Abstract
The breakthroughs achieved in green solvents promote the emergence of therapeutic deep eutectic solvents (THEDES), which possess intriguing possible applications in the biomedical field. Herein, the main aim was to unravel the biomedical potential of hydrophobic THEDES based in menthol and saturated fatty acids with different chain lengths (e.g., stearic acid (SA), myristic acid (MA), and lauric acid (LA)). Our comprehensive strategy resulted in the thermophysical characterization of different formulations, which allow one to identify the most suitable molar ratio, as well as the intermolecular interactions behind the successful formation of THEDES. The evaluation of their biological performance was also performed toward bacteria and HaCaT cells. Among the different formulations of THEDES, the one based on menthol and SA establishes stronger hydrogen bonding interactions, being also the most promising formulation because it did not elicit any relevant cytotoxicity, and potentiated wound healing, while presenting antibacterial properties against Staphylococcus epidermis and Staphylococcus aureus strains, some of which were methicillin resistant. This work provides clues on the future use of THEDES based on menthol:SA in wound dressings.
Keywords: deep eutectic solvents, green chemistry, wound healing, antibacterial properties, menthol, saturated fatty acids
Introduction
Introduced by Abbot and co-workers in a pioneering work, the interest in deep eutectic solvents (DES) has risen over the past decade due to their unique and attractive properties.1−3 DES can easily be obtained by mixing at certain molar ratios the counterparts, which by self-association lead to a eutectic mixture with the lowest melting point when compared with the counterparts.3−5 The depression on the melting point can be ascribed to hydrogen bond interactions between the hydrogen bond pairs.1,2,6 Among the remarkable properties of DES are their low preparation costs, straightforward and green synthesis, no need of postsynthesis purification, environmental disposal, nonflammability, broad range of polarity, low volatility, dipolar nature, chemical and thermal stability, water compatibility, biodegradability, and negligible toxicity profiles.6−9 Although DES present core characteristics similar to their analogues ionic liquids (ILs), DES fully represent the green chemistry metrics, which makes them highly desirable and a promising alternative to their former solvents.1,6,7 One of the most attractive features of DES is the large numbers of possible combinations, up to 106, that turn them into an environmentally friendly designer solvent. The tailoring of DES properties can be performed by changing the hydrogen bond pairs, molar ratio, polarizability, temperature, and water content.10−13
DES have claimed attention in several fields, such as in organic synthesis, separation processes, biocatalysis, nanomaterials, electrochemistry, polymer fabrication, CO2 capture, foods, cosmetics, pharmaceutics, and biomedical applications.14−17 In biomedical applications, DES can be used to improve solubility, permeation, and absorption of active pharmaceutical ingredients (APIs).18−22 The APIs can be used as a DES counterpart, being hence called therapeutic deep eutectic systems (THEDES).6,19,23 Herein, insights on therapeutic effects of the THEDES based on menthol and different saturated fatty acids will be addressed. Even though the THEDES herein reported have been mentioned in previously published works,11,24 their biological and pharmaceutical activity was not evaluated and their use in biomedical applications not explored. Menthol is a terpene that can been extracted from Mentha species, and it has been already used for THEDES preparation in combination with a wide range of compounds including ibuprofen, lidocaine, fluconazole, and captopril, among others.19,23−26 In this work, it was mostly used due to their effectiveness as permeation enhancer together with their well-known anti-inflammatory and antimicrobial properties.27−29 On the other side, fatty acids are commonly extracted from vegetal and animal fats and their potent antimicrobial properties have been extensively reported, including their important role of self-disinfection power of human skin.30−32 The preparation of THEDES from fatty acid blends was reported by Silva and co-workers, who evaluated the biological performance of the systems, namely the antimicrobial activity and the possibility to prepare gauzes loaded with the blend.33 Thereby, our interests have mainly focused on the study of the physicochemical and biological activity of THEDES based on menthol and fatty acids. This study will provide clues and relevant information on the thermophysical properties of these THEDES as well as on their potential use for therapeutic purposes, namely, in wound treatment.
Materials and Methods
THEDES Production and Characterization
During the preparation of the THEDES, menthol (Sigma-Aldrich, ref M2772) was mixed with different saturated fatty acids, including lauric acid (LA; Sigma-Aldrich, ref. W261408-SAMPLE-K), stearic acid (SA; Sigma-Aldrich, ref 175366), and myristic acid (MA; Sigma-Aldrich, ref. 70082). The systems were constantly stirred and heated to 70 °C during 30 min, until formation of a clear liquid solution. Optical characterization of different formulations of THEDES was carried out at room temperature (RT) by polarized optical microscopy (POM), as elsewhere reported.20,33 The viscosity was measured under controlled stress (shear rate, 10 s–1) using a Kinexus Prot rheometer (Malvern, MaL 1097376). A temperature scan was performed from 50 to 15 °C, adapting previous methodology in the literature.20,33 The differential scanning calorimetry (DSC; thermal analysis and analyzers) experiments of the powders and THEDES were also performed. The formulations were equilibrated at 40 °C, followed by cooling to −40 °C, an isothermal period of 5 min, and a heating to 100 °C at 5 °C/min. NMR experiments were recorded when the systems were in equilibrium, and no further change in their properties were observed, as elsewhere reported.34
Assessment of Cytotoxicity
HaCaT cell line (German Cancer Research Center (DKFZ), Germany) was cultured according to the manufacturer’s instructions in supplemented Dulbecco’s modified Eagle’s medium (Sigma-Aldrich). The assay was made using confluent and differentiated HaCaT cells, which represent 80% epidermal cells. HaCaT cells were seeded at a density of 4.5 × 104 cells/well and allowed to grow during 72 h. The cytotoxic effect of the individual counterparts and THEDES was performed by analyzing the effects of each formulation extract on the cellular metabolism, as established in ISO/EN 10993 and usually performed with THEDES.20,33−35 The extracts were prepared by overnight incubation of THEDES and powders at concentrations ranging from 0 to 32 mM. Then, cells were washed and cell viability was assessed using MTS colorimetric assay, cell viability being expressed in terms of percentage of living cells relative to the control. The half-maximal effective concentration (EC50) was also obtained using best-fitted trend lines.
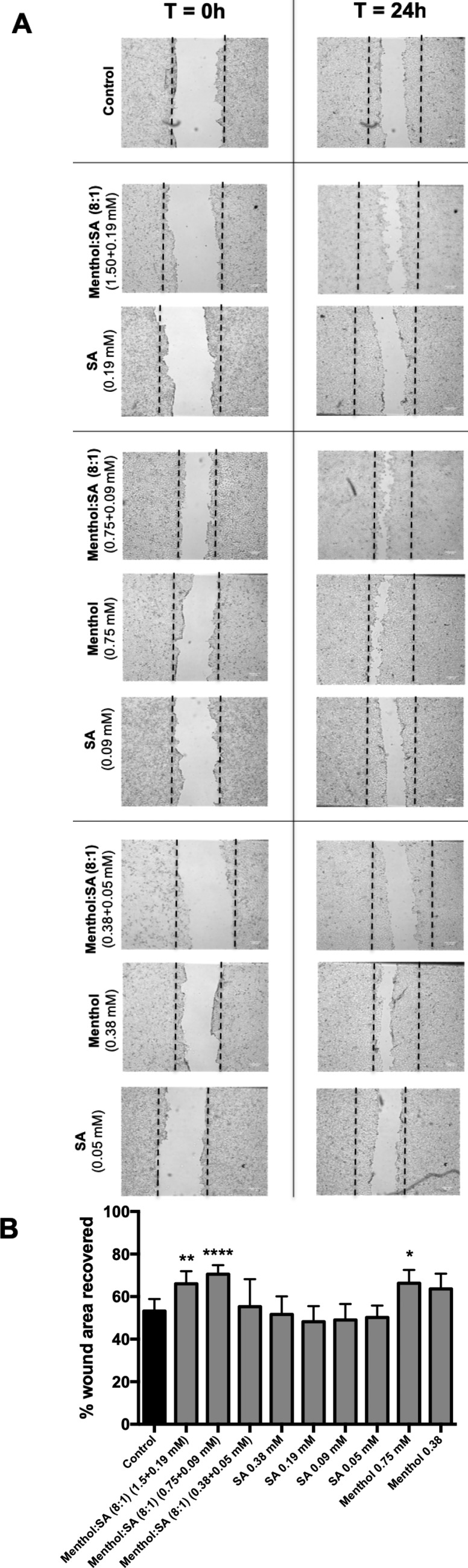
Wound Healing Assay
HaCaT cells were seeded cells at a density of 1 × 105 cell/cm2 in a 12-well plate and allowed to grow until 100% confluence (48 h). Afterward, the wound was formed with a 200 μL pipet tip and each well washed twice with warm PBS in order to remove the nonadherent cells. Then, menthol:SA (8:1), menthol, and SA were incubated for 24 h. Micrographs were taken by optic microscopy (Leica DM6000, Germany) at two different time points: 0 and 24 h. Image analysis was performed using ImageJ software and the wound area measured between borderlines. The wound area recovered was expressed in terms of percentage with the following equation using six isolated experiments performed in duplicate:
Antibacterial Assay
THEDES antimicrobial activity was tested by applying the agar diffusion assay. The antibacterial activity of eutectic blends was determined using Staphylococcus aureus (S. aureus) ATCC 25923 and ATCC 700698 (methicillin-resistant strain), S. epidermis ATCC 35984 (methicillin-resistant strain), Pseudomonas aeruginosa (P. aeruginosa) ATCC 27853, and Escherichia coli (E. coli) ATCC 25922, according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI), adapting a methodology previously reported33 with slight modifications, namely, the use of DMSO at 1% (v/v) concentration to help solubilize the compounds without affecting microbial growth significantly. Furthermore, due to the inherent volatility of menthol for MIC/MBC determination instead of the traditional 96-well plate, microtubes were used and volumes up-scaled accordingly (500 μL of formulations + 500 μL of bacterial suspension) for optimal conditions.
Statistical Analysis
The data were expressed as mean ± standard errors (SD). GraphPad Prism 6 software was used to calculate EC50 values and to analyze significant differences between data sets through one-way analysis of variance (ANOVA) followed by Tukey post hoc test. A p-value < 0.05 was considered significant.
Results and Discussion
THEDES Production and Characterization
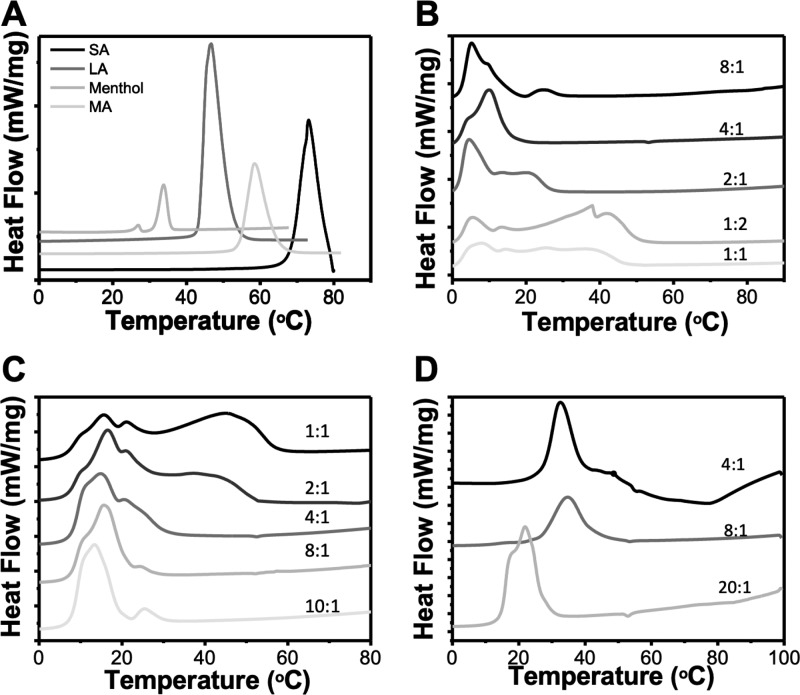
In this work, THEDES were prepared from readily available materials, such as menthol and saturated fatty acids. Different molar ratios of the compounds in their solid state have been prepared, by simple mixing of the constituents at either equimolar or imbalanced ratios (Table 1). The liquid phase is dependent on several factors, including the chemical nature of the counterparts and also the molar ratio used, which impair the eutectic point.36 The eutectic point is the point in the phase diagram with the lowest melting when compared with the counterparts.2,37 Thereby, DSC analysis was also performed to assess thermal events (Figure 1), namely, the variations on the melting point of THEDES when compared with the parent species, as a depression on the melting point of counterparts is a strong indicator of the successful formation of THEDES. The thermogram of racemic menthol presents two melting points at ≈28 and ≈33 °C, which have been ascribed to α and β polymorphs and is in good agreement with previous reported data.24,38,39 In the thermogram of each saturated fatty acid a well-defined and sharp endothermic peak was obtained at ≈46.6, ≈58.6, and ≈73 °C, for LA, MA, and SA, respectively. The thermograms of the individual starting materials corroborated previous data in the literature.24,38−41
Table 1. Different THEDES Formulations, Their Respective Visual Aspects, and POM Micrographsa.
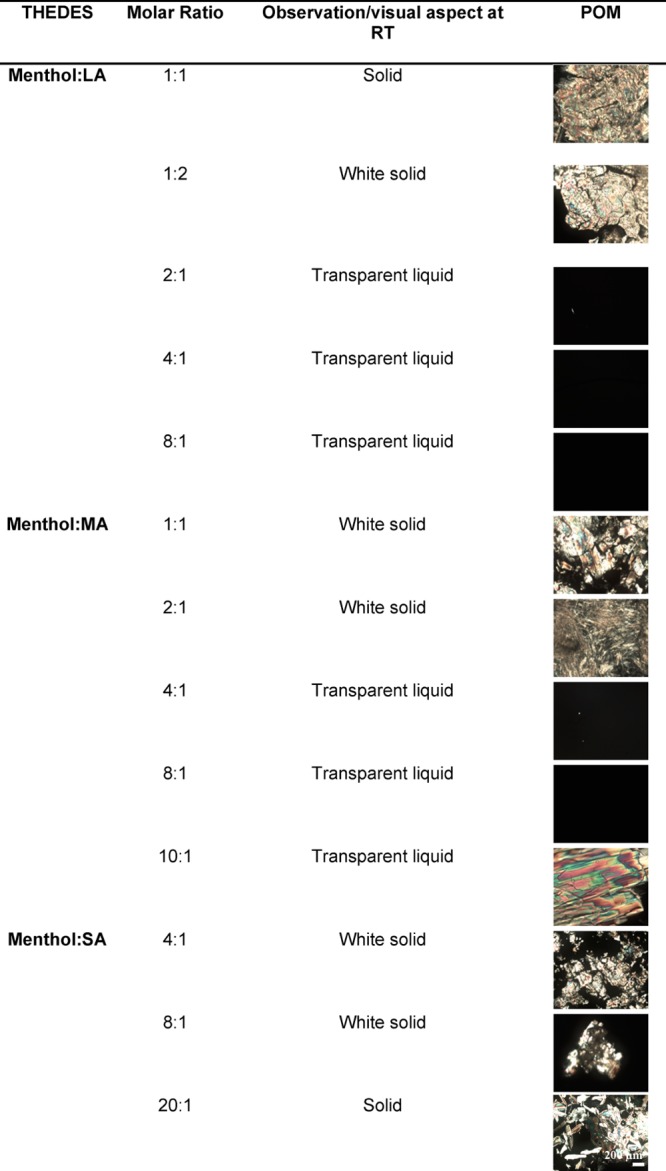
The scale bar is 200 μm.
Figure 1.
DSC thermograms obtained for powders (A) and THEDES, including menthol:LA (B) menthol:MA (C) and menthol:SA (D). Peaks arising above the baseline represent endothermic peaks.
The peaks obtained in THEDES are different from the ones of the parent species, which further suggests the supramolecular rearrangement while the compounds are in THEDES form. Additionally, a clear depression on the melting point of the parent species can be observed. The thermograms of THEDES indicate that the molar ratio strongly affects the intensity and shift of the peaks, which was previously reported for other THEDES.20,26,42,43 The DSC data together with POM were both used as easily accessible tools to assess the potential of particular molar ratios to originate THEDES. After this initial assessment, menthol was combined with LA, MA, and SA at 4:1, 8:1, and 8:1, respectively. In each case, a full black background was obtained and a thermogram with an endothermic peak was also achieved.
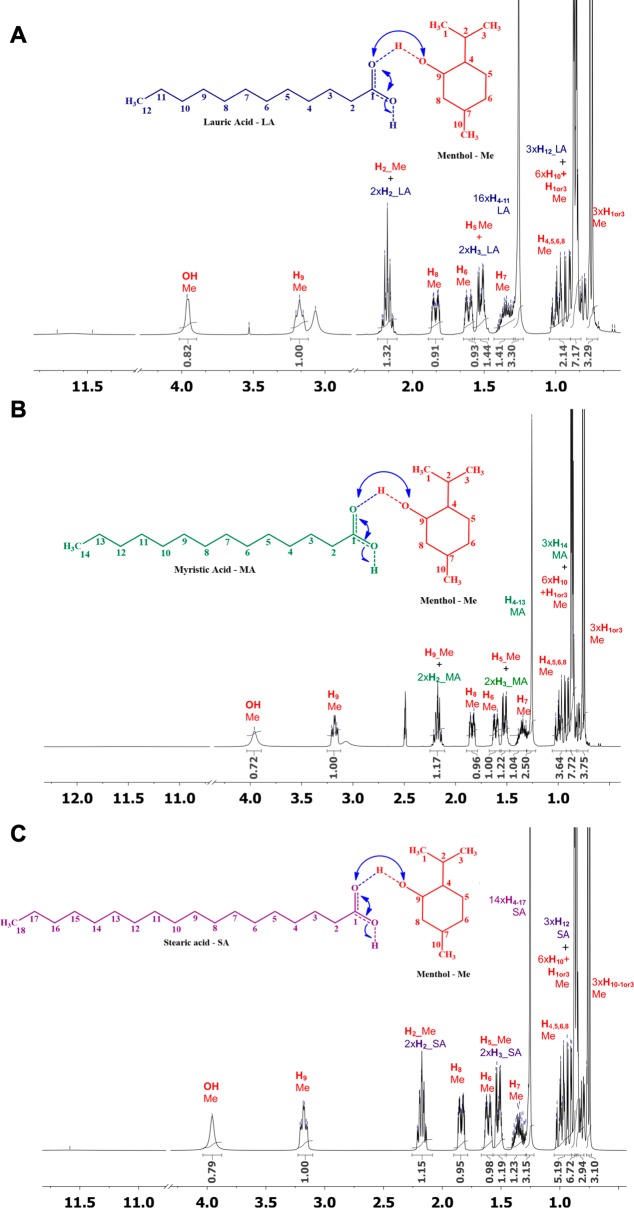
The establishment of hydrogen bonding between each parent species was then assessed by using NMR spectroscopy. NMR is commonly used to elucidate the types of interactions, as well as the atoms of each counterpart involved, allowing one to get insights into the hydrogen bonding network.10,44 In Figures 2 and 3, the 1 H NMR spectra of powders and THEDES are presented, as well as the integrals of different signals.
Figure 2.
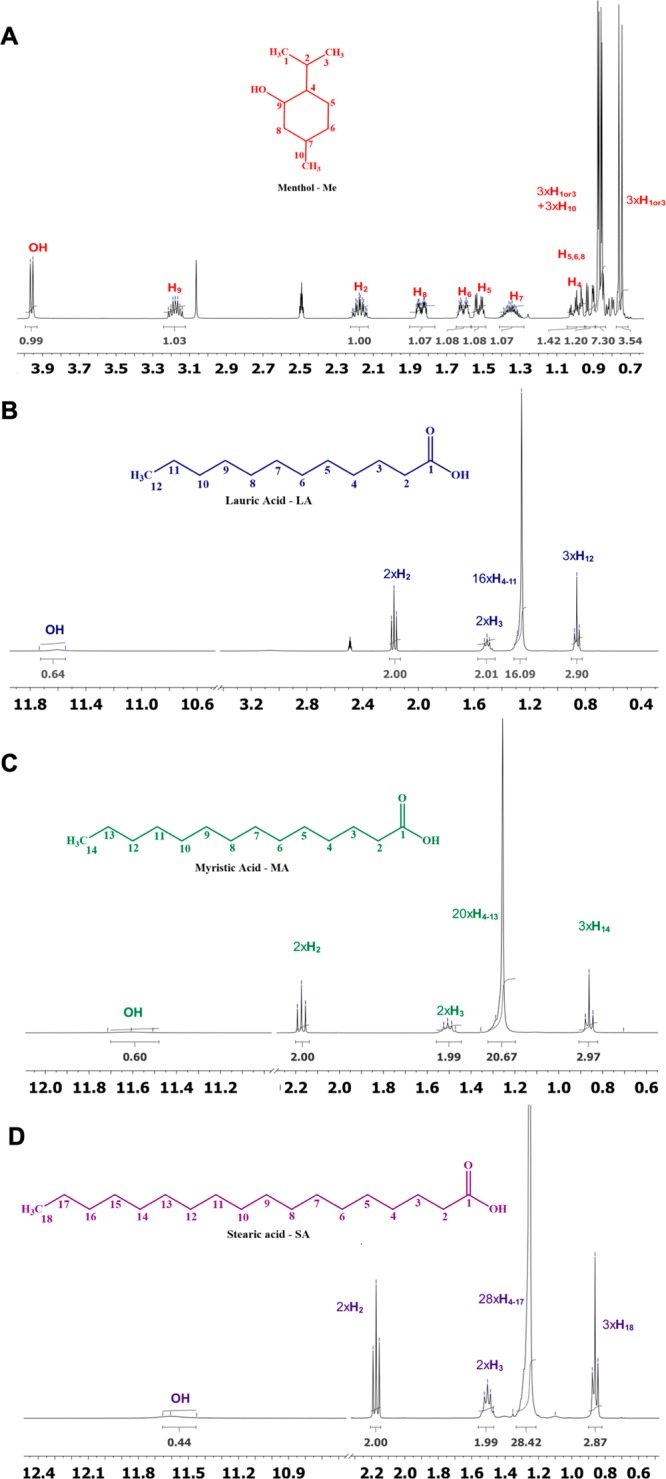
1H NMR spectra of the powders; (A) menthol, (B) LA, (C) MA, and (D) SA. All of the resonances are attributed.
Figure 3.
1H NMR spectra of the THEDES: (A) menthol:LA (4:1), (B) menthol:MA (8:1), and (C) menthol:SA (8:1). All of the resonances are attributed.
One of the differences between the spectrum of powders and the one of THEDES is the chemical signals ascribed to the hydroxyl groups of menthol. The powder spectrum (Figure 2A) of menthol presents a well-defined doublet (δ = 3.9 ppm), whereas in the THEDES spectrum (Figure 3) a larger singlet was obtained, without any further upfield or downfield chemical displacement. Additionally, the other evidence of the establishment of hydrogen bonding is the signal from proton (H9) bonded to the same carbon (C9) of the hydroxyl group from menthol. In the 1H NMR spectrum of the powder, this signal presents -H- resonance at a chemical shift of 3.14–3.22 ppm, being, as expected, a multiplet. However, in the THEDES 1H NMR spectra, besides no detectable shift, the signals are not anymore a well-defined multiplet, which further suggests that the H9 of menthol is affected by hydrogen bond interactions between the parent molecules. The establishment of hydrogen bonding is further proven by the disappearance of the hydroxyl group of saturated fatty acids in the THEDES spectrum comparison, while in the powder’s spectrum of fatty acids the sharp and defined signals were obtained in the expected chemical shift (δ = 11.5–11.8 ppm). Since these systems are viscous, at the bottom of the peaks in NMR spectra the line width is slightly broad due to the inter- and intradipolar interactions. The overall data indicate that the hydrogen bonds are established between the hydroxyl groups from menthol (hydrogen bond donor) and the carboxyl group from saturated fatty acids (hydrogen bond acceptor). The NMR data support the POM and DSC, as an extensive hydrogen bonding network was observed for the evaluated molar ratios.
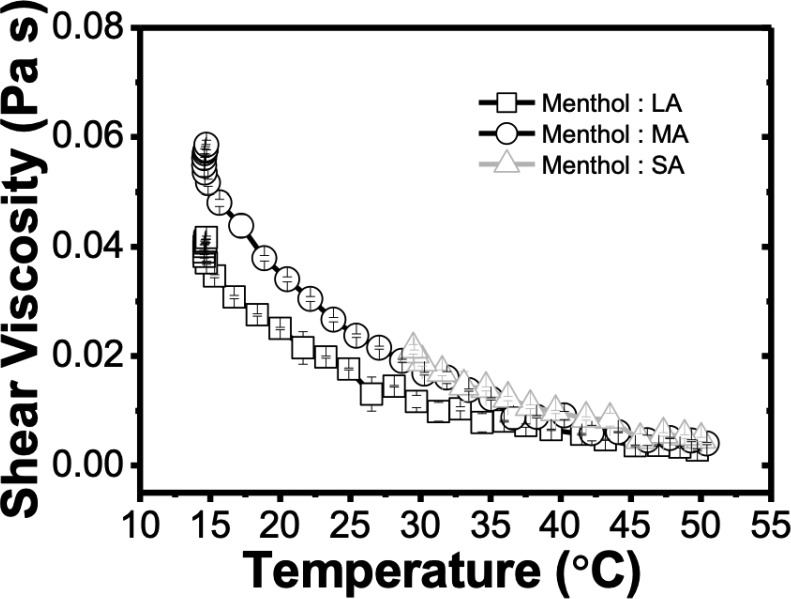
After this initial screening, the viscosity of the different THEDES was evaluated at constant shear rate and as a function of temperature (Figure 4). As expected, as the temperature increases, the viscosity of the systems decreases, which is in accordance with the Arrhenius equation.26,43,45 The menthol systems with lower viscosity were the one with LA, followed by those with MA and SA. Thereby, one can conclude that it highly depends on the chain length of saturated fatty acids. In menthol:SA, it was only possible to evaluate the viscosity until 30 °C; at lower temperatures the system is in solid phase, as shown in the DSC thermogram. The higher viscosity of menthol:SA can be attributed to their extensive hydrogen bonding interactions, which corroborated 1H NMR data.
Figure 4.
Variation of the shear viscosity of the different formulations of THEDES as a function of the temperature.
The data obtained also corroborated the one in the literature, where the ability to tune the viscosity of THEDES by the nature of starting compounds and temperature has been described.11,21,46−49 Additionally, the values of viscosity of these THEDES are relatively low, which is a valuable feature as it allows their manipulation and facilitates their potential applications without the need to, for example, add water in the formulations. The tailoring of viscosity of THEDES is recurrently performed by water content, chemical nature, and working temperature.12,35,48,50−52
The main advantage of using THEDES concerns in the formation of a supramolecular arrangement established due to intermolecular interactions, including hydrogen bonding interactions and van der Waals ones. Thereby, the solubility of these poorly water-soluble compounds is strongly increased by a phenomenon known as hydrotropy, where hydrotopes are capable of enhancing the solubility of hydrophobic molecules by means other than micellar solubilization.53−56 This is in fact a major advantage of the THEDES which can be further explored in biomedical/pharmaceutical field.18−21,23,26,57,58 Thereby, when using a physical mixture of compounds, different behaviors are obtained, as in that situation a simple dissolution of each compound in a certain medium occurred without any supramolecular arrangement of THEDES.
Bioactivity of THEDES
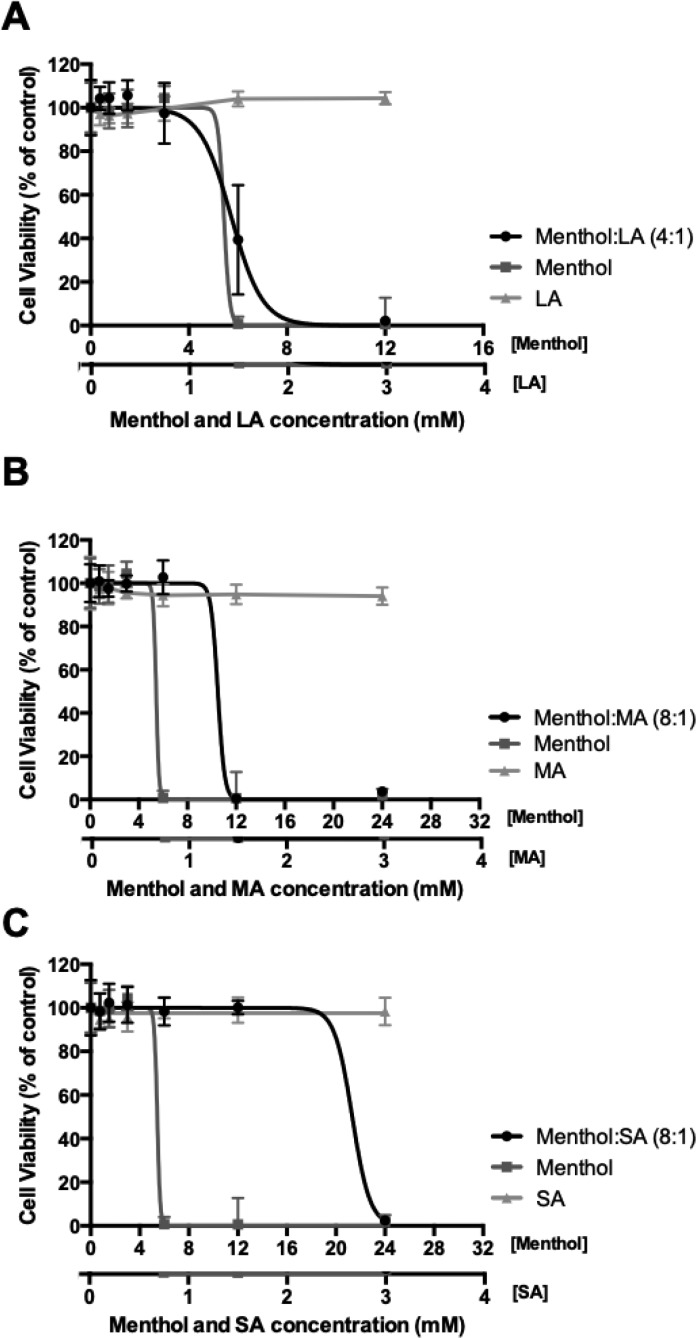
Menthol-based THEDES were initially tested in terms of cytotoxicity effect in order to select the range of concentrations that will be used in further assays. Comparing the effect of isolated compounds, none of the saturated fatty acids showed cytotoxicity in the range of concentration evaluated, as Figure 5 shows. Assessing THEDES cytotoxicity, menthol:LA (4:1) showed higher cytotoxicity with EC50 value of 5.569 ± 0.326 mM of equivalent menthol (Figure 5A). Moreover, this system showed cytotoxicity similar to that of pure menthol. Comparing menthol with other formulations of THEDES (menthol:MA (8:1) and menthol:SA (8:1)), the isolated compound had higher cytotoxic activity (Figure 5B,C). The presence of MA and SA compounds in the systems decreases the cytotoxicity, which further suggests the supramolecular arrangement between both counterparts while in THEDES form. These data corroborated several studies in the literature where it has been reported that hydrogen-bonded supramolecular arrangements established in DES may lead to synergetic or additive effects between the counterparts. .35,59−62 However, the synergetic/additivity effects may lead in some cases in more or less toxic systems in comparison with their constituents.13
Figure 5.
Cytotoxic effect of menthol:LA (4:1) (A), menthol:MA (8:1) (B), and menthol:SA (8:1) (C) with use of HaCaT cell model treated for 24 h. Results were expressed relative to the control as mean ± SD of three independent experiments performed in triplicate.
THEDES based on menthol:SA (8:1) present the lowest cytotoxicity, being the system selected to evaluate its wound healing properties. The bioactive properties of fatty acids and menthol have been reported,63−65 but from the best of our knowledge there is no information while in THEDES form. Three noncytotoxic concentrations of menthol:SA (8:1) were selected, and the ability of HaCaT cells to migrate was assessed using the wound healing assay. As panels A and B of Figure 6 show, the two highest menthol:SA (8:1) concentrations significantly induce cell migration, leading to higher wound enclosure (areas of 66.00 ± 5.92% and 70.50 ± 4.28%) compared to that of the control (53.23 ± 5.35%), representing an increase of nearly 40%. Moreover, isolated menthol and SA did not show a strong effect on cell migration, with the exception of menthol at a concentration of 0.75 mM. The THEDES (0.75 menthol + 0.09 SA mM) were more effective than isolated menthol, which might suggest that the hydrogen bonding interaction of SA with menthol potentiates its activity. Menthol is a terpene highly explored as anti-inflammatory, antiseptic, and antipruritic, therefore having a strong application in skin disorders.66 These results may reflect the potential bioactive properties of menthol:SA (8:1) over menthol for topical applications by its high capacity of inducing cell migration.
Figure 6.
Wound healing assay. (A) Migration assessment of HaCaT cells after the treatment with menthol:SA (8:1) and menthol and SA at 0 and 24 h postscratch. The lines indicated the boundary lines of the scratch at 0 h. (B) Results were expressed in terms of percentage of wound closure relative to the control using the mean ± SD of six independent experiments performed in duplicate.
Antibacterial Properties
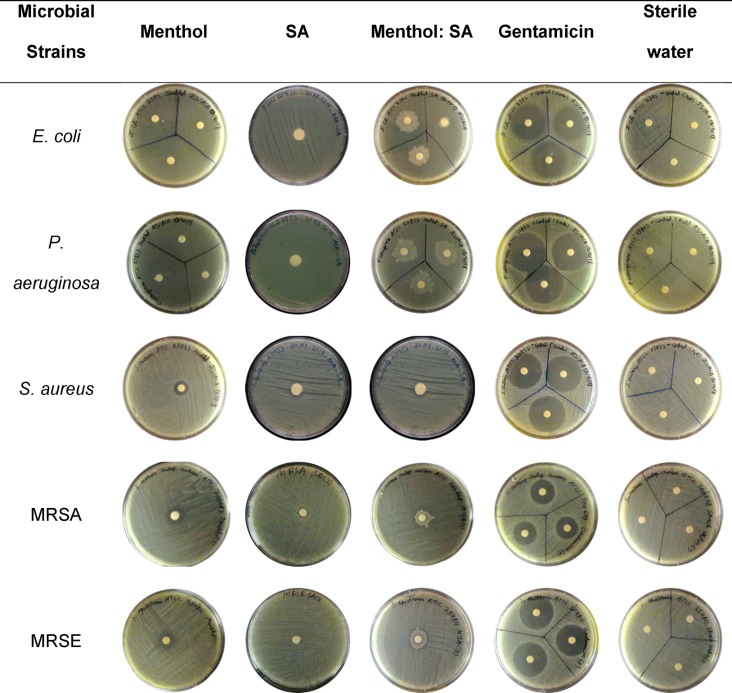
After confirming the biological activity of menthol:SA in wound healing by migration of HaCaT cells, the antibacterial properties were screened out against several microorganisms with disk diffusion assay (DDA). The results obtained for each bacteria and compounds are presented in Table 2. For both, the tested THEDES and pure compounds, no significant inhibition halo was observed in either the E. coli or P. aeruginosa strains tested, with instances of deposit being observable in menthol:SA plates which deterred correct assessment of inhibition halo diameter. Nevertheless, since for both isolated menthol and SA no inhibition halo was observable, these strains were deemed resistant. These results are not surprising, as several instances in literature report greater difficulty in dealing with Gram-negative bacteria due to their more complex membrane structure.31,67,68 Regarding the Gram-positive strains tested, SA showed no apparent inhibition and deposit formation was observed in menthol:SA, once again interfered with correct determination of inhibition halo. However, isolated menthol showed a significant inhibition halo in all cases.
Table 2. Representative Images of Disk Diffusion Assay Plates Obtained for Individual Counterparts, THEDES, and Controlsa.
Plates are presented by bacterial strains tested.
It should be noted that the lack of antibacterial activity of SA, as well as, the formation of deposit in all cases for menthol:SA is most likely a consequence of this fatty acid’s low solubility and, consequently, low diffusion rate. However, since the major component of the tested THEDES is menthol which showed relevant antibacterial activity in the DDA against the Gram-positive bacteria strains tested, these were subjected to MIC/MBC determination to try and accurately assess THEDES antibacterial potential. According to the performed experiments, menthol has a MIC value for S. aureus of 4 mM. Once again, SA did not show any antibacterial activity, which might be due to its low solubility, which hinders the complete dissolution of the compound in the selected conditions. Except for SA, all compositions demonstrate antimicrobial activity, being more efficient at higher concentrations, as expected. The methicillin-resistant strains tested show greater resilience, having a higher resistance to both menthol and the THEDES than S. aureus ATCC 25923. Nevertheless, MIC/MBC values for the methicillin-resistant strains are still within noncytotoxic values shown to promote wound healing. These results are presented in Table 3.
Table 3. MIC/MBC Values of Individual Counterparts and THEDESa.
| MIC (mM) |
MBC (mM) |
|||||
|---|---|---|---|---|---|---|
| microbial strain | menthol | SA | menthol:SA | menthol | SA | menthol:SA |
| S. aureus | 4 | ND | 3.26 + 1.79 | 8 | ND | 6.52 + 3.58 |
| MRSA | 8 | ND | 6.52 + 3.58 | 16 | ND | 13.03 + 7.16 |
| MRSE | 8 | ND | 6.52 + 3.58 | 16 | ND | 13.03 + 7.16 |
Results are presented by bacterial strains tested. ND, not dissolved.
A point of note in the results obtained lies in the fact that, in all cases, the MIC/MBC values of menthol:SA match those of its major component menthol. However, as already mentioned previously according to the molar ratio, 1 mol of menthol:SA (8:1) is composed of about 0.82 mol of menthol and 0.18 mol of stearic acid. As such, even though the MIC/MBC values of menthol:SA and menthol overlap, the THEDES effectively contains, in all cases, a lower amount of menthol per defect which strongly suggests a synergistic interaction between menthol and SA that potentiates antibacterial activity, highlighting the advantages of the studied THEDES.
Conclusion
Herein, the potential of THEDES based on menthol and fatty acid were unveiled, starting from comprehensive data on the thermophysical properties of these solvents and going up to their potential therapeutic application. The fundamental knowledge provides clues for a deeper understanding of these solvents, being crucial to understanding their properties at different operating conditions. The fundamental characterization is hence essential for choosing the right system for a certain application. The thermophysical characterization of THEDES was followed by the evaluation of their biological performance in terms of toxicity toward HaCaT cells and also by their antibacterial properties. Remarkably, among the different formulations, menthol:SA was the most promising formulation and the wound healing assay further suggests their ability to promote the migration of HaCaT. Thereby, the generated data in this work will fulfill gaps in the fundamental knowledge and biological performance of the THEDES based on menthol and fatty acids which fasten the pace to predicting their positive effects in biomedical applications. On the whole, we envisage that the present work opens new intriguing possibilities on the use of these THEDES in wound treatments. Additionally, these results emphasize the huge versatility of THEDES as a new approach toward the development of more effective therapies.
Acknowledgments
The research leading to these results has received funding from Horizon 2020 through ERC-2016-CoG-725034 Des.Solve (ERC Consolidator Grant). We also acknowledge the financial support by the Portuguese Foundation for Science and Technology (FCT) through the postdoctoral grant with Reference No. SFRH/BPD/116779/2016, the PTDC/BBB-490 EBB/1676/2014 Project, the PEst-OE/EQB/LA0004/2011 Grant, iNOVA4Health-UID/Multi/04462/2013, IF Starting Grant GRAPHYT (IF/00723/2014), FCT/MCTES (UID/QUI/50006/2019), FCT/MCTES (UID/Multi/04378/2019) and IF Starting Grant IF/01146/2015.
Author Present Address
○ LAQV-REQUIMTE, Chemistry Department, Faculty of Science and Technology, Nova University of Lisbon, 2829-516 Caparica, Portugal.
The authors declare no competing financial interest.
References
- Domínguez de María P. D.; Maugeri Z. Ionic liquids in biotransformations: from proof-of-concept to emerging deep-eutectic-solvents. Curr. Opin. Chem. Biol. 2011, 15 (2), 220–225. 10.1016/j.cbpa.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Smith E. L.; Abbott A. P.; Ryder K. S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114 (21), 11060–11082. 10.1021/cr300162p. [DOI] [PubMed] [Google Scholar]
- Tang B.; Row K. H. Recent developments in deep eutectic solvents in chemical sciences. Monatsh. Chem. 2013, 144 (10), 1427–1454. 10.1007/s00706-013-1050-3. [DOI] [Google Scholar]
- Xu P.; Zheng G.-W.; Zong M.-H.; Li N.; Lou W.-Y. Recent progress on deep eutectic solvents in biocatalysis. Bioresour. Bioprocess. 2017, 4 (1), 34. 10.1186/s40643-017-0165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.; De Oliveira Vigier K.; Royer S.; Jerome F. Deep eutectic solvents: syntheses, properties and applications. Chem. Soc. Rev. 2012, 41 (21), 7108–7146. 10.1039/c2cs35178a. [DOI] [PubMed] [Google Scholar]
- Paiva A.; Craveiro R.; Aroso I.; Martins M.; Reis R. L.; Duarte A. R. C. Natural deep eutectic solvents–solvents for the 21st century. ACS Sustainable Chem. Eng. 2014, 2 (5), 1063–1071. 10.1021/sc500096j. [DOI] [Google Scholar]
- Mbous Y. P.; Hayyan M.; Hayyan A.; Wong W. F.; Hashim M. A.; Looi C. Y. Applications of deep eutectic solvents in biotechnology and bioengineering—promises and challenges. Biotechnol. Adv. 2017, 35 (2), 105–134. 10.1016/j.biotechadv.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Mouden S.; Klinkhamer P. G.; Choi Y. H.; Leiss K. A. Towards eco-friendly crop protection: natural deep eutectic solvents and defensive secondary metabolites. Phytochem. Rev. 2017, 16 (5), 935–951. 10.1007/s11101-017-9502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radošević K.; Ćurko N.; Gaurina Srček V.; Cvjetko Bubalo M.; Tomašević M.; Kovačević Ganić K. K.; Radojčić Redovniković I. R. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT-Food Sci. Technol. 2016, 73, 45–51. 10.1016/j.lwt.2016.05.037. [DOI] [Google Scholar]
- Florindo C.; McIntosh A.; Welton T.; Branco L.; Marrucho I. A closer look into deep eutectic solvents: exploring intermolecular interactions using solvatochromic probes. Phys. Chem. Chem. Phys. 2018, 20 (1), 206–213. 10.1039/C7CP06471C. [DOI] [PubMed] [Google Scholar]
- Martins M. A. R.; Crespo E. A.; Pontes P. V.; Silva L. P.; Bülow M.; Maximo G. J.; Batista E. A. C.; Held C.; Pinho S. P.; Coutinho J. A. Tunable hydrophobic eutectic solvents based on terpenes and monocarboxylic acids. ACS Sustainable Chem. Eng. 2018, 6 (7), 8836–8846. 10.1021/acssuschemeng.8b01203. [DOI] [Google Scholar]
- Dai Y.; Witkamp G.-J.; Verpoorte R.; Choi Y. H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. 10.1016/j.foodchem.2015.03.123. [DOI] [PubMed] [Google Scholar]
- van Osch D. J.; Zubeir L. F.; van den Bruinhorst A.; Rocha M. A.; Kroon M. C. Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem. 2015, 17 (9), 4518–4521. 10.1039/C5GC01451D. [DOI] [Google Scholar]
- Abo-Hamad A.; Hayyan M.; AlSaadi M. A.; Hashim M. A. Potential applications of deep eutectic solvents in nanotechnology. Chem. Eng. J. 2015, 273, 551–567. 10.1016/j.cej.2015.03.091. [DOI] [Google Scholar]
- Alonso D. A.; Baeza A.; Chinchilla R.; Guillena G.; Pastor I. M.; Ramón D. J. Deep eutectic solvents: the organic reaction medium of the century. Eur. J. Org. Chem. 2016, 2016 (4), 612–632. 10.1002/ejoc.201501197. [DOI] [Google Scholar]
- Dai Y.; van Spronsen J.; Witkamp G.-J.; Verpoorte R.; Choi Y. H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. 10.1016/j.aca.2012.12.019. [DOI] [PubMed] [Google Scholar]
- Tang B.; Zhang H.; Row K. H. Application of deep eutectic solvents in the extraction and separation of target compounds from various samples. J. Sep. Sci. 2015, 38 (6), 1053–1064. 10.1002/jssc.201401347. [DOI] [PubMed] [Google Scholar]
- Stott P. W.; Williams A. C.; Barry B. W. Transdermal delivery from eutectic systems: enhanced permeation of a model drug, ibuprofen. J. Controlled Release 1998, 50 (1–3), 297–308. 10.1016/S0168-3659(97)00153-3. [DOI] [PubMed] [Google Scholar]
- Duarte A. R. C.; Ferreira A. S. D.; Barreiros S.; Cabrita E.; Reis R. L.; Paiva A. A comparison between pure active pharmaceutical ingredients and therapeutic deep eutectic solvents: Solubility and permeability studies. Eur. J. Pharm. Biopharm. 2017, 114, 296–304. 10.1016/j.ejpb.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Silva J. M.; Reis R. L.; Paiva A.; Duarte A. R. C. Design of functional therapeutic deep eutectic solvents based on choline chloride and ascorbic acid. ACS Sustainable Chem. Eng. 2018, 6 (8), 10355–10363. 10.1021/acssuschemeng.8b01687. [DOI] [Google Scholar]
- Morrison H. G.; Sun C. C.; Neervannan S. Characterization of thermal behavior of deep eutectic solvents and their potential as drug solubilization vehicles. Int. J. Pharm. 2009, 378 (1–2), 136–139. 10.1016/j.ijpharm.2009.05.039. [DOI] [PubMed] [Google Scholar]
- Santos F.; P. S. Leitão M.; C. Duarte A. Properties of Therapeutic Deep Eutectic Solvents of L-Arginine and Ethambutol for Tuberculosis Treatment. Molecules 2019, 24 (55), 55. 10.3390/molecules24010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroso I. M.; Craveiro R.; Rocha Â.; Dionísio M.; Barreiros S.; Reis R. L.; Paiva A.; Duarte A. R. C. Design of controlled release systems for THEDES—therapeutic deep eutectic solvents, using supercritical fluid technology. Int. J. Pharm. 2015, 492 (1–2), 73–79. 10.1016/j.ijpharm.2015.06.038. [DOI] [PubMed] [Google Scholar]
- Ribeiro B. D.; Florindo C.; Iff L. C.; Coelho M. A.; Marrucho I. M. Menthol-based eutectic mixtures: hydrophobic low viscosity solvents. ACS Sustainable Chem. Eng. 2015, 3 (10), 2469–2477. 10.1021/acssuschemeng.5b00532. [DOI] [Google Scholar]
- Jeevan R.; Venkat R.; Khan M. A.; Kunta; Goskonda; Brotherton H. O.; Reddy I. K. Effect of menthol and related terpenes on the percutaneous absorption of propranolol across excised hairless mouse skin. J. Pharm. Sci. 1997, 86 (12), 1369–1373. 10.1021/js970161+. [DOI] [PubMed] [Google Scholar]
- Aroso I. M.; Silva J. C.; Mano F.; Ferreira A. S.; Dionísio M.; Sá-Nogueira I.; Barreiros S.; Reis R. L.; Paiva A.; Duarte A. R. C. Dissolution enhancement of active pharmaceutical ingredients by therapeutic deep eutectic systems. Eur. J. Pharm. Biopharm. 2016, 98, 57–66. 10.1016/j.ejpb.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Juergens U.; Stöber M.; Vetter H. The anti-inflammatory activity of L-menthol compared to mint oil in human monocytes in vitro: a novel perspective for its therapeutic use in inflammatory diseases. Eur. J. Med. Res. 1998, 3 (12), 539–545. [PubMed] [Google Scholar]
- Patel T.; Ishiuji Y.; Yosipovitch G. Menthol: a refreshing look at this ancient compound. J. Am. Acad. Dermatol. 2007, 57 (5), 873–878. 10.1016/j.jaad.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Tsuk A. G.Menthol enhancement of transdermal drug delivery. U.S. Pat. Appl. US 07/031,077, 1990.
- Nakatsuji T.; Kao M. C.; Fang J.-Y.; Zouboulis C. C.; Zhang L.; Gallo R. L.; Huang C.-M. Antimicrobial property of lauric acid against Propionibacterium acnes: its therapeutic potential for inflammatory acne vulgaris. J. Invest. Dermatol. 2009, 129 (10), 2480–2488. 10.1038/jid.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaw L.; Jäger A.; Van Staden J.; Houghton P. Antibacterial effects of fatty acids and related compounds from plants. S. Afr. J. Bot. 2002, 68 (4), 417–423. 10.1016/S0254-6299(15)30367-7. [DOI] [Google Scholar]
- Desbois A. P.; Smith V. J. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85 (6), 1629–1642. 10.1007/s00253-009-2355-3. [DOI] [PubMed] [Google Scholar]
- Silva J. M.; Akkache S.; Araújo A. C.; Masmoudi Y.; Reis R. L.; Badens E.; Duarte A. R. C. Development of innovative medical devices by dispersing fatty acid eutectic blend on gauzes using supercritical particle generation processes. Mater. Sci. Eng., C 2019, 99, 599–610. 10.1016/j.msec.2019.02.012. [DOI] [PubMed] [Google Scholar]
- Castro V. I.; Craveiro R.; Silva J. M.; Reis R. L.; Paiva A.; C. Duarte A. R. Natural deep eutectic systems as alternative nontoxic cryoprotective agents. Cryobiology 2018, 83, 15–26. 10.1016/j.cryobiol.2018.06.010. [DOI] [PubMed] [Google Scholar]
- Hayyan M.; Looi C. Y.; Hayyan A.; Wong W. F.; Hashim M. A. In vitro and in vivo toxicity profiling of ammonium-based deep eutectic solvents. PLoS One 2015, 10 (2), e0117934 10.1371/journal.pone.0117934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnham E. R.; Drylie E. A.; Wheatley P. S.; Slawin A. M.; Morris R. E. Ionothermal materials synthesis using unstable deep-eutectic solvents as template-delivery agents. Angew. Chem. 2006, 118 (30), 5084–5088. 10.1002/ange.200600290. [DOI] [PubMed] [Google Scholar]
- Abbott A. P.; Capper G.; Gray S. Design of improved deep eutectic solvents using hole theory. ChemPhysChem 2006, 7 (4), 803–806. 10.1002/cphc.200500489. [DOI] [PubMed] [Google Scholar]
- Corvis Y.; Négrier P.; Massip S.; Leger J.-M.; Espeau P. Insights into the crystal structure, polymorphism and thermal behavior of menthol optical isomers and racemates. CrystEngComm 2012, 14 (20), 7055–7064. 10.1039/c2ce26025e. [DOI] [Google Scholar]
- Corvis Y.; Wurm A.; Schick C.; Espeau P. New menthol polymorphs identified by flash scanning calorimetry. CrystEngComm 2015, 17 (29), 5357–5359. 10.1039/C5CE00697J. [DOI] [Google Scholar]
- Wang L.; Meng D. Fatty acid eutectic/polymethyl methacrylate composite as form-stable phase change material for thermal energy storage. Appl. Energy 2010, 87 (8), 2660–2665. 10.1016/j.apenergy.2010.01.010. [DOI] [Google Scholar]
- Yuan Y.; Zhang N.; Tao W.; Cao X.; He Y. Fatty acids as phase change materials: a review. Renewable Sustainable Energy Rev. 2014, 29, 482–498. 10.1016/j.rser.2013.08.107. [DOI] [Google Scholar]
- Castro V. n. I.; Mano F.; Reis R. L.; Paiva A.; Duarte A. R. C. Synthesis and Physical and Thermodynamic Properties of Lactic Acid and Malic Acid-Based Natural Deep Eutectic Solvents. J. Chem. Eng. Data 2018, 63 (7), 2548–2556. 10.1021/acs.jced.7b01037. [DOI] [Google Scholar]
- Craveiro R.; Aroso I.; Flammia V.; Carvalho T.; Viciosa M.; Dionísio M.; Barreiros S.; Reis R.; Duarte A. R. C.; Paiva A. Properties and thermal behavior of natural deep eutectic solvents. J. Mol. Liq. 2016, 215, 534–540. 10.1016/j.molliq.2016.01.038. [DOI] [Google Scholar]
- D’Agostino C.; Harris R. C.; Abbott A. P.; Gladden L. F.; Mantle M. D. Molecular motion and ion diffusion in choline chloride based deep eutectic solvents studied by 1 H pulsed field gradient NMR spectroscopy. Phys. Chem. Chem. Phys. 2011, 13 (48), 21383–21391. 10.1039/c1cp22554e. [DOI] [PubMed] [Google Scholar]
- Aroso I. M.; Paiva A.; Reis R. L.; Duarte A. R. C. Natural deep eutectic solvents from choline chloride and betaine–Physicochemical properties. J. Mol. Liq. 2017, 241, 654–661. 10.1016/j.molliq.2017.06.051. [DOI] [Google Scholar]
- Passos H.; Tavares D. J.; Ferreira A. M.; Freire M. G.; Coutinho J. o. A. Are aqueous biphasic systems composed of deep eutectic solvents ternary or quaternary systems?. ACS Sustainable Chem. Eng. 2016, 4 (5), 2881–2886. 10.1021/acssuschemeng.6b00485. [DOI] [Google Scholar]
- Yadav A.; Pandey S. Densities and viscosities of (choline chloride+ urea) deep eutectic solvent and its aqueous mixtures in the temperature range 293.15 to 363.15 K. J. Chem. Eng. Data 2014, 59 (7), 2221–2229. 10.1021/je5001796. [DOI] [Google Scholar]
- Abbott A. P.; Ahmed E. I.; Harris R. C.; Ryder K. S. Evaluating water miscible deep eutectic solvents (DESs) and ionic liquids as potential lubricants. Green Chem. 2014, 16 (9), 4156–4161. 10.1039/C4GC00952E. [DOI] [Google Scholar]
- AlOmar M. K.; Hayyan M.; Alsaadi M. A.; Akib S.; Hayyan A.; Hashim M. A. Glycerol-based deep eutectic solvents: physical properties. J. Mol. Liq. 2016, 215, 98–103. 10.1016/j.molliq.2015.11.032. [DOI] [Google Scholar]
- Jeong K. M.; Lee M. S.; Nam M. W.; Zhao J.; Jin Y.; Lee D.-K.; Kwon S. W.; Jeong J. H.; Lee J. Tailoring and recycling of deep eutectic solvents as sustainable and efficient extraction media. Journal of Chromatography A 2015, 1424, 10–17. 10.1016/j.chroma.2015.10.083. [DOI] [PubMed] [Google Scholar]
- AlOmar M. K.; Alsaadi M. A.; Hayyan M.; Akib S.; Ibrahim R. K.; Hashim M. A. Lead removal from water by choline chloride based deep eutectic solvents functionalized carbon nanotubes. J. Mol. Liq. 2016, 222, 883–894. 10.1016/j.molliq.2016.07.074. [DOI] [Google Scholar]
- Zhekenov T.; Toksanbayev N.; Kazakbayeva Z.; Shah D.; Mjalli F. S. Formation of type III Deep Eutectic Solvents and effect of water on their intermolecular interactions. Fluid Phase Equilib. 2017, 441, 43–48. 10.1016/j.fluid.2017.01.022. [DOI] [Google Scholar]
- Cláudio A. F. M.; Neves M. C.; Shimizu K.; Canongia Lopes J. N.; Freire M. G.; Coutinho J. A. The magic of aqueous solutions of ionic liquids: ionic liquids as a powerful class of catanionic hydrotropes. Green Chem. 2015, 17, 3948–3963. 10.1039/C5GC00712G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cláudio A. F. M.; Neves M. C.; Shimizu K.; Canongia Lopes J. N.; Freire M. G.; Coutinho J. A. The magic of aqueous solutions of ionic liquids: ionic liquids as a powerful class of catanionic hydrotropes. Green Chem. 2015, 17 (7), 3948–3963. 10.1039/C5GC00712G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sintra T. E.; Shimizu K.; Ventura S. P.; Shimizu S.; Canongia Lopes J. N.; Coutinho J. A. Enhanced dissolution of ibuprofen using ionic liquids as catanionic hydrotropes. Phys. Chem. Chem. Phys. 2018, 20 (3), 2094–2103. 10.1039/C7CP07569C. [DOI] [PubMed] [Google Scholar]
- Soares B.; Tavares D. J.; Amaral J. L.; Silvestre A. J.; Freire C. S.; Coutinho J. A. Enhanced solubility of lignin monomeric model compounds and technical lignins in aqueous solutions of deep eutectic solvents. ACS Sustainable Chem. Eng. 2017, 5 (5), 4056–4065. 10.1021/acssuschemeng.7b00053. [DOI] [Google Scholar]
- Mano F.; Aroso I. M.; Barreiros S.; Borges J. o. P.; Reis R. L.; Duarte A. R. C.; Paiva A. Production of poly (vinyl alcohol)(PVA) fibers with encapsulated natural deep eutectic solvent (NADES) using electrospinning. ACS Sustainable Chem. Eng. 2015, 3 (10), 2504–2509. 10.1021/acssuschemeng.5b00613. [DOI] [Google Scholar]
- Yong C. S.; Jung S. H.; Rhee J.-D.; Choi H.-G.; Lee B.-J.; Kim D.-C.; Choi Y. W.; Kim C.-K. Improved solubility and in vitro dissolution of Ibuprofen from poloxamer gel using eutectic mixture with menthol. Drug Delivery 2003, 10 (3), 179–183. 10.1080/713840406. [DOI] [PubMed] [Google Scholar]
- Hayyan M.; Mbous Y. P.; Looi C. Y.; Wong W. F.; Hayyan A.; Salleh Z.; Mohd-Ali O. Natural deep eutectic solvents: cytotoxic profile. SpringerPlus 2016, 5 (1), 913. 10.1186/s40064-016-2575-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneidi I.; Hayyan M.; Hashim M. A. Evaluation of toxicity and biodegradability for cholinium-based deep eutectic solvents. RSC Adv. 2015, 5 (102), 83636–83647. 10.1039/C5RA12425E. [DOI] [Google Scholar]
- Radošević K.; Cvjetko Bubalo M. C.; Gaurina Srček V. G.; Grgas D.; Landeka Dragičević T. L.; Radojčić Redovniković I. R. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53. 10.1016/j.ecoenv.2014.09.034. [DOI] [PubMed] [Google Scholar]
- Radošević K.; Ćanak I.; Panić M.; Markov K.; Bubalo M. C.; Frece J.; Srček V. G.; Redovniković I. R. Antimicrobial, cytotoxic and antioxidative evaluation of natural deep eutectic solvents. Environ. Sci. Pollut. Res. 2018, 25 (14), 14188–14196. 10.1007/s11356-018-1669-z. [DOI] [PubMed] [Google Scholar]
- Hou C. T. New bioactive fatty acids. Asia Pac. J. Clin. Nutr. 2008, 17 (S1), 192–195. [PubMed] [Google Scholar]
- McKay D. L.; Blumberg J. B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. 2006, 20 (8), 619–633. 10.1002/ptr.1936. [DOI] [PubMed] [Google Scholar]
- Muniyan R.; Gurunathan J. Lauric acid and myristic acid from Allium sativum inhibit the growth of Mycobacterium tuberculosis H37Ra: in silico analysis reveals possible binding to protein kinase B. Pharm. Biol. 2016, 54 (12), 2814–2821. 10.1080/13880209.2016.1184691. [DOI] [PubMed] [Google Scholar]
- Barros A. A.; Silva J. M.; Craveiro R.; Paiva A.; Reis R. L.; Duarte A. R. C. Green solvents for enhanced impregnation processes in biomedicine. Current Opinion in Green and Sustainable Chemistry 2017, 5, 82–87. 10.1016/j.cogsc.2017.03.014. [DOI] [Google Scholar]
- Kitahara T.; Koyama N.; Matsuda J.; Aoyama Y.; Hirakata Y.; Kamihira S.; Kohno S.; Nakashima M.; Sasaki H. Antimicrobial activity of saturated fatty acids and fatty amines against methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 2004, 27 (9), 1321–1326. 10.1248/bpb.27.1321. [DOI] [PubMed] [Google Scholar]
- Ouattara B.; Simard R. E.; Holley R. A.; Piette G. J.-P.; Bégin A. Antibacterial activity of selected fatty acids and essential oils against six meat spoilage organisms. Int. J. Food Microbiol. 1997, 37 (2–3), 155–162. 10.1016/S0168-1605(97)00070-6. [DOI] [PubMed] [Google Scholar]