Abstract
Background:
Induction chemoradiation for resectable N2 non-small cell lung cancer (NSCLC) is used with the intent to optimize locoregional control, whereas induction chemotherapy given in systemic doses is meant to optimally target potential distant disease. However, the optimal preoperative treatment regimen is still unknown and practice patterns continue to vary widely. We compared multi-institutional oncologic outcomes for N2 NSCLC from four experienced lung cancer treatment centers.
Methods:
This collaborative retrospective study unites four major thoracic oncology centers. Patients with N2 NSCLC undergoing surgical resection after induction chemotherapy (CxT) or concurrent chemoradiation (CxRT) were included. Primary outcomes were overall and disease-free survival (OS and DFS).
Results:
822 patients were identified (CxT = 662 and CxRT = 160). There were no differences in 5-year OS (CxT 39.9% vs CxRT 42.9%, p=0.250) nor in DFS (CxT 28.7% vs 29.8%, p=0.207). Recurrence rates (CxT 46.8% vs CxRT 51.6%, p=0.282) and recurrence patterns were not significantly different (Local: CxT 9.8% vs CxRT 9.7%; and Distant: CxT 30.4% vs CxRT 33.1%, p=0.764). There was no differencein perioperative mortality. In the analyses of patients who underwent pretreatment invasive mediastinal staging (N=555), there were still no significant differences in OS (p=0.341) and DFS (p=0.455) between the two treatment strategies.
Conclusions:
Both treatment strategies produce equivalent and better than expected outcomes compared to historical controls for N2 NSCLC, with no differences in recurrence patterns. How these conventional therapeutic strategies will compare to those involving immunotherapy combined with surgical locoregional disease control for N2 disease remains to be determined.
Classification: neoadjuvant induction therapy, lung, lung cancer surgery, non-small cell lung cancer, multicenter approach
Ipsilateral mediastinal nodal (N2) disease represents a unique type of stage III non-small cell lung cancer (NSCLC), and in this setting, the optimal treatment regimen remains controversial. The debate regarding the management of N2 NSCLC has continued for decades; therapeutic players have included combinations of conventional therapy in the form of chemotherapy, radiation therapy and/or surgery. The Intergroup 0139 trial attempted to address the knowledge gap regarding the role of surgery in the management of stage IIIA N2 NSCLC within the context of concurrent chemoradiation (CxRT) [1]. Although there was improved progression free survival (PFS) with CxRT followed by resection, there was no change in overall survival (OS). However, a subgroup analysis showed improved survival among patients who underwent lobectomy following CxRT compared to patients who underwent CxRT alone. It is safe to say that current multi-modality strategies that include surgery for N2 NSCLC are largely based on retrospective reports and this subgroup analysis of the Intergroup 0139 trial. The relative paucity of well-designed randomized trials along with the inherent heterogeneity of AJCC 7th edition stage III disease has led to significant variation in practice patterns around the world; the new AJCC 8th edition subclassifies N2 disease in greater detail and upstages T3N2 to IIIB. Even amongst high-volume experienced centers, divergent opinions exist with respect to whether induction CxRT or chemotherapy alone (CxT) is best prior to surgical resection [2].
To enhance the literature on this subject, we chose to collaboratively compile data of surgically managed patients with N2 lung cancer from four high volume lung cancer centers across North America. All centers have well established radiation therapy, medical oncology and surgical programs for thoracic oncology and have individually published some of the best reported outcomes for lung cancer patients. However, each center has chosen to favor one approach or the other for their population of N2 NSCLC patients and has therefore developed considerable expertise with its own treatment algorithms. We hypothesized CxT or CxRT may show differing recurrence patterns or survival for N2 NSCLC.
Patients and Methods
Study population
This is a retrospective review of prospectively maintained data from consecutively treated patients at four major thoracic surgery centers from North America. Patient demographics, clinico-pathological, procedural, perioperative, and survival variables were obtained from each of the institutional databases using a standardized data collection form. The data was de-identified, encrypted and housed at MD Anderson Cancer Center in Houston, Texas. Ethical review board approval was obtained and data sharing agreements were signed between the participating institutions.
The inclusion criteria were as follows: patients with clinical stage III N2 NSCLC who underwent surgical resection after induction CxT or concurrent CxRT from 1997 – 2015. Positive mediastinal (N2) nodal disease was determined by a combination of CT, PET/CT or invasive mediastinal staging. We performed a separate analysis only on those patients with N2 disease proven by endobronchial ultrasound, mediastinoscopy or a Chamberlain procedure.
Study outcomes
The primary study outcomes were overall and disease-free survival (OS and DFS), while the secondary study outcomes were 30-and 90-day perioperative mortality, as well as local and distant recurrence rate. OS was defined as the time from date of surgery to death from any cause. DFS was defined as the time from surgery until the time of tumor recurrence or death. Patients without known evidence of an event (death or recurrence) at the last contact were censored at the date of last follow-up. Surveillance strategies at all centers were based on NCCN guidelines for the care of surgically resected NSCLC.
Statistical analysis
Pearson chi-square and Fisher exact tests were used to analyze differences between groups for categorical variables, while T-test and non-parametric Mann-Whitney U test were used to compare continuous variables. Kaplan-Meier with log-rank test was used to compare survival. Cox proportional hazards regression was used to test the association between various covariates with OS or DFS. Variables with a p-value < 0.25 on univariable analysis were further evaluated by multivariable analysis after backward stepwise Wald elimination with a p=0.10 as the entry and removal probability taking into consideration clinical plausibility and testing for clinically meaningful interactions. Diagnostics were performed to test for proportionality of the hazards function, as well as linearity between the log-hazard and independent variables. P<0.05 was considered statistically significant. All the statistical analyses were performed using Stata, version 13 (StataCorp, College Station, TX).
Results
Demographics and Clinico-Pathologic Characteristics
Of the total 822 patients identified, 662 patients received induction CxT and 160 patients received induction CxRT. Median follow-up time after surgery in patients who received CxT was 25 months (range 0–170 months) and that in patients who received CxRT was 34 months (range 0–189 months). Patients who received CxT were older with worse lung function, although the demographic differences were relatively minor between the groups (Table 1). The rate of invasive mediastinal staging was significantly higher in the group that received induction CxRT (61.0% CxT vs 94.4% CxRT, p<0.001). Patients who underwent CxRT had an increased rate of nodal downstaging (ypN0 status) compared to patients who received CxT only (44.4% vs 35.2%, p=0.076), and also increased rate of pathologic complete response within the tumor (ypT0 status, 10.7% vs 5.6%, p=0.064).
Table 1.
Demographics and Tumor Characteristics
| Variables | Induction chemotherapy only (N=662) | Induction chemo-radiation (N=160) | P value |
|---|---|---|---|
| Age, years; mean(range) | 64(31–86) | 62(37–90) | 0.004* |
| Sex(Male) | 316(47.7) | 78(48.7) | 0.817 |
| Any Smoking history | 582(87.9) | 134(84.8) | 0.292 |
| Pack years, mean(median) | 39.4(35.0) | 35.2(35.5) | 0.049* |
| FEV <60% | 33(5.1) | 10(6.9) | 0.383 |
| DLCO <80% | 390(61.5) | 58(50.9) | 0.033* |
| COPD | 143(21.6) | 17(10.8) | 0.002* |
| Invasive mediastinal staging | 404(61.0) | 151(94.4) | <0.001* |
| Clinical T status | <0.001* | ||
| 1 | 239(36.4) | 48(30.0) | |
| 2 | 291(44.4) | 87(54.4) | |
| 3 | 120(18.3) | 17(10.6) | |
| 4 | 6(0.9) | 8(5.0) | |
| Pathological T status | 0.064 | ||
| 0 | 37(5.6) | 16(10.7) | |
| 1 | 242(36.7) | 55(36.9) | |
| 2 | 254(38.5) | 60(40.3) | |
| 3 | 96(14.6) | 12(8.1) | |
| 4 | 30(4.6) | 6(4.0) | |
| Pathological N status | 0.076 | ||
| 0 | 233(35.2) | 68(44.4) | |
| 1 | 87(13.1) | 14(9.2) | |
| 2 | 342(51.7) | 71(46.4) | |
| Histology | 0.162 | ||
| Adenocarcinoma | 430(65.0) | 107(67.3) | |
| Squamous Cell | 137(20.7) | 28(17.6) | |
| Adenosquamous | 12(1.8) | 1(0.6) | |
| Large cell | 24(3.6) | 2(1.3) | |
| Others | 59(8.9) | 21(13.2) |
Note: Data are no. of patients (%) unless otherwise indicated.
p <0.05 considered statistically significant
Operative and Perioperative Outcomes
Most patients had a right-sided lung resection (67.2% CxT, 65.8% CxRT) and an open surgical approach (94.3% CxT, 91.7% CxRT), with no differences between the two induction treatment strategies (Table 2). However, one in ten patients who received CxT also had a sublobar resection, whereas there were no such patients among CxRT recipients (p<0.001). The R0 resection rate was significantly higher after CxRT as opposed to CxT alone (90.0% CxT vs 95.6% CxRT, p=0.045). Patients who underwent induction CxT received adjuvant radiation, either given alone or concurrently with chemotherapy more often (39.3% vs 13.4% p <0.001). On the other hand, the group with induction CxRT received more adjuvant chemotherapy as compared to induction CxT group (66.4% vs 16.6%, p<0.001). There were no differences between the groups in 30-day mortality (1.5% CxT vs 1.9% CxRT, p=0.740) or 90-day mortality (3.2% CxT vs 4.4% CxRT, p=0.452). For patients who underwent pneumonectomy, 30-day mortality was 1.4% (1/71) in the CxT cohort versus 8% (2/25) in the CxRT cohort (p = 0.103) and 90-day mortality was 5.6% (4/71) in the CxT cohort versus 12% (3/25) in the CxRT cohort (p = 0.292).
Table 2.
Operative and Perioperative Outcomes
| Variables | Induction chemotherapy only (N=662) | Induction chemo-radiation (N=160) | P value |
|---|---|---|---|
| Type of lung resection | <0.001* | ||
| Wedge | 55(8.3) | 0(0.0) | |
| Segmentectomy | 12(1.8) | 0(0.0) | |
| Lobectomy | 524(79.2) | 135(84.4) | |
| Pneumonectomy | 71(10.7) | 25(15.6) | |
| Surgical approach | 0.224 | ||
| Open | 615(94.3) | 144(91.7) | |
| Other | 37(5.7) | 13(8.3) | |
| Laterality | 0.737 | ||
| Left | 217(32.8) | 54(34.2) | |
| Right | 445(67.2) | 104(65.8) | |
| Extent of resection | 0.045* | ||
| R0 | 591(90.0) | 151(95.6) | |
| R1 | 37(5.6) | 6(3.8) | |
| R2 | 29(4.4) | 1(0.6) | |
| Adjuvant Therapy | <0.001* | ||
| None | 337(52.0) | 47(31.5) | |
| Chemo only | 56(8.6) | 82(55.0) | |
| Radiation only | 201(31.0) | 3(2.0) | |
| Chemoradiation | 54(8.3) | 17(11.4) | |
| Mortality | |||
| 30-day | 10(1.5) | 3(1.9) | 0.740 |
| 90-day | 21(3.2) | 7(4.4) | 0.452 |
Note: Data are no. of patients (%) unless otherwise indicated
p <0.05 considered statistically significant
Recurrence Patterns
Site of first cancer recurrence was similar between the groups for local, distant, and combined recurrences, Table 3. Overall, there were 310/662 (46.8%) recurrences after CxT and 82/160 (51.6%) recurrences after CxRT. The recurrences rates between CxT and CxRT groups were 9.8% vs 9.7% for local recurrence, 30.4% vs 33.1% for distal recurrence, and 6.5% vs 7.1% for synchronous local and distal recurrences respectively.
Table 3.
Oncologic outcomes
| Variables | Induction chemotherapy only (N=662) | Induction chemo-radiation (N=160) | P value |
|---|---|---|---|
| Recurrence | 310(46.8) | 82(51.6) | 0.282 |
| Recurrence pattern | 0.891 | ||
| None | 352(53.2) | 77(50.0) | |
| Local | 15(9.7) | ||
| Distant | 65(9.8) | 51(33.1) | |
| Local and Distant | 201(30.4) | 11(7.1) | |
| 43(6.5) | |||
| Overall survival (%) | 0.250 | ||
| 3-year rate | 51.7 | 57.2 | |
| 5-year rate | 39.9 | 42.9 | |
| Median survival time, months | 37.5 | 48.0 | |
| Disease-free survival (%) | 0.207 | ||
| 3-year rate | 35.3 | 44.6 | |
| 5-year rate | 28.7 | 29.8 | |
| Median survival time, months | 19.6 | 30.1 | |
| Patients with mediastinal staging | (N=404) | (N=151) | |
| Overall survival (%) | 0.341 | ||
| 3-year rate | 53.3 | 57.9 | |
| 5-year rate | 41.5 | 43.8 | |
| Median survival time, months | 39.9 | 48.2 | |
| Disease-free survival (%) | 0.455 | ||
| 3-year rate | 38.2 | 46.1 | |
| 5-year rate | 29.9 | 30.5 | |
| Median survival time, months | 21.5 | 31.2 |
Note: Data are no. of patients (%) unless otherwise indicated.
Overall and Disease-Free Survival
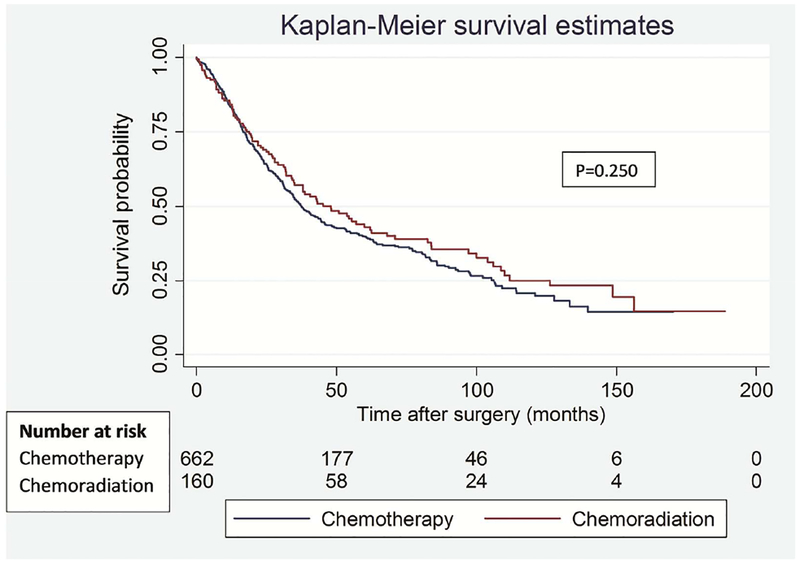
There were no statistically significant differences in OS (p=0.250) or DFS (p=0.207) between the groups (Figure 1). Five-year OS was 39.9% vs 42.9%, with median overall survival time of 37.5 (95% CI: 34.0 – 44.3 months) vs 48 months (95% CI: 34.7 – 62.6 months) in CxT and CxRT groups respectively. 5-year DFS was 28.7% vs 29.8%, with median disease-free survival duration of 19.6 (95% CI: 15.4 – 22.8 months) vs 30.1 months (95% CI: 18.4 – 41.7 months) in CxT and CxRT groups respectively. Hence, while there were no statistically significant differences in the rates of OS and DFS, there was a clinically important difference in median OS and DFS of 10.5 months favoring the CxRT cohort in this unsupervised analysis.
Figure 1.
Overall (A) and disease-free (B) survival in patients treated with induction chemotherapy versus induction chemoradiation.
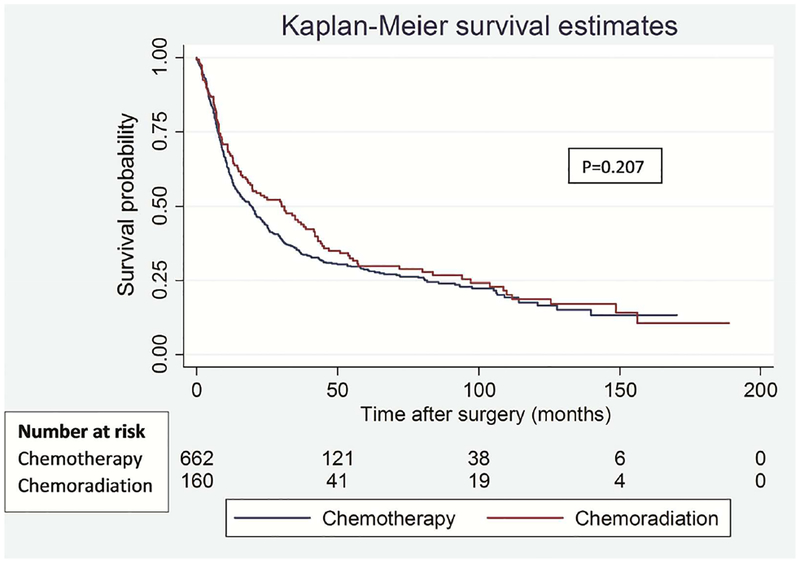
One of the primary differences noted between the two cohorts was the rate of invasive mediastinal staging prior to commencement of neo-adjuvant therapy (61.0% CxT vs 94.4% CxRT, p<0.001). Given the potential impact of understaging in the CxT cohort with almost 40% of patients not undergoing invasive staging, we conducted a priori analysis to exclude patients without pre-treatment invasive staging. In this separate analysis of patients with invasive mediastinal staging we observed no significant differences in OS (p=0.341) and DFS (p=0.455) between the groups (Figure 2). The median survival time remained mostly unchanged at 8.3 months for OS and 9.7 months for DFS (Table 3).
Figure 2.
Overall (A) and disease-free (B) survival in patients who underwent invasive mediastinal staging prior to induction chemotherapy versus induction chemoradiation.
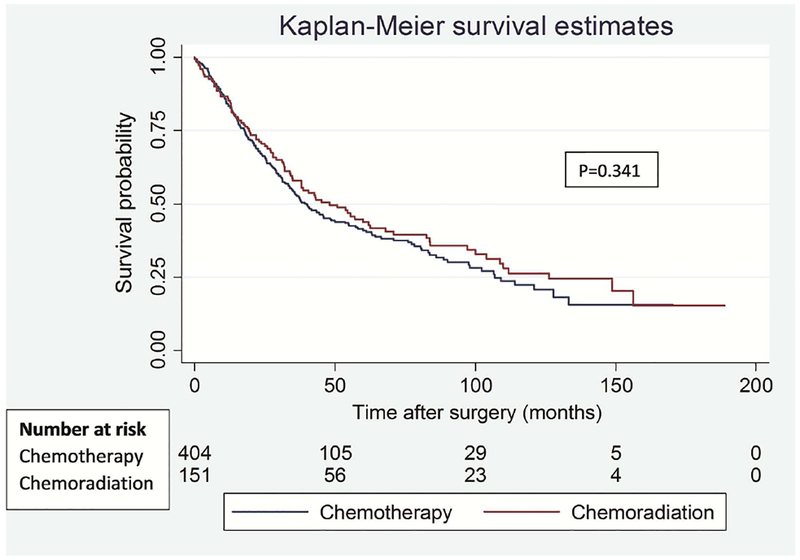
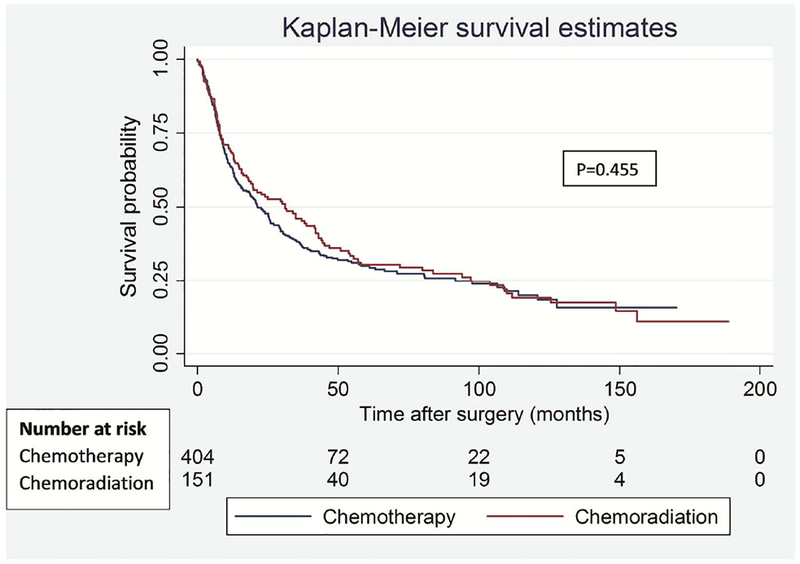
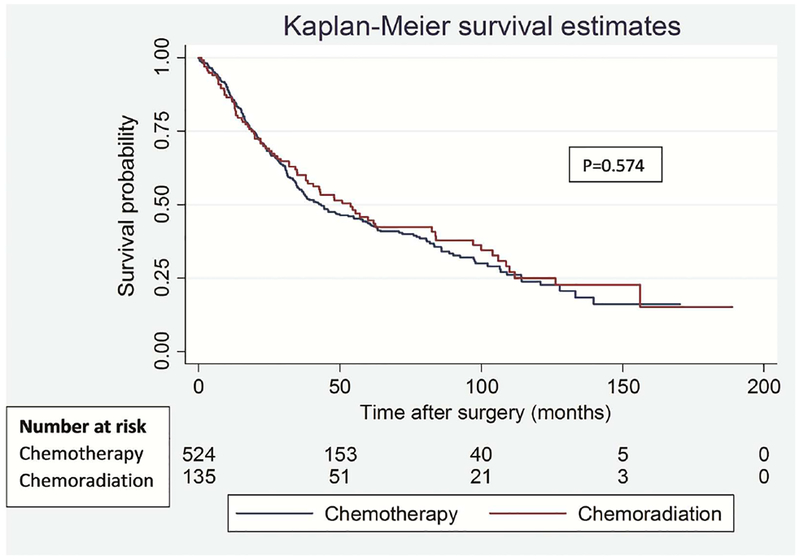
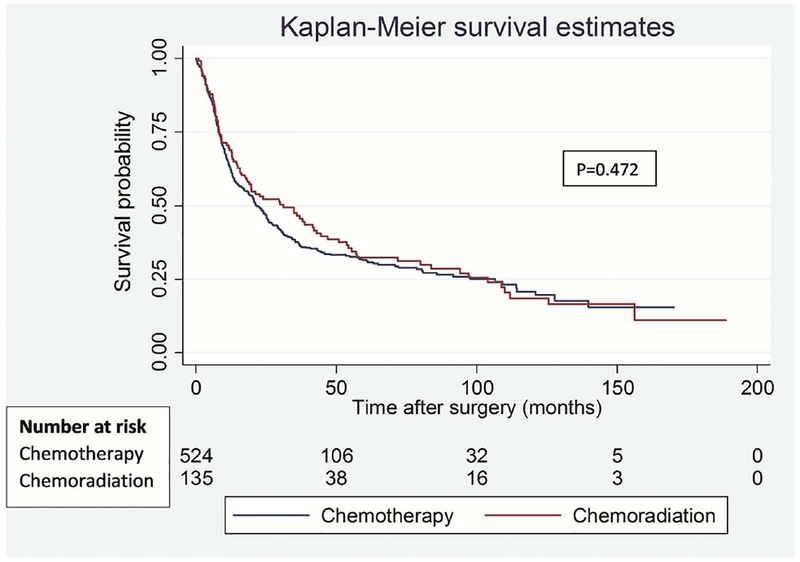
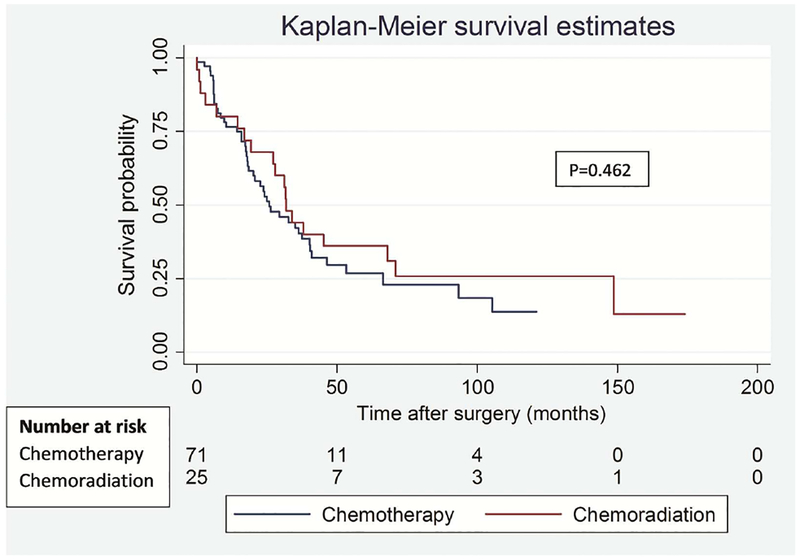
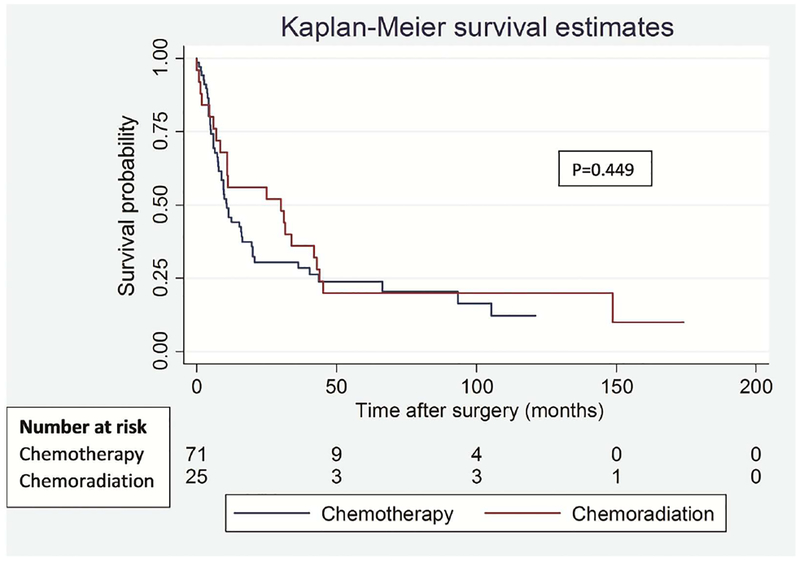
To address the potential impact of difference in type of lung resection on survival outcomes between the groups, we performed a separate analysis for lobectomy and pneumonectomy patients. Among lobectomy patients, there was no significant difference in OS (p=0.574) and DFS (p=0.472) between the groups (Figure 3). Similarly, among pneumonectomy patients, there was no difference in OS (p=0.462) and DFS (p=0.449) between the groups (Figure 4).
Figure 3.
Overall (A) and disease-free (B) survival in patients who underwent lobectomy segregated by induction treatment.
Figure 4.
Overall (A) and disease-free (B) survival in patients who underwent pneumonectomy segregated by induction treatment.
Factors Associated with Overall Survival
Multivariable analysis identified ypN2 status (HR 1.98), pneumonectomy (HR 1.51), and positive margin status (HR 1.66) to be negatively associated with overall survival. Adjuvant chemotherapy (HR 0.63) or chemo-radiation (HR 0.51) was favorably associated with OS (Table 4). Age and ypT status were associated with worse OS on univariable analysis, but this difference disappeared after multivariable adjustment. Induction CxT or CxRT were not associated with OS on either univariable or multivariable analysis. To investigate the possibility that induction CxRT could mediate improvement in OS through changing the R0 rate, improving ypN2 status, or changing the rate of pneumonectomy, a separate multivariable analysis was performed that removed these potential causal mediators; the analysis showed that administration of induction CxRT was not associated with OS (data not shown). Results of univariable analyses for OS and DFS are presented in Supplemental Table 1. Predictors of DFS were identical and results from the multivariable analysis are presented in Supplemental Table 2.
Table 4.
Factors Associated with Overall Survival
| Variables | N | Multivariable cox regression | |||
|---|---|---|---|---|---|
| HR | 95% C.I for HR | P value | |||
| Lower | Upper | ||||
| Pathological N stage | <0.001 | ||||
| N0* | 301 | 1.00 | |||
| N1 | 101 | 1.13 | 0.81 | 1.58 | 0.465 |
| N2 | 413 | 1.98 | 1.57 | 2.48 | <0.001 |
| Type of lung resection | 0.005 | ||||
| Lobectomy or sublobar resection* | 726 | 1.00 | |||
| Pneumonectomy | 96 | 1.51 | 1.13 | 2.00 | |
| Type of Adjuvant therapy | <0.001 | ||||
| None* | 384 | 1.00 | |||
| Chemotherapy | 138 | 0.63 | 0.48 | 0.82 | 0.001 |
| Radiation therapy | 204 | 0.80 | 0.62 | 1.02 | 0.077 |
| Chemoradiation | 71 | 0.51 | 0.35 | 0.74 | <0.001 |
| Completeness of resection | 0.001 | ||||
| R0* | 742 | 1.00 | |||
| R1–R2 | 80 | 1.66 | 1.24 | 2.23 | |
Reference group; HR indicates the hazard ratio; CI, confidence interval.
Comment
In this multi-center study of patients with N2 positive NSCLC treated with either induction CxT or CxRT, completeness of resection, pathologic nodal status, and a lesser than pneumonectomy resection were associated with favorable OS. Both overall and disease-free survival outcomes were similar at 5 years regardless of the induction strategy. Furthermore, despite improved downstaging and R0 resection rate in CxRT group, and hypothesized improved systemic control following induction CxT, neither CxT nor CxRT were associated with significantly different local nor distant recurrence rates. From an oncologic standpoint, both treatment strategies combined with surgery showed a similar impact.
Historically, surgery alone was used to treat locally advanced lung cancer. However, since early 1990s, evidence from randomized trails emerged to demonstrate substantial superiority for surgery with perioperative chemotherapy for locally advanced lung cancers [3–6]. Studies from the 1980s and 1990s highlighted that careful patient selection for locally advanced N2 NSCLC is critical to deriving benefit. Specifically, bulk of N2 disease is predictive of outcome. Those treated with surgery alone and non-bulky disease result in 5-year survival near 15%, while those with bulky lymphadenopathy experience 5-year survival close to 0% [7]. With regard to non-surgical therapy, multiple regimens have been studied. Concurrent definitive chemoradiation has demonstrated 20% 5-year survival (Intergroup 0139) whereas sequential chemo-radiation regimen achieved 14% 5-year survival (EORTC) [1, 7].
To date, Intergroup 0139 has been the most influential study, which established the “standard of care” for locally advanced N2 NSCLC. Based on the subgroup analysis, which demonstrated improved survival in concurrent CxRT and lobectomy group as compared to a matched cohort of concurrent CxRT alone, many experts support trimodality therapy as the most optimal approach. Since then, further subgroup analyses and single series studies have suggested that patients with single station N2 disease are those most likely to benefit from surgery [5, 8–13], leaving those with resectable multi-station disease in a gray area where the tendency is to favor definitive concurrent chemoradiation with no surgical intervention. This recommendation for sole locoregional disease control with CxRT is based on the high distant recurrence rates in this group, and motivation to avoid surgical incisions and potential surgical morbidity. However, in the rapidly evolving era of immunotherapy and significantly improved distal disease control, durable locoregional lung cancer control may become much more important. Complete surgical resection of primary lung cancer and mediastinal lymph nodes should therefore not be discarded as a treatment modality even in more advanced locoregional disease and should continue to be evaluated in clinical trials.
The importance of much improved systemic therapy is perhaps best demonstrated by the encouraging results of the PACIFIC trial for unresectable stage IIIA-B NSCLC. Definitive chemoradiation was followed by 12 months of maintenance immunotherapy with durvalumab (PD-L1 inhibitor) and demonstrated significantly prolonged progression-free survival (16.8 versus 5.6 months) [14]. This concept of prolonged adjuvant immunotherapy after surgical loco-regional intrathoracic disease control will hopefully be soon tested in clinical trials, along with a number of novel lone immunotherapy and combined chemo-immunotherapy induction trials, which are already under way [15–17].
Following systemic induction therapy and surgical disease control, consolidative post-operative radiation therapy (PORT) can be reserved on a case-by-case basis depending on amount of residual ypN2 disease and presence of positive surgical margins [18, 19]. A recent trial randomized patients with stage IIIA N2 disease to either sequential CxRT or CxT alone followed by surgery and showed no differences in event free survival [20]. Other randomized trials were closed early, and either went unpublished or showed no differences in survival with small numbers of patients [21][22]. [27]A recent meta-analysis comparing induction CxT versus CxRT for stage III NSCLC showed improvements in tumor downstaging, mediastinal nodal pathological complete response, and local control with induction CxRT, but no change in 5-year survival or progression-free survival [23]. Likewise, most large database studies have concluded that there is no difference in survival outcomes between these treatment approaches [24, 25]. However, these databases compile data from numerous centers where treatment standards may differ and lack locoregional and distant recurrence patterns. Such variables are critical to determining the relative benefit of double-dose local therapy (CxRT + surgery) versus higher dose systemic therapy combined with surgery. In addition, perioperative outcomes are known to be dictated by treatment center volume, particularly in the case of patients who have undergone CxRT, where a significant learning curve exists to achieve optimal results [26].
The surgical mortality rates both overall and specifically post-pneumonectomy patients in this study compares favorably to data from prior clinical trials. In the INT0139 trial, the overall 30-day mortality rate for CxRT patients was 5%. In addition, 14/16 patients died after pneumonectomy from non-cancer related causes, although the timeframe of these deaths is not reported in the manuscript [24]. In the German Lung Cancer Cooperative Group trial comparing chemotherapy/surgery/adjuvant radiation versus chemotherapy/chemoradiation/surgery for IIIA and IIIB NSCLC resulted in a treatment-related mortality rate of 14% for those who received chemoradiation versus 6% for the chemotherapy cohort [27]. In our series, there was no statistical difference between the two treatment strategies; however, the likelihood of a type 2 error is high given the small sample size of patients having undergone pneumonectomy. It remains likely that pneumonectomy after CxRT confers a higher mortality risk as compared to induction CxT.
This retrospective study has several limitations, including differing care or selection effects at each center, as well as the risk of being underpowered to detect a difference within smaller subgroups. We also lacked information on the extent of bulky or multistation N2 disease or specific chemotherapy regimens. Furthermore, many patients received either adjuvant radiation or chemotherapy further confounding our observations. However, strengths of this study include [32]relatively well annotated, prospectively recorded institutional data from high volume centers.
How our findings will influence the use of surgery as well as combinations and timing of chemotherapy and radiation for locoregional control in N2 disease in the era of immunotherapy remains to be determined. Several clinical trials investigating the use of immunotherapy in the context of locally advanced NSCLC will be critical additions to our understanding [15–19]. What seems to be apparent, is that the choice of conventional induction regimen (CxT or CxRT) does not seem to impact long-term survival in a significant fashion especially if additional XRT or chemotherapy can be added in the adjuvant setting. Our progressive understanding of immune-oncology and anticipated results of currently ongoing induction immunotherapy therapy clinical trials in NSCLC will likely soon change treatment paradigms for loco-regional lung cancers.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albain KS, et al. , Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. The Lancet, 2009. 374(9687): p. 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocco G, et al. , Management of stage IIIA (N2) non-small cell lung cancer: A transatlantic perspective. J Thorac Cardiovasc Surg, 2016. 151(5): p. 1235–8. [DOI] [PubMed] [Google Scholar]
- 3.Roth JA, et al. , A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage iiia non-small-cell lung cancer. Journal of the National Cancer Institute, 1994. 86(9): p. 673–680. [DOI] [PubMed] [Google Scholar]
- 4.Andre F, et al. , Survival of patients with resected N2 non-small-cell lung cancer: Evidence for a subclassification and implications. Journal of Clinical Oncology, 2000. 18(16): p. 2981–2989. [DOI] [PubMed] [Google Scholar]
- 5.Rosell R, et al. , A Randomized Trial Comparing Preoperative Chemotherapy Plus Surgery with Surgery Alone in Patients with Non-Small-Cell Lung Cancer. New England Journal of Medicine, 1994. 330(3): p. 153–158. [DOI] [PubMed] [Google Scholar]
- 6.Nagai K, et al. , A randomized trial comparing induction chemotherapy followed by surgery with surgery alone for patients with stage IIIA N2 non-small cell lung cancer (JCOG 9209). J Thorac Cardiovasc Surg, 2003. 125(2): p. 254–60. [DOI] [PubMed] [Google Scholar]
- 7.van Meerbeeck JP, et al. , Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. Journal of the National Cancer Institute, 2007. 99(6): p. 442–450. [DOI] [PubMed] [Google Scholar]
- 8.Goldstraw P, et al. , Surgical management of non-small-cell lung cancer with ipsilateral mediastinal node metastasis (N2 disease). Journal of Thoracic and Cardiovascular Surgery, 1994. 107(1): p. 19–28. [PubMed] [Google Scholar]
- 9.Martini N and Flehinger BJ, The role of surgery in N2 lung cancer. Surgical Clinics of North America, 1987. 67(5): p. 1037–1049. [DOI] [PubMed] [Google Scholar]
- 10.Mountain CF, Surgery for stage IIIa-N2 non-small cell lung cancer. Cancer, 1994. 73(10): p. 2589–2598. [DOI] [PubMed] [Google Scholar]
- 11.Inoue M, et al. , Results of surgical intervention for p-stage IIIA (N2) non-small cell lung cancer: Acceptable prognosis predicted by complete resection in patients with single N2 disease with primary tumor in the upper lobe. Journal of Thoracic and Cardiovascular Surgery, 2004. 127(4): p. 1100–1106. [DOI] [PubMed] [Google Scholar]
- 12.Vansteenkiste JF, et al. , Survival and prognosis factors in resected N2 non-small cell lung cancer: A study of 140 cases. Annals of Thoracic Surgery, 1997. 63(5): p. 1441–1450. [DOI] [PubMed] [Google Scholar]
- 13.Ichinose Y, et al. , Completely resected stage IIIA non-small cell lung cancer: The significance of primary tumor location and N2 station. Journal of Thoracic and Cardiovascular Surgery, 2001. 122(4): p. 803–808. [DOI] [PubMed] [Google Scholar]
- 14.Antonia SJ, et al. , Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. New England Journal of Medicine, 2017. 377(20): p. 1919–1929. [DOI] [PubMed] [Google Scholar]
- 15.ClinicalTrials.gov. National Library of Medicine (U.S.). Neo - Adjuvant Chemo / Immunotherapy for the Treatment of Resectable Stage IIIA Non-Small Cell Lung Cancer (NSCLC): A Phase II Multicenter Exploratory Study. Identifier Retrieved June 8, 2018 from: https://clinicaltrials.gov/ct2/show/NCT03081689
- 16.ClinicalTrials.gov. National Library of Medicine (U.S.). Pembrolizumab Prior to Surgery for Stage 1B, 2 or 3A Non-small Cell Lung Cancer (NSCLC): A Phase II Study. Identifier Retrieved June 8, 2018 from: https://clinicaltrials.gov/ct2/show/NCT02818920
- 17.ClinicalTrials.gov. National Library of Medicine (U.S.). Phase II Study of Induction Checkpoint Blockade for Untreated Stage I-IIIA Non-Small Cell Lung Cancers Amenable For Surgical Resection, NEOSTAR/INDUCTION - Strategic Alliance: BMS. Identifier Retrieved June 8, 2018 from: https://clinicaltrials.gov/ct2/show/NCT03158129
- 18.Billiet C, et al. , Outcome after PORT in ypN2 or R1/R2 versus no PORT in ypN0 stage IIIN2 NSCLC after induction chemotherapy and resection. Journal of Thoracic Oncology, 2016. 11(11): p. 1940–1953. [DOI] [PubMed] [Google Scholar]
- 19.Stephens R, et al. , The role of post-operative radiotherapy in non-small-cell lung cancer: a multicentre randomised trial in patients with pathologically staged T 1–2, N 1–2, M 0 disease. British journal of cancer, 1996. 74(4): p. 632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pless M, et al. , Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet, 2015. 386(9998): p. 1049–56. [DOI] [PubMed] [Google Scholar]
- 21.Katakami N, et al. , A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer (WJTOG9903). Cancer, 2012. 118(24): p. 6126–35. [DOI] [PubMed] [Google Scholar]
- 22.ClinicalTrials.gov. National Library of Medicine (U.S.). Phase III Randomized Trial of Preoperative Chemotherapy Versus Preoperative Concurrent Chemotherapy and Thoracic Radiotherapy Followed by Surgical Resection and Consolidation Chemotherapy in Favorable Prognosis Patients With Stage IIIA (N2) Non-Small Cell Lung Cancer. Retrieved June 8, 2018 from: https://clinicaltrials.gov/ct2/show/study/NCT00113386
- 23.Guo S.x., et al. , Neoadjuvant Chemoradiotherapy vesus Chemotherapy alone Followed by Surgery for Resectable Stage III Non-Small-Cell Lung Cancer: a Meta-Analysis. Scientific Reports, 2016. 6: p. 34388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang C-FJ, et al. , Adding Radiation to Induction Chemotherapy Does Not Improve Survival of Patients with Operable Clinical N2 Non-Small Cell Lung Cancer. The Journal of thoracic and cardiovascular surgery, 2015. 150(6): p. 1484–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno AC, et al. , Concurrent versus sequential chemoradiation therapy in completely resected pathologic N2 non-small cell lung cancer: propensity-matched analysis of the National Cancer Data Base. Annals of surgical oncology, 2018: p. 1–9. [DOI] [PubMed] [Google Scholar]
- 26.Eaton BR, et al. , Institutional enrollment and survival among NSCLC patients receiving chemoradiation: NRG Oncology Radiation Therapy Oncology Group (RTOG) 0617. JNCI: Journal of the National Cancer Institute, 2016. 108(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas M, et al. , Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. The lancet oncology, 2008. 9(7): p. 636–648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.