Abstract
We present the case of a 60-year-old man with unresectable cutaneous squamous cell carcinoma (cSCC) of the sternal area, which was not amenable to radiation therapy. The treatment history of this patient is remarkable as the disease had progressed through all lines of conventional therapy established in the literature. We decided to initiate treatment with epidermal growth factor receptor (EGFR) inhibitor cetuximab and we reassessed the patient after 12 weeks with a whole-body CT scan, documenting stability in the size and radiologic features of the disease. Cetuximab, like all current treatments for advanced cSCC, is administered off-label and proved effective in preventing further progression of disease in our patient.
Keywords: cutaneous squamous cell carcinoma, cetuximab, EGFR, non-melanoma skin cancer
Introduction
This case describes the effective use of cetuximab in an extensive thoracic cutaneous squamous cell carcinoma resistant to all previous lines of chemotherapy.
Non-melanoma skin cancer (NMSC) is the most common malignant neoplasm affecting Caucasian individuals, the main types of which are basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). SCC has a lower incidence than BCC and the gold standard of treatment is surgical excision. Between 1–5% of SCCs exhibit biologically aggressive behavior and are resistant to surgery 1.
The management of metastatic or locally advanced SCC includes radiotherapy (alone or in combination with surgery) and systemic chemotherapy, but a consensus for the treatment of cutaneous SCC (cSCC) is still lacking. cSCCs that don’t respond to conventional treatments pose a further challenge and may benefit from the use of target therapy, such as inhibitors of the epidermal growth factor receptor (EGFR) pathway and immunotherapy with checkpoint inhibitors 2.
Cetuximab is a monoclonal antibody first approved for metastatic colon-rectal carcinoma with EGFR expression. EGFR is also overexpressed in the majority of SCCs and the drug is indicated, in association with radiotherapy, for locally advanced head-neck SCC, or with chemotherapy for recurrent/metastatic disease. It is normally used at the standard weekly dosage of 250mg/m2. Cetuximab use is currently off-label for cSCC in different skin sites. Current literature supports the use of monoclonal antibodies and oral agents targeting EGFR in advanced cSCC 3.
Case presentation
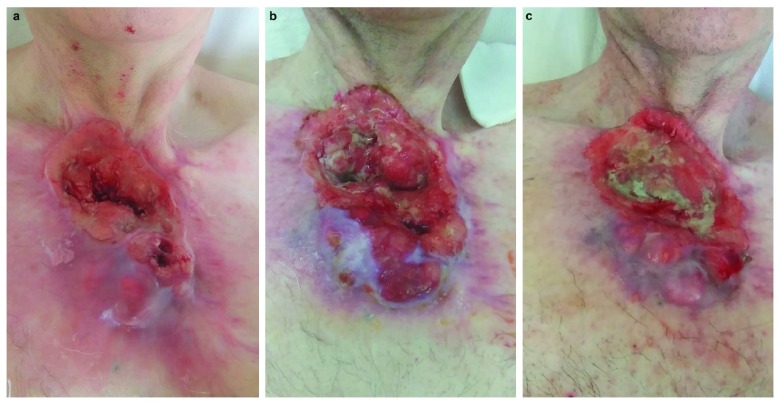
A 60-year-old Caucasian man, currently unemployed, presented to our dermatology department complaining of the recurrence of a thoracic cSCC. Physical examination revealed an extensive ulcerative skin lesion of the sternal area covered by necrotic and fibrinous tissue. The patient reported intermittent pain and bleeding ( Figure 1).
Figure 1.
Clinical presentation before cetuximab ( a) and after six ( b) and 12 weeks of therapy ( c).
The onset of a nodular skin lesion in the same site dated back to 2000, but an initial diagnosis of BCC was made only in 2013, when a single biopsy was performed (see Table 1 for timeline). A computerized tomography (CT) scan followed, demonstrating a high local burden of disease, with destructive osteo-muscular infiltration, preventing a surgical or radiation approach, and the patient was treated with vismodegib (150 mg daily). After 12 months of apparent clinical remission, a local relapse was observed, and the histologic examination of an excisional biopsy diagnosed SCC. Surgical removal of the tumor was not radical, and the patient was referred for adjuvant chemotherapy, failing four consecutive cytotoxic regimens, until the personal decision of the patient to withdraw from treatment. The four regimens were as follows: cisplatinum 100mg/m2 on day one with fluorouracil 1000mg/m2 on days 1–4 of 21-day cycles for three cycles; radio-chemotherapy with gemcitabine 3000mg/m2 on days one and 15 of 28-day-cycles; cisplatinum 100mg/m2 and docetaxel 75mg/m2 on day one of 21-day-cycles; and monotherapy with gemcitabine 3000mg/m2 on days one and 15 of 28-day-cycles for eight cycles.
Table 1. Timeline of interventions and outcomes.
| Timeline | Medical history and past interventions | |
|---|---|---|
| No family history of skin cancer
1999: total gastrectomy for gastric adenocarcinoma |
||
| Diagnostic testing and interventions | ||
| Past Interventions
2013 – 2017 |
2000: Patient reports onset of nodular skin lesion
2013: Incisional biopsy: BCC CT scan (23-Jan-2013): high local burden of disease (5.0cm AP diameter), with destructive osteo-muscular infiltration - vismodegib 150mg daily from Feb to Nov-2013 2014: relapse of nodular skin lesion Excision biopsy (Feb-2014): SCC Wide surgical excision (22-May-2014): not radical Adjuvant chemotherapy: - cisplatinum/fluorouracil for 3 cycles, Aug – Sep-2014 - radio-chemotherapy with gemcitabine Dec-2014 – Jan-2015 - cisplatinum/docetaxel Aug-2016 – Nov-2016 - gemcitabine monotherapy for 8 cycles Dec-2016 – Jul-2017 |
|
| 31-Jan-2018 | Baseline assessment | Immunohistochemistry: low/no PD-L1 expression
CT scan (31-Jan-2018): 6.2cm AP diameter |
| 19-Apr-2018 | Initiation of therapy | - cetuximab initial single dose of 400mg/m2, then 250mg/m2 weekly |
| 6 weeks | Reassessment | CT scan (28-May-2018): 7.2cm AP diameter
- cetuximab 250mg/m2 every two weeks |
| 12 weeks | Final outcome | CT scan (12-July-2018): 7.0cm AP diameter
- cetuximab 250mg/m2 every two weeks |
AP, anterior-posterior; BCC, basal cell carcinoma; CT, computerized tomography; SCC, squamous cell carcinoma; PD-L1, programmed cell death ligand-1.
A stage III-disease (T3N0M0, Figure 2a) 4 progressing through several lines of conventional chemotherapy advised the use of targeted and immunological therapies. First, immunohistochemistry for tissue levels of programmed cell death ligand 1 (PD-L1) was performed on the previous biopsy sample documenting no/low expression. We resorted to cetuximab, the use of which is off-label for cSCC. We administered cetuximab at an initial single dose of 400mg/m2, followed by 250mg/m2 every week, the standard dosing approved for SCC of the head-neck district, for seven cycles and every two weeks for six more cycles. The patient was staged after six and 12 weeks with a whole-body CT scan, documenting stability in the size and radiologic features of the disease. ( Figure 2b and 2c).
Therapy with cetuximab is ongoing and we plan to restage the patient after three months. Future management of our patient includes ongoing treatment with cetuximab or evaluation for therapy with programmed cell death protein-1 (PD-1) inhibitors.
Figure 2.
CT scan performed at baseline ( a), after six ( b) and 12 weeks of therapy ( c), highlighting the anterior-posterior diameter of the tumor.
Discussion
The results of our report encourage the use of cetuximab in this setting. However, data on long-term efficacy is lacking and we are not able to predict duration of response.
Treatment options for locally invasive or metastatic SCC include systemic chemotherapy, adjuvant chemo-radiotherapy, as well as inhibitors of the EGFR pathway and immunotherapy with checkpoint inhibitors, which are the latest additions 2. Systemic drugs for the treatment of cSCC are used off-label and the established regimens, mainly cisplatinum-based combinations, are those that were administered to our patient 5, 6.
Response to second line-therapy, after failure of the first line regimen, is generally uncommon and evidence of prolonged survival is lacking. Prior treatment history, the patient’s general condition and toxicity profiles guide the choice of second-line cytotoxic agent. Gemcitabine has shown activity in previously treated subjects and was employed in our patient with radiotherapy and as single agent 7.
We were challenged to select an effective treatment in this advanced case and resorted to EGFR inhibitor therapy on the biological notion that EGFR is overexpressed in over 90% of cSCC. A phase II study of unresectable cSCC treated with cetuximab for at least six weeks registered 25% objective response and 42% disease stabilization 8. Cetuximab is approved for the treatment of locally or regional advanced SCC of the head and neck region (in combination with radiation) or for recurrent or metastatic disease (alone or in association with platinum). Its use in cSCC of other regions is currently off-label but our choice of drug was extensively supported by evidence in published literature 3.
A novel attractive approach is immune checkpoint inhibition in the context of cSCC, with monoclonal antibody cemiplimab currently undergoing registration for the treatment of cSCC 9. Cancer immune surveillance is crucial to the development of cSCC, as demonstrated by the high frequency of cSCC in immunosuppressed patients. PD-1 inhibitors stimulate an anti-cancer immune response and their use in dermatology is established for the treatment of metastatic melanoma. In this case, PD-L1 expression was assessed through immunohistochemistry and low/no expression was found. Mucosal head-neck SCCs display high expression of PD-L1 in the majority of cases 10; however, the role of tumor PD-L1 expression in predicting response to therapy has not been demonstrated and low expression does not contraindicate immunotherapy, even if PD-L1 negative tumors correlate to poorer prognosis and lower response to checkpoint inhibitors.
In accordance with published literature 11, we support the necessity of taking serial biopsies of extensive epithelial neoplasms to exclude foci of multiple differentiation and believe that a focus of SCC existed prior to therapy with vismodegib and was responsible for the subsequent recurrence and evolution of disease.
Conclusions
Serial biopsies are mandatory for advanced BCC candidates prior to vismodegib treatment.
No drugs are currently approved specifically for cSCC, so all treatments are administered off-label.
The potential efficacy of cetuximab is based on the biological similarity of cSCC to mucosal SCCs of the head-neck district.
Low PD-L1 expression does not preclude the efficacy of checkpoint inhibitors.
Data availability
All data underlying the results are available as part of the article and no additional source data are required.
Consent
Written informed consent for publication of their clinical details and clinical images was obtained from the patient.
Funding Statement
Associazione Romana Ricerca Dermatologica covered the publication fees of this article as support to the authors.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 1 approved with reservations]
References
- 1. Que SKT, Zwald FO, Schmults CD: Cutaneous squamous cell carcinoma: Management of advanced and high-stage tumors. J Am Acad Dermatol.United States;2018;78(2):249–61. 10.1016/j.jaad.2017.08.058 [DOI] [PubMed] [Google Scholar]
- 2. Stratigos A, Garbe C, Lebbe C, et al. : Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer.England;2015;51(14):1989–2007. 10.1016/j.ejca.2015.06.110 [DOI] [PubMed] [Google Scholar]
- 3. Jalili A, Pinc A, Pieczkowski F, et al. : Combination of an EGFR blocker and a COX-2 inhibitor for the treatment of advanced cutaneous squamous cell carcinoma. J Dtsch Dermatol Ges.Wiley/Blackwell (10.1111);2008;6(12):1066–9. 10.1111/j.1610-0387.2008.06861.x [DOI] [PubMed] [Google Scholar]
- 4. Amid M, editor: Part II Head and Neck. In: AJCC Cancer Staging Manual. 8th ed. New York, NY, USA: Springer;2018;53. [Google Scholar]
- 5. Tanvetyanon T, Padhya T, McCaffrey J, et al. : Postoperative concurrent chemotherapy and radiotherapy for high-risk cutaneous squamous cell carcinoma of the head and neck. Head Neck.United States;2015;37(6):840–5. 10.1002/hed.23684 [DOI] [PubMed] [Google Scholar]
- 6. Martinez JC, Otley CC, Okuno SH, et al. : Chemotherapy in the management of advanced cutaneous squamous cell carcinoma in organ transplant recipients: theoretical and practical considerations. Dermatol Surg.Wiley/Blackwell (10.1111);2004;30(4 Pt 2):679–86. 10.1111/j.1524-4725.2004.30156.x [DOI] [PubMed] [Google Scholar]
- 7. Raguse JD, Gath HJ, Bier J, et al. : Gemcitabine in the treatment of advanced head and neck cancer. Clin Oncol (R Coll Radiol).England;2005;17(6):425–9. 10.1016/j.clon.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 8. Maubec E, Petrow P, Scheer-Senyarich I, et al. : Phase II study of cetuximab as first-line single-drug therapy in patients with unresectable squamous cell carcinoma of the skin. J Clin Oncol.United States;2011;29(25):3419–26. 10.1200/JCO.2010.34.1735 [DOI] [PubMed] [Google Scholar]
- 9. Papadopoulos KP, Owonikoko TK, Johnson ML, et al. : REGN2810: A fully human anti-PD-1 monoclonal antibody, for patients with unresectable locally advanced or metastatic cutaneous squamous cell carcinoma (CSCC)—Initial safety and efficacy from expansion cohorts (ECs) of phase I study. J Clin Oncol. American Society of Clinical Oncology. 2017;35(15_suppl):9503 10.1200/JCO.2017.35.15_suppl.9503 [DOI] [Google Scholar]
- 10. Malm IJ, Bruno TC, Fu J, et al. : Expression profile and in vitro blockade of programmed death-1 in human papillomavirus-negative head and neck squamous cell carcinoma. Head Neck.United States;2015;37(8):1088–95. 10.1002/hed.23706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu GA, Sundram U, Chang ALS: Two different scenarios of squamous cell carcinoma within advanced Basal cell carcinomas: cases illustrating the importance of serial biopsy during vismodegib usage. JAMA Dermatol.United States;2014;150(9):970–3. 10.1001/jamadermatol.2014.583 [DOI] [PubMed] [Google Scholar]