Abstract
Platelets play a crucial role in wound healing as they are reservoirs of growth factors and cytokines which play a fundamental role in homeostasis and tissue remodeling. Recently, fields such as dermatology and plastic and reconstructive surgery have become interested in the tissue regenerative properties of these compounds, especially since it promotes wound healing, improves scar outcomes and has rejuvenating effects on the skin and other tissues. We evaluated the effects of Platelet Rich Fibrin (PRF) in full thickness skin graft healing. Our study included 40 male Wistar rats. Skin grafts were assessed macroscopically using planimetry. The full thickness skin grafts in the test group, displayed a lower necrosis rate compared to the control group. Our study displays the potential benefits of using Platelet Rich compounds to facilitate wound healing and integration of full thickness skin grafts.
Keywords: Full thickness skin graft, platelet rich fibrin, dermal necrosis, planimetry
Introduction
Platelet concentrate. Growth factors
Platelets play a crucial role in wound healing as they are reservoirs of growth factors and cytokines which play a fundamental role in homeostasis and tissue remodeling.
First described in hematology, Platelet Rich Plasma (PRP) describes plasma that has a higher platelet count than peripheral blood.
A first-generation platelet concentrate, it was initially used as a blood transfusion product to treat patients suffering from thrombocytopenia and later used for enhancing wound healing [1].
Years later, the short duration of cytokine release (obtaining a peak concentration 15-60 minutes after administration) and its poor mechanical properties, prompted the development of another Platelet Rich compound prepared from an anticoagulant-free blood sample, without exogenous thrombin, in order to obtain Platelet Rich Fibrin (PRF)-a fibrin based concentrated biomaterial which polymerizes during centrifugation, entrapping platelets and growth factors for a continual and gradual release of growth factors over a 10-day period.
This was done because of the hemostatic properties that fibrin displays, as well as the favorable architecture it provides and the demonstrated effects that platelet derived growth factors exhibit in the wound process [2,3,4,5,6].
A natural source of molecules that play a role in cell signaling, stimulation of cellular growth, proliferation, and differentiation, Platelet Rich compounds have a significant role in modifying the pericellular environment by releasing numerous growth factors such as VEGF, FGF, PDGF, EGF, IGF-1 and IGF-2 [6,7,8,9,10,11,12].
Recently, fields such as dermatology and plastic and reconstructive surgery have become interested in the tissue regenerative properties of these compounds, especially since it promotes wound healing, improves scar outcomes and has rejuvenating effects on the skin and other tissues [2,8,9,12,13,14,15].
Historically, it has been proven that fibrin plays a critical role in the adherence of biological dressings and autografts to wounds, the presence of fibrin being associated with a high graft success rate [16].
Matherial and Method
For our in vivo study, adult male Wistar rats were selected as our experimental model for full-thickness skin wounds covered with full-thickness skin grafts.
This animal model was selected because of its ease of handling, histological similarities to human skin and numerous previous experiments performed on the skin of the rat [8,9,10,17].
The University of Medicine and Pharmacy of Craiova supplied 40 rats of at least 52 weeks of age, weighing between 460-550g per specimen.
The study was approved by the Animal Care and Use Committee and the Ethics Committee of the University of Medicine and Pharmacy of Craiova, Romania.
The rats were divided into two groups randomly: a control group and a test group (PRF treated).
The rats were housed individually in cages, in order to avoid any interaction or trauma to the surgical site, provided with typical living conditions, allowed access to food and water ad libitum.
Wound healing time was recorded, and the residual unhealed wound area was calculated on the 21st day.
Electing the experimental animal models of similar age and weight, we were able to consistently and reliably induce identical dorsal lesions on all specimens.
All surgical procedures were conducted with minimal variation in performance, under sterile conditions, at the animal research center of University of Medicine and Pharmacy of Craiova.
Anesthesia
The rats were anesthetized by means of an intraperitoneal injection of a solution of Ketamine and Xylazine (50mg/kg and 5mg/kg respectively). Successful anesthesia was evaluated by using a toe pinch test [18,19].
Preparation
Prior to the surgical procedure, the rats’ hair on the dorsum was clipped with electrical clippers and shaved with a razor.
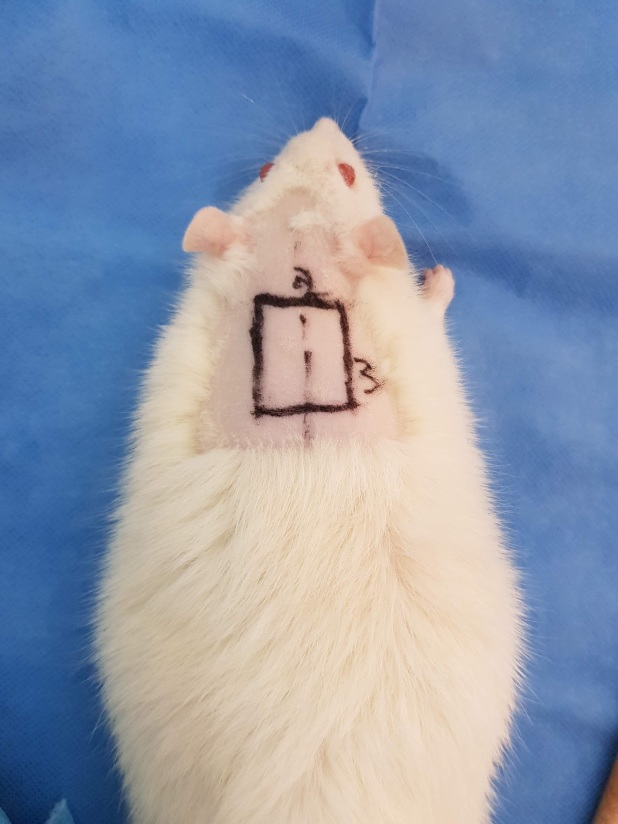
We performed rectangular 3 by 2 centimeters preoperative surgical markings on the dorsum of the rats, after placing them in a prone position on the surgical platform (Fig. 1).
Figure 1.
Preoperative surgical markings
We surgically scrubbed the skin of the animals with a topical aqueous solution of 7.5% povidone-iodine and draped with sterile surgical fields.
Surgery
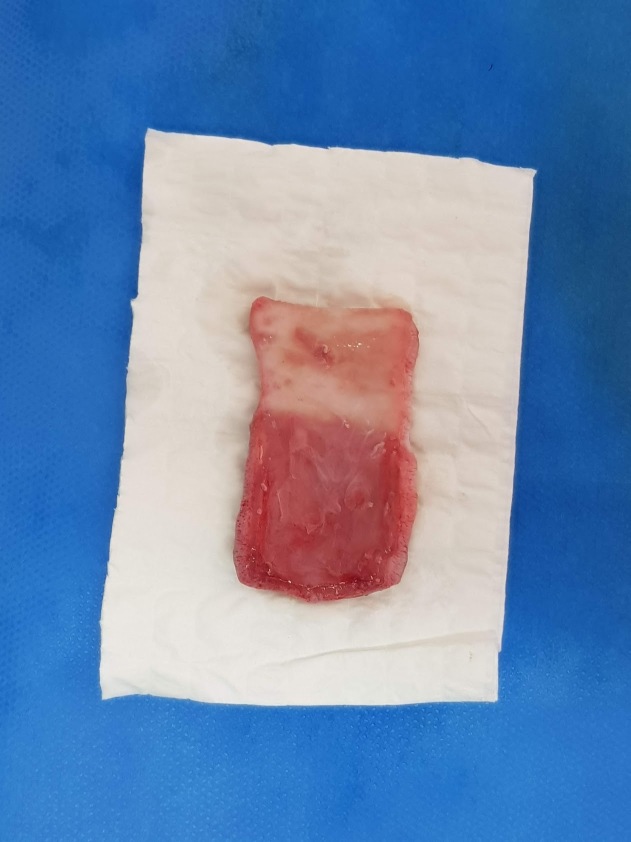
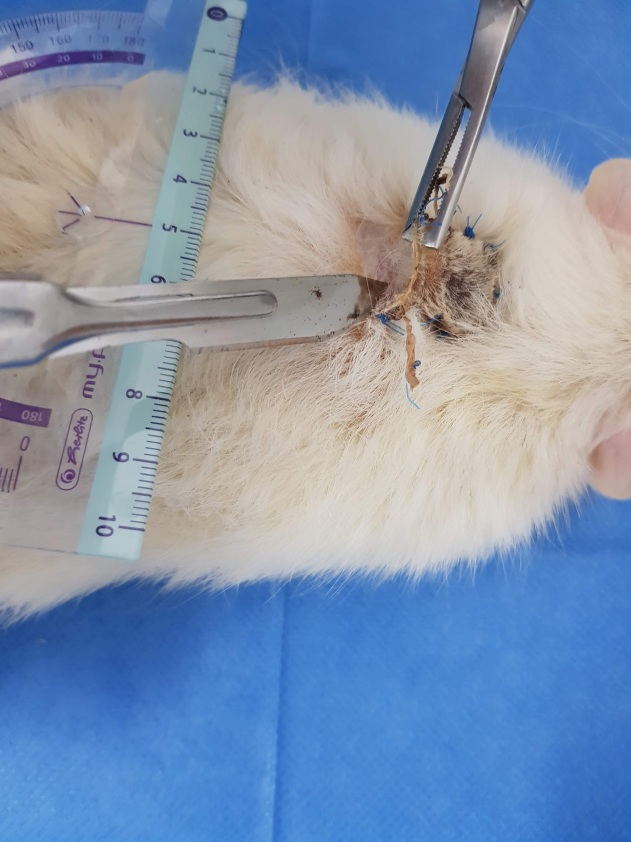
The incisions were made on the outlined preoperatory marks and the skin was dissected from the deep muscular fascia and harvested (Fig.2). The panniculus carnosus muscle was detached from the overlying dermis (Fig. 3).
Figure 2.
Deep muscular fascia and flap with panniculus carnosus muscle
Figure 3.
Full thickness skin graft with Panniculus carnosus muscle partially removed
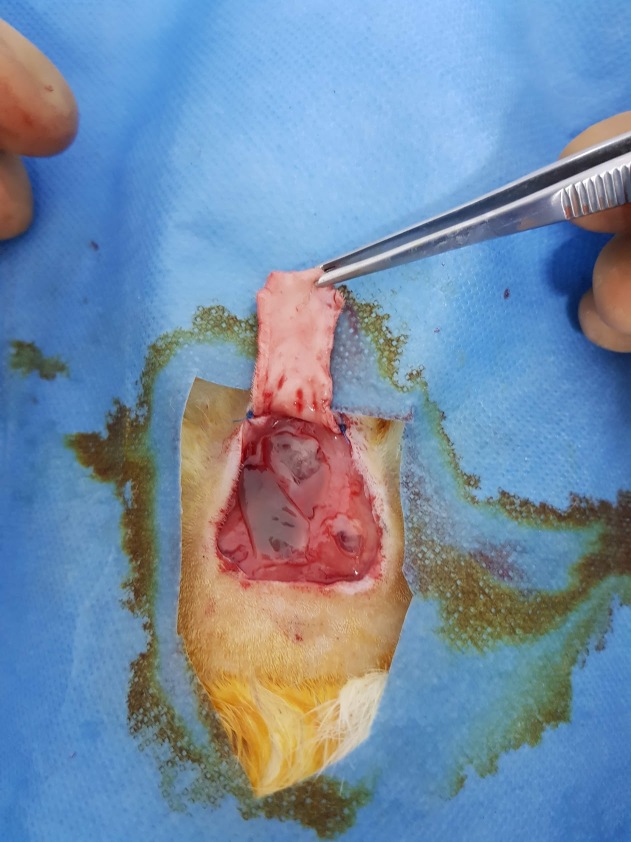
After scrubbing the wound again with povidone-iodine and cleansing with saline solution, the skin graft was then sutured with 4-0 polypropylene surgical sutures (Fig. 4, 5).
Figure 4.
PRF on deep muscular fascia
Figure 5.
Sutured skin graft
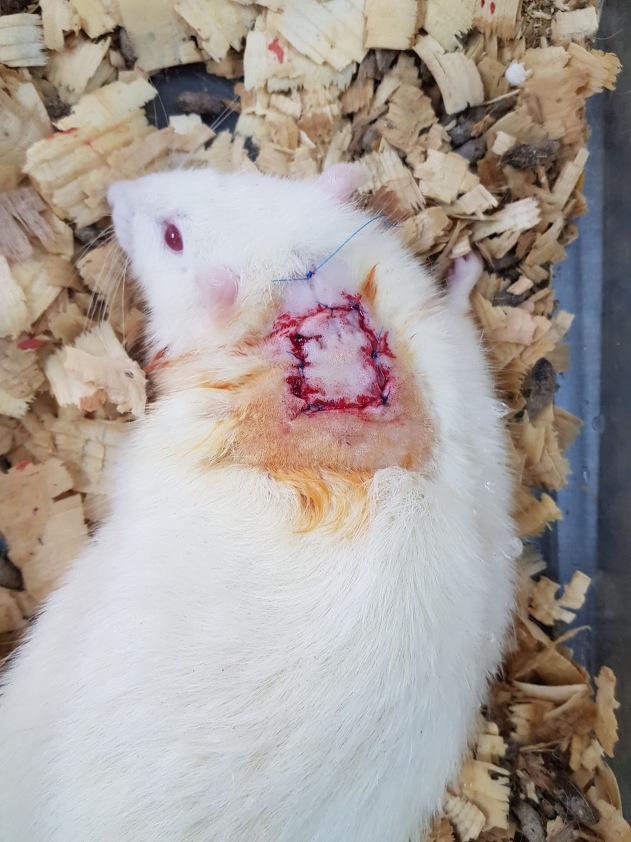
A tie-over dressing was used in order to prevent the formation of a hematoma and protect against trauma and infection (Fig. 6).
Figure 6.
Tie over dressing
We harvested the blood needed to produce PRF from the control group rats, on the 21st day, at the finalization of the experiment, in order to limit the number of animals needed.
Because of the large quantities of blood required for the experiment and the necessity for rapid harvesting to prevent clotting before centrifugation, the blood had to be collected via cardiac punctures.
The rats were deeply anesthetized for the terminal procedure and the required quantity of blood was obtained.
Using this technique, we were able to harvest 9-12ml of blood from each rat. The blood samples were harvested on plain vacuum tubes and processed according to our protocol-centrifuged at a relative centrifugal force (RCF) of 450g for 12 minutes [5,19,20,21].
During the centrifugation process, a different rat, part of the test group was anesthetized. After the dissection and harvesting of the full thickness skin graft, PRF was applied on the deep muscular fascia (Fig.4). The graft was applied on the wound bed and fixed with 4-0 polypropylene surgical suture and covered with a tie over dressing.
Healing was monitored daily and, on the 21st postoperative day, the rats were humanely terminated. Wound healing was evaluated by the residual wound area.
Planimetry
21 days after the surgical procedure, the skin grafts were assessed macroscopically by planimetry. This period was chosen because it allows for an objective assessment of the viability of the graft result.
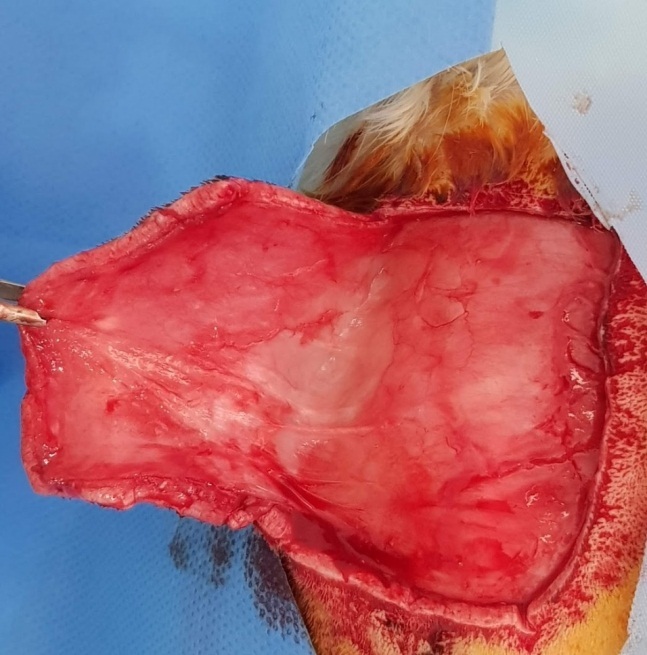
After the removal of the epidermal scab, which was present in all specimens (Fig.7), the dermal tissue was assessed. We calculated the area of dermal necrosis as a percentage of the total surface area in the skin graft sample (Fig.8).
Figure 7.
Removing the epidermolysis scab
Figure 8.
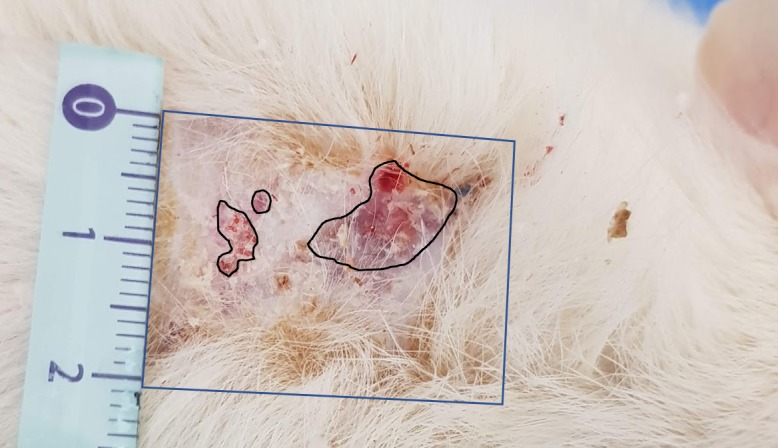
Areas of necrosis characterized with distinctive demarcation
Our statistics and data analysis were conducted using Microsoft Excel and GraphPad Prism 8. The images were processed using ImageJ medical image processing software, by using the selection tools and analyze particle functions [22].
Results
Of the 40 male Wistar rats, equally divided in 2 groups (Control and Test), included in the study, 2 died before the assessment of the results (1 from each group).
We also encountered 2 instances of PRF preparation failure, because of prolonged blood harvesting time which lead to the blood clotting while in the plain vacuum tubes, before centrifugation. As the surgical procedure was already underway on the Test group rats, we decided to continue with the procedure without PRF, and place the rats in the control group. Thus, we were left with a control group comprised of 21 rats, and a test group of 17 rats.
Using the planimetric assessment of the graft areas we observed a statistically significant difference between the control group and test group (p<0.01).
Epidermal necrosis was discovered in 100% of the skin grafts, in both the control and test groups. The epidermal scab was removed before the evaluation of the underlying dermal graft. The healthy and necrotic areas of the graft were subsequently calculated.
Even though epidermal necrosis was observed in all test subjects, it did not reflect the quality of the underlying dermis.
Full thickness skin graft dermal necrosis rates were compared between the control and test groups by a 2-tailed t-test. The statistical significance was determined at p<0.01. The mean percentage of dermal necrosis in full thickness skin grafts augmented with PRF (14.9%, SD=5.1) was significantly lower than that in control grafts (28.5%, SD=9.2)-p<0.01 (Fig.9,10).
Figure 9.
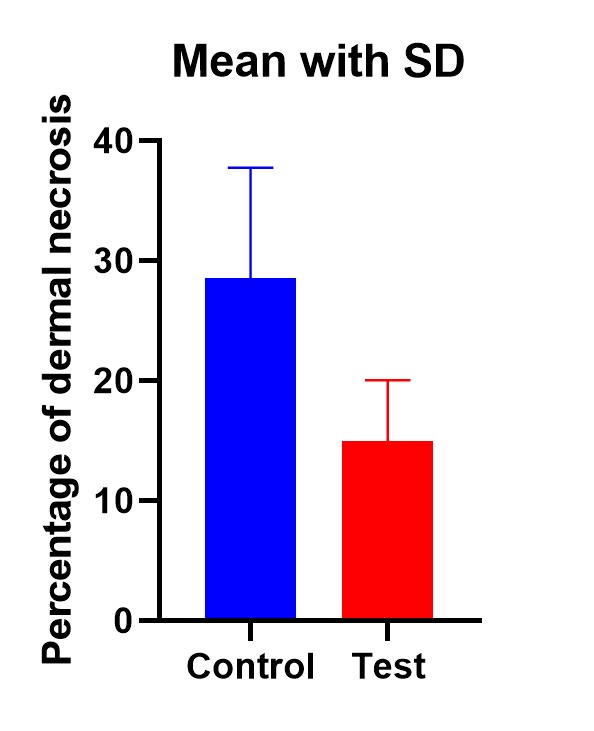
Significantly lower necrosis rate in the test group (p<0.01)
Figure 10.
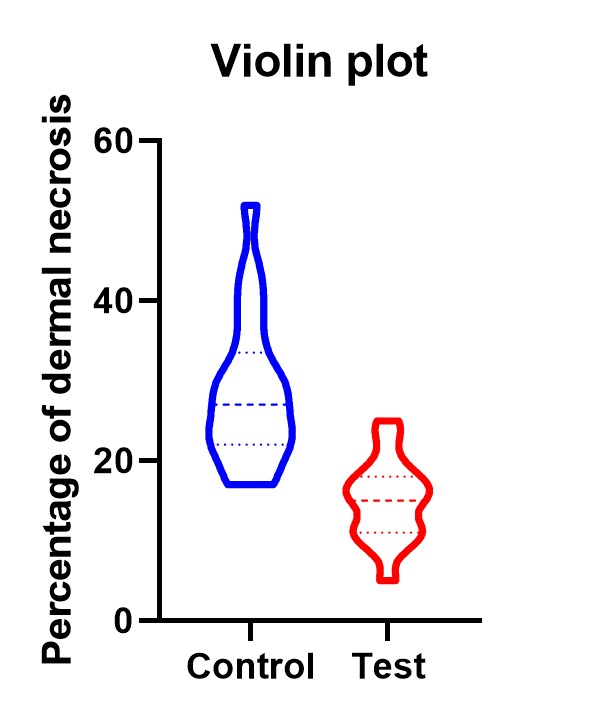
Data distribution
Discussion
Skin grafts are a vital tool for reconstructive surgery with a wide range of applications in a wide variety of pathological situations. The high incidence of burns that require medical care (30,000/day or about 11 million per year) [23], other post-traumatic skin defect and skin tumors, makes skin grafting a very common operative procedure, widely used in plastic, reconstructive, and aesthetic surgery.
Non-lethal burn complications are the main cause of morbidity and lead to prolonged hospital stay, disfigurement, disability and ultimately a high level of social stigma and social rejection, even if a functional recovery is made.
Skin grafts are the most efficient and simple method that a plastic surgeon can employ in order to repair skin defects. While effective, the functional and aesthetic outcomes have a wide range of unpredictability due to the nature of the injury, as well as the variable post-surgical healing process. This process is determined by a number of factors that have to do with both the quality of care and also the specific particularities of the patient and case. Often, complications may lead to a lack of adherence of the skin graft, hypertrophic and keloid scarring which may induce a high level of functional impairment and aesthetic discomfort.
A way to offer a higher benefit rate to patients would be to ensure a reliable graft outcome regardless of patient and injury related factors. For this reason, the notion of PRF adjuvant therapy could constitute a new support pylon in order to improve the overall standard of care. PRF is a second generation PRP which contains a resorbable fibrin network that abounds with cytokines and growth factors which are steadily released, improving skin graft adherence and supporting new tissue formation [2,3,6].
Cutaneous wound healing summarizes the complex and fragile assembly of biological and biochemical processes that are initiated in different tissues following injury. Because of its innate susceptibility of failure and interruption, the complex healing process may be augmented by providing the tissues with the building blocks for new cell formation. These stages are finely regulated by a plethora of growth factors and cytokines produced by a wide range of cells present in the extracellular matrix [6,8,9,17,24].
By establishing a reproducible model of study to evaluate the multiple stages of tissue remodeling and repair following injury, this experiment aimed to study one possible method of augmentation of the healing process.
Although full thickness skin grafts generally have a low percentage of graft failure, we speculate that augmenting wound beds with growth factors, can accelerate healing and aid the natural biological process of wound repair, may improve scarring in the skin, as well as in other organs and tissues.
Another advantage of its usage is an increase in availability and relatively high ease of use, as well as a low cost of usage. It can then be theorized that it would be a valid method to assist skin grafting for a wide spectrum of patients as well as a wide array of injury types, particularly small injuries that concern articular surfaces, such as the interphalangeal joints or other highly mechanically strained areas.
New studies are warranted to detail possible ways of accelerating wound healing, improving the architecture of the newly formed tissue and furthermore evaluate the possible epithelial-mesenchymal interactions that contribute to the transformations of tissues affected by lesions.
Conclusions
Our study displays the potential benefits of using Platelet Rich compounds to facilitate wound healing and integration of full thickness skin grafts.
The translational potential of this study involves the development of immediate, cheap and readily available augmentations for the full thickness skin graft and we believe that our study has elaborated the potential benefits of using PRF to treat suboptimal wound beds in order to promote healing.
References
- 1.Wandt H, Schäfer-Eckart K, Greinacher A. Platelet Transfusion in Hematology, Oncology and Surgery. Dtsch Arztebl Int. 2014;111(48):809–815. doi: 10.3238/arztebl.2014.0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naik B, Karunakar P, Jayadev M, Marshal VR. Role of Platelet rich fibrin in wound healing: A critical review. J Conserv Dent. 2013;16(4):284–293. doi: 10.4103/0972-0707.114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi E, Flückiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, et al. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 2016;20(9):2353–2360. doi: 10.1007/s00784-016-1719-1. [DOI] [PubMed] [Google Scholar]
- 4.Bansal S, Garg A, Khurana R, Chhabra P. Platelet-rich fibrin or platelet-rich plasma-which one is better? an opinion. Indian J Dent Sci. 2017;9(5):49–52. [Google Scholar]
- 5.Yazigi Junior JA, Dos Santos JBG, Xavier BR, Fernandes M, Valente SG, Leite VM. Quantification of platelets obtained by different centrifugation protocols in SHR rats. Rev Bras Ortop. 2015;50(6):729–738. doi: 10.1016/j.rboe.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khiste SV, Naik Tari R. Platelet-Rich Fibrin as a Biofuel for Tissue Regeneration. ISRN Biomater. 2013;2013:1–6. [Google Scholar]
- 7.Kronemann N, Bouloumié A, Bassus S, Kirchmaier CM, Busse R, Schini-Kerth VB. Aggregating Human Platelets Stimulate Expression of Vascular Endothelial Growth Factor in Cultured Vascular Smooth Muscle Cells Through a Synergistic Effect of Transforming Growth Factor-β 1 and Platelet-Derived Growth Factor AB. Circulation. 1999;100:855–860. doi: 10.1161/01.cir.100.8.855. [DOI] [PubMed] [Google Scholar]
- 8.Zhang F, Oswald TM, Lin L, Wang S, Lin S, Lineaweaver WC. Improvement of Full-Thickness Skin Graft Survival by Application of Vascular Endothelial Growth Factor in Rats. Ann Plast Surg. 2008;60(5):589–593. doi: 10.1097/SAP.0b013e31816d78fe. [DOI] [PubMed] [Google Scholar]
- 9.Richter GT, Fan CY, Ozgursoy O, McCoy J, Vural E. Effect of Vascular Endothelial Growth Factor on Skin Graft Survival in Sprague-Dawley Rats. Arch Otolaryngol Neck Surg. 2006;132(6):637–641. doi: 10.1001/archotol.132.6.637. [DOI] [PubMed] [Google Scholar]
- 10.Kryger Z, Zhang F, Lineaweaver WC, Dogan T, Cheng C, Buncke HJ. The effects of VEGF on survival of a random flap in the rat: examination of various routes of administration. Br J Plast Surg. 2000;53(3):234–239. doi: 10.1054/bjps.1999.3315. [DOI] [PubMed] [Google Scholar]
- 11.Su CY, Kuo YP, Tseng YH, Su C-H, Burnouf T. In vitro release of growth factors from platelet-rich fibrin (PRF): a proposal to optimize the clinical applications of PRF. Oral Surgery Oral Med Oral Pathol Oral Radiol Endodontology. 2009;108(1):56–61. doi: 10.1016/j.tripleo.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Vahabi S, Vaziri S, Torshabi M, Rezaei Esfahrood Z. Effects of Plasma Rich in Growth Factors and Platelet-Rich Fibrin on Proliferation and Viability of Human Gingival Fibroblasts. J Dent (Tehran) 2015;12(7):504–512. [PMC free article] [PubMed] [Google Scholar]
- 13.Lin H, Yang Y, Wang Y, Wang L, Zhou X, Liu J, Peng D. Effect of Mixed Transplantation of Autologous and Allogeneic Microskin Grafts on Wound Healing in a Rat Model of Acute Skin Defect. PLoS One. 2014;9(1):e85672–e85672. doi: 10.1371/journal.pone.0085672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietrich F, Duré GL, Klein CP, Bampi VF, Padoin AV, Silva VD, Braga-Silva J. Platelet-Rich Fibrin Promotes an Accelerated Healing of Achilles Tendon When Compared to Platelet-Rich Plasma in Rat. World J Plast Surg. 2015;4(2):101–109. [PMC free article] [PubMed] [Google Scholar]
- 15.Nica O, Grecu A, Dincă EA, Marinescu D, Ciurea ME. A Rare Case of Upper Calf Swelling and Necrosis-The Morel-Lavallée Lesion. Curr Heal Sci J. 2017;44(3):311–315. doi: 10.12865/CHSJ.44.03.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tavis MJ, Thornton JW, Harney JH, Woodroof EA, Bartlett RH. Graft adherence to de-epithelialized surfaces: a comparative study. Ann Surg. 1976;184(5):594–600. doi: 10.1097/00000658-197611000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richter GT, Bowen T, Boerma M, Fan C-Y, Hauer-Jensen M, Vural E. Impact of Vascular Endothelial Growth Factor on Skin Graft Survival in Irradiated Rats. Arch Facial Plast Surg. 2009;11(2):110–113. doi: 10.1001/archfacial.2008.526. [DOI] [PubMed] [Google Scholar]
- 18.Xu Q, Ming Z, Dart AM, Du X-J. Optimizing Dosage of Ketamine and Xylazine in Murine Echocardiography. Clin Exp Pharmacol Physiol. 2007;34(5-6):499–507. doi: 10.1111/j.1440-1681.2007.04601.x. [DOI] [PubMed] [Google Scholar]
- 19.Parasuraman S, Raveendran R, Kesavan R. Blood sample collection in small laboratory animals. J Pharmacol Pharmacother. 2010;1(2):87–93. doi: 10.4103/0976-500X.72350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dohan Ehrenfest DM, Pinto NR, Pereda A, Jiménez P, Corso MD, Kang B-S, Nally M, Lanata N, Wang HL, Quirynen M. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets. 2018;29(2):171–184. doi: 10.1080/09537104.2017.1293812. [DOI] [PubMed] [Google Scholar]
- 21.Grecu AF, Grecu DC, Nica O, Ciuca EM, Camen A, Ciurea ME. A Novel Method of Obtaining Platelet Rich Fibrin from Rats and Quantifying Platelet Count. Curr Heal Sci J. 2019;45(1):104–110. doi: 10.12865/CHSJ.45.01.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schindelin J, Rueden CT, Hiner MC, Eliceiri KW. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol Reprod Dev. 2015;82(7-8):518–529. doi: 10.1002/mrd.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stokes MAR, Johnson WD. Burns in the Third World: an unmet need. Ann Burns Fire Disasters. 2017;30(4):243–236. [PMC free article] [PubMed] [Google Scholar]
- 24.Seppä H, Grotendorst G, Seppä S, Schiffmann E, Martin GR. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982;92(2):584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]