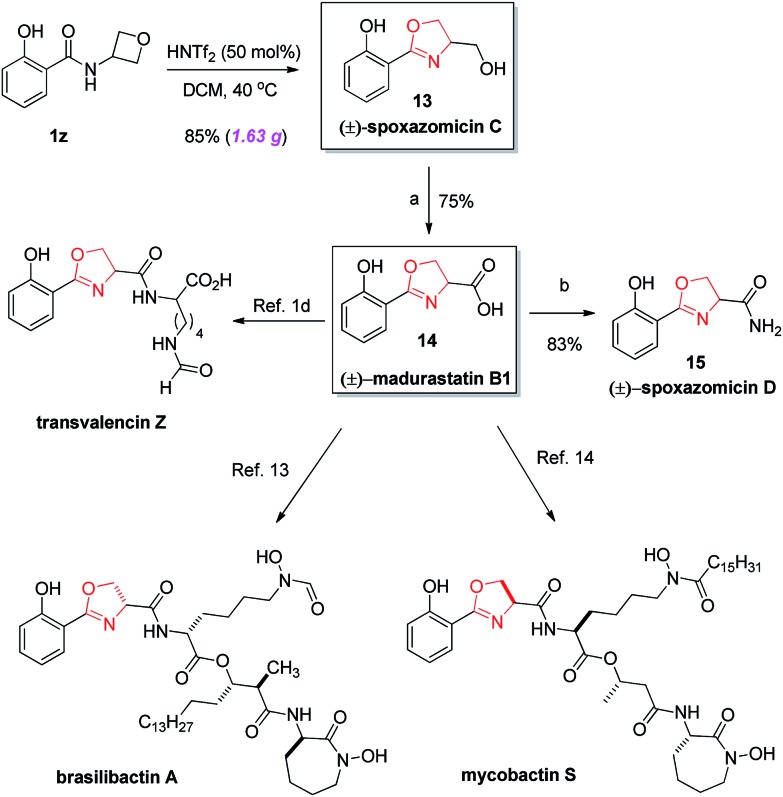
Scheme 2. Rapid (formal) synthesis of diverse natural products. (a) CrO3, H5IO6, CH3CN, rt, 30 min, 75%; (b) NH4HCO3, (Boc)2O, pyridine, CH3CN, rt, 22 h, 83%.
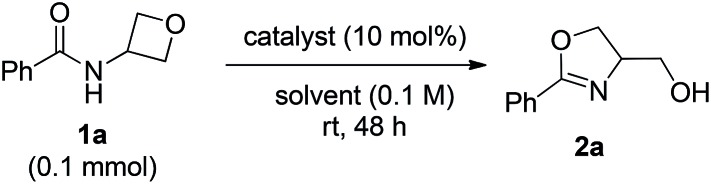
We started our study with model substrate 1a, which was obtained by acylation of commercially available 3-amino oxetane. To activate the oxetane motif, we evaluated various oxophilic Lewis acids (Table 1, entries 1–6). Although most of them showed catalytic activity toward the desired product 2a, Sc(OTf)3 and In(OTf)3 exhibited much higher catalytic efficiency, with the latter being slightly better (entries 1–2). Thus, in the presence of 10 mol% of In(OTf)3, the reaction of 1a in toluene proceeded smoothly at room temperature to form oxazoline 2a in 75% yield (entry 2). In contrast, Brønsted acids, such as TsOH and HNTf2, showed extremely low catalytic turnover (entries 7–8). Next, different solvents were compared with In(OTf)3 as the optimal catalyst (entries 9–12). While polar and protic solvents, such as MeCN and MeOH, led to low reactivity, DCM could improve the reaction efficiency (86% yield, entry 12). Finally, at 40 °C, the reaction time could be shortened to 24 h and the yield could be further improved to 93% (entry 13).
Table 1. Evaluation of reaction conditions.
| |||
| Entry | Catalyst | Solvent | Yield a (%) |
| 1 | Sc(OTf)3 | Toluene | 70 |
| 2 | In(OTf)3 | Toluene | 75 |
| 3 | Zn(OTf)3 | Toluene | 46 |
| 4 | AlCl3 | Toluene | 20 |
| 5 | TMSOTf | Toluene | 20 |
| 6 | BF3·OEt2 | Toluene | 51 |
| 7 | TsOH·H2O | Toluene | 15 |
| 8 | HNTf2 | Toluene | 16 |
| 9 | In(OTf)3 | MeCN | 45 |
| 10 | In(OTf)3 | MeOH | 60 |
| 11 | In(OTf)3 | THF | 69 |
| 12 | In(OTf)3 | CH2Cl2 | 86 |
| 13 b , c | In(OTf)3 | CH2Cl2 | 93 |
aYield was determined by 1H NMR analysis of the crude mixture with CH2Br2 as an internal standard.
bRun at 40 °C.
cRun for 24 h.
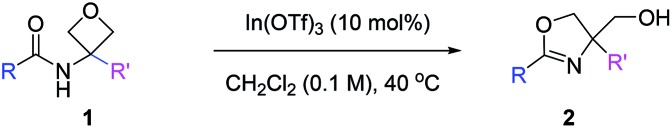
With the above optimal conditions, we next examined the generality of this protocol. As shown in Table 2, a wide array of 3-amido oxetanes with different electronic properties participated in this mild intramolecular cyclization process to form the corresponding 2-oxazolines with high efficiency. Both aromatic and aliphatic amides were suitable substrates. Moreover, α,β-unsaturated amides and carbamates could also react to form the desired oxazolines with vinylic and alkoxy substituents at the 2-position, respectively (e.g., 2o–p). This cyclization was not sensitive to steric hindrance on the amide functionality (2n). Heterocycles could also be incorporated into the products. Furthermore, given the versatility of oxazolines as ligands, we also evaluated the possibility for the synthesis of bidentate ligands. For example, with free and protected amino groups and phosphine oxide in close proximity, the corresponding oxazolines (2q–s) could still be formed with good efficiency, although the latter case required slightly higher temperature. The structure of 2s was confirmed by X-ray crystallography. In contrast, it is worth mentioning that In(OTf)3 failed to catalyze the reaction of pyridine-incorporated substrate 1t, presumably due to competing binding of the pyridine motif to In(OTf)3, thus leading to catalyst deactivation. Nevertheless, after further optimization with (super)stoichiometric amounts of acids, such as HNTf2 and HCl, the desired oxazoline products (2t and 2t′) could be successfully obtained as pyridinium salts. Finally, 3,3-disubstituted oxetanes could also react to provide 4,4-disubstituted oxazolines (2u–2y). Overall, this mild protocol could tolerate a wide range of functional groups, including ether, nitro, nitrile, aryl halide, amine, ester, alkene, and alkyne groups. The robustness of this protocol was also demonstrated by a highly efficient gram-scale reaction of 2a. It is notable that, compared to the antibacterial natural product spoxazomicin C (shown in Fig. 1), these oxazoline products should also have antibacterial activity and could be directly used for “structure–activity relationship” (SAR) studies, further highlighting the synthetic efficiency of this reaction.
Table 2. Reaction scope a .
|
|
aReaction scale: oxetane 1 (0.50 mmol), In(OTf)3 (10 mol%), CH2Cl2 (5.0 mL); isolated yield.
bRun with 5 mol% of In(OTf)3.
cRun in DCE solvent at 60 °C.
dRun with 20 mol% of In(OTf)3.
eRun with HNTf2 (1 equiv.) in place of In(OTf)3.
fRun with HCl (2.5 equiv., 4 M solution in MeOH) in place of In(OTf)3.
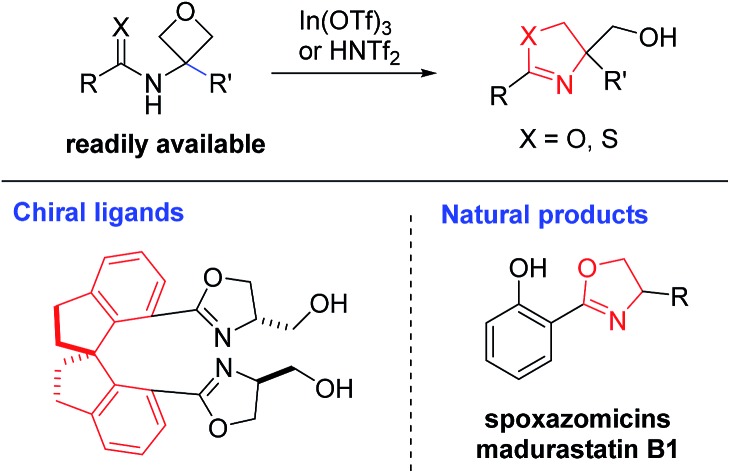
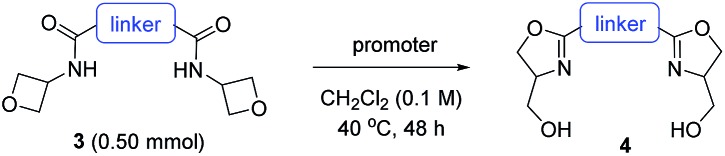
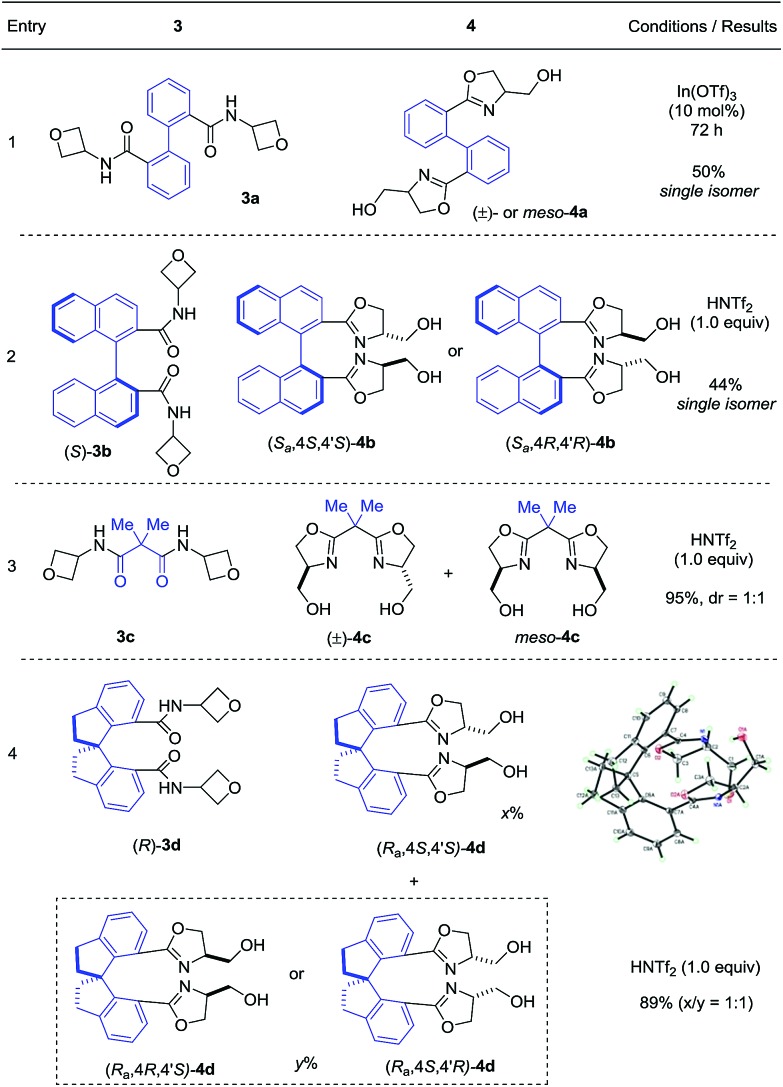
Next, we evaluated the ability of this protocol in the synthesis of bis(oxazoline) compounds, in view of their proven superior synthetic utility as bidentate ligands. As shown in Table 3, various bis(oxazoline) products 4 with different linkers could be synthesized via double cyclization of the bis(amide) substrates 3. Among them, 4b and 4d were obtained in their enantioenriched forms as the bis(amide) substrates were grafted on enantiopure chiral backbones. It is worth noting that 4a and 4b were obtained as a single isomer.9 While we do not have a clear explanation at this point, it is presumably because the spatial orientation of the initially formed oxazoline unit has certain influence on the subsequent ring formation. Since products 4b–d are known superior bidentate ligands that can bind metals, In(OTf)3 proved less effective than HNTf2.10 It is worth noting that, depending on the optical purity of the linker, these products could be formed in enantiopure form and directly used as chiral ligands (vide infra).
Table 3. Synthesis of bis(oxazoline) compounds.
|
|
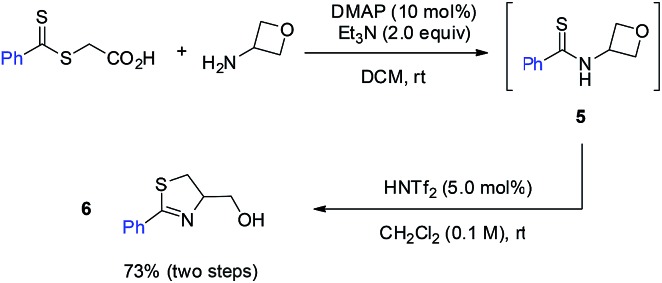
By changing the amide unit to thioamide, we were able to further extend this approach to the synthesis of 2-thiazoline ring, another important heterocycle in natural products, medicinal chemistry, and organic synthesis.11 While thioamide 5 was found to be unstable, we managed to generate it in situ from 2-((phenylcarbonothioyl)thio)acetic acid and 3-aminooxetane. Subsequent treatment with HNTf2 without purification of thioamide 5 afforded the desired 2-thiazoline 6 in 73% yield over two steps (eqn (1)).
 |
1 |
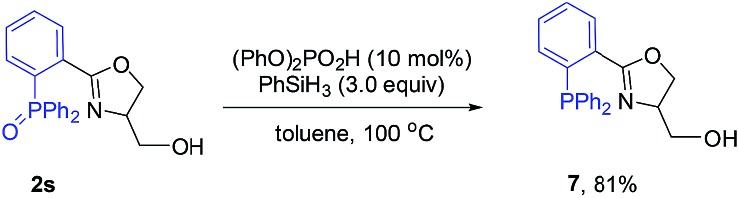
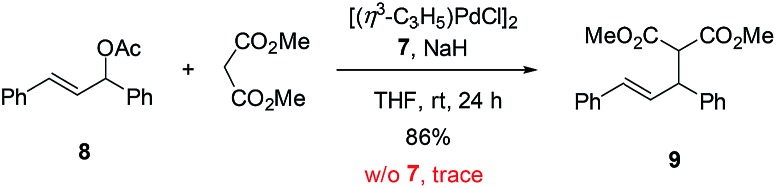
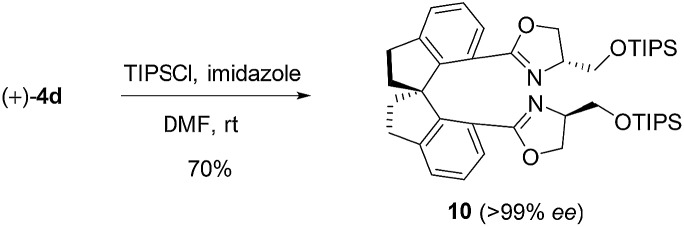
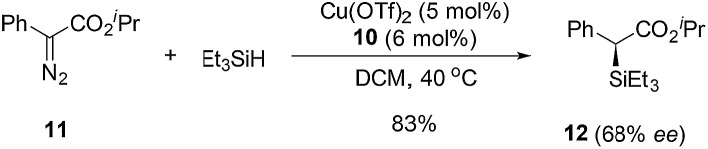
To further demonstrate the utility of this reaction, the oxazoline products were employed as ligands for synthesis. For example, a P,N-ligand 7 could be obtained after simple reduction of the phosphine oxide 2s (eqn (2)). With this ligand, palladium-catalyzed allylic substitution of 8 by malonate proceeded efficiently to form product 9 in 86% yield (eqn (3)).12 In contrast, without ligand, trace product was formed. In another example, the enantiopure spirocyclic bis(oxazoline) product 4d was protected and then used as a chiral ligand for asymmetric carbene insertion to the Si–H bond of triethylsilane (eqn (4) and (5)). Without condition optimization, the α-silyl ester 12 was formed in 83% yield and 68% ee.13 Further optimization to tune other reaction parameters might be able to improve the outcome.
 |
2 |
 |
3 |
 |
4 |
 |
5 |
Finally, the power of this protocol was further proved by rapid access to various natural products (Scheme 2). For example, natural product (±)-spoxazomicin C (13)1a could be directly obtained via the standard cyclization of amide 1z. Next, a simple oxidation step delivered the corresponding acid 14, another natural product (±)-madurastatin B1.1b Further transformation to amide in the presence of NH4HCO3 gave another natural product (±)-spoxazomicin D in 83% yield.1b Moreover, acid 14 is a known precursor to other natural products, including transvalencin Z,1c–d brasilibactin A,14 and mycobactin S,15 and a potential intermediate toward oxachelin C,1b thereby representing an expedient formal synthesis of these natural molecules. These natural products exhibit interesting antibacterial or antimicrobial activities. Our reaction not only provided a uniquely effective pathway for their collective synthesis, but also allowed easy modification of these structures for medicinal studies.
In summary, we have developed a new catalytic protocol for the expedient synthesis of oxazolines from oxetanes. It represents not only a powerful complement to the conventional oxazoline synthetic strategies based on non-catalytic cyclization of β-hydroxy or unsaturated amides, but also a new expansion to the reactivity profile of oxetanes, particularly for the synthesis of heterocycles. The suitable choice of a superior Lewis acid catalyst In(OTf)3 allowed a wide range of readily available 3-amido oxetanes to cyclize efficiently to form the corresponding oxazolines under mild conditions. This protocol is also amenable to the synthesis of various bidentate ligands, including those bis(oxazoline) compounds with proved utility. Moreover, the obtained products, typically bearing a pendant 4-hydroxymethyl substituent, perfectly match the structures of a diverse family of natural products and antibacterial molecules. Ongoing studies to extend this process to its catalytic asymmetric variant is underway.16
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
Financial support was provided by National Natural Science Foundation of China (21572192, 21490570), Hong Kong RGC (16302617, 16302318), Shenzhen Science and Technology Innovation Committee (JCYJ20170818113708560) and Jiangsu specially appointed professors program. We thank Jianlei Wu for experimental assistance.
Footnotes
†Electronic supplementary information (ESI) available. CCDC 1908430 and 1908431. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc03843d
References
- (a) Inahashi Y., Iwatsuki M., Ishiyama A., Namatame M., Nishihara-Tsukashima A., Matsumoto A., Hirose T., Sunazuka T., Yamada H., Otoguro K., Takahashi Y., Omura S., Shiomi K. J. Antibiot. 2011;64:303–307. doi: 10.1038/ja.2011.16. [DOI] [PubMed] [Google Scholar]; (b) Shaaban K. A., Saunders M. A., Zhang Y., Tran T., Elshahawi S. I., Ponomareva L. V., Wang X., Zhang J., Copley G. C., Sunkara M., Kharel M. K., Morris A. J., Hower J. C., Tremblay M. S., Prendergast M. A., Thorson J. S. J. Nat. Prod. 2017;80:2–11. doi: 10.1021/acs.jnatprod.6b00948. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Mukai A., Fukai T., matsumoto Y., Ishikawa J., Hoshino Y., Yazawa K., Harada K.-i., Mikami Y. J. Antibiot. 2006;59:366–369. doi: 10.1038/ja.2006.53. [DOI] [PubMed] [Google Scholar]; (d) Nelson K. M., Salomon C. E., Aldrich C. C. J. Nat. Prod. 2012;75:1037–1043. doi: 10.1021/np200972s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Onishi H. R., Pelak B. A., Gercken L. S., Silver L. L., Kahan F. M., Chen M.-H., Patchett A. A., Galloway S. M., Hyland S. A., Anderson M. S., Raetz C. R. H. Science. 1996;274:980–982. doi: 10.1126/science.274.5289.980. [DOI] [PubMed] [Google Scholar]
- For recent reviews on oxazolines in organic synthesis, particularly as ligands: . For an example of using PyBox VII as an optimal ligand, see: ; (a) Hargaden G. C., Guiry P. J. Chem. Rev. 2009;109:2505–2550. doi: 10.1021/cr800400z. [DOI] [PubMed] [Google Scholar]; (b) Desimoni G., Faita G., Jørgensen K. A. Chem. Rev. 2011;111:PR284–PR437. doi: 10.1021/cr100339a. [DOI] [PubMed] [Google Scholar]; (c) Wang L., Zhou J., Tang Y. Chin. J. Chem. 2018;36:1123–1129. [Google Scholar]; (d) Yang G., Zhang W. Chem. Soc. Rev. 2018;47:1783–1810. doi: 10.1039/c7cs00615b. [DOI] [PubMed] [Google Scholar]; (e) Zhang K., Lu L.-Q., Yao S., Chen J.-R., Shi D.-Q., Xiao W.-J. J. Am. Chem. Soc. 2017;139:12847–12852. doi: 10.1021/jacs.7b08207. [DOI] [PubMed] [Google Scholar]
- Selected other utilities in organic synthesis: ; (a) Nakafuku K. M., Fosu S. C., Nagib D. A. J. Am. Chem. Soc. 2018;140:11202–11205. doi: 10.1021/jacs.8b07578. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Byrne C. M., Church T. L., Kramer J. W., Coates G. W. Angew. Chem., Int. Ed. 2008;47:3979–3983. doi: 10.1002/anie.200705310. [DOI] [PubMed] [Google Scholar]; (c) Chen K., Li Z.-W., Shen P.-X., Zhao H.-W., Shi Z.-J. Chem.–Eur. J. 2015;21:7389–7393. doi: 10.1002/chem.201406528. [DOI] [PubMed] [Google Scholar]; (d) Shang M., Wang M.-M., Saint-Denis T. G., Li M.-H., Dai H.-X., Yu J.-Q. Angew. Chem., Int. Ed. 2017;56:5317–5321. doi: 10.1002/anie.201611287. [DOI] [PubMed] [Google Scholar]
- Selected examples from β-hydroxyamides, which typically need stoichiometric reagents (like DAST) and/or strong conditions (like heating): ; (a) Burrell G., Evans J. M., Jones G. E., Stemp G. Tetrahedron Lett. 1990;31:3649–3652. [Google Scholar]; (b) Phillips A. J., Uto Y., Wipf P., Reno M. J., Williams D. R. Org. Lett. 2000;2:1165–1168. doi: 10.1021/ol005777b. [DOI] [PubMed] [Google Scholar]; (c) You S.-L., Kelly J. W. Chem.–Eur. J. 2004;10:71–75. [Google Scholar]; (d) Thiede S., Wosniok P. R., Herkommer D., Schulz-Fincke A.-C., Gütschow M., Menche D. Org. Lett. 2016;18:3964–3967. doi: 10.1021/acs.orglett.6b01724. [DOI] [PubMed] [Google Scholar]; (e) Li X., Taechalertpaisarn J., Xin D., Burgess K. Org. Lett. 2015;17:632–635. doi: 10.1021/ol5036547. [DOI] [PubMed] [Google Scholar]
- Selected examples from β-unsaturated amides (with stoichiometric oxidant): ; (a) Deng Q.-H., Chen J.-R., Wei Q., Zhao Q.-Q., Lu L.-Q., Xiao W.-J. Chem. Commun. 2015;51:3537–3540. doi: 10.1039/c4cc10217g. [DOI] [PubMed] [Google Scholar]; (b) Liu G.-Q., Yang C.-H., Li Y.-M. J. Org. Chem. 2015;80:11339–11350. doi: 10.1021/acs.joc.5b01832. [DOI] [PubMed] [Google Scholar]; (c) Gilbert A., Bertrand X., Paquin J.-F. Org. Lett. 2018;20:7257–7260. doi: 10.1021/acs.orglett.8b03170. [DOI] [PubMed] [Google Scholar]; (d) Haupt J. D., Berger M., Waldvogel S. R. Org. Lett. 2019;21:242–245. doi: 10.1021/acs.orglett.8b03682. [DOI] [PubMed] [Google Scholar]
- For examples of other approaches: ; (a) Shao P.-L., Liao J.-Y., Ho Y. A., Zhao Y. Angew. Chem., Int. Ed. 2014;53:5435–5439. doi: 10.1002/anie.201402788. [DOI] [PubMed] [Google Scholar]; (b) Cai A.-J., Zheng Y., Ma J.-A. Chem. Commun. 2015;51:8946–8949. doi: 10.1039/c5cc02749g. [DOI] [PubMed] [Google Scholar]; (c) Punk M., Merkley C., Kennedy K., Morgan J. B. ACS Catal. 2016;6:4694–4698. doi: 10.1021/acscatal.6b01400. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wang H., Zhang J., Tan J., Xin L., Li Y., Zhang S., Xu K. Org. Lett. 2018;20:2505–2508. doi: 10.1021/acs.orglett.8b00165. [DOI] [PubMed] [Google Scholar]; (e) Luo H., Yang Z., Lin W., Zheng Y., Ma S. Chem. Sci. 2018;9:1964–1969. doi: 10.1039/c7sc04079b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Wu F., Alom N.-E., Ariyarathna J. P., Naß J., Li W. Angew. Chem., Int. Ed. 2019;58:11676–11680. doi: 10.1002/anie.201904662. [DOI] [PubMed] [Google Scholar]
- For leading reviews on oxetanes in medicinal chemistry and organic synthesis: ; (a) Burkhard J. A., Wuitschik G., Rogers-Evans M., Müller K., Carreira E. M. Angew. Chem., Int. Ed. 2010;49:9052–9067. doi: 10.1002/anie.200907155. [DOI] [PubMed] [Google Scholar]; (b) Mack D. J., Njardarson J. T. ACS Catal. 2013;3:272–286. [Google Scholar]; (c) Wang Z., Chen Z., Sun J. Org. Biomol. Chem. 2014;12:6028–6032. doi: 10.1039/c4ob00920g. [DOI] [PubMed] [Google Scholar]; (d) Malapit C. A., Howell A. R. J. Org. Chem. 2015;80:8489–8495. doi: 10.1021/acs.joc.5b01255. [DOI] [PubMed] [Google Scholar]; (e) Bull J. A., Croft R. A., Davis O. A., Doran R., Morgan K. F. Chem. Rev. 2016;116:12150–12233. doi: 10.1021/acs.chemrev.6b00274. [DOI] [PubMed] [Google Scholar]
- For recent examples on the synthesis of heterocycles from oxetanes: For our efforts: ; (a) Burkhard J. A., Tchitchanov B. H., Carreira E. M. Angew. Chem., Int. Ed. 2011;50:5379–5382. doi: 10.1002/anie.201100260. [DOI] [PubMed] [Google Scholar]; (b) Guo B., Schwarzwalder G., Njardarson J. T. Angew. Chem., Int. Ed. 2012;51:5675–5678. doi: 10.1002/anie.201201367. [DOI] [PubMed] [Google Scholar]; (c) Ruider S. A., Müller S., Carreira E. M. Angew. Chem., Int. Ed. 2013;52:11908–11911. doi: 10.1002/anie.201306563. [DOI] [PubMed] [Google Scholar]; (d) White A. R., Kozlowski R. A., Tsai S.-C., Vanderwal C. D. Angew. Chem., Int. Ed. 2017;56:10525–10529. doi: 10.1002/anie.201704119. [DOI] [PubMed] [Google Scholar]; (e) Zhao W., Wang Z., Sun J. Angew. Chem., Int. Ed. 2012;51:6209–6213. doi: 10.1002/anie.201200513. [DOI] [PubMed] [Google Scholar]; (f) Chen Z., Wang B., Wang Z., Zhu G., Sun J. Angew. Chem., Int. Ed. 2013;52:2027–2031. doi: 10.1002/anie.201206481. [DOI] [PubMed] [Google Scholar]; (g) Wang Z., Chen Z., Sun J. Angew. Chem., Int. Ed. 2013;52:6685–6688. doi: 10.1002/anie.201300188. [DOI] [PubMed] [Google Scholar]; (h) Yang W., Sun J. Angew. Chem., Int. Ed. 2016;55:1868–1871. doi: 10.1002/anie.201509888. [DOI] [PubMed] [Google Scholar]; (i) Yang W., Wang Z., Sun J. Angew. Chem., Int. Ed. 2016;55:6954–6958. doi: 10.1002/anie.201601844. [DOI] [PubMed] [Google Scholar]
- The relative stereochemistry of the products 4 was based on analysis of their molecular symmetry derived from the NMR spectra. See the ESI for more details.
- Zhao W., Sun J. Chem. Rev. 2018;118:10349–10392. doi: 10.1021/acs.chemrev.8b00279. [DOI] [PubMed] [Google Scholar]
- Gaumont A.-C., Gulea M., Levillain J. Chem. Rev. 2009;109:1371–1401. doi: 10.1021/cr800189z. [DOI] [PubMed] [Google Scholar]
- Aït-Haddou H., Hoarau O., Cramailÿre D., Pezet F., Daran J. C., Balavoine G. G. A. Chem.–Eur. J. 2004;10:699–707. doi: 10.1002/chem.200204649. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-Z., Zhu S.-F., Wang L.-X., Zhou Q.-L. Angew. Chem., Int. Ed. 2008;47:8496–8498. doi: 10.1002/anie.200803192. [DOI] [PubMed] [Google Scholar]
- Mitchell J. M., Shaw J. T. Org. Lett. 2007;9:1679–1681. doi: 10.1021/ol070355o. [DOI] [PubMed] [Google Scholar]
- Hu J., Miller M. J. J. Am. Chem. Soc. 1997;119:3462–3468. [Google Scholar]
- Simple extension of the current catalytic system together with a chiral ligand led to low enantioselecitivity.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.