Abstract
Marine mollusks are commonly subjected to heat stress. To evaluate the effects of heat stress on the physiological metabolism of the ark shell Scapharca subcrenata, clams were exposed to different high temperatures (24, 28 and 32 °C) for 72 h. The oxygen consumption and ammonia excretion rates were measured at 2, 12, 24, 48 and 72 h. The results indicated that the metabolic rates of the ark shell significantly increased with increasing heat stress, accompanied by mortalities in response to prolonged exposure. A metabolomics approach based on gas chromatography coupled with mass spectrometry was further applied to assess the changes of metabolites in the mantle of the ark shell at 32 °C. Moreover, multivariate and pathway analyses were conducted for the different metabolites. The results showed that the heat stress caused changes in energy metabolism, amino acid metabolism, osmotic regulation, carbohydrate metabolism and lipid metabolism through different metabolic pathways. These results are consistent with the significant changes of oxygen consumption rate and ammonia excretion rate. The present study contributes to the understanding of the impacts of heat stress on intertidal bivalves and elucidates the relationship between individual-level responses and underlying molecular metabolic dynamics.
Keywords: Heat stress, Oxygen consumption rate, Ammonia excretion rate, Metabolomics, Scapharca subcrenata, Marine invertebrate
Introduction
Temperature is one of the more prominent abiotic factors that influences the physiological metabolism of animals and determines the ecological niche of a species (Pörtner et al., 2006; Pörtner, 2010; Ezgeta-Balić et al., 2011). Temperature has also become an increasingly severe source of environmental stress due to the increase of seawater temperature as a result of global climate changes. The global water surface temperature has increased by approximately 0.7 °C during the last century (Hansen et al., 2006) and a continued increase has been predicted (Wang et al., 2015). In response to environmental changes, organisms typically adjust their metabolic physiology to adapt to new energy requirements (Cheung & Lam, 1995; Lagerspetz, 2006; Zhang et al., 2017). It has been reported that temperature can affect the metabolic rates of marine invertebrates, thus influencing the energy available for growth (González et al., 2002). Intertidal bivalves frequently face extreme heat stress (Han et al., 2013), and form reliable models to investigate the adaptations to highly fluctuating environments (Davenport & Davenport, 2005; Wang et al., 2015). Thus, studying the underlying metabolic alterations can help to understand the physiological changes that happen in bivalves in response to thermal stress.
The effects of heat stress on the energy metabolism of marine bivalves have been widely studied in many species (Sokolova et al., 2012), such as Mytilus galloprovincialis (Anestis et al., 2007), the limpet Cellana toreuma (Han et al., 2013), Mercenaria mercenaria (Ivanina et al., 2013) and the eastern oyster Crassostrea virginica (Casas et al., 2018). Among the many indicators of physiological responses to thermal challenge, the respiration behavior and individual-level metabolic rates (especially oxygen consumption rate) have been widely used to assess the physiological state in response to stress tolerance or adaptation during exposure to heat stress (Sobral & Widdows, 1997; Saucedo et al., 2004; Sarà et al., 2008; Dowd & Somero, 2013; Frisk, Steffensen & Skov, 2013; Wang et al., 2015; Casas et al., 2018). Successful persistence or tolerance requires molecular adaptations to compensate for the impaired metabolism, triggered by changes of temperature (Lim et al., 2016). Moreover, investigating the correlation between individual-level responses and molecular changes is useful toward a better understanding of the responses and regulating mechanisms from an overall perspective. Recently, research has increasingly focused on molecular adaptation or tolerance of marine bivalves to heat stress, and new analytic techniques, such as transcriptomics (Lim et al., 2016; Nie et al., 2017; Yang et al., 2017; Juárez et al., 2018; Zhang et al., 2019) and metabolomics (Ellis et al., 2014; Digilio et al., 2016), were used. The mantle tissue of mollusks has multiple functions, which include ligament secretion and sensorial activities; moreover, this tissue is very responsive to external stimuli (Artigaud et al., 2015). The mantle tissue has been used for transcriptome, proteomic, or metabolomic analyses in many studies (Artigaud et al., 2014, 2015; Wei et al., 2015).
Metabolomics refers to the systematic study of chemical processes that involve metabolites. By measuring the levels of endogenous low-molecular-weight metabolites, metabolomics can be used to identify biomarkers that are indicative of physiological responses of living samples to specific environmental or culture conditions (Alfaro & Young, 2018). Many analytical platforms have been used for metabolomics, including raman spectroscopy, infrared spectroscopy, nuclear magnetic resonance (NMR) and many mass spectrometry (MS) techniques, of which NMR and MS are the most widely applied analytical tools for their sufficient high throughput and resolution properties (Young & Alfaro, 2018). Gas chromatography coupled with mass spectrometry (GC/MS) is a well-established analytical method that can provide a comprehensive and systematic understanding of all metabolites in biological samples (Tsugawa et al., 2011; Nguyen & Alfaro, 2019a). GC/MS-based metabolomics has been widely applied to study the physiological responses of aquatic organisms to environmental stressors, including pathogen infection, water contaminants and aerial exposure, and many metabolites and associated pathways have been successfully identified (Guo et al., 2014; Ji et al., 2016; Chen et al., 2015; Nguyen et al., 2018a, 2018c; 2019, Alfaro, Nguyen & Mellow, 2019).
The ark shell Scapharca subcrenata inhabits the muddy sediments of the shallow coasts of China, Japan and Korea (Nakamura, 2005) and is widely cultured and consumed as a popular foodstuff in China and Korea (Jin, Ahn & Je, 2018). Due to their large geographic distribution, ark shell populations are exposed to strongly differing thermal conditions such as diurnal temperature fluctuations and extreme high temperature during summer. For example, the water temperature of the S. subcrenata habitat Xiangshan Bay (China) varies between 6.7 °C and 33.0 °C (You & Jiao, 2011). However, little information is available on the physiological responses of S. subcrenata to such pronounced heat stress.
Understanding the response of organisms to heat stress requires an in-depth understanding of both their acute responses and their compensatory acclimatization responses to the elevated temperature (Pörtner et al., 2006). In the present study, the oxygen consumption and ammonia excretion rates of S. subcrenata in response to heat stress were measured. Furthermore, the metabolic profile in the mantle was characterized using GC/MS to identify biomarkers for the responses to heat stress.
Materials and Methods
Animals and heat stress
Adult S. subcrenata individuals were collected in Xiangshan Bay, East China Sea in November by a fishing trawler and transported in buckets containing seawater to the laboratory at the seaside of the Bay. The water temperature at the sampling site was 19.6 °C. The clams were maintained in a glass aquarium at 20 ± 0.5 °C, provided with constant aeration. To minimize the effects of body size on metabolic responses to heat stress, only individuals with similar shell length were used (28.11 ± 1.36 mm).
The clams were randomly divided into four groups (70 clams for 20, 24 and 28 °C treatment, and 80 clams for 32 °C treatment) and were transferred to four 60 L water baths, filled with aerated seawater, that were connected to a temperature controller. The seawater temperature was gradually increased from 20 to 24, 28 and 32 °C over 2, 4 and 6 h periods, respectively. The temperature was then maintained constant for further 72 h. At 2, 12, 24, 48 and 72 h of exposure to different heat stress levels, both the oxygen consumption rate and ammonia excretion rate of S. subcrenata were measured. During the experiment, the seawater in each tank was renewed daily.
Based on the results of oxygen consumption and ammonia excretion measurements, six replicates of mantle samples were taken 2 h and 24 h after the seawater temperature was gradually increased from 20 °C to 32 °C. All samples were immediately frozen in liquid nitrogen and stored at −80 °C for further metabolomic analysis.
Measurement of oxygen consumption rate and ammonia excretion rate
Both the oxygen consumption and ammonia excretion rates were determined in a 1,500 mL glass respiration chamber. Two clams were sealed for 2 h in a chamber filled with oxygen saturated seawater. The oxygen concentration in this chamber was measured according to standard procedure (Stickland & Parsons, 1968). The NH4+-N concentration was determined with the phenol-hypochlorite method (Solorzano, 1969). Individual clams were sampled for measurement at 1, 11, 23, 47 and 71 h after heat stress challenge. Since each measurement cycle spanned a 2 h period, the measured values represent the averages of 1–3, 11–13, 23–25, 47–49 and 71-73 h, respectively. The results are presented as the rates at 2, 12, 24, 48 and 72 h. Each measurement was performed in three replicates and a chamber without clams served as control. After measurements, the dry weight of the soft parts of each clam was determined after drying at 65 °C for 24 h.
Metabolite extraction
For metabolite extraction, 30 mg accurately weighed wet sample was transferred into a 1.5 mL Eppendorf tube with two small steel balls (with diameters of 1.50 mm) for crushing. An aliquot of 20 μl of 2-chloro-l-phenylalanine (0.3 mg/mL), dissolved in methanol as internal standard and 600 μl mixture of methanol and water (4/1, vol/vol) were added to each sample. All samples were cooled to −80 °C for 2 min and then ground at 60 Hz for 2 min. After vortexing, the ground samples were ultrasonicated for 10 min at ambient temperature and cooled to −20 °C for 30 min. The samples were then centrifuged at 13,000 rpm, 4 °C for 15 min (Eppendorf Centrifuge 5427 R; Hamburg, Germany). The supernatant (400 μl) was dried in a freeze concentration centrifugal dryer (Christ RVC 2-33IR; Osterode, Germany), and 80 μl of 15 mg/mL methoxylamine hydrochloride in pyridine was added subsequently. The resulting mixture was vortexed for 2 min and incubated at 37 °C for 90 min. Then, 80 μl of bis(trimethylsilyl)trifluoroacetamide (BSTFA) (with 1% trimethylchlorosilane) and 20 μl n-hexane was added, which was vortexed for 2 min, and then derivatized at 70 °C for 60 min. The resultant mixture was exposed to ambient temperature for 30 min before GC-MS analysis.
Quality control (QC) samples were prepared by pooling all samples together, with the volume same as the analytic samples, and then analyzed using the same method. The QCs were injected at regular intervals (every 10 samples) throughout the analytical process to ensure the reproducibility of the GC-MS measurement.
GC/MS analysis
The derivatized samples were analyzed using an Agilent 7890A GC system coupled with an Agilent 5975C MSD system (Agilent, Santa Clara, CA, USA). A HP-5MS fuzed-silica capillary column (30 m × 0.25 mm × 0.25 μm, Agilent) was utilized to separate the derivatives. Helium (>99.999%) was used as carrier gas at a constant flow rate of 6.0 mL/min through the column. The injector temperature was maintained at 280 °C and the injection volume was one μl in splitless mode. The initial oven temperature was 60 °C, was increased to 125 °C at a rate of 8 °C/min, to 190 °C at a rate of 10 °C/min, to 210 °C at a rate of 4 °C/min, to 310 °C at a rate of 20 °C/min, and finally, a temperature of 310 °C was sustained for 8.5 min. The temperatures of the MS quadrupole and ion source electron impact (EI) were set to 150 °C and 230 °C, respectively, and the collision energy was 70 eV. Mass data was acquired in full-scan mode (m/z 50–600), and the solvent delay time was set to 5 min.
Statistical analysis
For the results of oxygen consumption rates and ammonia excretion rates, One-way ANOVA and multiple comparisons were performed with SPSS 11.5 statistical software, and a P value less than 0.05 was considered to be statistically significant.
The acquired MS data from GC-MS were analyzed by ChromaTOF software (v4.34; LECO, St. Joseph, MI, USA). The metabolites were identified by the Fiehn database using the method described by Mishra, Gong & Kelly (2017). Briefly, after alignment with the Statistic Compare component, the CSV file was obtained with three-dimensional data sets including sample information, peak name, retention time, m/z and peak intensities. The internal standard was used for QC. The internal standards and any identified pseudo positive peaks, such as peaks caused by noise, column bleed and the BSTFA derivatization procedure, were removed from the data set. Peaks from the same metabolites were combined.
The resulting data were normalized to the total peak area of each sample using Excel 2007 (Microsoft, Redmond, Washington D.C., USA) and were imported into the SIMCA software package (v14.0; Umetrics, Umeå, Sweden), where principal component analysis (PCA), partial least-squares discriminant analysis (PLS-DA), and orthogonal partial least-squares discriminant analysis (OPLS-DA) were performed. The Hotelling’s T2 region, shown as an ellipse in score plots of the models, was used to define the 95% confidence interval of the modeled variation. The quality of the models was described by the R2X or R2Y and Q2 values. R2X or R2Y are defined as the proportion of variance in the data that can be explained by the models and indicates goodness of fit. Q2 is defined as the proportion of variance in the data that is, predicted by the model and indicates predictability, as calculated by a cross-validation procedure. A default seven-round cross-validation in SIMCA was performed to determine the optimal number of principal components and to avoid model overfitting. The OPLS-DA models were also validated by permutation analysis (200 times).
The different metabolites were selected based on the combination of a statistically significant threshold of variable influence on projection (VIP) values obtained from the OPLS-DA model and on P values from a two-tailed Student’s t test on the normalized peak areas. Metabolites with VIP values exceeding 1.0 and P values of less than 0.05 were included.
Results
Survival rates
Enhanced heat stress increased the mortality of experimental clams. The survival rates of S. subcrenata exposed to 20, 24, 28 and 32 °C seawater temperature were 97.14%, 92.85%, 84.28% and 75%, respectively. Most mortalities occurred within 24 h after thermal challenge.
Metabolic rates
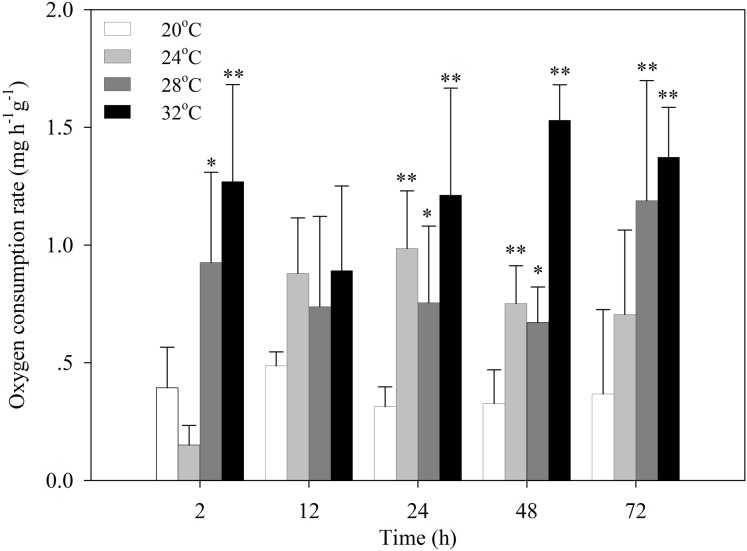
After the temperature was increased to 24 °C, the oxygen consumption rate of S. subcrenata decreased for 2 h, followed by a significant increase at 24 h and 48 h (P < 0.05 and P < 0.01, respectively; Fig. 1). After exposure to 28 °C and 32 °C stress, the oxygen consumption rates increased significantly at 2, 24, 48 and 72 h (P < 0.05, 0.01). The increase was not significant for 12 h, although the oxygen consumption rate was still higher than that of the control group.
Figure 1. Oxygen consumption rates of S. subcrenata after exposed to different temperatures.
Asterisks indicate significant differences (*P < 0.05; **P < 0.01) between the stressed and control group.
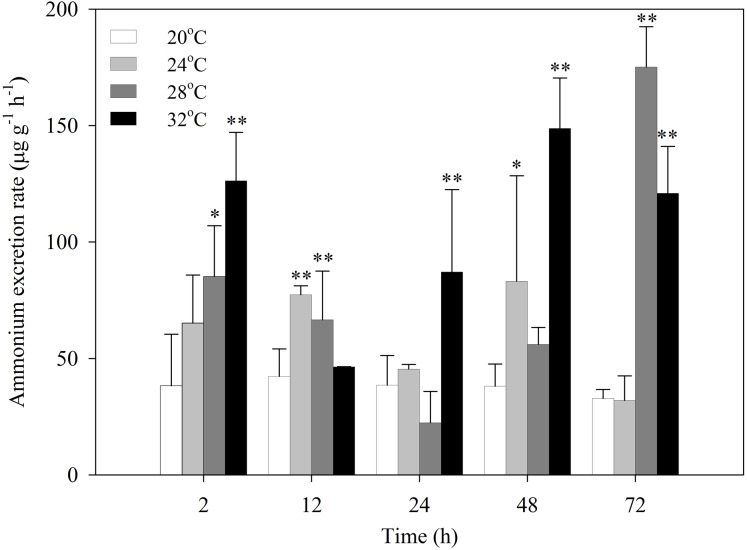
After exposure to heat stress, the ammonia excretion rates of S. subcrenata increased first, then decreased, and increased again (Fig. 2). S. subcrenata exposed to heat stress at 24 °C demonstrated higher ammonia excretion at 12 h compared with the control group (P < 0.01). The clams exposed to 28 °C heat stress showed significantly higher ammonia excretion rates at 2, 12 and 72 h (P < 0.05 and P < 0.01, respectively). The ammonia excretion rate of S. subcrenata exposed to 32 °C was significantly higher than that of the control group at 2, 24, 48 and 72 h (P < 0.01).
Figure 2. Ammonia excretion rates of S. subcrenata after exposed to different temperatures.
Asterisks indicate significant differences (*P < 0.05; **P < 0.01) between the stressed and control group.
Metabolic profiles analyzed by GC-MS
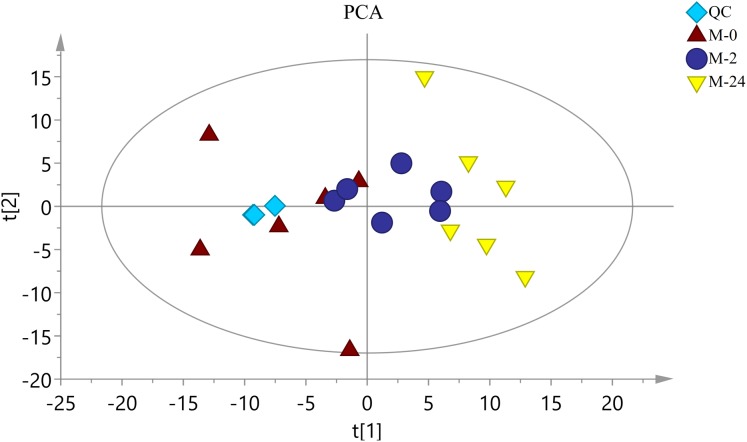
The typical total ion chromatograms (TIC) of both S. subcrenata mantle samples and QC samples displayed stable retention times (Fig. S1). Thus, the TIC could directly reflect the differences of metabolite profiles among groups. The PCA score plot is shown in Fig. 3. The three groups were generally separated, especially the control group and the M-24 group. The R2X value of the PCA model, representing the explained variance for the groups, was 0.484. All groups were within the Hotelling ellipse of 95% confidence, indicating that the analyzed samples contained no outlier.
Figure 3. The score plot of PCA.
QC is the quality control sample. M-0 is the control group, and M-2 and M-24 are the samples taken at 2 h and 24 h, respectively. The Hotelling ellipse indicating 95% Confidence Interval.
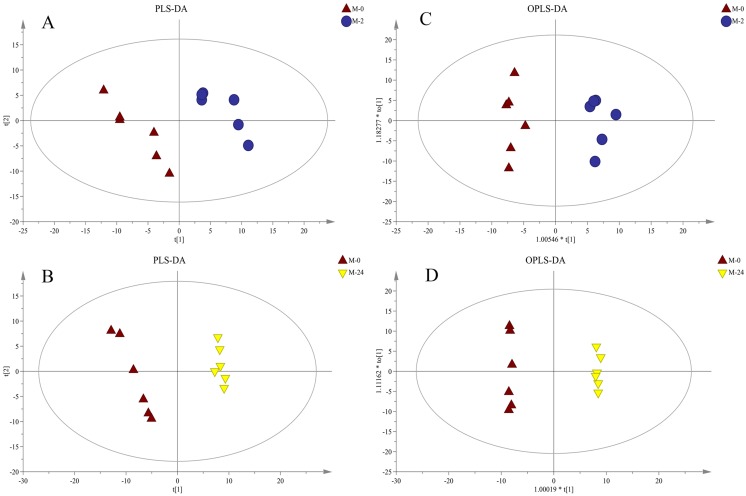
Further supervised pattern recognitions, PLS-DA and OPLS-DA, were performed to obtain a better explanation of different metabolic patterns (Fig. 4). The classification parameters for the PLS-DA and OPLS-DA model are shown in Table 1, indicating the robustness of the models. The pairwise groups in each subplot were clearly separated into two sides of the Hotelling T2 ellipse, indicating that both models could identify differences among groups.
Figure 4. PLS-DA and OPLS-DA score plots derived from metabolite profiles of S. subcrenata.
M-0 is the control group, and M-2 and M-24 are the samples taken at 2 h and 24 h, respectively. (A) PLS-DA score plot of the M-0 group and M-2 group, (B) PLS-DA score plot of the M-0 group and M-24 group, (C) OPLS-DA score plot of the M-0 group and M-2 group, (D) OPLS-DA score plot of the M-0 group and M-24 group.
Table 1. Multivariate analysis of metabolite profiles of S. subcrenata after exposure to heat stress.
Two principal components for the PCA model, two principal components for the PLS-DA model, and one principal component and one orthogonal component for the OPLS-DA model were used.
| Model type | 0–2 h | 0–24 h | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2X (cum) | R2Y (cum) | Q2 (cum) | R2 | Q2 | R2X (cum) | R2Y (cum) | Q2 (cum) | R2 | Q2 | |
| PLS-DA | 0.3 | 0.97 | 0.445 | 0.36 | 0.991 | 0.841 | ||||
| OPLS-DA | 0.3 | 0.97 | 0.381 | 0.963 | −0.033 | 0.468 | 0.999 | 0.833 | 0.993 | −0.003 |
Metabolite identification and comparison
A total of 345 metabolites were identified, including amino acids (e.g., aspartic acid, glutamic acid and histidine), organic acids (e.g., creatine, gluconic acid, malic acid and oxalic acid), and energy metabolism-related metabolites (e.g., glucose-6-phosphate and erythrose). At 2 h after heat stress, 39 metabolites showed significant changes, including 13 downregulated and 26 upregulated metabolites (Table S1). At 24 h, 90 metabolites showed significant changes, including 39 downregulated and 51 upregulated (Table S2).
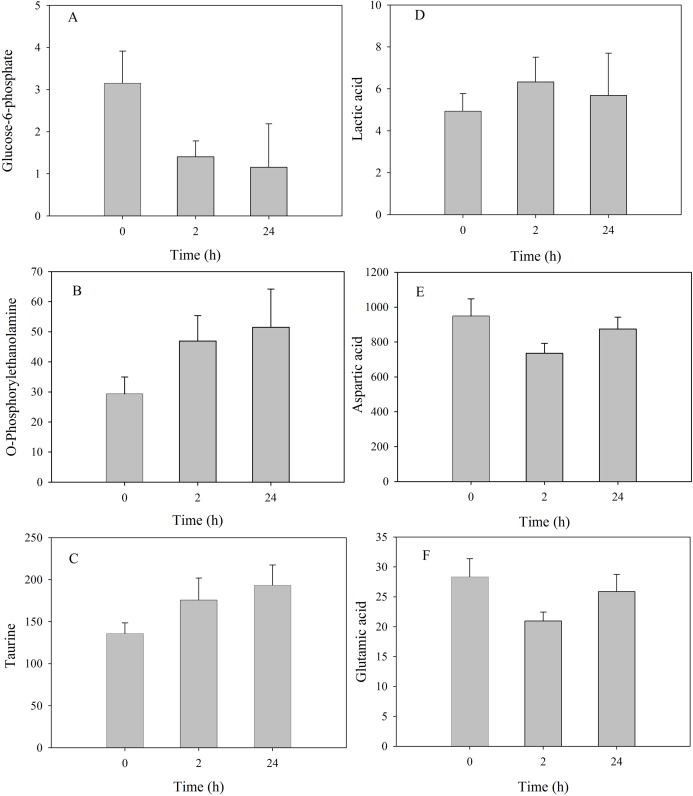
Among metabolites that showed significant changes, the relative concentration of glucose-6-phosphate presented a continuous decrease at 2 h and 24 h, in contrast to the continuous increase of o-phosphorylethanolamine and taurine (Fig. 5). Latic acid was significantly upregulated at 2 h but decreased to the control level at 24 h; aspartic acid and glutamic acid were significantly downregulated at 2 h but increased to the control level at 24 h.
Figure 5. Metabolite levels of metabolites in the mantle of S. subcrenata after exposure to 32 °C.
(A) Glucose-6-phosphate, (B) O-phosphorylethanolamine, (C) taurine, (D) lactic acid, (E) aspartic acid and (F) glutamic acid.
Metabolic pathway of common metabolites
KEGG pathway analysis was performed using MetaboAnalyst 3.0 software. Metabolic pathways were identified against the Danio rerio KEGG (zebrafish) library. Pathway topology analysis was performed based on the relative betweenness to calculate the important values. The metabolites were mapped onto 15 pathways for samples taken at 2 h (Table 2; Fig. S2). Of these, the histidine metabolism pathway was significantly affected by thermal stress (P < 0.05). The D-glutamine metabolism, alanine, aspartate and glutamate metabolism exhibited higher impact values and was also affected even though no significant difference was observed. A total of 15 pathways were obtained when metabolites were imported into KEGG for samples taken at 24 h, and no pathway appeared to be significantly affected (Table 3). Interestingly, five pathways (glutathione metabolism, histidine metabolism, beta-alanine metabolism, nitrogen metabolism, alanine, aspartate and glutamate metabolism, aminoacyl-tRNA biosynthesis) showed significant changes between 2h and 24h (Table S3).
Table 2. Pathway enrichment analysis of the metabolites at 2 h after exposed to 32 °C.
| Pathway | P | −log(P) | Impact |
|---|---|---|---|
| Histidine metabolism | 0.026555 | 3.62 | 0.0 |
| Alanine, aspartate and glutamate metabolism | 0.071896 | 2.63 | 0.45253 |
| D-Glutamine and D-glutamate metabolism | 0.090061 | 2.41 | 1.0 |
| Taurine and hypotaurine metabolism | 0.12386 | 2.09 | 0.2 |
| Nitrogen metabolism | 0.15645 | 1.86 | 0.0 |
| Arginine and proline metabolism | 0.19022 | 1.66 | 0.09612 |
| Nicotinate and nicotinamide metabolism | 0.23292 | 1.46 | 0.0 |
| beta-Alanine metabolism | 0.2616 | 1.34 | 0.0 |
| Sphingolipid metabolism | 0.32888 | 1.11 | 0.01504 |
| Pyruvate metabolism | 0.34161 | 1.07 | 0.0 |
| Butanoate metabolism | 0.34161 | 1.07 | 0.0 |
| Aminoacyl-tRNA biosynthesis | 0.35866 | 1.0254 | 0.0 |
| Glycolysis or Gluconeogenesis | 0.39025 | 0.94097 | 0.0 |
| Glutathione metabolism | 0.39025 | 0.94097 | 0.02968 |
| Porphyrin and chlorophyl metabolism | 0.40186 | 0.91166 | 0.0 |
Table 3. Pathway enrichment analysis of the metabolites at 24 h after exposed to 32 °C.
| Pathway | P | −log(P) | Impact |
|---|---|---|---|
| Glyoxylate and dicarboxylate metabolism | 0.18143 | 1.7069 | 0.03704 |
| Synthesis and degradation of ketone bodies | 0.19888 | 1.615 | 0.0 |
| Cyanoamino acid metabolism | 0.23371 | 1.4537 | 0.0 |
| Butanoate metabolism | 0.2462 | 1.4016 | 0.0 |
| Taurine and hypotaurine metabolism | 0.26706 | 1.3203 | 0.2 |
| Biosynthesis of unsaturated fatty acids | 0.2724 | 1.3005 | 0.0 |
| Alanine, aspartate and glutamate metabolism | 0.27904 | 1.2764 | 0.0 |
| Nitrogen metabolism | 0.32952 | 1.1101 | 0.0 |
| Riboflavin metabolism | 0.38674 | 0.94999 | 0.16667 |
| Glycine, serine and threonine metabolism | 0.39216 | 0.93608 | 0.0 |
| Valine, leucine and isoleucine biosynthesis | 0.43916 | 0.82289 | 0.0 |
| Histidine metabolism | 0.46369 | 0.76853 | 0.2381 |
| beta-Alanine metabolism | 0.50963 | 0.67406 | 0.0 |
| Selenoamino acid metabolism | 0.53113 | 0.63275 | 0.0 |
| Pyrimidine metabolism | 0.53865 | 0.61869 | 0.08825 |
Discussion
Energy metabolism
The present study found a positive correlation between the oxygen consumption rate of S. subcrenata and the temperature of the surrounding environment. An increase in environmental temperature will result in the elevation of physiological rates and biochemical reactions, such as activities of mitochondria and metabolic enzymes, and other oxygen- and energy-demanding processes (Ivanina et al., 2013). The significant increase of oxygen consumption rate indicated the upregulation of both aerobic metabolism and energy demand (Morley et al., 2012). Many studies have indicated that the oxygen consumption rates of organisms typically increase with temperature until reaching a threshold temperature (Shumway, 1982; Yukihira, Lucas & Klumpp, 2000). Beyond this threshold, physiological rates can drastically decrease and anaerobic metabolic end products accumulate (Sommer, Klein & Pörtner, 1997; Zhang et al., 2004). This threshold is often referred to as the Arrhenius break temperature (ABT) (Jansen, Hummel & Bonga, 2009). In the present study, the oxygen consumption rate at 32 °C was always higher than at other temperatures, suggesting that the ABT for S. subcrenata is higher than 32 °C. However, the higher mortality (25%) at 32 °C suggests that such elevated heat stress might exceed the self-regulation capacity of this species despite the 6 h acclimation the clams experienced.
Variations in energy loss through respiration due to heat stress have been confirmed to influence the energy balance (González et al., 2002). Ammonia production is a result of the deamination of amino acids, and was also used to evaluate the energy loss of organisms when faced with environmental stress (Wang et al., 2011; Shin, Chan & Cheung, 2014). Evidence showed that amino acids may be catabolized after being released from cells, resulting in the increase of blood ammonia concentrations and external ammonia excretion rate (Pierce, 1982; Vitale & Friedl, 1984). Thus, the rate of ammonia-nitrogen excretion reflects the rate of protein catabolism (Widdows, 1978). The increased ammonia excretion rate in the present study indicated that amino acids might be catabolized, suggesting increased energy demand during heat stress.
The metabolomics approach, coupled with multivariate analysis, allowed the successful investigation of metabolic changes in response to environmental stress (Cappello et al., 2013). Multivariate analyses identified a clear separation between the control and heat stressed groups, suggesting the existence of significant metabolic differences in the metabolic profile. Metabolic profiling and functional analysis of key metabolic pathways provided an overview of the metabolic status both before and after heat stress (Digilio et al., 2016; Hao et al., 2018). The present data indicated that higher temperatures caused a wide array of changes in the metabolite profiles of S. subcrenata. The metabolites with significant changes and their pathways differed greatly between clams that were exposed to heat-stress for 2 h and 24 h, which could be due to changes of metabolic substrates with prolonged stress exposure.
Amino acid metabolism
Many studies have highlighted that heat stress could influence both the energy balance and energy homeostasis of aquatic invertebrates (Sokolova et al., 2012; Han et al., 2013). As shown by the pathway enrichment analysis, most metabolites that showed significant changes participated in amino acid metabolism (alanine, aspartate, glutamate, taurine, histidine and beta-alanine). Free amino acids account for a large fraction of the metabolome of marine invertebrates (Cappello et al., 2013) and can be oxidized to supply energy in the Krebs cycle. When the oyster (Crassostrea sikamea) was exposed to metal pollution, amino acids including threonine, alanine, arginine, glutamate, beta-alanine, aspartate and glycine decreased significantly (Ji et al., 2016). Exposure to Cu2+ could result in alterations of 25 metabolites involved in oxidative stress responses and apoptosis processes in mussel Perna canaliculus (Nguyen et al., 2018a). In the present study, the relative concentrations of both aspartic acid and glutamic acid were significantly downregulated 2 h after heat stress, suggesting that these amino acids were possibly oxidized. This downregulation is consistent with the significant increase of the oxygen consumption rate. The oxidation of amino acids for energy expenditure is typically achieved via deamination (McVeigh et al., 2006), which would also explain the observed significant changes of the ammonia excretion rate at 32 °C. Taurine is also an osmolyte and plays an important role in osmotic regulation (Preston, 1993; Cappello et al., 2013). Elevation of taurine levels indicates a disorder in osmotic regulation of S. subcrenata under heat stress and similar responses have been reported for the abalone Haliotis diversicolor (Lu et al., 2016).
In addition to the elevated aerobic metabolism, changes of anaerobic metabolites involved in the energy metabolism were also observed in the present study. Glucose-6-phosphate lies at the start of two major metabolic pathways: the glycolysis pathway and the pentose phosphate pathway. Significant depletion of glucose-6-phosphate and activation of the glycolysis pathway suggested that heat stress led to an enhancement of the anaerobic metabolism of S. subcrenata. Moreover, the accumulation of lactic acid and Krebs cycle intermediate (pyruvic acid) suggested that the Krebs cycle was disrupted by a switch towards anaerobic respiration. A similar result was also found in mussel P. canaliculus infected Vibrio sp (Nguyen et al., 2018b; Nguyen & Alfaro, 2019b) and surf clam Crassula aequilatera exposed to thermal stress (Alfaro, Nguyen & Mellow, 2019). When the ambient temperature exceeded the ABT, anaerobic metabolism in the limpet C. toreuma was enhanced via the opine pathway to provide energy (Han, Zhang & Dong, 2017). In stonefly nymphs, accumulation of anaerobic metabolites (lactate, acetate and alanine) was observed when the animals reached critical temperature (Verberk et al., 2013). This transition to partial anaerobiosis at high temperature is likely a compensation for insufficient aerobic energy production and can be attributed to the limited capacity of oxygen uptake (Sokolova et al., 2012). In ectotherms, when the ratio of oxygen supply to oxygen demand decreases and shortages of oxygen arise, both the cardiac and respiration activities were insufficient to meet the elevated oxygen demand at higher temperature (Frederich & Pörtner, 2000; Verberk et al., 2013).
Carbohydrate metabolism and lipid metabolism
In the present study, the significant variation of phospho-ethanolamine, fatty acids, cytosine and adenine indicated that the lipid and nucleotide metabolisms were also involved into responses to heat stress. Lipids play both functional and structural roles in biological processes such as for energy supply and the maintenance of biological membranes (Lee, Park & Lee, 2018). The oxidation of fatty acids produces acetyl CoA, which is an important intermediate metabolite of the Krebs cycle (Roznere et al., 2014). The significant decreases of azelaic acid, adipic acid, oleic acid, palmitic acid and pentadecanoic acid suggest that the clams used lipid energy reserves for the production of acetyl CoA. Phospho-ethanolamine is an intermediate of the phospholipid metabolism. Ethanolamine is part of phosphatidylethanolamine (PE), which forms the cytomembrane of animal cells (McMaster, Tardi & Choy, 1992). Ethanolamine is phosphorylated and enters the cytidine diphosphate pathway for PE synthesis (Cheng et al., 2012). It has been reported that variations in temperature could result in membrane lipid remodeling in the blue mussel Mytilus edulis and the oyster C. virginica (Pernet et al., 2007, 2008). The present study found a significant upregulation of phospho-ethanolamine. This is inconsistent with the elevation of taurine, indicating that the membrane permeability might be influenced by exposure to heat stress. The intracellular concentrations of adenine nucleotides have been proposed as indicators of stress in aquatic organisms (Vetter, Hwang & Hodson, 1986). In the present study, the concentrations of both adenine and cytosine significantly increased 2 h after exposure to 32 °C, followed by increases of adenosine and cytidine-monophosphate at 24 h, indicating enhanced nucleotide synthesis. However, no significant change was observed in the adenosine 5′-monophosphate (AMP) levels. The thymine concentration was significantly increased at 24 h, while the thymidine concentration was significantly decreased, suggesting that the nucleotide metabolism during heat stress requires further investigation.
Conclusions
In summary, the oxygen consumption and ammonia excretion rates were determined during stress caused by different elevated temperatures in S. subcrenata. A GC/MS-based metabolomics approach was applied to assess changes of metabolites at 32 °C. The results demonstrated that the clams increased their metabolic rates with elevated temperature, and mortality was observed under heat stress. Metabolite and functional analyses indicated that heat stress induced disturbances in energy metabolism, osmotic regulation, amino acid metabolism, carbohydrate metabolism and lipid metabolism via different metabolic pathways. The present study provides an important contribution to the understanding of the impacts of heat stress on the clam S. subcrenata and elucidates the relationship between whole-organism responses and molecular metabolic dynamics. To better understand the physiological response of bivalves to environmental stresses, the integration of different omics approaches, including transcriptomics, proteomics and metabolomics, may be adopted in the future research.
Supplemental Information
(A): the control (B): 2 h (C): 24 h. The ordinate shows the relative mass abundance, and the abscissa shows the retention time.
A: 2 h, B: 24 h.
Acknowledgments
We appreciate very much the assistance from Ms. Wenchao Liu and Mr. Peibo Bao in the lab measurement.
Funding Statement
This work was supported by the Special Fund for Agro-scientific Research in the Public Interest of China (201303047), the Natural Science Foundation of Shanghai (18ZR1450000), and the National Natural Science Foundation of China (41576167). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Yazhou Jiang conceived and designed the experiments, performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Haifeng Jiao performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Peng Sun prepared figures and/or tables, and approved the final draft.
Fei Yin analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Baojun Tang conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw measurements of metabolic rates, data matrix of metabolomics analysis, the metabolites with significant changes, pathway enrichment analysis of the metabolites between 2 h and 24 h, and all pathways enriched after exposure to 32 °C are available in the Supplemental Files.
References
- Alfaro, Nguyen & Mellow (2019).Alfaro AC, Nguyen TV, Mellow D. A metabolomics approach to assess the effect of storage conditions on metabolic processes of New Zealand surf clam (Crassula aequilatera) Aquaculture. 2019;498:315–321. doi: 10.1016/j.aquaculture.2018.08.065. [DOI] [Google Scholar]
- Alfaro & Young (2018).Alfaro AC, Young T. Showcasing metabolomic applications in aquaculture: a review. Reviews in Aquaculture. 2018;10(1):135–152. doi: 10.1111/raq.12152. [DOI] [Google Scholar]
- Anestis et al. (2007).Anestis A, Lazou A, Pörtner HO, Michaelidis B. Behavioral, metabolic, and molecular stress responses of marine bivalve Mytilus galloprovincialis during long-term acclimation at increasing ambient temperature. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2007;293(2):R911–R921. doi: 10.1152/ajpregu.00124.2007. [DOI] [PubMed] [Google Scholar]
- Artigaud et al. (2015).Artigaud S, Lacroix C, Richard J, Flye-Sainte-Marie J, Bargelloni L, Pichereau V. Proteomic responses to hypoxia at different temperatures in the great scallop (Pecten maximus) PeerJ. 2015;3(3):e871. doi: 10.7717/peerj.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigaud et al. (2014).Artigaud S, Thorne MAS, Richard J, Lavaud R, Jean F, Flye-Sainte-Marie J, Peck LS, Pichereau V, Clark MS. Deep sequencing of the mantle transcriptome of the great scallop Pecten maximus. Marine Genomics. 2014;15:3–4. doi: 10.1016/j.margen.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Cappello et al. (2013).Cappello T, Mauceri A, Corsaro C, Maisano M, Parrino V, Paro GL, Giuseppe LP, Giuseppe M, Salvatore F. Impact of environmental pollution on caged mussels Mytilus galloprovincialis, using NMR-based metabolomics. Marine Pollution Bulletin. 2013;77(1–2):132–139. doi: 10.1016/j.marpolbul.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Casas et al. (2018).Casas SM, Filgueira R, Lavaud R, Comeau LA, La Peyre MK, La Peyre JF. Combined effects of temperature and salinity on the physiology of two geographically-distant eastern oyster populations. Journal of Experimental Marine Biology and Ecology. 2018;506:82–90. doi: 10.1016/j.jembe.2018.06.001. [DOI] [Google Scholar]
- Chen et al. (2015).Chen S, Zhang C, Xiong Y, Tian X, Liu C, Jeevithan E, Wu W. A GC-MS-based metabolomics investigation on scallop (Chlamys farreri) during semi-anhydrous living-preservation. Innovative Food Science & Emerging Technologies. 2015;31:185–195. doi: 10.1016/j.ifset.2015.07.003. [DOI] [Google Scholar]
- Cheng et al. (2012).Cheng J-S, Niu Y-H, Lu S-H, Yuan Y-J. Metabolome analysis reveals ethanolamine as potential marker for improving lipid accumulation of model photosynthetic organisms. Journal of Chemical Technology and Biotechnology. 2012;87(10):1409–1418. doi: 10.1002/jctb.3759. [DOI] [Google Scholar]
- Cheung & Lam (1995).Cheung SG, Lam SW. Effect of salinity, temperature and acclimation on oxygen consumption of Nassarius festivus (Powys, 1835) (Gastropoda: Nassariidae) Comparative Biochemistry and Physiology Part A: Physiology. 1995;111(4):625–631. doi: 10.1016/0300-9629(95)00051-8. [DOI] [Google Scholar]
- Davenport & Davenport (2005).Davenport J, Davenport JL. Effects of shore height, wave exposure and geographical distance on thermal niche width of intertidal fauna. Marine Ecology Progress Series. 2005;292(1):41–50. doi: 10.3354/meps292041. [DOI] [Google Scholar]
- Digilio et al. (2016).Digilio G, Sforzini S, Cassino C, Robotti E, Oliveri C, Marengo E, Musso D, Osella D, Viarengo A. Haemolymph from Mytilus galloprovincialis: response to copper and temperature challenges studied by 1H-NMR metabonomics. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2016;183-184:61–71. doi: 10.1016/j.cbpc.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Dowd & Somero (2013).Dowd WW, Somero GN. Behavior and survival of Mytilus congeners following episodes of elevated body temperature in air and seawater. Journal of Experimental Biology. 2013;216(3):502–514. doi: 10.1242/jeb.076620. [DOI] [PubMed] [Google Scholar]
- Ellis et al. (2014).Ellis RP, Spicer JI, Byrne JJ, Sommer U, Viant MR, White DA, Widdicombe S. 1H NMR metabolomics reveals contrasting response by male and female mussels exposed to reduced seawater pH, increased temperature, and a pathogen. Environmental Science & Technology. 2014;48(12):7044–7052. doi: 10.1021/es501601w. [DOI] [PubMed] [Google Scholar]
- Ezgeta-Balić et al. (2011).Ezgeta-Balić D, Rinaldi A, Peharda M, Prusina I, Montalto V, Niceta N, Sarà G. An energy budget for the subtidal bivalve Modiolus barbatus (Mollusca) at different temperatures. Marine Environmental Research. 2011;71(1):79–85. doi: 10.1016/j.marenvres.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Frederich & Pörtner (2000).Frederich M, Pörtner HO. Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in spider crab, Maja squinado. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2000;279(5):R1531–R1538. doi: 10.1152/ajpregu.2000.279.5.R1531. [DOI] [PubMed] [Google Scholar]
- Frisk, Steffensen & Skov (2013).Frisk M, Steffensen JF, Skov PV. The effects of temperature on specific dynamic action and ammonia excretion in pikeperch (Sander lucioperca) Aquaculture. 2013;404-405:65–70. doi: 10.1016/j.aquaculture.2013.04.005. [DOI] [Google Scholar]
- González et al. (2002).González ML, López DA, Pérez MC, Castro JM. Effect of temperature on the scope for growth in juvenile scallops Argopecten purpuratus (Lamark, 1819) Aquaculture International. 2002;10(4):339–348. doi: 10.1023/A:1022429209469. [DOI] [Google Scholar]
- Guo et al. (2014).Guo C, Huang X-Y, Yang M-J, Wang S, Ren S-T, Li H, Peng X-X. GC/MS-based metabolomics approach to identify biomarkers differentiating survivals from death in crucian carps infected by Edwardsiella tarda. Fish & Shellfish Immunology. 2014;39(2):215–222. doi: 10.1016/j.fsi.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Han, Zhang & Dong (2017).Han GD, Zhang S, Dong YW. Anaerobic metabolism and thermal tolerance: the importance of opine pathways on survival of a gastropod after cardiac dysfunction. Integrative Zoology. 2017;12(5):361–370. doi: 10.1111/1749-4877.12229. [DOI] [PubMed] [Google Scholar]
- Han et al. (2013).Han G-D, Zhang S, Marshall DJ, Ke C-H, Dong Y-W. Metabolic energy sensors (AMPK and SIRT1), protein carbonylation and cardiac failure as biomarkers of thermal stress in an intertidal limpet: linking energetic allocation with environmental temperature during aerial emersion. Journal of Experimental Biology. 2013;216(17):3273–3282. doi: 10.1242/jeb.084269. [DOI] [PubMed] [Google Scholar]
- Hansen et al. (2006).Hansen J, Sato M, Ruedy R, Lo K, Lea DW, Medina-Elizade M. Global temperature change. Proceedings of the National Academy of Sciences of the United States. 2006;103(39):14288–14293. doi: 10.1073/pnas.0606291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao et al. (2018).Hao R, Wang Z, Yang C, Deng Y, Zheng Z, Wang Q, Du X. Metabolomic responses of juvenile pearl oyster Pinctada maxima to different growth performances. Aquaculture. 2018;491:258–265. doi: 10.1016/j.aquaculture.2018.03.050. [DOI] [Google Scholar]
- Ivanina et al. (2013).Ivanina AV, Dickinson GH, Matoo OB, Bagwe R, Dickinson A, Beniash E, Sokolova IM. Interactive effects of elevated temperature and CO2 levels on energy metabolism and biomineralization of marine bivalves Crassostrea virginica and Mercenaria mercenaria. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2013;166(1):101–111. doi: 10.1016/j.cbpa.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Jansen, Hummel & Bonga (2009).Jansen JM, Hummel H, Bonga SW. The respiratory capacity of marine mussels (Mytilus galloprovincialis) in relation to the high temperature threshold. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2009;153(4):399–402. doi: 10.1016/j.cbpa.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Ji et al. (2016).Ji C, Li F, Wang Q, Zhao J, Sun Z, Wu H. An integrated proteomic and metabolomic study on the gender-specific responses of mussels Mytilus galloprovincialis, to tetrabromobisphenol A (TBBPA) Chemosphere. 2016;144:527–539. doi: 10.1016/j.chemosphere.2015.08.052. [DOI] [PubMed] [Google Scholar]
- Jin, Ahn & Je (2018).Jin J-E, Ahn C-B, Je J-Y. Purification and characterization of antioxidant peptides from enzymatically hydrolyzed ark shell (Scapharca subcrenata) Process Biochemistry. 2018;72:170–176. doi: 10.1016/j.procbio.2018.06.001. [DOI] [Google Scholar]
- Juárez et al. (2018).Juárez OE, Cruz FL, Leyva-Valencia I, López-Landavery E, García-Esquivel Z, Díaz F, Re-Araujo D, Vadopalas B, Galindo-Sánchez CE. Transcriptomic and metabolic response to chronic and acute thermal exposure of juvenile geoduck clams Panopea globosa. Marine Genomics. 2018;42:1–13. doi: 10.1016/j.margen.2018.09.003. [DOI] [PubMed] [Google Scholar]
- Lagerspetz (2006).Lagerspetz KYH. What is thermal acclimation? Journal of Thermal Biology. 2006;31(4):332–336. doi: 10.1016/j.jtherbio.2006.01.003. [DOI] [Google Scholar]
- Lee, Park & Lee (2018).Lee M-C, Park JC, Lee J-S. Effects of environmental stressors on lipid metabolism in aquatic invertebrates. Aquatic Toxicology. 2018;200:83–92. doi: 10.1016/j.aquatox.2018.04.016. [DOI] [PubMed] [Google Scholar]
- Lim et al. (2016).Lim H-J, Kim B-M, Hwang IJ, Lee J-S, Choi I-Y, Kim Y-J, Rhee J-S. Thermal stress induces a distinct transcriptome profile in the pacific oyster Crassostrea gigas. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics. 2016;19:62–70. doi: 10.1016/j.cbd.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Lu et al. (2016).Lu J, Shi Y, Wang S, Chen H, Cai S, Feng J. NMR-based metabolomic analysis of Haliotis diversicolor exposed to thermal and hypoxic stresses. Science of the Total Environment. 2016;545-546:280–288. doi: 10.1016/j.scitotenv.2015.12.071. [DOI] [PubMed] [Google Scholar]
- McMaster, Tardi & Choy (1992).McMaster CR, Tardi PG, Choy PC. Modulation of phosphatidylethanolamine biosynthesis by exogenous ethanolamine and analogues in the hamster heart. Molecular and Cellular Biochemistry. 1992;116(1–2):69–73. doi: 10.1007/BF01270571. [DOI] [PubMed] [Google Scholar]
- McVeigh et al. (2006).McVeigh A, Moore M, Allen JI, Dyke P. Lysosomal responses to nutritional and contaminant stress in mussel hepatopancreatic digestive cells: a modelling study. Marine Environmental Research. 2006;62(Suppl. 1):S433–S438. doi: 10.1016/j.marenvres.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Mishra, Gong & Kelly (2017).Mishra P, Gong Z, Kelly BC. Assessing biological effects of fluoxetine in developing zebrafish embryos using gas chromatography-mass spectrometry based metabolomics. Chemosphere. 2017;188:157–167. doi: 10.1016/j.chemosphere.2017.08.149. [DOI] [PubMed] [Google Scholar]
- Morley et al. (2012).Morley SA, Hirse T, Thorne MAS, Pörtner HO, Peck LS. Physiological plasticity, long term resistance or acclimation to temperature, in the Antarctic bivalve, Laternula elliptica. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2012;162(1):16–21. doi: 10.1016/j.cbpa.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Nakamura (2005).Nakamura Y. Suspension feeding of the ark shell Scapharca subcrenata as a function of environmental and biological variables. Fisheries Science. 2005;71(4):875–883. doi: 10.1111/j.1444-2906.2005.01040.x. [DOI] [Google Scholar]
- Nguyen & Alfaro (2019a).Nguyen TV, Alfaro AC. Applications of omics to investigate responses of bivalve haemocytes to pathogen infections and environmental stress. Aquaculture. 2019a doi: 10.1016/j.aquaculture.2019.734488. Epub ahead of print 13 September 2019. [DOI] [Google Scholar]
- Nguyen & Alfaro (2019b).Nguyen TV, Alfaro AC. Targeted metabolomics to investigate antimicrobial activity of itaconic acid in marine molluscs. Metabolomics. 2019b;15(7):97. doi: 10.1007/s11306-019-1556-8. [DOI] [PubMed] [Google Scholar]
- Nguyen et al. (2018a).Nguyen TV, Alfaro AC, Merien F, Lulijwa R, Young T. Copper-induced immunomodulation in mussel (Perna canaliculus) haemocytes. Metallomics. 2018a;10(7):965–978. doi: 10.1039/C8MT00092A. [DOI] [PubMed] [Google Scholar]
- Nguyen et al. (2018b).Nguyen TV, Alfaro AC, Merien F, Young T, Grandiosa R. Metabolic and immunological responses of male and female New Zealand Greenshell™ mussels (Perna canaliculus) infected with Vibrio sp. Journal of Invertebrate Pathology. 2018b;157:80–89. doi: 10.1016/j.jip.2018.08.008. [DOI] [PubMed] [Google Scholar]
- Nguyen et al. (2019).Nguyen TV, Alfaro AC, Young T, Merien F. Tissue-specific immune responses to Vibrio sp. infection in mussels (Perna canaliculus): a metabolomics approach. Aquaculture. 2019;500:118–125. doi: 10.1016/j.aquaculture.2018.09.061. [DOI] [Google Scholar]
- Nguyen et al. (2018c).Nguyen TV, Alfaro AC, Young T, Ravi S, Merien F. Metabolomics study of immune responses of New Zealand greenshell™ mussels (Perna canaliculus) infected with pathogenic Vibrio sp. Marine Biotechnology. 2018c;20(3):396–409. doi: 10.1007/s10126-018-9804-x. [DOI] [PubMed] [Google Scholar]
- Nie et al. (2017).Nie HT, Liu LH, Huo ZM, Chen P, Ding JF, Yang F, Yan XW. The HSP70 gene expression responses to thermal and salinity stress in wild and cultivated Manila clam Ruditapes philippinarum. Aquaculture. 2017;470:149–156. doi: 10.1016/j.aquaculture.2016.12.016. [DOI] [Google Scholar]
- Pernet et al. (2007).Pernet F, Tremblay R, Comeau L, Guderley H. Temperature adaptation in two bivalve species from different thermal habitats: energetics and remodelling of membrane lipids. Journal of Experimental Biology. 2007;210(17):2999–3014. doi: 10.1242/jeb.006007. [DOI] [PubMed] [Google Scholar]
- Pernet et al. (2008).Pernet F, Tremblay R, Redjah I, Sevigny JM, Gionet C. Physiological and biochemical traits correlate with differences in growth rate and temperature adaptation among groups of the eastern oyster Crassostrea virginica. Journal of Experimental Biology. 2008;211(6):969–977. doi: 10.1242/jeb.014639. [DOI] [PubMed] [Google Scholar]
- Pierce (1982).Pierce S. Invertebrate cell volume control mechanisms: a coordinated use of intracellular amino acids and inorganic ions as osmotic solute. Biological Bulletin. 1982;163(3):405–419. doi: 10.2307/1541452. [DOI] [Google Scholar]
- Preston (1993).Preston RL. Transport of amino-acids by marine-invertebrates. Journal of Experimental Zoology. 1993;265(4):410–421. doi: 10.1002/jez.1402650410. [DOI] [Google Scholar]
- Pörtner (2010).Pörtner HO. Oxygen- and capacity-limitation of thermal tolerance: amatrix for integrating climate-related stressor effects in marine ecosystems. Journal of Experimental Biology. 2010;213(6):881–893. doi: 10.1242/jeb.037523. [DOI] [PubMed] [Google Scholar]
- Pörtner et al. (2006).Pörtner HO, Bennett AF, Bozinovic F, Clarke A, Lardies MA, Lucassen M, Pelster B, Schiemer F, Stillman JH. Trade-offs in thermal adaptation: the need for a molecular to ecological integration. Physiological and Biochemical Zoology. 2006;79(2):295–313. doi: 10.1086/499986. [DOI] [PubMed] [Google Scholar]
- Roznere et al. (2014).Roznere I, Watters GT, Wolfe BA, Daly M. Nontargeted metabolomics reveals biochemical pathways altered in response to captivity and food limitation in the freshwater mussel Amblema plicata. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics. 2014;12:53–60. doi: 10.1016/j.cbd.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Sarà et al. (2008).Sarà G, Romano C, Widdows J, Staff FJ. Effect of salinity and temperature on feeding physiology and scope for growth of an invasive species (Brachidontes pharaonis—MOLLUSCA: BIVALVIA) within the Mediterranean sea. Journal of Experimental Marine Biology and Ecology. 2008;363(1–2):130–136. doi: 10.1016/j.jembe.2008.06.030. [DOI] [Google Scholar]
- Saucedo et al. (2004).Saucedo PE, Ocampo L, Monteforte M, Bervera H. Effect of temperature on oxygen consumption and ammonia excretion in the Calafia mother-of-pearl oyster, Pinctada mazatlanica (Hanley, 1856) Aquaculture. 2004;229(1–4):377–387. doi: 10.1016/S0044-8486(03)00327-2. [DOI] [Google Scholar]
- Shin, Chan & Cheung (2014).Shin PKS, Chan CSK, Cheung SG. Physiological energetics of the fourth instar of Chinese horseshoe crabs (Tachypleus tridentatus) in response to hypoxic stress and re-oxygenation. Marine Pollution Bulletin. 2014;85(2):522–525. doi: 10.1016/j.marpolbul.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Shumway (1982).Shumway SE. Oxygen consumption in oysters: an overview. Marine Biology Letters. 1982;3(1):1–23. [Google Scholar]
- Sobral & Widdows (1997).Sobral P, Widdows J. Effects of elevated temperatures on the scope for growth and resistance to air exposure of the clam Ruditapes decussatus (L.), from southern Portugal. Scientia Marina. 1997;61(2):163–171. doi: 10.1023/A:1018435711128. [DOI] [Google Scholar]
- Sokolova et al. (2012).Sokolova IM, Frederich M, Bagwe R, Lannig G, Sukhotin AA. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Environmental Research. 2012;79:1–15. doi: 10.1016/j.marenvres.2012.04.00. [DOI] [PubMed] [Google Scholar]
- Solorzano (1969).Solorzano L. Determination of ammonia in natural waters by the phenolhypochlorite method. Limnology and Oceanography. 1969;14(5):799–801. doi: 10.4319/lo.1969.14.5.0799. [DOI] [Google Scholar]
- Sommer, Klein & Pörtner (1997).Sommer A, Klein B, Pörtner HO. Temperature induced anaerobiosis in two populations of the polychaete worm Arenicola marina (L.) Journal of Comparative Physiology B. 1997;167(1):25–35. doi: 10.1007/s003600050044. [DOI] [Google Scholar]
- Stickland & Parsons (1968).Stickland J, Parsons T. A practical handbook of seawater analysis. Fisheries Research Board of Canada Bulletin. 1968;55(1):167. doi: 10.1002/iroh.19700550118. [DOI] [Google Scholar]
- Tsugawa et al. (2011).Tsugawa H, Tsujimoto Y, Arita M, Bamba T, Fukusaki E. GC/MS based metabolomics: development of a data mining system for metabolite identification by using soft independent modeling of class analogy (SIMCA) BMC Bioinformatics. 2011;12(1):131. doi: 10.1186/1471-2105-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberk et al. (2013).Verberk WCEP, Sommer U, Davidson RL, Viant MR. Anaerobic metabolism at thermal extremes: a metabolomic test of the oxygen limitation hypothesis in an aquatic insect. Integrative and Comparative Biology. 2013;53(4):609–619. doi: 10.1093/icb/ict015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter, Hwang & Hodson (1986).Vetter RD, Hwang HM, Hodson RE. Comparison of glycogen and adenine nucleotides as indicators of metabolic stress in mummichogs. Transactions of the American Fisheries Society. 1986;115(1):47–51. doi: 10.1577/1548-8659(1986)115<47:COGAAN>2.0.CO;2. [DOI] [Google Scholar]
- Vitale & Friedl (1984).Vitale MA, Friedl FE. Ammonia production by the freshwater bivalve Elliptio buckleyi (LEA): intact and monovalve preparations. Comparative Biochemistry and Physiology Part A: Physiology. 1984;77(1):113–116. doi: 10.1016/0300-9629(84)90021-5. [DOI] [Google Scholar]
- Wang et al. (2011).Wang Y, Hu M, Wong WH, Shin PK, Cheung SG. The combined effects of oxygen availability and salinity on physiological responses and scope for growth in the green-lipped mussel Perna viridis. Marine Pollution Bulletin. 2011;63(5–12):255–261. doi: 10.1016/j.marpolbul.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2015).Wang YJ, Li L, Hu M, Lu W. Physiological energetics of the thick shell mussel Mytilus coruscus exposed to seawater acidification and thermal stress. Science of the Total Environment. 2015;514:261–272. doi: 10.1016/j.scitotenv.2015.01.092. [DOI] [PubMed] [Google Scholar]
- Wei et al. (2015).Wei L, Wang Q, Ning X, Mu C, Wang C, Cao R, Wu H, Cong M, Li F, Ji C, Zhao J. Combined metabolome and proteome analysis of the mantle tissue from pacific oyster Crassostrea gigas exposed to elevated pCO2. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics. 2015;13:16–23. doi: 10.1016/j.cbd.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Widdows (1978).Widdows J. Physiological indices of stress in Mytilus edulis. Journal of the Marine Biological Association of the UK. 1978;58(1):125–142. doi: 10.1017/S0025315400024450. [DOI] [Google Scholar]
- Yang et al. (2017).Yang C, Gao Q, Liu C, Wang L, Zhou Z, Gong C, Zhang A, Zhang H, Qiu L, Song L. The transcriptional response of the pacific oyster, Crassostrea gigas, against acute heat stress. Fish & Shellfish Immunology. 2017;68:132–143. doi: 10.1016/j.fsi.2017.07.016. [DOI] [PubMed] [Google Scholar]
- You & Jiao (2011).You ZJ, Jiao HF. Research on Xiangshan Bay ecological environmental protection and restoration technology. Beijing: China Ocean Press; 2011. pp. 9–10. [in Chinese] [Google Scholar]
- Young & Alfaro (2018).Young T, Alfaro AC. Metabolomic strategies for aquaculture research: a primer. Reviews in Aquaculture. 2018;10(1):26–56. doi: 10.1111/raq.12146. [DOI] [Google Scholar]
- Yukihira, Lucas & Klumpp (2000).Yukihira H, Lucas JS, Klumpp DW. Comparative effects of temperature on suspension feeding and energy budgets of the pearl oysters Pinctada margaritifera and P. maxima. Marine Ecology-Progress Series. 2000;195:179–188. doi: 10.3354/meps195179. [DOI] [Google Scholar]
- Zhang et al. (2004).Zhang JH, Fang JG, Hawkins AJS, Pascoe PL. The effect of temperature on clearance rate and oxygen consumption of scallops, Chlamys farreri. Journal of Shellfish Research. 2004;23(3):715–721. [Google Scholar]
- Zhang et al. (2019).Zhang X, Shi J, Sun Y, Habib YJ, Yang H, Zhang Z, Wang Y. Integrative transcriptome analysis and discovery of genes involving in immune response of hypoxia/thermal challenges in the small abalone Haliotis diversicolor. Fish & Shellfish Immunology. 2019;84:609–626. doi: 10.1016/j.fsi.2018.10.044. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2017).Zhang Y, Wu H, Wei L, Xie Z, Guan B. Effects of hypoxia in the gills of the manila clam Ruditapes philippinarum using NMR-based metabolomics. Marine Pollution Bulletin. 2017;114(1):84–89. doi: 10.1016/j.marpolbul.2016.08.066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A): the control (B): 2 h (C): 24 h. The ordinate shows the relative mass abundance, and the abscissa shows the retention time.
A: 2 h, B: 24 h.
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements of metabolic rates, data matrix of metabolomics analysis, the metabolites with significant changes, pathway enrichment analysis of the metabolites between 2 h and 24 h, and all pathways enriched after exposure to 32 °C are available in the Supplemental Files.