Abstract
The effects of cystic fibrosis (CF) transmembrane conductance regulator (CFTR) modulators on lung function, pulmonary exacerbations, and quality of life have been well documented. However, CF is a multiorgan disease, and therefore an evidence base is emerging on the systemic effects of CFTR modulators beyond the pulmonary system. This is of great clinical importance, as many of these studies provide proof of concept that CFTR modulators might be used one day to prevent or treat extrapulmonary manifestations stemming from CFTR dysfunction. In this concise review of the literature, we summarize the results of key publications that have evaluated the effects of CFTR modulators on weight and growth, pancreatic function, the gastrointestinal and hepatobiliary systems, sinus disease, bone disease, exercise tolerance, fertility, mental health, and immunity. Although many of these studies have reported beneficial extrapulmonary effects related to the use of ivacaftor (IVA) in patients with CF with at least one gating mutation, most of the evidence is low or very low quality, given the limited number of patients evaluated and the lack of control groups. Based on an even smaller number of studies evaluating the extrapulmonary effects of lumacaftor-IVA, the benefits are less clear. Although limited, these studies may provide the basis for future clinical trials to evaluate CFTR modulators on the extrapulmonary manifestations of CF.
Keywords: cystic fibrosis, cystic fibrosis transmembrane conductance regulator, ivacaftor, lumicaftor–ivacaftor, tezacaftor–ivacaftor
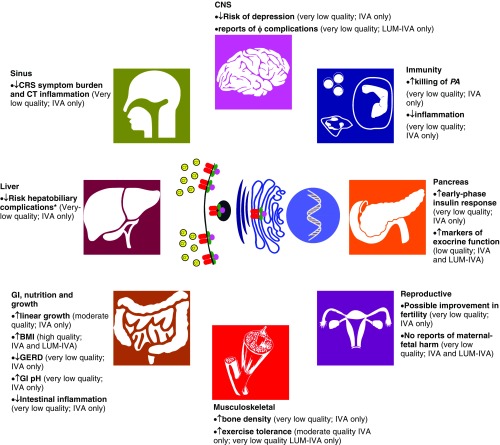
Cystic fibrosis (CF) transmembrane conductance regulator (CFTR) modulators represent a paradigm shift in the treatment landscape of CF (1). The effects of CFTR modulators on respiratory function, pulmonary exacerbations, and quality of life have been well documented, as these important clinical endpoints form the basis for regulatory agency approval (2). However, CF is a multiorgan disease, and therefore an evidence base is emerging on the effects of CFTR modulators beyond the pulmonary system, which is important both clinically and scientifically. Targeted CFTR therapy provides a unique opportunity to study the pathophysiology of CF and further our understanding of CF as a multisystem disease. In this concise review, we have systematically reviewed the literature to summarize the extrapulmonary effects of CFTR modulators reported to date, recognizing that the clinical effects may vary depending on the effectiveness of the CFTR modulator and age group under investigation (Figure 1).
Figure 1.
Cystic fibrosis (CF) transmembrane conductance regulator (CFTR) modulators and extrapulmonary effects. Reported associations between CFTR modulator use and extrapulmonary clinical outcomes graded by quality of evidence according to the American Thoracic Society (75) with reference to the effects of specific CFTR modulators. American Thoracic Society quality of evidence rating based on study methodology: high = randomized controlled trial (RCT); low = well-done observational studies with control groups; moderate = downgraded RCT or upgraded observational study; very low = others (e.g., case reports and case series). *Hepatobiliary complications: as reported by the Cystic Fibrosis Foundation Patient Registry (CFFPR) and UK Cystic Fibrosis Registry (CFR); the U.S. CFFPR includes gallstones, gallstones requiring surgery/procedure, liver disease (cirrhosis), cirrhosis complications (esophageal varices, gastric varices, gastrointestinal bleed, splenomegaly, hypersplenism, and ascites), liver disease (noncirrhosis), hepatic steatosis, liver disease (other), and abnormal liver enzymes (UK CFR only). φ = psychiatric; BMI = body mass index; CNS = central nervous system; CRS = chronic rhinosinusitis; CT = computed tomography; GERD = gastroesophageal reflux disease; GI = gastrointestinal; IVA = ivacaftor; LUM = lumacaftor; PA = Pseudomonas aeruginosa.
Weight and Growth
Improvements in weight and body mass index (BMI) are well-recognized benefits of CFTR modulator therapy, but the effect can be variable depending on the mutation-specific CFTR modulator used. Randomized, placebo-controlled trials and observational studies consistently demonstrate significant improvements in weight and BMI after ivacaftor (IVA) treatment in patients with the G551D-CFTR mutation (3–5). Increased BMI has also been reported for F508del-CFTR homozygous individuals after 24 weeks of lumacaftor (LUM)-IVA compared with placebo, but the effects are relatively modest for individuals 12 years of age or older and not significant for 6–11 year olds (6, 7). Similarly, BMI increased in F508del-CFTR homozygous individuals treated with tezacaftor (TEZ)-IVA over 24 weeks, but the changes are not significantly different from placebo (8). Based on these results, longer-term studies might be required to observe an improvement in BMI with less effective CFTR modulators.
The underlying mechanisms for weight gain related to IVA is likely multifactorial. A comprehensive study of 23 children and adults identified reduced resting energy expenditure, decreased gut inflammation, and decreased fat malabsorption as key factors most contributory to IVA-associated weight gain (9). In another study of patients on IVA, patient perceptions of appetite, body image, and ability to gain weight improved based on the CF Questionnaire-Revised (3).
Linear growth is restricted in CF, likely due to defective CFTR-mediated insulin-like growth factor (IGF-1) signaling coupled with the effects of lung disease and poor nutritional status on growth (10). Based on the combined results of two studies evaluating a total of 83 patients between 6 and 11 years old with at least one copy of the G551D-CFTR gating mutation followed for up to 12 months, IVA promoted linear growth over 1 year (11). In adults, one study assessed the impact of LUM-IVA on the function of the growth hormone (GH)–insulin-like growth factor-1 axis in a cohort of patients with CF with GH deficiency (GHD). LUM-IVA initiation was associated with improved GH secretion in response to arginine stimulation, including two cases of biochemical resolution of severe GHD (12). Longer-term studies are needed to determine whether growth in treated children and adolescents can catch up to achieve reference standards for the general population by adulthood.
Pancreas
Exocrine Function
Pancreatic destruction typically occurs early in life, often in utero and, in most cases, by 4 years of age. Consequently, CFTR modulator therapy will most likely need to be started early in life to prevent the onset of pancreatic insufficiency. However, it is believed that as little as 1–2% of residual pancreatic function is required to maintain exocrine pancreatic sufficiency, raising the possibility that even patients with borderline pancreatic insufficiency can be rescued. As such, restoring flow of pancreatic ductal secretions containing pancreatic enzymes with CFTR modulator therapy may enhance pancreatic function (3, 13). Fecal elastase (FE)-1, a marker of pancreatic function, increased significantly among children aged 2–5 years, with at least one CFTR gating mutation followed for 24 weeks on IVA (5), an observation that has similarly been noted in several case reports (14, 15). Furthermore, the proportion of patients who attained an FE-1 level of greater than 200 μg/g (a threshold that correlates with pancreatic sufficiency) was higher at week 24 of IVA (7 of 31, 23%) compared with baseline (1 of 27, 4%), but the sample size was small and the within-group change was not statistically significant (5). Among F508del-CFTR-homozygous individuals treated with LUM-IVA, 3 of 48 (6%) children aged 2 to 5 years with baseline FE-1 levels less than 100 μg/g improved to 200 μg/g or greater after therapy with reduction back to baseline after therapy, suggestive of transient improvement in pancreatic function (6). However, limited conclusions can be drawn from these findings owing to small sample sizes, unknown variability in FE levels over time, and the lack of a control group. Other markers of improved pancreatic function have been reported, including decreased serum immunoreactive trypsinogen, a surrogate marker of pancreatic damage, as well as progressive reduction in the required weight-adjusted dose of lipase supplementation during the course of CFTR modulator therapy (6, 16, 17). Future studies should continue investigating the effect of CFTR modulator therapy on exocrine pancreatic function and, in particular, elucidate factors influencing the degree of pancreatic recovery and circumstances in which sufficiency can be achieved (7).
In patients with mild CF disease and residual pancreatic function, mucous plugging of the pancreatic ducts can lead to recurrent pancreatitis, and therefore CFTR modulator therapy has the potential to reduce the risk of pancreatitis. A small retrospective study conducted by Carrion and colleagues (18) evaluated the frequency of pancreatitis requiring hospitalization before and after IVA among patients with CF and an IVA-responsive gating mutation. This study demonstrated a reduction in the pancreatitis-related hospitalization rate and decreased opioid use. Despite being a relatively small study, this observation suggests that CFTR modulators may decrease episodes of pancreatitis among individuals with CF with residual pancreatic function, possibly via the reduction of pancreatic ductal mucoid obstruction. This finding is important, as there are no other therapies available currently that can reduce episodes of pancreatitis. Consequently, minimizing bouts of acute pancreatitis may prevent or delay the onset of pancreatic insufficiency.
Endocrine Function
CF-related diabetes (CFRD) is a chronic, progressive condition characterized by the development of insulin insufficiency due to progressive destruction of β islet cells of the pancreas. Based on a large observational analysis of joint U.S. and UK CF registry data selected for patients with a variety of gating mutations on IVA, a lower prevalence and relative risk of CFRD was observed for the IVA-treated group compared with an untreated control group with a similar genotype severity (19). However, this study did not account for potential differences in the rates of CFRD between groups before starting therapy, and it is therefore difficult to conclude a treatment effect. A few small studies and case reports have demonstrated improvements in glucose tolerance or reduced insulin requirement when IVA is used in patients with G551D and non-G551D gating mutations, but this effect is not observed in all patients, and one study showed that this improvement might be related to improved early-phase insulin secretion (20–25). In the largest and most detailed study performed to date evaluating 12 patients with IVA-responsive mutations and normal-to-mildly-impaired glucose tolerance treated with IVA over 4 months, arginine-induced insulin secretion (based on C-peptide measurement) improved, consistent with a positive effect on β islet cell function and possibly α cell function as well (26). Whether CFTR modulation is having a direct or indirect effect on β cell function (e.g., mediated via incretins that enhance β cell function) remains unclear, but, collectively, these studies suggest improvements in early insulin response in patients with CF with gating mutations on IVA therapy. However, despite the possibility of a more rapid insulin response, studies thus far have failed to demonstrate consistent improvement in serum glucose or HbA1c (26, 27).
For patients homozygous for F508del treated with LUM-IVA, there are comparatively fewer studies, but no study has demonstrated an improvement in glucose tolerance or insulin secretion with treatment. Li and colleagues (28) examined 9 pediatric patients on LUM-IVA for a median of 29 months and found no meaningful difference in serum glucose before and after treatment. However, there was a trend toward lower amplitude of glycemic excursion in male patients only after treatment. When LUM-IVA was evaluated in a small adult CF cohort over 6–8 weeks, no statistically significant improvements in glucose tolerance or insulin secretion were observed (27).
Intestinal Tract
Several small observational studies suggest that effective CFTR modulator therapy with IVA has the potential to improve intestinal function. In a small study by Zeybel and colleagues (29), patient-reported gastroesophageal reflux symptoms decreased over 52 weeks of IVA. No objective physiologic testing was performed, such as esophageal pH monitoring, and therefore the mechanism for this potential benefit of IVA is unclear. Based on one small study by Gelfond and colleagues (30) evaluating gastrointestinal motility before and after IVA with a wireless motility capsule, no change in gastric emptying or intestinal transit time was observed to suggest that this benefit might be related to improved gastric emptying. However, based on data generated from the same cohort using a wireless motility capsule, IVA has been shown to nearly normalize intestinal pH via CFTR-mediated bicarbonate secretion, and therefore this might provide a mechanism leading to reduced acid reflux symptoms (4). Alkalization of intestinal pH in response to IVA may also enhance pancreatic enzyme function, allowing for improved intestinal nutrient absorption. In the study by Gelfond and colleagues (30), weight gain in patients taking IVA was associated with increased proximal small intestinal pH. Lastly, Ooi and colleagues (31) have investigated changes to the CF gut microbiota and intestinal inflammation after IVA treatment. They observed lower fecal calprotectin levels reflective of reduced intestinal inflammation, which was associated with increased abundance of Akkermansia, a gram-negative, mucin-degrading bacterium, linked to reduced intestinal inflammation. Intestinal inflammation in CF has been associated with small bowel pathology, including mucosal changes that may contribute to intestinal malabsorption, and therefore reduced chronic intestinal inflammation with IVA might improve nutritional status and chronic abdominal symptoms, and possibly even reduce gastrointestinal malignancy risk that is increased in the CF population, but further study is required (32).
Hepatobiliary
CF-associated liver disease is a nonspecific diagnosis with a complex pathophysiological presentation, including biliary tract disease (cholangiopathy, cholestasis, and gallbladder disease), focal biliary cirrhosis, acute-on-chronic liver function abnormalities, and histological changes spanning mild steatosis to multilobular cirrhosis with or without portal hypertension (33). Although the mechanism remains poorly understood, CFTR is solely expressed by cholangiocytes lining the bile duct epithelium, suggesting an underlying pathology related to ductal occlusion and downstream complications, such as biliary cirrhosis and portal hypertension (34). To date, investigations on the effects of CFTR modulators on hepatobiliary outcomes remain limited. A large observational analysis of data from the U.S. and UK CF registries indicated that patients treated with IVA had lower reporting of hepatobiliary complications (defined by gallstones, liver disease, cirrhosis, cirrhosis complications, hepatic steatosis, and abnormal liver enzymes) compared with their untreated counterparts, but a direct treatment effect was not clearly established (19). Furthermore, there is one case report in the literature of a 17-year-old female (F508del-CFTR/G551D) with findings of hepatic steatosis on liver biopsy who experienced complete resolution after IVA therapy over a 2-year span (35). Although these studies would support the notion that CF hepatobiliary disease might be prevented or reversed by CFTR modulator therapy, larger and more detailed studies are required.
Musculoskeletal/PhysicalActivity
CF bone disease (CFBD) is characterized by low bone mineral density (osteopenia and osteoporosis) and its etiology is multifactorial, in part due to reduced bone formation and increased resorption due to primary CFTR dysfunction, pancreatic insufficiency leading to deficiencies in vitamin D and K, which are important for bone mineralization, as well as increased osteoclastic bone resorption due to systemic inflammation. Based on a large observational analysis of joint U.S. and UK CF registry data selected for patients with a variety of gating mutations on IVA, a lower prevalence and relative risk of CFBD was observed for the IVA-treated group compared with untreated controls with similar genotype profiles (19). However, as mentioned previously here, this study did not account for potential differences in the rates of disease between groups before starting therapy, and therefore it is difficult to draw definitive conclusions from these data. In a small series by Sermet-Gaudelus and colleagues (36) involving seven adults with CF with the G551D mutation treated with IVA for a mean of 1.7 years, a significant improvement in lumbar spine z-scores was observed, suggesting that CFTR modulator therapy may improve bone mineralization and density. The mechanism of this benefit is likely multifactorial and related to improved nutritional status, reduced systemic inflammation, and/or increased physical activity levels. However, based on data from accompanying in vitro experiments, the authors speculated that this benefit could be due to improvements in osteoblastic CFTR activity leading to reduced receptor activator of nuclear factor κ-B ligand (RANK-L) production, and, hence, less osteoclast formation leading to reduced bone resorption. Future studies are required to confirm this finding.
CFTR modulators may also directly increase exercise tolerance and physical activity levels. In a double-blind, placebo-controlled crossover study, Edgeworth and colleagues (37) showed that IVA treatment increased mean exercise time (an indicator of fitness) during cardiopulmonary exercise testing. Interestingly, this occurred without a corresponding improvement in ventilation parameters (maximal oxygen uptake or minute ventilation) and therefore the improvements in exercise time on IVA could be attributed to extrapulmonary factors, such as enhanced skeletal muscle function due to improved conditioning or CFTR-related changes in cellular energetics or mitochondrial function. A few smaller studies have evaluated the effects of LUM-IVA on exercise tolerance. Wark and colleagues (38) demonstrated that 6-minute walk distance improved significantly among 10 F508del homozygous adults with severe lung disease (percent-predicted [pp] forced expiratory volume in 1 second [FEV1] < 40%) treated with LUM-IVA over 1 year compared with untreated control subjects. Interestingly, the improvements preceded changes in ppFEV1 for this severe lung disease group, whereby lung function does not vary as much with health status given the floor effects of ppFEV1. In another study evaluating three adult patients treated with LUM-IVA over 2 years, improvements in physical activity measurements and oxygen uptake values during cardiopulmonary exercise testing were observed compared with baseline, and this correlated with improved function with activities (39).
Fertility
Based on data from the CF Foundation Patient Registry, Heltshe and colleagues (40) reported changes in pregnancy incidence among women with the G551D mutation with a rate of 34/1,000 woman-years before IVA therapy, 14.4/1,000 woman-years during the phase-3 trials, and an increase to 38.4/1,000 woman-years after trial. The increased incidence after trial is likely due to a concerted effort to delay conception during the clinical trial, recovering to a similar rate as before IVA therapy, but it also raises the question of whether CFTR modulator therapy can improve fertility. Jones and colleagues (41) highlight a series of patients who previously required in vitro fertilization spontaneously becoming pregnant or having normalized menstrual cycles after IVA treatment. The authors theorize that this may be due to improvement in the viscoelastic properties of cervical secretions along with alterations in the hypothalamic pituitary adrenal (HPA) axis. The potential impact on male fertility is less clear due to a lack of studies. However, as the underlying pathophysiology of CF male infertility is due to congenital bilateral absence of the vas deferens and obstructive azoospermia, CFTR modulation is likely to have minimal impact after fetal development.
With regard to safety, IVA is classified as category B pregnancy risk by the U.S. Food and Drug Administration (FDA) despite limited safety data in humans, but animal studies have not demonstrated teratogenecity (42). Certainly, a handful of reports describe unremarkable term births while on IVA (43–47). A few case reports also describe patients who continued on LUM-IVA with no ill effects on the pregnancy or infant development up to 5 months of life (48, 49). Both LUM and IVA can cross the placenta, having been detected in cord blood, breast milk, and infant plasma (49). Much fewer data are available on the safety of TEZ during pregnancy. TEZ has been detected in the placental fluid and breast milk of peripartum rats (50). Although no evidence of harm has been detected with TEZ given at 0.2× the maximum recommended human dose in a rabbit model, lower fetal body weights were noted at the maximum recommended human dose (50). How these observations translate to human response has yet to be determined. Consequently, TEZ is classified as category N (pregnancy risk not assigned).
Neurocognitive Effects
CFTR is also expressed in the central and peripheral nervous systems, with some theorizing a wide-ranging role for CFTR from the regulation of hypothalamic and neuroendocrine function to modulating the activation of peripheral nerves in the airways and the gastrointestinal tract (51). In vitro studies have demonstrated affinity of IVA and its metabolites with 5-hydrozytryptaine (5HT), the 5HTT2c receptor, the B3 adrenergic receptor, the μ-opioid receptor, and the dopamine transporter. This translates into in vivo improvement in immobility and increased locomotor activity rivaling the effect of fluoxetine in a murine depression model (52). However, the effects in humans are less clear. Bessonova and colleagues (19) demonstrated a relative risk reduction in the prevalence of depression in patients with gating mutations treated with IVA compared with those who did not receive treatment, but this study did not control for differential rates of depression before starting therapy. However, other reports have yielded troubling findings of increased severity or new-onset depression, anxiety, or bipolar disorder associated in some with suicidal ideation and attempt (53, 54). As CFTR modulators can alter the pharmacodynamics of psychotropic medications, it is possible that the worsening of these psychiatric conditions may be attributable to changes in the therapeutic drug levels. However, there were a number of reports of new-onset depression and anxiety, which reversed in the majority of cases once LUM-IVA was discontinued (55, 56). Therefore, although mental health concerns have been understudied to date, possibly in part due to the controversy of whether these outcomes should be considered drug-related serious adverse events, these studies highlight the critical need for ongoing monitoring and more systematic and detailed mental health evaluation.
Chronic Rhinosinusitis
Two observational studies and several case reports have demonstrated a beneficial association between IVA and chronic rhinosinusitis severity. The first study noted improved patient-reported outcomes using the validated 20-item Sino-Nasal Outcome Test (SNOT-20) questionnaire, with significant improvement with respect to rhinological symptoms (e.g., rhinorrhea, postnasal drip, and thick nasal discharge) and psychological symptoms (e.g., fatigue, reduced concentration, and sadness) (57). A smaller prospective observational study assessed the appearance of sinus disease on computed tomography before and after IVA initiation. Improvement was noted in all but one patient, whereas 4/12 patients moved from a “severe” to a “mild” category (58). Several case reports have noted more dramatic improvements, including complete reversal of chronic rhinosinusitis (59, 60) and a complete resolution of symptoms (61). To date, no studies have assessed the effect of CFTR correctors (LUM-IVA or TEZ-IVA) on sinus disease.
Immune Function
Several groups have leveraged the opportunity to use CFTR modulators as a tool to explore the pathophysiological mechanisms linking CFTR dysfunction to dysregulated immunity. These studies suggest that CFTR modulation may improve clinical outcomes by correcting dysregulated immune function that characterize CF not only through enhanced antibacterial function of leukocytes, but also through decreased chronic inflammation. One of the earliest ex vivo studies evaluating neutrophils collected from patients with CF taking IVA demonstrated enhanced neutrophil killing of Pseudomonas aeruginosa that was mediated through a partially restored degranulation mechanism (62). A more recent ex vivo study found increased neutrophilic activity, mediated through enhanced expression of hydrogen voltage-gated channel-1, resulting in increased oxidative burst in patients treated with IVA (63). However, this effect appeared to be transient. Interestingly, an ex vivo study using monocyte-derived macrophages from patients treated with IVA also demonstrated improved phagocytosis and killing of P. aeruginosa (64). As the same effect was not seen in monocyte-derived macrophages from patients treated with LUM-IVA, it is not yet clear if these enhancements are drug specific, class specific, or dependent on the extent to which the CFTR modulator increases CFTR activity.
CFTR modulators may also have antiinflammatory effects. One study demonstrated reduced neutrophil–epithelial cell binding (which may result in decreased diapedesis) and decreased endoplasmic reticulum stress in neutrophils isolated from patients treated with IVA in the context of decreased circulating levels of inflammatory mediators (CXCL7, CXCL8, and sTNFR1) (65). These results are consistent with the findings of Hisert and colleagues (66) who performed a proteomic analysis of leukocytes from patients treated with IVA and showed a decrease in the expression of markers that mediate diapedesis. A more recent study by Gray and colleagues (67) showed increased apoptosis of neutrophils from patients treated with IVA, an important antiinflammatory mechanism that disposes of toxic and damaging neutrophils. Finally, Bratcher and colleagues (68) demonstrated decreased circulating inflammatory markers, as well as dampened stimulation-induced changes in the leukocyte inflammatory markers, CD11b and CXCR2. Collectively, these studies propose several mechanisms by which CFTR modulators may decrease inflammation. However, it is still not clear if these findings are directly related to the effects of CFTR modulators on immune cells (indeed, CFTR is expressed in leukocytes) or if these results are secondary to improved mucociliary clearance and reduced airway infection leading to decreased inflammation.
Lastly, underlying abnormalities in fatty acid metabolism predispose patients with CF to increased production of proinflammatory eicosanoids, including arachidonic acid and its downstream by-product, urine prostaglandin-E metabolite. IVA therapy in individuals with at least one gating mutation resulted in significantly decreased urine prostaglandin-E metabolite without detectable changes to the overall fatty acid profile, suggesting a unique mechanism by which IVA may reduce inflammation, independent of its effects in the lungs (69).
Miscellaneous
Several studies have reported unique and interesting extrapulmonary effects of CFTR modulators. One large cohort study using U.S. CF Foundation patient registry data showed that LUM-IVA and IVA was associated with higher mean blood hemoglobin levels (70). Although CFTR is expressed in erythrocytes, it is not clear if the increase in hemoglobin is mediated through the direct effects of CFTR modulators on normalizing the rates of erythrocyte turnover, liberation of iron stores, or reduced inflammation, leading to the reversal of anemia of chronic disease. Another potential benefit of CFTR modulators may be realized outside of CF, as LUM-IVA was shown to shorten the QT interval on electrocardiogram in two patients with Long QT syndrome type II (71). Finally, one case report demonstrated decreased aquagenic wrinkling of the palms after initiation of IVA, a phenomenon that occurs much quicker in patients with CF (72).
Conclusions and Future Directions
Although the main outcomes in CFTR modulator clinical trials have focused on improvements in pulmonary function and reductions in pulmonary exacerbations, there is mounting evidence that these medications may have significant extrapulmonary effects as well. Should these early findings be confirmed in larger scale and, preferably, placebo-controlled studies, the indication for CFTR modulators may be expanded to treat other CF complications, such as sinus disease, CFRD, pancreatic insufficiency, pancreatitis, and CFBD. The effects of CFTR modulators on other extrapulmonary manifestations, including distal intestinal obstruction syndrome, nephrolithiasis, cholelithiasis, and CF-related arthropathy, have not been reported to date, but might be detected in the future and should be systematically examined, as they are important comorbidities that impact the health and quality of life of individuals with CF. Furthermore, no studies to date have evaluated the extrapulmonary effects of CFTR modulators in the period after lung transplant, as treatment is often stopped after lung transplant, but certain conditions (e.g., sinus disease and distal intestinal obstruction syndrome) might be improved or prevented with treatment.
In addition, as the CFTR modulator development pipeline continues to evolve, more CFTR modulators will be available on the marketplace. Given that IVA was the first CFTR modulator to receive FDA approval, the bulk of available evidence to date is weighted toward those with gating mutations treated with IVA therapy. These findings may not be generalizable across different CFTR modulator compounds (i.e., other potentiators, correctors, and amplifiers) and among patients with different CFTR or gene modifier mutations. Indeed, the limited studies performed to date evaluating LUM-IVA therapy has, overall, failed to demonstrate major extrapulmonary benefits. Baseline CFTR function, the extent of recovery of CFTR function after modulator, and the pharmacokinetics of the CFTR modulator may play significant roles in the robustness of extrapulmonary response to CFTR modulation. Therefore, it will be imperative to continue efforts to study the effects of CFTR modulator therapy, not just based on drug classes, but also with a focus on the various types of CFTR mutations.
Finally, the timing of CFTR modulator initiation may also have a significant impact on the degree of extrapulmonary response. Earlier intervention with CFTR modulator therapy before the establishment of extrapulmonary disease might be able to alter the trajectory or even prevent the development of CF-related complications, such as pancreatic insufficiency. However, older patients with well-established extrapulmonary disease might be less responsive to CFTR modulation. For example, CFRD that has progressed to the stage of requiring insulin is unlikely to revert back to the prediabetic state. However, insulin response might improve and insulin requirements decrease while on CFTR modulation. Similarly, azoospermia is unlikely to reverse with therapy, but female fertility might improve with CFTR modulation through thinning of cervical mucus. With anticipated improvements in longevity due to CFTR modulators, certain age-related comorbidities, such as micro- and macrovascular complications of CFRD, osteoporosis, and gastrointestinal malignancy risk, might actually increase in prevalence.
In view of the promising clinical trial results of next-generation, triple-therapy modulators (73, 74) and the broadening of treatment indication by CFTR genetics and age, more and more patients will be eligible for CFTR therapy, giving researchers an unprecedented opportunity to conduct larger-scale and more rigorous studies to better appreciate CFTR modulator effects on both pulmonary and extrapulmonary outcomes.
Supplementary Material
Footnotes
Supported by a Michael Smith Foundation for Health Research Scholar Award (B.S.Q.) and by the Cystic Fibrosis Canada Clinical Fellowship Award (G.Y.L.).
CME will be available for this article at http://www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Jennings MT, Flume PA. Cystic fibrosis: translating molecular mechanisms into effective therapies. Ann Am Thorac Soc. 2018;15:897–902. doi: 10.1513/AnnalsATS.201802-075FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Habib A-RR, Kajbafzadeh M, Desai S, Yang CL, Skolnik K, Quon BS. A systematic review of the clinical efficacy and safety of CFTR modulators in cystic fibrosis. Sci Rep. 2019;9:7234. doi: 10.1038/s41598-019-43652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borowitz D, Lubarsky B, Wilschanski M, Munck A, Gelfond D, Bodewes F, et al. Nutritional status improved in cystic fibrosis patients with the G551D mutation after treatment with ivacaftor. Dig Dis Sci. 2016;61:198–207. doi: 10.1007/s10620-015-3834-2. [DOI] [PubMed] [Google Scholar]
- 4.Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, et al. GOAL Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190:175–184. doi: 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies JC, Cunningham S, Harris WT, Lapey A, Regelmann WE, Sawicki GS, et al. KIWI Study Group. Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in patients aged 2-5 years with cystic fibrosis and a CFTR gating mutation (KIWI): an open-label, single-arm study. Lancet Respir Med. 2016;4:107–115. doi: 10.1016/S2213-2600(15)00545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNamara JJ, McColley SA, Marigowda G, Liu F, Tian S, Owen CA, et al. Safety, pharmacokinetics, and pharmacodynamics of lumacaftor and ivacaftor combination therapy in children aged 2-5 years with cystic fibrosis homozygous for F508del-CFTR: an open-label phase 3 study. Lancet Respir Med. 2019;7:325–335. doi: 10.1016/S2213-2600(18)30460-0. [DOI] [PubMed] [Google Scholar]
- 7.Ratjen F, Hug C, Marigowda G, Tian S, Huang X, Stanojevic S, et al. VX14-809-109 investigator group. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6-11 years with cystic fibrosis homozygous for F508del-CFTR: a randomised, placebo-controlled phase 3 trial. Lancet Respir Med. 2017;5:557–567. doi: 10.1016/S2213-2600(17)30215-1. [DOI] [PubMed] [Google Scholar]
- 8.Taylor-Cousar JL, Munck A, McKone EF, van der Ent CK, Moeller A, Simard C, et al. Tezacaftor–ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med. 2017;377:2013–2023. doi: 10.1056/NEJMoa1709846. [DOI] [PubMed] [Google Scholar]
- 9.Stallings VA, Sainath N, Oberle M, Bertolaso C, Schall JI. Energy balance and mechanisms of weight gain with ivacaftor treatment of cystic fibrosis gating mutations. J Pediatr. 2018;201:229–237.e4. doi: 10.1016/j.jpeds.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Rogan MP, Reznikov LR, Pezzulo AA, Gansemer ND, Samuel M, Prather RS, et al. Pigs and humans with cystic fibrosis have reduced insulin-like growth factor 1 (IGF1) levels at birth. Proc Natl Acad Sci USA. 2010;107:20571–20575. doi: 10.1073/pnas.1015281107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stalvey MS, Pace J, Niknian M, Higgins MN, Tarn V, Davis J, et al. Growth in prepubertal children with cystic fibrosis treated with ivacaftor. Pediatrics. 2017;139:e20162522. doi: 10.1542/peds.2016-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pascucci C, De Biase RV, Savi D, Quattrucci S, Gnessi L, Lubrano C, et al. Impact of CFTR-modulating drugs on GH–IGF-1 axis impairment in adult patients with cystic fibrosis. J Endocrinol Invest. 2019;42:1361–1363. doi: 10.1007/s40618-019-01051-4. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld M, Cunningham S, Harris WT, Lapey A, Regelmann WE, Sawicki GS, et al. KLIMB study group. An open-label extension study of ivacaftor in children with CF and a CFTR gating mutation initiating treatment at age 2-5 years (KLIMB) J Cyst Fibros. 2019 doi: 10.1016/j.jcf.2019.03.009. pii:S1569-1993(19)30061-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Megalaa R, Gopalareddy V, Champion E, Goralski JL. Time for a gut check: pancreatic sufficiency resulting from CFTR modulator use. Pediatr Pulmonol. 2019;54:E16–E18. doi: 10.1002/ppul.24353. [DOI] [PubMed] [Google Scholar]
- 15.Kounis I, Lévy P, Rebours V. Ivacaftor CFTR potentiator therapy is efficient for pancreatic manifestations in cystic fibrosis beyond pancreatic cyst epithelium : evidence of ovarian-like stroma in EUS-guided through- forceps biopsy specimens. Am J Gastroenterol. 2018;113:1058–1059. doi: 10.1038/s41395-018-0123-7. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Pastrana D, Nwokoro C, McLean M, Brown S, Christiansen N, Pao CS. Real-world effectiveness of ivacaftor in children with cystic fibrosis and the G551D mutation [in Spanish] An Pediatr (Barc) 2019;90:148–156. doi: 10.1016/j.anpedi.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Rosenfeld M, Wainwright CE, Higgins M, Wang LT, McKee C, Campbell D, et al. ARRIVAL Study Group. Ivacaftor treatment of cystic fibrosis in children aged 12 to <24 months and with a CFTR gating mutation (ARRIVAL): a phase 3 single-arm study. Lancet Respir Med. 2018;6:545–553. doi: 10.1016/S2213-2600(18)30202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrion A, Borowitz DS, Freedman SD, Siracusa CM, Goralski JL, Hadjiliadis D, et al. Reduction of recurrence risk of pancreatitis in cystic fibrosis with ivacaftor: case series. J Pediatr Gastroenterol Nutr. 2018;66:451–454. doi: 10.1097/MPG.0000000000001788. [DOI] [PubMed] [Google Scholar]
- 19.Bessonova L, Volkova N, Higgins M, Bengtsson L, Tian S, Simard C, et al. Data from the US and UK cystic fibrosis registries support disease modification by CFTR modulation with ivacaftor. Thorax. 2018;73:731–740. doi: 10.1136/thoraxjnl-2017-210394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christian F, Thierman A, Shirley E, Allen K, Cross C, Jones K. Sustained glycemic control with ivacaftor in cystic fibrosis–related diabetes. J Investig Med High Impact Case Rep. 2019;7:2324709619842898. doi: 10.1177/2324709619842898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayes D, Jr, McCoy KS, Sheikh SI. Resolution of cystic fibrosis–related diabetes with ivacaftor therapy. Am J Respir Crit Care Med. 2014;190:590–591. doi: 10.1164/rccm.201405-0882LE. [DOI] [PubMed] [Google Scholar]
- 22.Bellin MD, Laguna T, Leschyshyn J, Regelmann W, Dunitz J, Billings J, et al. Insulin secretion improves in cystic fibrosis following ivacaftor correction of CFTR: a small pilot study. Pediatr Diabetes. 2013;14:417–421. doi: 10.1111/pedi.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dagan A, Cohen-Cymberknoh M, Shteinberg M, Levine H, Vilozni D, Bezalel Y, et al. Ivacaftor for the p.Ser549Arg (S549R) gating mutation—the Israeli experience. Respir Med. 2017;131:225–228. doi: 10.1016/j.rmed.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Tsabari R, Elyashar HI, Cymberknowh MC, Breuer O, Armoni S, Livnat G, et al. CFTR potentiator therapy ameliorates impaired insulin secretion in CF patients with a gating mutation. J Cyst Fibros. 2016;15:e25–e27. doi: 10.1016/j.jcf.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Mutyam V, Libby EF, Peng N, Hadjiliadis D, Bonk M, Solomon GM, et al. Therapeutic benefit observed with the CFTR potentiator, ivacaftor, in a CF patient homozygous for the W1282X CFTR nonsense mutation. J Cyst Fibros. 2017;16:24–29. doi: 10.1016/j.jcf.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly A, De Leon DD, Sheikh S, Camburn D, Kubrak C, Peleckis AJ, et al. Islet hormone and incretin secretion in cystic fibrosis after four months of ivacaftor therapy. Am J Respir Crit Care Med. 2019;199:342–351. doi: 10.1164/rccm.201806-1018OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomassen JC, Mueller MI, Alejandre Alcazar MA, Rietschel E, van Koningsbruggen-Rietschel S. Effect of lumacaftor/ivacaftor on glucose metabolism and insulin secretion in Phe508del homozygous cystic fibrosis patients. J Cyst Fibros. 2018;17:271–275. doi: 10.1016/j.jcf.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Li A, Vigers T, Pyle L, Zemanick E, Nadeau K, Sagel SD, et al. Continuous glucose monitoring in youth with cystic fibrosis treated with lumacaftor-ivacaftor. J Cyst Fibros. 2019;18:144–149. doi: 10.1016/j.jcf.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeybel GL, Pearson JP, Krishnan A, Bourke SJ, Doe S, Anderson A, et al. Ivacaftor and symptoms of extra-oesophageal reflux in patients with cystic fibrosis and G551D mutation. J Cyst Fibros. 2017;16:124–131. doi: 10.1016/j.jcf.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gelfond D, Heltshe S, Ma C, Rowe SM, Frederick C, Uluer A, et al. Impact of CFTR modulation on intestinal pH, motility, and clinical outcomes in patients with cystic fibrosis and the G551D mutation. Clin Transl Gastroenterol. 2017;8:e81–e86. doi: 10.1038/ctg.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ooi CY, Syed SA, Rossi L, Garg M, Needham B, Avolio J, et al. Impact of CFTR modulation with ivacaftor on gut microbiota and intestinal inflammation. Sci Rep. 2018;8:17834. doi: 10.1038/s41598-018-36364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werlin SL, Benuri-Silbiger I, Kerem E, Adler SN, Goldin E, Zimmerman J, et al. Evidence of intestinal inflammation in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2010;51:304–308. doi: 10.1097/MPG.0b013e3181d1b013. [DOI] [PubMed] [Google Scholar]
- 33.Flass T, Narkewicz MR. Cirrhosis and other liver disease in cystic fibrosis. J Cyst Fibros. 2013;12:116–124. doi: 10.1016/j.jcf.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van de Peppel IP, Bertolini A, Jonker JW, Bodewes FAJA, Verkade HJ. Diagnosis, follow-up and treatment of cystic fibrosis–related liver disease. Curr Opin Pulm Med. 2017;23:562–569. doi: 10.1097/MCP.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 35.Hayes D, Jr, Warren PS, McCoy KS, Sheikh SI. Improvement of hepatic steatosis in cystic fibrosis with ivacaftor therapy. J Pediatr Gastroenterol Nutr. 2015;60:578–579. doi: 10.1097/MPG.0000000000000765. [DOI] [PubMed] [Google Scholar]
- 36.Sermet-Gaudelus I, Delion M, Durieu I, Jacquot J, Hubert D. Bone demineralization is improved by ivacaftor in patients with cystic fibrosis carrying the p.Gly551Asp mutation. J Cyst Fibros. 2016;15:e67–e69. doi: 10.1016/j.jcf.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Edgeworth D, Keating D, Ellis M, Button B, Williams E, Clark D, et al. Improvement in exercise duration, lung function and well-being in G551D–cystic fibrosis patients: a double-blind, placebo-controlled, randomized, cross-over study with ivacaftor treatment. Clin Sci (Lond) 2017;131:2037–2045. doi: 10.1042/CS20170995. [DOI] [PubMed] [Google Scholar]
- 38.Wark PAB, Cookson K, Thiruchelvam T, Brannan J, Dorahy DJ. Lumacaftor/ivacaftor improves exercise tolerance in patients with cystic fibrosis and severe airflow obstruction. BMC Pulm Med. 2019;19:106. doi: 10.1186/s12890-019-0866-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savi D, Schiavetto S, Simmonds NJ, Righelli D, Palange P. Effects of lumacaftor/ivacaftor on physical activity and exercise tolerance in three adults with cystic fibrosis. J Cyst Fibros. 2019;18:420–424. doi: 10.1016/j.jcf.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Heltshe SL, Godfrey EM, Josephy T, Aitken ML, Taylor-Cousar JL. Pregnancy among cystic fibrosis women in the era of CFTR modulators. J Cyst Fibros. 2017;16:687–694. doi: 10.1016/j.jcf.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones GH, Walshaw MJ. Potential impact on fertility of new systemic therapies for cystic fibrosis. Paediatr Respir Rev. 2015;16:25–27. doi: 10.1016/j.prrv.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 42.Vertex Pharmaceuticals. Kalydeco (ivacaftor) Boston, MA: Vertex Pharmaceuticals Incorporated; 2019. [Google Scholar]
- 43.Hebestreit H, Sauer-Heilborn A, Fischer R, Käding M, Mainz JG. Effects of ivacaftor on severely ill patients with cystic fibrosis carrying a G551D mutation. J Cyst Fibros. 2013;12:599–603. doi: 10.1016/j.jcf.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Ladores S, Kazmerski TM, Rowe SM. A case report of pregnancy during use of targeted therapeutics for cystic fibrosis. J Obstet Gynecol Neonatal Nurs. 2017;46:72–77. doi: 10.1016/j.jogn.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 45.Marco A, Montales MT, Mittadodla P, Mukasa L, Bhaskar N, Bates J, et al. Tuberculosis reinfection in a pregnant cystic fibrosis patient. Respir Med Case Rep. 2015;16:57–59. doi: 10.1016/j.rmcr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nash EF, Brokaar E, Casey R, Castellani C, Cotton C, Doe S, et al. WS12-2-1 Pregnancy outcomes in women with cystic fibrosis on ivacaftor—an international survey. J Cyst Fibros. 2019;18:S22. [Google Scholar]
- 47.Taylor-Cousar JL, Jain R, Brown AW, Nash EF. Continuation of dual combination CFTR modulators during pregnancy in women with cystic fibrosis. J Cyst Fibros. 2019;18:S22. [Google Scholar]
- 48.Kaminski R, Nazareth D. A successful uncomplicated CF pregnancy while remaining on ivacaftor. J Cyst Fibros. 2016;15:133–134. doi: 10.1016/j.jcf.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 49.Trimble A, McKinzie C, Terrell M, Stringer E, Esther CR., Jr Measured fetal and neonatal exposure to lumacaftor and ivacaftor during pregnancy and while breastfeeding. J Cyst Fibros. 2018;17:779–782. doi: 10.1016/j.jcf.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vertex Pharmaceuticals. Boston, MA: Vertex Pharmaceuticals Incorporated; 2019. Symdeko (tezacaftor-ivacaftor) [Google Scholar]
- 51.Reznikov LR. Cystic fibrosis and the nervous system. Chest. 2017;151:1147–1155. doi: 10.1016/j.chest.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schneider EK, McQuade RM, Carbone VC, Reyes-Ortega F, Wilson JW, Button B, et al. The potentially beneficial central nervous system activity profile of ivacaftor and its metabolites. ERJ Open Res. 2018;4 doi: 10.1183/23120541.00127-2017. pii:00127–02017. [Published erratum appears in ERJ Open Res 4:pii: 00127-2017-ERR.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McKinzie CJ, Goralski JL, Noah TL, Retsch-Bogart GZ, Prieur MB. Worsening anxiety and depression after initiation of lumacaftor/ivacaftor combination therapy in adolescent females with cystic fibrosis. J Cyst Fibros. 2017;16:525–527. doi: 10.1016/j.jcf.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 54.Talwalkar JS, Koff JL, Lee HB, Britto CJ, Mulenos AM, Georgiopoulos AM. Cystic fibrosis transmembrane regulator modulators: implications for the management of depression and anxiety in cystic fibrosis. Psychosomatics. 2017;58:343–354. doi: 10.1016/j.psym.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 55.McKinzie CJ, Goralski JL, Noah TL, Retsch-Bogart GZ, Prieur MB. Worsening anxiety and depression after initiation of lumacaftor/ivacaftor combination therapy in adolescent females with cystic fibrosis. J Cyst Fibros. 2017;16:525–527. doi: 10.1016/j.jcf.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 56.Talwalkar JS, Koff JL, Lee HB, Britto CJ, Mulenos AM, Georgiopoulos AM. Cystic fibrosis transmembrane regulator modulators: implications for the management of depression and anxiety in cystic fibrosis. Psychosomatics. 2017;58:343–354. doi: 10.1016/j.psym.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 57.McCormick J, Cho DY, Lampkin B, Richman J, Hathorne H, Rowe SM, et al. Ivacaftor improves rhinologic, psychologic, and sleep-related quality of life in G551D cystic fibrosis patients. Int Forum Allergy Rhinol. 2019;9:292–297. doi: 10.1002/alr.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheikh SI, Long FR, McCoy KS, Johnson T, Ryan-Wenger NA, Hayes D., Jr Ivacaftor improves appearance of sinus disease on computerised tomography in cystic fibrosis patients with G551D mutation. Clin Otolaryngol. 2015;40:16–21. doi: 10.1111/coa.12310. [DOI] [PubMed] [Google Scholar]
- 59.Chang EH, Tang XX, Shah VS, Launspach JL, Ernst SE, Hilkin B, et al. Medical reversal of chronic sinusitis in a cystic fibrosis patient with ivacaftor. Int Forum Allergy Rhinol. 2015;5:178–181. doi: 10.1002/alr.21440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vreede CL, Berkhout MC, Sprij AJ, Fokkens WJ, Heijerman HGM. Ivacaftor and sinonasal pathology in a cystic fibrosis patient with genotype deltaF508/S1215N. J Cyst Fibros. 2015;14:412–413. doi: 10.1016/j.jcf.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 61.Hayes D, Jr, McCoy KS, Sheikh SI. Improvement of sinus disease in cystic fibrosis with ivacaftor therapy. Am J Respir Crit Care Med. 2014;190:468. doi: 10.1164/rccm.201403-0595IM. [DOI] [PubMed] [Google Scholar]
- 62.Pohl K, Hayes E, Keenan J, Henry M, Meleady P, Molloy K, et al. A neutrophil intrinsic impairment affecting Rab27a and degranulation in cystic fibrosis is corrected by CFTR potentiator therapy. Blood. 2014;124:999–1009. doi: 10.1182/blood-2014-02-555268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guerra L, D’Oria S, Favia M, Castellani S, Santostasi T, Polizzi AM, et al. CFTR-dependent chloride efflux in cystic fibrosis mononuclear cells is increased by ivacaftor therapy. Pediatr Pulmonol. 2017;52:900–908. doi: 10.1002/ppul.23712. [DOI] [PubMed] [Google Scholar]
- 64.Zhang S, Shrestha CL, Kopp BT. Cystic fibrosis transmembrane conductance regulator (CFTR) modulators have differential effects on cystic fibrosis macrophage function. Sci Rep. 2018;8:17066. doi: 10.1038/s41598-018-35151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.White MM, Geraghty P, Hayes E, Cox S, Leitch W, Alfawaz B, et al. Neutrophil membrane cholesterol content is a key factor in cystic fibrosis lung disease. EBioMedicine. 2017;23:173–184. doi: 10.1016/j.ebiom.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hisert KB, Schoenfelt KQ, Cooke G, Grogan B, Launspach JL, Gallagher CG, et al. Ivacaftor-induced proteomic changes suggest monocyte defects may contribute to the pathogenesis of cystic fibrosis. Am J Respir Cell Mol Biol. 2016;54:594–597. doi: 10.1165/rcmb.2015-0322LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gray RD, Hardisty G, Regan KH, Smith M, Robb CT, Duffin R, et al. Delayed neutrophil apoptosis enhances NET formation in cystic fibrosis. Thorax. 2018;73:134–144. doi: 10.1136/thoraxjnl-2017-210134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bratcher PE, Rowe SM, Reeves G, Roberts T, Szul T, Harris WT, et al. Alterations in blood leukocytes of G551D-bearing cystic fibrosis patients undergoing treatment with ivacaftor. J Cyst Fibros. 2016;15:67–73. doi: 10.1016/j.jcf.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Connor MG, Seegmiller A. The effects of ivacaftor on CF fatty acid metabolism: an analysis from the GOAL study. J Cyst Fibros. 2017;16:132–138. doi: 10.1016/j.jcf.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gifford AH, Heltshe SL, Goss CH. CFTR modulator use is associated with higher hemoglobin levels in individuals with cystic fibrosis. Ann Am Thorac Soc. 2019;16:331–340. doi: 10.1513/AnnalsATS.201807-449OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwartz PJ, Gnecchi M, Dagradi F, Castelletti S, Parati G, Spazzolini C, et al. From patient-specific induced pluripotent stem cells to clinical translation in long QT syndrome type 2. Eur Heart J. 2019;40:1832–1836. doi: 10.1093/eurheartj/ehz023. [DOI] [PubMed] [Google Scholar]
- 72.Grasemann H, Ratjen F, Solomon M. Aquagenic wrinkling of the palms in a patient with cystic fibrosis. N Engl J Med. 2013;369:2362–2363. doi: 10.1056/NEJMc1308349. [DOI] [PubMed] [Google Scholar]
- 73.Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, et al. VX16-445-001 Study Group. VX-445–tezacaftor–ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med. 2018;379:1612–1620. doi: 10.1056/NEJMoa1807120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davies JC, Moskowitz SM, Brown C, Horsley A, Mall MA, McKone EF, et al. VX16-659-101 Study Group. VX-659–tezacaftor–ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med. 2018;379:1599–1611. doi: 10.1056/NEJMoa1807119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schünemann HJ, Jaeschke R, Cook DJ, Bria WF, El-Solh AA, Ernst A, et al. ATS Documents Development and Implementation Committee. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. Am J Respir Crit Care Med. 2006;174:605–614. doi: 10.1164/rccm.200602-197ST. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.