Abstract
Background and purpose:
An ipsilateral mild carotid stenosis, defined as plaque with <50% luminal narrowing, is identified in nearly 40% of patients with embolic stroke of undetermined source (ESUS) and could represent an unrecognized source of athero-embolism. We aimed to summarize data regarding the frequency of mild carotid stenosis with high-risk features in ESUS.
Methods:
We searched Pubmed and Ovid-Embase for studies reporting carotid plaque imaging features in ESUS. The prevalence of ipsilateral and contralateral mild carotid stenosis with high-risk features was pooled using random-effect meta-analysis.
Results:
Eight studies enrolling 323 participants were included. The prevalence of mild carotid stenosis with high-risk features in the ipsilateral carotid was 32.5% (95% CI: 25.3 – 40.2) compared to 4.6 % (95% CI: 0.1 – 13.1) in the contralateral carotid. The odds ratio of finding a plaque with high-risk features in the ipsilateral versus the contralateral carotid was 5.5 (95% CI: 2.5 – 12.0).
Conclusions:
Plaques with high-risk features are five times more prevalent in the ipsilateral compared to the contralateral carotid in ESUS, suggesting a relationship to stroke risk.
Keywords: embolic stroke of undetermined source, carotid plaque, intraplaque hemorrhage, echolucency, ulceration, stroke etiology
Subject terms: ischemic stroke, atherosclerosis, stenosis, computed tomography, magnetic resonance imaging, ultrasound
INTRODUCTION
Embolic stroke of undetermined source (ESUS) represents 17% (9–25%) of all ischemic strokes [1]. An ipsilateral mild carotid stenosis (plaque with <50% luminal narrowing) is identified in nearly 40% of patients with ESUS and may represent a source of athero-embolism [2, 3]. Vascular imaging is used to assess carotid plaque features other than degree of stenosis that may be important to estimate the stroke risk, notably intraplaque hemorrhage, large lipid-rich necrotic core, thin or ruptured fibrous cap, silent embolic infarcts, progression, irregularity or ulceration, echolucency, neovascularization, inflammation, large juxta-liminal hypoechoic area, large plaque volume, microembolic signals, and impaired cerebrovascular reserve [4]. Patients with ESUS that have a high-risk plaque may benefit from specific interventions to prevent stroke. We aimed to summarize data on the frequency of mild carotid stenosis with high-risk features in ESUS.
METHODS
This report is compliant with the Preferred reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The data supporting the findings of this study are available from the corresponding author upon reasonable request.
We searched Medline and Ovid-Embase for observational studies reporting carotid plaque imaging results in ESUS, from inception to July 15, 2019 (Table I, please see https://www.ahajournals.org/journal/str). The titles and abstracts were screened, and full-texts of potentially eligible records were retrieved for further assessment. Disagreements regarding study inclusion were resolved through consensus (JKT and GJ). The risk of bias was assessed using the Risk of Bias Tool for Prevalence Studies (Table II, please see https://www.ahajournals.org/journal/str) with the aim of excluding all studies with high-risk of bias from the quantitative synthesis.
We extracted first author’s name, year of publication, study design, sample size, mean age, proportion of women, frequency of cardiovascular risk factors, type of index event (stroke or TIA), imaging modality, onset-to-imaging time, side and frequency of mild carotid stenosis with high-risk features.
Analyses were performed with STATA (version 13, StataCorp, College Station, TX, USA). Heterogeneity between studies was assessed using the χ2 test on the Cochran’s Q statistic and quantified by the I2 index. The prevalence of ipsilateral and contralateral mild carotid stenosis with high-risk features was pooled using random-effect meta-analysis after stabilizing the variance of each study with the Freeman-Tukey double arc-sine transformation. Small-study effect was assessed by visual inspection of funnel plots and formally tested using Egger’s test. Statistical tests were two-sided and statistical significance defined as p ≤ 0.05.
RESULTS
The initial search identified 181 records. Eight articles met the inclusion criteria [5–12] (Figure I, please see https://www.ahajournals.org/journal/str).
All studies were prospective and enrolled 323 participants with unilateral anterior circulation ischemic stroke (Table 1). Plaque imaging was performed within 14 days of stroke onset using MRI [5, 8–10], CTA [7] or ultrasound [6]. Ulceration, intraplaque hemorrhage, thrombus, fibrous cap rupture, echolucency, or plaque thickness ≥ 3 mm were the high-risk features considered.
Table 1:
Characteristics of the included studies
| PMID | Author | Year | Sample size | Age (mean) | Age (median) | Women % | HTN % | DM % | Smoking % | DLP % | CAD % | Plaque imaging | Imaging delay (days) | High-risk features | ROB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24330333 | Bayer-Karpinska [5] | 2013 | 32 | NA | 74 | 32 | 72 | 22 | 49 | 28 | 22 | MRI (HRBB) | < 7 | ulceration, intraplaque hemorrhage, thrombus | 9 |
| 29307510 | Buon [6] | 2018 | 44 | NA | 46.5 | 43 | 14 | 2 | 59 | 16 | NA | Carotid US | NA | ulceration, echolucency, thrombus | 9 |
| 27412144 | Coutinho [7] | 2016 | 85 | NA | 70 | 52 | 60 | 28 | NA | 34 | 20 | CTA | < 10 | plaque thickness ≥ 3 mm | 10 |
| 22498329 | Freilinger [8] | 2012 | 32 | 71.7 | NA | 31 | 59 | 22 | 63 | 47 | 22 | MRI (HRBB) | 5.8 | ulceration, intraplaque hemorrhage, thrombus | 10 |
| 26077590 | Gupta [9] | 2015 | 27 | 71 | NA | 48 | 78 | 22 | 4 | 56 | 11 | MRI (3D-TOF) | 2.6 | intraplaque hemorrhage | 8 |
| 29571754 | Singh [10] | 2018 | 35 | 74.3 | NA | 54 | 74 | 29 | 6 | 80 | 49 | MRI (HRBB) | NA | intraplaque hemorrhage | 9 |
| 26897689 | Gupta [11] | 2016 | 50 | 69.5 | NA | 50 | NA | NA | NA | NA | NA | MRI (3D-TOF) | 1 | intraplaque hemorrhage | 9 |
| 26433367 | Hyafil [12] | 2016 | 18 | 70 | NA | 63 | 72 | 22 | 17 | 28 | 22 | MRI (HRBB) | < 14 | fibrous cap rupture, intraplaque hemorrhage, thrombus | 9 |
3D-TOF = 3-dimensional time of flight, CAD = Coronary artery disease, DLP = Dyslipidemia, DM = Diabetes mellitus, HRBB = High-resolution black blood, HTN = Hypertension, MRI = Magnetic Resonance Imaging, NA = Not available, PMID = PubMed accession number, ROB = Risk of bias score (maximum 10, 8–10 = low risk of bias/high-quality, 5–7 = moderate risk of bias/moderate quality, ≤4 = high risk of bias/low quality), US = Ultrasound
The pooled prevalence of mild carotid stenosis with high-risk features was 32.5% (95% CI: 25.3 – 40.2) in the ipsilateral carotid (Figure 1) and 4.6 % (95% CI: 0.1 – 13.1) in the contralateral carotid (Figure II, please see https://www.ahajournals.org/journal/str). There was no small-study effect (Figure III, please see https://www.ahajournals.org/journal/str). The odds ratio of finding a mild carotid stenosis with high-risk features in the ipsilateral versus the contralateral carotid was 5.5 (95% CI: 2.5 – 12.0) (Figure 2). The odds ratio of finding a ruptured fibrous cap in the ipsilateral versus the contralateral carotid was 17.5 (95% CI: 2.2 – 140.1) (Table III, please see https://www.ahajournals.org/journal/str). In the sensitivity analysis, similar results were obtained after excluding studies with sample size < 20 or with potential population overlap [11, 12] (Figures IV and V, please see https://www.ahajournals.org/journal/str).
Figure 1: Prevalence of ipsilateral carotid plaque with high-risk features in ESUS.
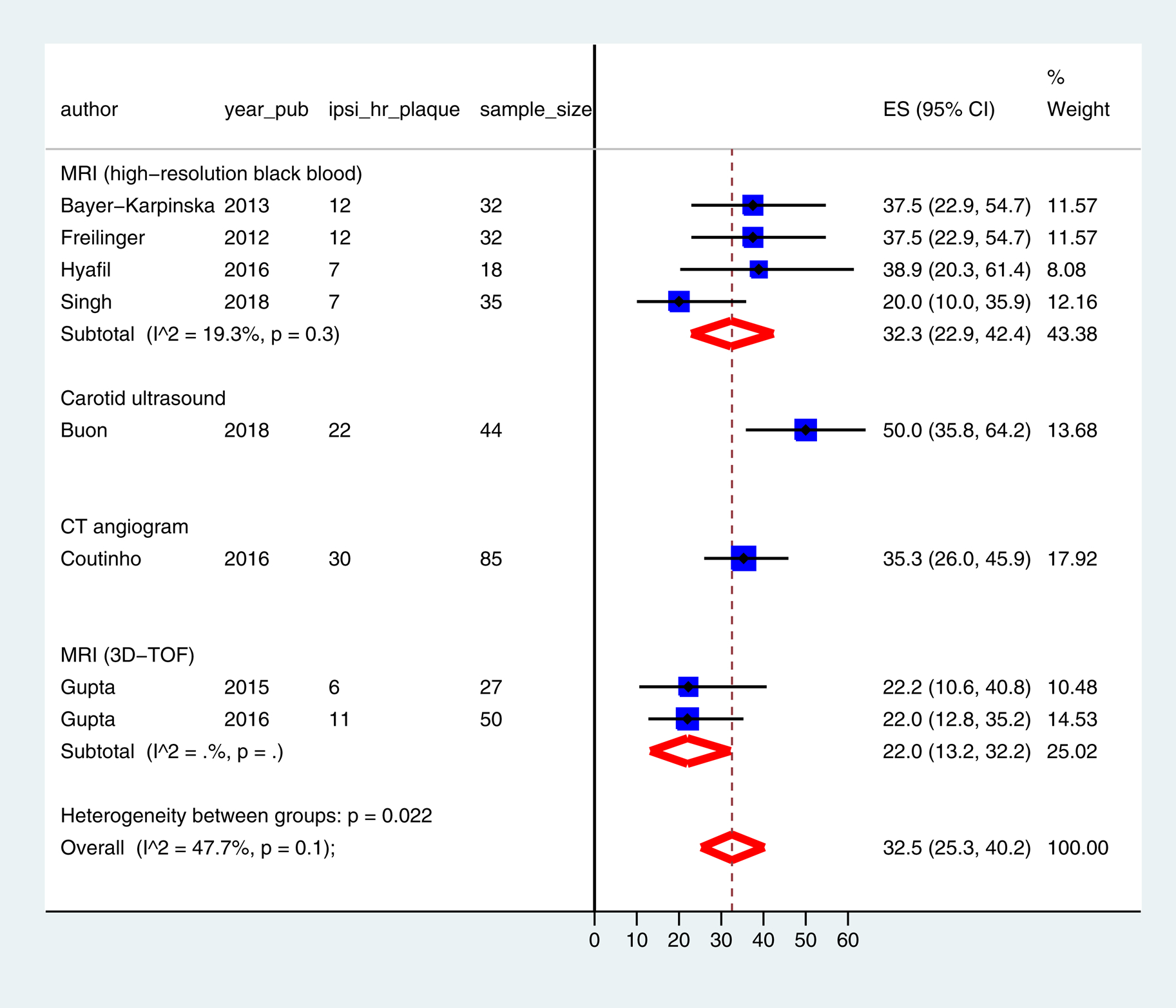
3D-TOF = 3-dimensional time of flight, CI = Confidence interval, CT = Computed tomography, ES = Effect size, ipsi_hr_plaque = ipsilateral carotid plaque with high-risk features, MRI = Magnetic resonance imaging, sample_size = number of participants in the study, year_pub = year of publication
Figure 2: Odds-ratio of finding plaque with high-risk features in the ipsilateral versus the contralateral carotid in ESUS.
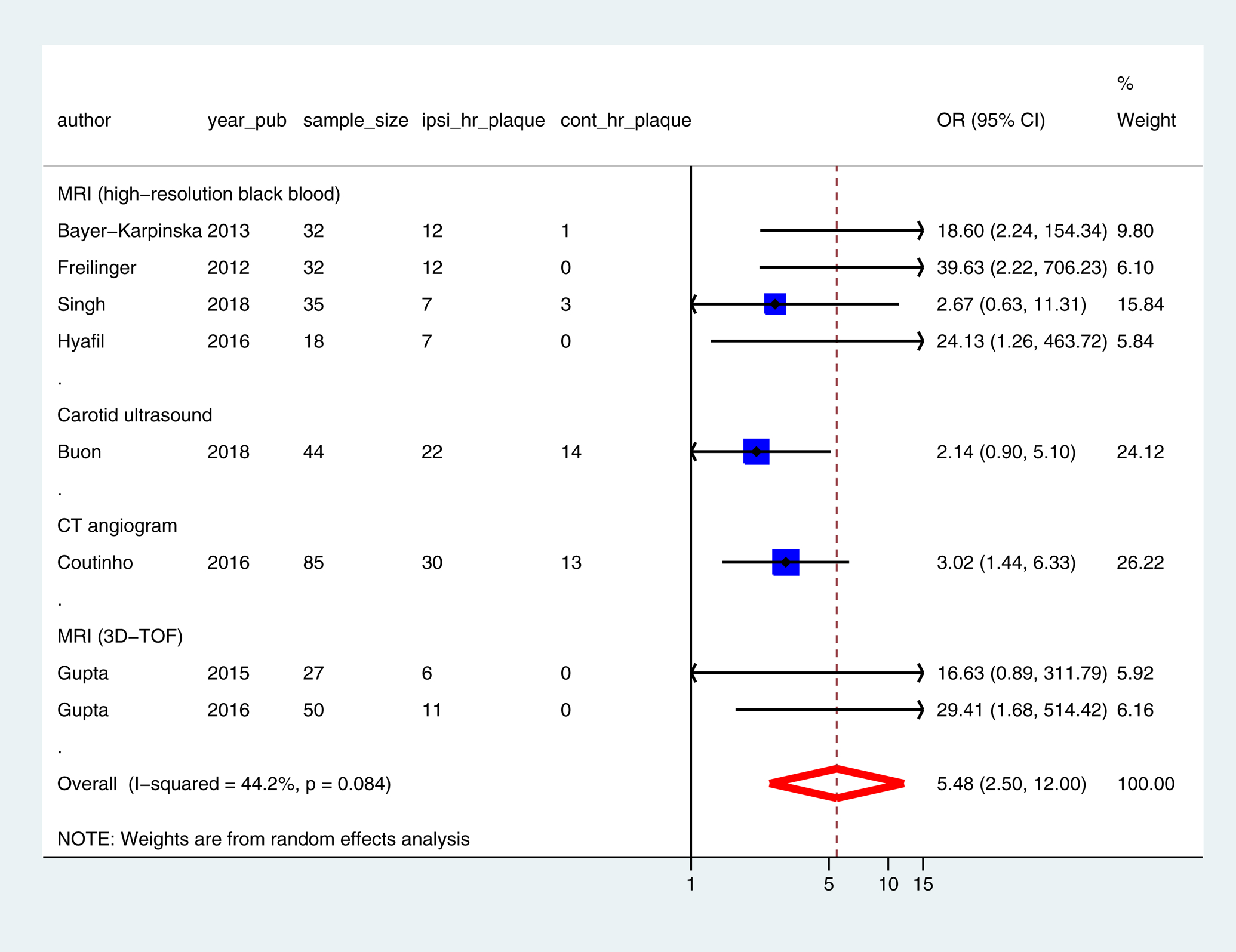
3D-TOF = 3-dimensional time of flight, CI = Confidence interval, CT = Computed tomography, cont_hr_plaque = contralateral carotid plaque with high-risk features, ipsi_hr_plaque = ipsilateral carotid plaque with high-risk features, MRI = Magnetic resonance imaging, OR = Odds ratio, sample_size = number of participants in the study, year_pub = year of publication
DISCUSSION
Mild stenosis with high-risk features were five times more prevalent in the ipsilateral compared to the contralateral carotid in ESUS, suggesting a relationship to stroke risk. Our findings align with the results of AF-ESUS study showing that patients with ESUS and ipsilateral mild carotid stenosis had a lower 10-year probability of atrial fibrillation detection, thus making a cardioembolic source less probable [2]. Moreover, in NAVIGATE-ESUS trial, patients with ESUS and ipsilateral mild carotid stenosis did not benefit from anticoagulation [3]. In COMPASS trial [13], Rivaroxaban-Aspirin combination was more effective than Aspirin or Rivaroxaban for prevention of non-cardioembolic strokes and represents a potential therapeutic option in patients with ESUS and an ipsilateral mild carotid stenosis. However, recent strokes were excluded and some participants had asymptomatic ≥ 50% carotid stenosis [14]. Therefore, further trials are needed to investigate the benefit of Rivaroxaban-Aspirin combination in patients with recent ESUS and an ipsilateral mild carotid stenosis. Dual antiplatelet therapy with high-dose statins, endarterectomy or stenting also represent potential treatment options.
All studies used a single plaque imaging modality which may have led to underestimation of the prevalence of high-risk plaques in ESUS since various imaging modalities have different sensitivity and specificity for detection of high-risk features [4]. Besides features visible on plaque MRI, high-risk features identified by other imaging modalities may be useful: microembolic signals (transcranial Doppler), large plaque volume (3D ultrasound), plaque neovascularization (contrast-enhanced ultrasound), and plaque inflammation (PET-CT) [4]. Combination of vascular imaging and blood biomarkers may also be useful to refine stroke risk stratification in patients with ESUS and ipsilateral mild carotid stenosis. RNA biomarker panels that predict stroke etiology with >90% sensitivity and specificity [15] can be integrated into multiparameter scores to predict causality of an ipsilateral mild carotid stenosis in ESUS and better stratify the risk of recurrence prior to inclusion in trials.
Supplementary Material
Funding:
GCJ receives research support from the Canadian Institutes of Health Research (CIHR), the National Institutes of Health (NIH), the Heart and Stroke Foundation (HSF), the Canada Foundation for Innovation (CFI), and the University Hospital Foundation.
Footnotes
Disclosures: none.
REFERENCES
- 1.Hart RG, Catanese L, Perera KS, Ntaios G, Connolly SJ. Embolic Stroke of Undetermined Source: A Systematic Review and Clinical Update. Stroke. 2017;48:867–872. [DOI] [PubMed] [Google Scholar]
- 2.Ntaios G, Perlepe K, Sirimarco G, Strambo D, Eskandari A, Karagkiozi E, et al. Carotid plaques and detection of atrial fibrillation in embolic stroke of undetermined source. Neurology. 2019;92:e2644–e2652. [DOI] [PubMed] [Google Scholar]
- 3.Ntaios G, Swaminathan B, Berkowitz SD, Gagliardi RJ, Lang W, Siegler JE, et al. Efficacy and Safety of Rivaroxaban Versus Aspirin in Embolic Stroke of Undetermined Source and Carotid Atherosclerosis. Stroke. 2019;50:2477–2485. [DOI] [PubMed] [Google Scholar]
- 4.Saba L, Saam T, Jager HR, Yuan C, Hatsukami TS, Saloner D, et al. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. 2019;18:559–572. [DOI] [PubMed] [Google Scholar]
- 5.Bayer-Karpinska A, Schwarz F, Wollenweber FA, Poppert H, Boeckh-Behrens T, Becker A, et al. The carotid plaque imaging in acute stroke (CAPIAS) study: protocol and initial baseline data. BMC Neurol. 2013;13:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buon R, Guidolin B, Jaffre A, Lafuma M, Barbieux M, Nasr N, et al. Carotid Ultrasound for Assessment of Nonobstructive Carotid Atherosclerosis in Young Adults with Cryptogenic Stroke. J Stroke Cerebrovasc Dis. 2018;27:1212–1216. [DOI] [PubMed] [Google Scholar]
- 7.Coutinho JM, Derkatch S, Potvin AR, Tomlinson G, Kiehl TR, Silver FL, et al. Nonstenotic carotid plaque on CT angiography in patients with cryptogenic stroke. Neurology. 2016;87:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freilinger TM, Schindler A, Schmidt C, Grimm J, Cyran C, Schwarz F, et al. Prevalence of nonstenosing, complicated atherosclerotic plaques in cryptogenic stroke. JACC Cardiovasc Imaging. 2012;5:397–405. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, Gialdini G, Lerario MP, Baradaran H, Giambrone A, Navi BB, et al. Magnetic resonance angiography detection of abnormal carotid artery plaque in patients with cryptogenic stroke. J Am Heart Assoc. 2015;4:e002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh N, Moody AR, Panzov V, Gladstone DJ. Carotid Intraplaque Hemorrhage in Patients with Embolic Stroke of Undetermined Source. J Stroke Cerebrovasc Dis. 2018;27:1956–1959. [DOI] [PubMed] [Google Scholar]
- 11.Gupta A, Gialdini G, Giambrone AE, Lerario MP, Baradaran H, Navi BB, et al. Association Between Nonstenosing Carotid Artery Plaque on MR Angiography and Acute Ischemic Stroke. JACC Cardiovasc Imaging. 2016;9:1228–1229. [DOI] [PubMed] [Google Scholar]
- 12.Hyafil F, Schindler A, Sepp D, Obenhuber T, Bayer-Karpinska A, Boeckh-Behrens T, et al. High-risk plaque features can be detected in non-stenotic carotid plaques of patients with ischaemic stroke classified as cryptogenic using combined (18)F-FDG PET/MR imaging. Eur J Nucl Med Mol Imaging. 2016;43:270–279. [DOI] [PubMed] [Google Scholar]
- 13.Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med. 2017;377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 14.Sharma M, Hart RG, Connolly SJ, Bosch J, Shestakovska O, Ng KKH, et al. Stroke Outcomes in the COMPASS Trial. Circulation. 2019;139:1134–1145. [DOI] [PubMed] [Google Scholar]
- 15.Jickling GC, Stamova B, Ander BP, Zhan X, Liu D, Sison SM, et al. Prediction of cardioembolic, arterial, and lacunar causes of cryptogenic stroke by gene expression and infarct location. Stroke. 2012;43:2036–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


