Abstract
Background. Fabry disease (FD) is a rare genetic lysosomal disease with an estimated prevalence of 1:100000. Mutations on the GLA gene lead to alpha-galactosidase deficiency and multiorgan involvement due to sphingolipid accumulation. Our aim was to present and analyze the demographic and clinical characteristics of the Fabry patients in Romania. Methods. All known Fabry patients in Romania between 2015-2018 were prospectively included in the study. Data on personal history, family history and clinical parameters were collected and statistically analyzed. Results. The study included 42 patients with a mean age of 47±15 years, of which 19 (45%) were men and 23 (55%) women. Women were significantly older (52±15 years vs. 40±13 years, p=0.006) and presented similar prevalence of cardiac, renal, neurologic, ophthalmologic and otologic burden. The majority of patients presented organ damage, most prevalent being cardiac (48%), cutaneous (45%) and neurologic (52%) involvements. There were 20 families in total, comprising on average of 2.1 members each. Of the 20 families, only two had the same pathogenic GLA mutation. Conclusion. FD patients in our country show a significant degree of multiorgan involvement with important psychological and social impact on the patients and their families. Women with Fabry disease show similar disease burden as men, but at a later age.
Keywords: Fabry disease, Genetic disease, Lysosomal disease
Introduction
Fabry disease (FD) is a rare genetic disorder caused by mutations in the GLA gene, located on the X chromosome. This gene encodes a lysosomal enzyme called alpha-galactosidase (AGAL), which is responsible of metabolizing the sphingolipid called globotriaosylceramide (Gb3). Thus, in FD there is accumulation of Gb3 in most tissues leading to multi-organ involvement [1]. Moreover, being an X-linked disease, Fabry mutations are mathematically twice as prevalent in women.
FD is best known for its characteristic skin lesions, called angiokeratoma; however, involvement of the heart, kidney and central nervous system determine the negative prognosis of the disease [2].
The importance of correctly diagnosing FD lies in the existence of specific treatment (enzyme replacement therapy), which has been demonstrated to increase the life expectancy of these patients when started early [3]. Thus, timely diagnosis is essential.
The aim of the present study was to describe the clinical characteristics in the Romanian population of FD patients.
Matherial and Methods
This is an observational, prospective study, which included all patients with a positive genetic diagnosis for FD in Romania, diagnosed before the end of 2018. There were no exclusion criteria once a clear Fabry diagnosis was established. The study was approved by the local ethics’ committee (Ethics’ Committee of the “Prof. Dr. C.C. Iliescu” Emergency Institute for Cardiovascular Diseases, Bucharest).
For each patient, 50 parameters were recorded regarding clinical data and personal history.
Anamnestic parameters included cardiologic symptomatology (angina, palpitations, syncope), organ involvement diagnosis related to FD (cutaneous, ophthalmologic, auditive, cardiac, renal, nervous system), number of relatives also diagnosed with FD and specific mutations for each family.
Organ involvement was defined as follows:
• Skin-angiokeratoma
• Heart-left ventricular hypertrophy (maximum wall thickness of >12mm) on echocardiography
• Kidney-albuminuria (30mg/24 hours, albumin/creatinine rate ≥30mg/g) and/or chronic kidney disease at least stage 3 (glomerular filtration rate <60ml/min/1.73m2)
• Nervous system-peripheral involvement (acroparesthesia, lack of sweating, heat intolerance) and/or central involvement (stroke, transient ischemic attack)
• Eye-cornea verticillata
• Ear-tinnitus and/or sudden hearing loss in one or both ears
In more than half of the patients (23), we also recorded the Mainz severity index, which is a score that attributes a number of points to each type of organ involvement. Afterwards, the sum of all the scores is calculated and severe involvement is defined as a total score of >40, as described in [4]. Moreover, some of these same patients (10) were asked to submit a disease impact questionnaire used in cardiomyopathies (Kansas City Cardiomyopathy Questionnaire [5]).
Clinical parameters included weight, height, body mass index (BMI), body surface area (BSA) and the presence of high blood pressure (HBP).
The database and statistical analysis were done using Microsoft Excel (Microsoft Corp., Redmond, WA, USA) and IBM SPSS Statistics 23.0 (IBM Corporation, Armonk, NY, USA).
Categorical variables are presented as total number and percentage. Continuous variables are presented as mean±standard deviation. For descriptive statistics, the standard frequencies method in SPSS was used for categorical variables and standard descriptive analysis for continuous variables. Chi2 test was used to determine significance for categorical variables according to a set criterion. ANOVA test was used to determine significance of continuous variables to differentiate two set groups. Correlation graphs along with Pearson’s correlation coefficient (r) were used to determine the relation between continuous variables. Statistical significance was defined as p <0.05.
Results
In total, 42 FD patients were included from all parts of Romania and evaluated in our center.
The average age of the population was 47±15 years. Almost half of the patients were between the ages of 40 and 60 years (48%). The age extremes were a 19 year-old man and an 80 year-old woman (Fig. 1).
Figure 1.
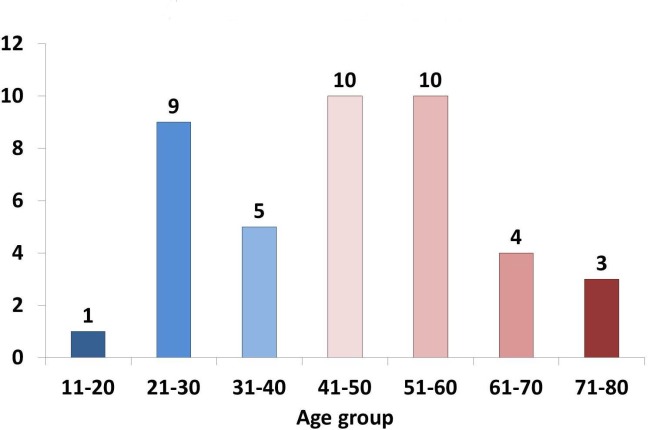
Age distribution of patients in 10-year groups
Sex distribution was relatively equal: 19 men (45%) and 23 women (55%). However, the women were significantly older, on average years older (52±15 years vs. 40±13 years, p=0.006).
The 42 patients in the study are distributed in 20 families, which results in an average of 2.1 diagnosed members per family. There was only one GLA mutation that was shared by 2 families (p.[N215S], c.[644A>G]), all the other families have their unique mutation in this Romanian Fabry population. Also, in total there were 7 families of only one member diagnosed with FD, each. Table 1 details the specific GLA mutations and family distribution of these patients, as well as their age and sex.
Table 1.
The genetic mutations and family distribution of all Fabry disease patients in Romania in 2018
|
No. |
Family |
Sex (M=1/F=0) |
Age (years) |
GLA Mutation (c) |
GLA Mutation (p) |
|
1 |
1 |
0 |
62 |
c.[1031T>C] |
p.[L344P] |
|
2 |
1 |
0 |
40 |
c.[1031T>C] |
p.[L344P] |
|
3 |
2 |
0 |
55 |
c.[1224del66] |
- |
|
4 |
2 |
1 |
28 |
c.[1224del66] |
- |
|
5 |
3 |
1 |
40 |
c.[141G>A] |
p.[W47*] |
|
6 |
3 |
0 |
60 |
c.[141G>A] |
p.[W47*] |
|
7 |
4 |
1 |
44 |
c.[154T>A] |
p.[C52S] |
|
8 |
4 |
0 |
67 |
c.[154T>A] |
p.[C52S] |
|
9 |
5 |
1 |
54 |
c.[295C>T] |
p.[Q99*] |
|
10 |
6 |
1 |
29 |
c.[334C>T] |
p.[R112C] |
|
11 |
6 |
0 |
50 |
c.[334C>T] |
p.[R112C] |
|
12 |
7 |
1 |
65 |
c.[416A>G] |
p.[N139S] |
|
13 |
8 |
1 |
35 |
c.[485G>A] |
p.[W162*] |
|
14 |
8 |
0 |
34 |
c.[485G>A] |
p.[W162*] |
|
15 |
8 |
0 |
54 |
c.[485G>A] |
p.[W162*] |
|
16 |
9 |
0 |
68 |
c.[548delG] |
- |
|
17 |
9 |
1 |
44 |
c.[548delG] |
- |
|
18 |
9 |
0 |
47 |
c.[548delG] |
- |
|
19 |
10 |
0 |
59 |
c.[644A>G] |
p.[N215S] |
|
20 |
11 |
0 |
27 |
c.[671A>G] |
p.[N224S] |
|
21 |
11 |
0 |
30 |
c.[671A>G] |
p.[N224S] |
|
22 |
11 |
0 |
28 |
c.[671A>G] |
p.[N224S] |
|
23 |
11 |
1 |
60 |
c.[671A>G] |
p.[N224S] |
|
24 |
11 |
1 |
43 |
c.[671A>G] |
p.[N224S] |
|
25 |
12 |
1 |
19 |
c.[779G>A] |
p.[G260E] |
|
26 |
12 |
0 |
48 |
c.[779G>A] |
p.[G260E] |
|
27 |
13 |
0 |
49 |
c.[797A>C] |
p.[D266A] |
|
28 |
13 |
1 |
29 |
c.[797A>C] |
p.[D266A] |
|
29 |
13 |
1 |
30 |
c.[797A>C] |
p.[D266A] |
|
30 |
14 |
1 |
49 |
c.[831G>A] |
p.[W277*] |
|
31 |
15 |
1 |
26 |
c.[836A>G] |
p.[Q279R] |
|
32 |
15 |
1 |
41 |
c.[836A>G] |
p.[Q279R] |
|
33 |
15 |
0 |
51 |
c.[836A>G] |
p.[Q279R] |
|
34 |
16 |
1 |
41 |
c.[863C>A] |
p.[A288D] |
|
35 |
16 |
0 |
74 |
c.[863C>A] |
p.[A288D] |
|
36 |
17 |
0 |
55 |
c.[937G>T] |
p.[D313Y] |
|
37 |
18 |
0 |
59 |
c.[1121_1123delAAG] |
- |
|
38 |
18 |
1 |
29 |
c.[1121_1123delAAG] |
- |
|
39 |
18 |
0 |
37 |
c.[1121_1123delAAG] |
- |
|
40 |
19 |
0 |
80 |
c.[644A>G] |
p.[N215S] |
|
41 |
19 |
1 |
55 |
c.[644A>G] |
p.[N215S] |
|
42 |
20 |
0 |
78 |
* |
* |
*Mutation for patient no. 42 could not be obtained from their home center at this time
Most patients (55%) had normal weight (BMI 18.5-24.9kg/m2), while 35% were overweight (BMI 25-29.9kg/m2), 10% obese (BMI >30kg/m2) and only 7% underweight (BMI <18.5kg/m2). Patient measurements averages are presented in Table 2.
Table 2.
Table 2. Patient measurements
BMI, body mass index; BSA, body surface area
|
Parameter |
Total (42) |
Men (19) |
Women (23) |
p |
|
Height (cm) |
167±9 |
173±7 |
162±6 |
<0.001 |
|
Weight (kg) |
69±14 |
72±15 |
65±13 |
0.112 |
|
BMI (kg/m2) |
24.5±4.6 |
24.0±4.6 |
24.9±4.7 |
0.599 |
|
BSA (m2) |
1.77±0.20 |
1.86±0.20 |
1.69±0.16 |
0.005 |
When comparing organ involvement according to sex, the only involvement that was significantly higher in men was the cutaneous one, 15 (79%) vs. 4 (17%) (Fig. 2).
Figure 2.
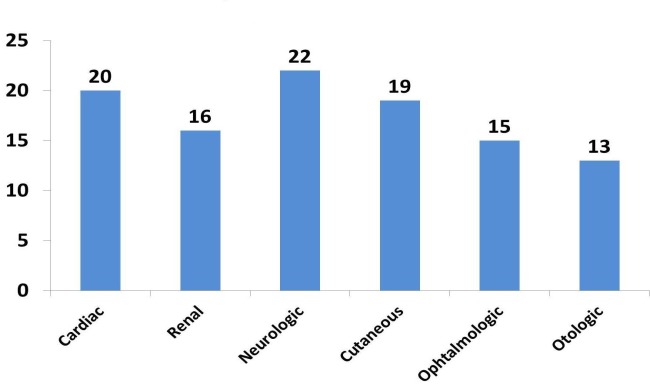
Distribution of organ involvements
Cardiac pacemaker was present in 7 (16%) patients, of which 5 were women and 2 men, while none of the patients had a defibrillator implanted. Myectomy had been performed on 2 (5%) patients. Renal transplantation had been performed on 2 (5%) patients due to renal failure in the context of Fabry nephropathy at young ages (18 and 36 years, respectively), while 2 others (5%) were currently on dialysis therapy at the time of inclusion in this study.
Cardiac symptomatology was present in a part of the patients. They most commonly described fatigability or dyspnea (38%) or palpitations (26%); however, there were also cases some presenting angina (14%) or fainting/syncope (16%) (Fig. 3).
Figure 3.
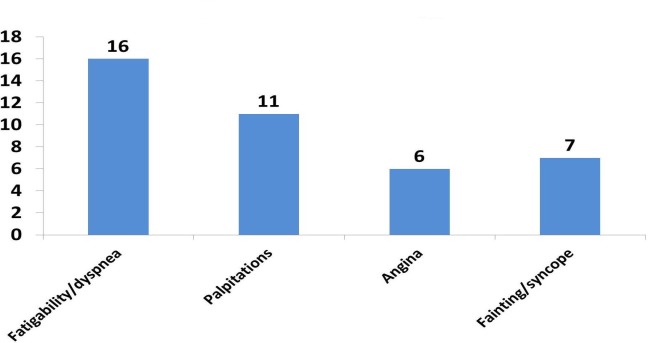
Distribution of cardiac symptoms
Mainz severity index was available in 23 patients, having an average score of 16±9.
Ten of these patients also had the Kansas City Cardiomyopathy Questionnaire available. The two scores presented moderate correlation (r=0.5) (Fig. 4).
Figure 4.
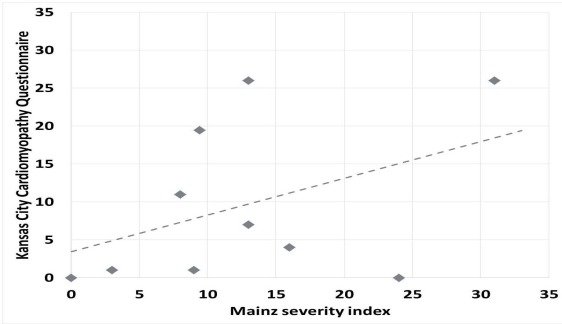
Correlation graph between Mainz Severity Index and Kansas City Cardiomyopathy Questionnaire
Discussion
To our knowledge this is the first paper including an overview of the clinical characteristics of Romanian FD patients. Firstly, the data presented reflects a deficiency in early diagnosis in many of the patients. On the other hand, the data sheds light on the natural history of this disorder, which, when left untreated, greatly diminishes life expectancy [1].
Indeed, enzyme replacement therapy has been shown to slow or stop disease progression when started before permanent alterations to organs occur [6,7].
The prevalence of FD is estimated to be approximately 1:100000 [8], which would mean that in a country of ~20 million people (like Romania) there should be about 200 patients diagnosed. The total number of Fabry patients in Romania is lower than expected, compared to published data on prevalence [1]. However, the total number of cases has considerably increased from the beginning to the end of this study (from 14 to 42 known patients between 2015 and 2018). This important increase has partially been realized through the constant efforts of our center in family screening and raising awareness. However, the average was ~2 FD members per family, which is lower than the estimated average published before [9].
The relatively equal distribution of men/women (19/23) is and indicator of neglect of FD in women, as mathematically, an X-linked disease should have twice as many women than men. Also, analyzing individual cases and families, many of the women in the study are more frequently the mothers of the male patients, rather than sisters or daughters, which is also reflected in the 12-year difference in average age.
Additionally, it has been shown that women are not mere carriers, as it was thought in the past, but they usually develop multiorgan involvement with a delay of 10-15 years compared to men [10]. Moreover, in our study, women had a similar proportion of organ involvement, except for cutaneous lesions. Also, they had an important amount of pacemakers, which is a sign of advanced Fabry cardiomyopathy [11].
Perhaps the most notable feature of the studied population is their heterogeneity in terms of disease burden. These differences can be partially explained by age, sex and time of enzyme therapy initiation. However, there can be phenotypic variability within the same family, as blood relatives and carriers of the same mutation can show predominant involvement of different organs [12]. Also, there were only 2 unrelated families which shared the same GLA mutation, this diversity of genotype is to be expected however in a population of 20 families [8].
Cardiac symptomatology is non-specific in FD [12] and in our study, a good proportion of patients presented fatigability or palpitations. Signs of organ involvement, were present in the majority of the patients, the most prevalent being the neurologic, cardiac and cutaneous ones.
Lastly, the correlation between the scores of the disease severity index and the score of the patient perception of the disease was only moderate. However, the number of patients that had both scores available was low, and the disease perception score refers to cardiac disease [5], while the other one takes into account all the organs [14]. Moreover, the relation between severity scores and quality of life questionnaires (QoL) has been established in other disorders [15,16]. In FD this relationship is yet to be established to the best of our knowledge and comparison between Mainz severity index and QoL would be a compelling objective for future studies.
Conclusion
The current work describes the clinical characteristics of Romanian Fabry patients for the first time, as part of the continuous study on-going in our institution.
FD patients in our country show a significant degree of multiorgan involvement with important psychological and social impact on the patients and their families.
While there is still room for progress, big steps have been taken towards diagnosing and treating this rare disease in the past years in Romania.
References
- 1. Mehta A, Widmer U. Natural history of Fabry disease. In: Mehta A , Beck M, Sunder-Plassmann G, editors. Fabry disease -perspectives from 5 years of FOS. Oxford s: Oxford PharmaGenesis; 2006 . pp. 70 – 75 . [PubMed] [Google Scholar]
- 2.MacDermot KD, Holmes A, Miners AH. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J Med Genet. 2001;38(11):750–760. doi: 10.1136/jmg.38.11.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Germain DP, Charrow J, Desnick RJ, Guffon N, Kempf J, Lachmann RH, Lemay R, Linthorst GE, Packman S, Scott CR, Waldek S, Warnock DG, Weinreb NJ, Wilcox WR. Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease. J Me. Genet. 2015;52(5):353–358. doi: 10.1136/jmedgenet-2014-102797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whybra c, Bähner F, Baron K. Measurement of disease severity and progression in Fabry disease. In: Mehta A , Beck M , Sunder-Plassmann G , editors. Fabry disease-perspectives from 5 years of FOS. Oxford : Oxford PharmaGenesis; 2006. pp. 101– 105. [PubMed] [Google Scholar]
- 5.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 6.Weidemann F, Niemann M, Breunig F, Herrmann S, Beer M, Störk S, Voelker W, Ertl G, Wanner C, Strotmann J. Long-Term Effects of Enzyme Replacement Therapy on Fabry Cardiomyopathy: Evidence for a Better Outcome With Early Treatment. Circulation. 2009;119(4):524–529. doi: 10.1161/CIRCULATIONAHA.108.794529. [DOI] [PubMed] [Google Scholar]
- 7.Branton MH, Schiffmann R, Sabnis SG, Murray GJ, Quirk JM, Altarescu G, Goldfarb L, Brady RO, Balow JE, Austin Iii HA, J undefined. B. Kopp. Natural history of Fabry renal disease: influence of alpha-galactosidase A activity and genetic mutations on clinical course. Medicine (Baltimore) 2002;81(2):122–138. doi: 10.1097/00005792-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Germain DP. Fabry disease. Orphanet J Rare Dis. 2010;22(5):30–30. doi: 10.1186/1750-1172-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laney DA, Fernhoff PM. Diagnosis of Fabry Disease via Analysis of Family History. J Genet Couns. 2008;17(1):79–83. doi: 10.1007/s10897-007-9128-x. [DOI] [PubMed] [Google Scholar]
- 10.Niemann M, Herrmann S, Hu K, Breunig F, Strotmann J, Beer M, Machann W, Voelker W, Ertl G, Wanner C, F undefined. Weidemann. Differences in Fabry Cardiomyopathy Between Female and Male Patients. JACC Cardiovasc Imaging. 2011;4(6):592–601. doi: 10.1016/j.jcmg.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 11. Linhart A. The heart in Fabry disease . In: Mehta A , Beck M , Sunder-Plassmann G , editors. Fabry disease-perspectives from 5 years of FOS. Oxford : Oxford PharmaGenesis; 2006. pp. 76– 81. [PubMed] [Google Scholar]
- 12.Militaru S, Saftoiu A, Streubel B, Jurcut R. New Fabry disease mutation confirms cardiomyopathy aetiology: a case report. Eur Hear J-Case Reports. 2018;2(4):yty133–yty133. doi: 10.1093/ehjcr/yty133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linhart A, Elliott PM. The heart in Anderson-Fabry disease and other lysosomal storage disorders. Heart. 2007;93(4):528–535. doi: 10.1136/hrt.2005.063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck M. The Mainz Severity Score Index (MSSI): development and validation of a system for scoring the signs and symptoms of Fabry disease. Acta Paediatr. 2007;95:43–46. doi: 10.1111/j.1651-2227.2006.tb02388.x. [DOI] [PubMed] [Google Scholar]
- 15.Sampogna F, Sera F, Abeni D. Measures of Clinical Severity, Quality of Life, and Psychological Distress in Patients with Psoriasis: A Cluster Analysis. J Invest Dermatol. 2004;122(3):602–607. doi: 10.1046/j.0022-202X.2003.09101.x. [DOI] [PubMed] [Google Scholar]
- 16.Fortin M, Bravo G, Hudon C, Lapointe L, Almirall J, Dubois MF, Vanasse A. Relationship Between Multimorbidity and Health-Related Quality of Life of Patients in Primary Care. Qual Life Res. 2006;15(1):83–91. doi: 10.1007/s11136-005-8661-z. [DOI] [PubMed] [Google Scholar]


