Abstract
Heat stress is an increasing concern in poultry industry as it can cause a rise in the body temperature of chickens. Recently, we reported that l-citrulline (l-Cit) is a potential hypothermic agent that could improve thermotolerance in chicks. However, synthetic l-Cit has not yet been approved for inclusion in animal diets. l-Cit was first isolated from watermelon. Watermelon rind (WR), an agricultural waste product, contains more l-Cit than the flesh of the fruit. In the current study, the chemical composition and l-Cit content of WR dried powder (WRP) were determined. WRP was mixed with water at a ratio of 4:5 (wt/v) to make WRP mash, and then mixed with a commercial starter diet to prepare a 9% WRP mash diet. The WRP mash diet was fed to 3- to 15-day-old chicks and daily food intake, body weight, and changes in rectal temperature were measured. At the end of the experiment, blood was collected from the chicks to analyze plasma l-Cit and other free amino acids. The chemical analysis of WRP revealed a variety of components including 19.1% crude protein. l-Cit was the most abundant free amino acid in WRP (3.18 mg/g). Chronic supplementation of the WRP mash diet significantly increased compensatory food intake, plasma l-Cit, l-ornithine, and l-tyrosine in chicks. WRP mash diet did not affect the body temperature of the chicks. In conclusion, WRP mash diet supplementation increased plasma l-Cit concentration in chicks. The increase in plasma l-Cit concentrations suggest that WR could be used as a natural source of l-Cit in chicks to ameliorate the adverse effects of heat stress.
Keywords: body temperature, chicks, food intake, plasma l-citrulline, watermelon rind
Introduction
Body temperature is an important parameter for assessing the homeostatic status of an organism. Ambient temperature can affect body temperature in chickens. In particular, high ambient temperatures cause body temperature increases and induce heat stress in chicks (Chowdhury et al., 2012; Ito et al., 2014) because the birds do not have the ability to sweat due to the absence of sweat glands (Marder and Arad, 1989; Ensminger et al., 1990). Moreover, prolonged high body temperature negatively affects oxidative status and growth performance in chickens (Savory, 1986; Azad et al., 2010; Chowdhury et al., 2014). Therefore, reducing body temperature in chickens is an effective approach to protecting them from the potentially adverse effects of heat stress.
l-Citrulline (l-Cit) is an endogenous amino acid in most living systems (Curis et al., 2005). It is a non-protein amino acid (Angela et al., 2011) that is metabolized to l-arginine (l-Arg), which is then converted to l-ornithine (l-Orn) by arginase (Tamir and Ratner, 1963; Suenaga et al., 2008). Recently, it was found that plasma l-Cit levels are significantly reduced by heat stress in chicks (Chowdhury et al., 2014). Interestingly, it was also reported that oral administration of l-Cit, but not l-Arg or l-Orn, lowered body temperature in chicks (Chowdhury et al., 2015) and afforded a degree of thermotolerance (Chowdhury et al., 2017). Therefore, the provision of l-Cit may offer a novel nutritional means of reducing body temperature in chickens under heat stress. Although the inclusion of synthetic l-Cit in poultry rations has not yet been approved (Food and Agricultural Materials Inspection Center, Japan, 1953), an alternative approach might be the use of a natural source of l-Cit, which might offer a means to increase plasma content of l-Cit in chickens to ameliorate heat stress effects and improve poultry production.
l-Cit was first identified as a constituent of watermelon (Citrullus vulgaris) in the early twentieth century (Koga and Ohtake, 1914; Wada, 1930), and watermelon is believed to be a natural source of l-Cit (Rimando and Perkins-Veazie, 2005; Tarazona-Díaz et al., 2011). Interestingly, watermelon rind (WR), an agricultural waste product, contains a high amount of l-Cit in comparison with its flesh (Rimando and Perkins-Veazie, 2005). Following absorption of l-Cit from watermelon, plasma l-Arg levels have been found to increase in humans (Mandel et al., 2005; Collins et al., 2007). Consumption of watermelon combined with exercise was shown to reduce arterial blood pressure compared with a placebo (Figueroa et al., 2011). Thus, there are indications that l-Cit in watermelon juice may affect physiological functions. However, to the best of our knowledge, the effect of using WR as a natural source of l-Cit on plasma l-Cit levels or body temperatures has not been studied in any species.
In the present study, the chemical composition and free amino acid contents of WR dried powder (WRP) were examined. In addition, the effects of WR on plasma l-Cit level and body temperature in chicks were examined. Chick blood was analyzed to evaluate plasma levels of l-Cit and other free amino acid concentrations following long-term feeding with a diet supplemented with WRP.
Materials and Methods
Animals
Day-old male layer chicks(Julia strain; Gallus gallus domesticus) were purchased from a local hatchery (Murata Hatchery, Fukuoka, Japan) and housed together in metal cages (50×35×33 cm) in a group (14 birds) at a constant temperature of 30±1°C with continuous light. Chicks had free access to food [Adjust diets (metabolizable energy (ME): > 12.55 MJ/kg, protein: > 23%); Toyohashi Feed and MillsCo. Ltd., Aichi, Japan] and water during the whole experimental period. This study was performed in accordance with the guidelines for animal experiments of the Faculty of Agriculture and of the Graduate Course of Kyushu University, and adhered to Law no. 105 and Notification no. 6 of the Japanese government.
Preparation of WRP Mash
Fresh watermelons were obtained from Suika-no-Meisan, (Kumamoto, Japan). The rind was separated from the flesh and dried in an oven (Matsui MFG CO., Ltd., Japan) at 60°C for 96 h. After complete drying, the WR was ground for 1 min using an electric grinder [Wonder blender (KT.WB-1), Kastech, Japan] to produce WRP. The WRP was stored in airtight plastic bags at room temperature until it was used in the experiments. To produce a WRP mash diet, 100 g of WRP was mixed with 125 ml deionized distilled water. Then, the WRP mash (225 g) was mixed with a commercial starter diet (900 g) to produce a 9% WRP mash diet. Since the maximum recommended level of feed additives in poultry diet is 15% (Banerjee, 1998), the additive level used here is relatively low.
Proximate Analysis of WRP
The chemical composition of WRP was analyzed by the Japanese Functional Food Analysis and Research Center (Fukuoka, Japan). The moisture, crude protein, ether extract, crude fiber and ash contents were determined. In brief, moisture was determined by weight loss on heating at 135°C for 2 h. The Kjeldahl method was applied to measure crude protein. Hydrolysis and ether extraction methods were used for the analysis of crude fat. Crude fiber analysis was performed using H2SO4 and NaOH. Ash was determined by direct baking of WRP at 600°C for 2 h.
Analysis of Free Amino Acid Concentrations in WRP
Free amino acid concentrations in WRP were analyzed by ultra performance liquid chromatography (UPLC) based on the method of Ohmori et al. (2011) and Furudate and Meguro (2002) with some modifications. Forty mg of WRP was homogenized in a 15 ml centrifuge tube with 200 µl of 99% ethanol for 30 seconds. After homogenization the WRP sample was kept at room temperature for 30 min. The WRP homogenate was then filtered through 70 mm filter paper (Advantec, Toyo Roshi Kaisha, Ltd., Japan). Any remaining WRP homogenate in the centrifuge tube was washed out using 1200 µl of 80% ethanol and subjected to filtration as above. The filtrate was dried under vacuum at −100 kPa (Centrifugal Vaporizer, CVE-200D, Eyela, Japan). The dried residue was dissolved in 400 µl deionized distilled water and filtered through a 0.20 µm filter (Millipore, Bedford, MA, USA). Twenty µl WRP sample was mixed with 2 µl 1 M NaOH and vortexed. Then 10 µl WRP was transferred to a UPLC tube, and 20 µl N-acetylcysteine/O-phthalaldehyde, and 70 µl borate buffer were added and mixed; the mixture was kept for 2 min in a dark room. Both l- and the d-amino acid contents were measured using a UPLC system (Acquity™ UPLC system, consisting of a Waters Binary Solvent Manager, Water Sample Manager, and a Waters FLR Detector) with an ACCQ-TAG™ ULTRA C18 1.7 µm 2.1×100 mm column (Waters Corporation, USA). The excitation and emission wavelengths for the fluorescent detection of amino acids were 350 and 450 nm, respectively. The system was controlled with a flow rate of 0.25 ml/min at 30°C. The UPLC gradient system (A = 50 mM sodium acetate [pH 5.9], B=methanol) was 10–20% B over 3.2 min, 20% B for 1 min, 20–40% B for 3.6 min, 40% B for 1.2 min, 40–60% B for 3.8 min, 60% B for 1 min and 60–10% B for 0.01 min. The same method was used for the standard solutions containing 18 l-amino acids, 16 d-amino acids, glycine, and taurine. The concentrations of free amino acids in WRP were determined and expressed as nmol/mg sample.
Experimental Design
A total of 14 chicks (2-day-old) were gradually separated into groups of 2 chicks per cage (21×10×14 cm); 3-day-old chicks were individually isolated into two groups (n=7). We used gradual isolation (1st day, 14 chicks/cage; 2nd day, 2 chicks/cage; and 3rd day, 1 chick/cage) to separate chicks individually to minimize isolation stress. The starter diet was replaced by 9% WRP mash diet in the treatment group, while the control group continued the starter diet during the experimental period, from 3 to 15 days old. Daily recording of food intake, body weight, and rectal temperature was carried out. Rectal temperature was measured with a digital thermometer with an accuracy of ±0.1°C (Thermalert TH-5, Physitemp Instruments Inc., USA) by inserting the thermistor probe into the rectum through the cloaca to a depth of around 2 cm from the anus. At the end of the experiment, birds were euthanized by exposure to isoflurane (Mylan Inc., Tokyo, Japan). Blood was immediately collected from the jugular vein into heparinized tubes and centrifuged at 10,000×g for 4 min at 4°C (MX-307, Tommy, Japan) to collect plasma. The plasma was stored at −80°C until analysis of free amino acids was carried out.
Analysis of Free Amino Acid Concentrations in Plasma
Free amino acid concentrations were analyzed by UPLC according to the method of Ohmori et al. (2011) with some modifications. Plasma was obtained by centrifuging it at 14,000×g for 15 min at 4°C (MX-307, Tommy, Japan). Plasma was then filtered through ultrafiltration tubes (Millipore, Bedford, USA). Samples (10 µl) of plasma were transferred to UPLC tubes, and 20 µl N-acetylcysteine/Ophthalaldehyde, and 70 µl borate buffer were added and mixed; the tubes were left for 2 min in a dark room. The samples and standards were applied to UPLC as described above for the analysis of WRP. Plasma amino acid concentrations were expressed in nmol/µl.
Statistical Analysis
Changes in food intake, body weight, and rectal temperature were statistically analyzed by two-way ANOVA, where the main effects were the WRP treatment and days/time. Plasma free amino acids were analyzed by Student's t-test. Statistical analyses were performed using StatView Version 5.0 software (SAS Institute, Cary, NC, USA, 1998). Values are presented as means±S.E.M.
Results
The results of the chemical analysis of WRP are presented in Table 1 and the free amino acid contents are shown in Table 2. l-Cit (18.2 nmol/mg or 3.18 mg/g) was the most abundant free amino acid in WRP, and l-Arg was the next most abundant (2.72 nmol/mg) (Table 2). The concentrations of l-alanine, l-glutamine, l-valine, l-phenylalanine, l-isoleucine, l-serine, l-tyrosine, and GABA are also shown in Table 2.
Table 1. Chemical composition of watermelon rind powder.
| Composition | Content |
|---|---|
| Moisture (%) | 15.7 |
| Crude protein (%) | 19.1 |
| Crude fat (%) | 1.22 |
| Crude fiber (%) | 14.5 |
| Ash (%) | 15.4 |
| Nitrogen-free extract1 (%) | 34.1 |
| Gross energy2 (kcal/100 g) | 336 |
| Metabolizable energy3 (kcal/100 g) | 196 |
Nitrogen-free extract: 100−(Moisture+Crude protein+Crude fat +Crude fiber);
Gross energy: Crude protein×5.67+Crude fat ×9.68+Crude fiber×4.9+Nitrogen-free extract×4.25;
Metabolizable energy: Crude protein×34.92+Crude fat×62.16+Nitrogen-free extract×35.61 (Janssen, 1989).
Table 2. Free amino acid contents in watermelon rind powder.
| Amino acids | Content (nmol/mg) |
|---|---|
| L-Citrulline | 18.2±0.71 |
| L-Arginine | 2.72±0.10 |
| L-Alanine | 0.97±0.04 |
| GABA | 0.85±0.09 |
| L-Glutamine | 0.84±0.03 |
| L-Valine | 0.78±0.03 |
| L-Phenylalanine | 0.76±0.03 |
| L-Isoleucine | 0.47±0.02 |
| L-Serine | 0.37±0.01 |
| L-Tyrosine | 0.32±0.02 |
Nine samples were analyzed. Values are means±S.E.M in nmol/mg.
Chicks fed the 9% WRP mash diet had a significantly increased food intake in all periods (Fig. 1A; F(1,12) = 61.4, P<0.0001). The length of time into the experiment (‘days’) also had a significant effect on food intake (F(11,132)=82.9, P<0.0001). A significant interaction on food intake was also found between treatment and days (F(11,132)=2.35, P<0.05), indicating that the increase in food intake was greater with time in the WRP mash diet-treated group compared with the control. The WRP mash diet did not affect total weight gain throughout the feeding test (WRP, 106.1±2.9 g; control, 112.4±3.4 g; F(1,12)=1.42, P=0.256; Fig. 1 B). There was a significant effect of time (days) on body weight (e.g., WRP, 45.6±1.4 g and control, 46.8±1.4 g on day 3; WRP, 151.7±3.6 g and control, 159.1±2.9 g on day 15; F(12,144)=2063, P<0.0001) in both the WRP mash diet group and the control group. Rectal temperature was not changed significantly (F(1,12)=2.94, P=0.112) by chronic dietary supplementation with the WRP mash diet (Fig. 1 C). Time (days of feeding) had a significant effect on changes in body temperature caused by chronic treatment with the WRP mash diet (F(12,144)=13.7, P<0.0001). Plasma l-Cit, l-Orn, and l-Tyr were significantly increased by administration of the chronic WRP mash supplement (Fig. 2 A–C; P<0.05). Other analyzed plasma free amino acids were not changed by the chronic WRP mash supplementation (data not shown).
Fig. 1.
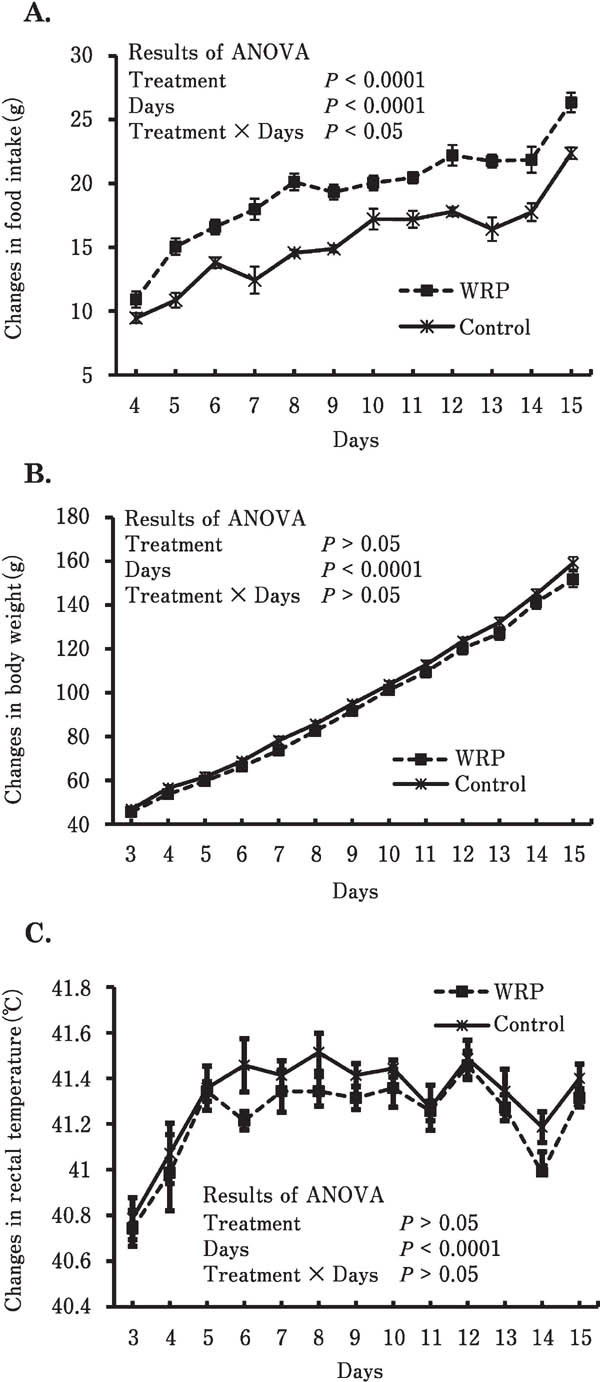
Effects of chronic WRP mash diet supplementation on changes in food intake (A), body weight (B), and rectal temperature (C) in chicks from 3 to 15 days old. Seven chicks were used in each group. Values are means±S.E.M. WRP, watermelon rind powder.
Fig. 2.
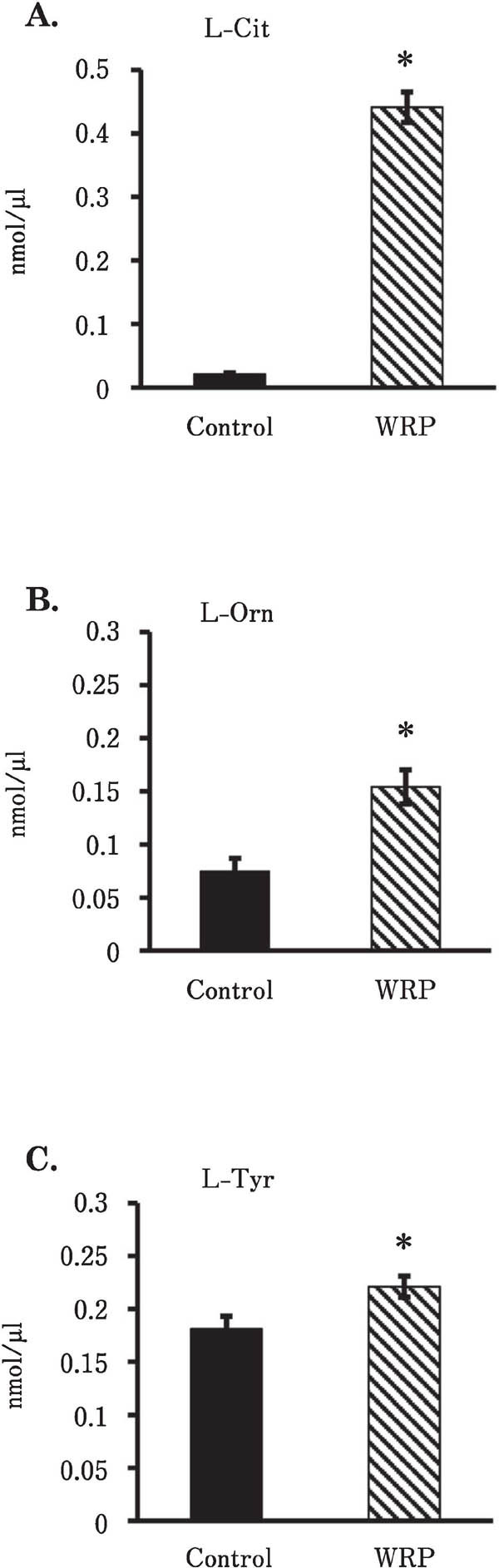
Effects of chronic WRP mash diet supplementation on changes in plasma L-Cit (A), L-Orn (B), and L-Tyr (C) in chicks. Seven chicks were used in each group. Values are means±S.E.M. *, P<0.05 by Student's t-test. L-Cit, L-citrulline; L-Orn, L-ornithine; L-Tyr, L-tyrosine; WRP, watermelon rind powder.
Discussion
In the current study, we found that WRP mash diet supplementation increased the daily food intake of chicks compared with a control group. The ME content of WRP was calculated as approximately 196 kcal/100 g (8 MJ/kg). Therefore, the ME content in the treatment diet was estimated as 10.8 MJ/kg considering the 9% inclusion of WRP; this is much lower than the ME content in the standard diet (calculated value, 12.6 MJ/kg). Hence, it could be predicted that the lower energy in the WRP diet might have caused the increase food intake by the chicks to satisfy their energy demands. WR contains several bioactive compounds, aromatic compounds, lycopene, phenolic compounds, and antioxidants (Tarazona-Díaz et al., 2011). Although we did not identify the bioactive compounds in WRP, it could be assumed that the WRP mash diet was sufficient because of the presence of such compounds, and this might have played a positive role in enhancing the compensatory food intake. We found that the actual intake of starter diet was not different between the WRP group (186.1±3.8 g) and control group (184.7±3.8 g) over the whole experimental period.
Rectal temperature did not change significantly after WRP supplementation. Previously, it was shown that acute oral administration of <15 mmol/kg body weight of l-Cit did not lower rectal temperature in 6-day-old chicks given an oral administration of 1.03 mmol l-Cit (Chowdhury et al., 2015). However, the WRP mash diet contained 0.0016 mmol of l-Cit per mg of food. Based on our calculation of food intake in the current study, chicks consumed 0.03 and 0.04 mmol of l-Cit when 6- and 15-day-old, respectively. The amount of l-Cit consumed was not enough to reduce rectal temperature. It is not yet known how much plasma l-Cit is required to cause a reduction in body temperature; however, plasma l-Cit significantly increased in the WRP mash diet-treated group to 0.44 nmol/µl compared with the control group in the present study.
Plasma l-Arg levels increase after ingestion of l-Cit from watermelon flesh and watermelon juice in humans (Mandel et al., 2005; Collins et al., 2007). Our chemical analysis here confirmed that l-Arg was the second most abundant free amino acid in WRP (Table 2). However, plasma levels of l-Arg did not increase significantly in chicks in the WRP mash-supplemented group compared with the control diet group (WRP, 0.64±0.04 nmol/µl; control, 0.55±0.06 nmol/µl). Chickens can synthesize l-Orn from l-Arg but cannot synthesize l-Cit from l-Orn (Tamir and Ratner, 1963). Thus, in the present study, the increased level of plasma l-Orn might have been the result of accumulation of l-Orn due to the different metabolic pathways between mammals and birds.
In conclusion, WRP, which is an agricultural waste product could be a natural source of l-Cit in chicks. Increased plasma l-Cit concentrations through chronic dietary supplementation with WRP at a level of 9% was not enough to induce a hypothermic effect in chicks. Further research will clarify a suitable level of inclusion of WR to reduce the effects of heat stress in chicks.
Acknowledgments
We would like to thank the JICA project and the Vietnam International Education Development program for offering a scholarship under the Can Tho University Improvement Project to LTNN, who came from the Department of Animal Sciences, College of Agriculture and Applied Biology, Can Tho University, Vietnam to study at Kyushu University. The authors are very grateful to Mr. Junya Harada, Suika-no-Meisan, Ueki, Kumamoto (http://www.suika-meisan.com/) for the generous donation of fresh watermelons that were needed to conduct the study. This work was partly supported by JSPS KAKENHI Grant NumbersJP15K07694 and JP18 K19721 to VSC.
References
- Angela RD, Charles L, Webber, Fish WW. L-Citrulline levels in watermelon cultigens tested in two environments. Horticultural Science, 46: 1572-1575. 2011. [Google Scholar]
- Azad MA, Kikusato M, Maekawa T, Shirakawa H, Toyomizu M. Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 155: 401-406. 2010. [DOI] [PubMed] [Google Scholar]
- Banerjee GC. In: Poultry. 3rd ed. pp. 104 Oxford and IBH Publishing Co. Pvt. Ltd. New Delhi, Bombay, Calcutta: 1998. [Google Scholar]
- Chowdhury VS, Tomonaga S, Nishimura S, Tabata S, Furuse M. Physiological and behavioral responses of young chicks to high ambient temperature. Journal of Poultry Science, 49: 212-218. 2012. [Google Scholar]
- Chowdhury VS, Tomonaga S, Ikegami T, Erwan E, Ito K, Cockrem JF, Furuse M. Oxidative damage and brain concentrations of free amino acid in chicks exposed to high ambient temperature. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 169: 70-76. 2014. [DOI] [PubMed] [Google Scholar]
- Chowdhury VS, Shigemura A, Erwa E, Ito K, Mohammad AB, Tran PV, Furuse M. Oral admission of L-citruline, but not Larginine or L-ornithine, acts as a hypothermic agent in chicks. Journal of Poultry Science, 52: 331-335. 2015. [Google Scholar]
- Chowdhury VS, Han G, Bahry MA, Phuong VT, Phong HD, Yang H, Furuse M. L-Citrulline acts as potential hypothermic agent to afford thermotolerance in chicks. Journal of Thermal Biology, 69: 163-170. 2017. [DOI] [PubMed] [Google Scholar]
- Collins J, Wu G, Perkins-Veazie P, Spears K, Claypool P, Baker R, Clevidence B. Watermelon consumption increases plasma arginine concentrations in adults. Nutrition, 23: 261-266. 2007. [DOI] [PubMed] [Google Scholar]
- Curis E, Nicolis C, Moinard S, Osowska N, Zerrouk S, Benazeth S, Cynober L. Almost all about citrulline in mammals. Amino Acids, 29: 177-205. 2005. [DOI] [PubMed] [Google Scholar]
- Ensminger ME, Oldfield JE, Heinemann WW. Feeding poultry. In: Feeds and Nutrition (2nd Edition). The Ensminger Publishing Company, Clovis, California, USA: 1009-1064. 1990. [Google Scholar]
- Figueroa A, Sanchez-Gozalez MA, Perkins Veazie PM, Arjmandi BH. Effects of watermelon supplementation on aortic blood pressure and wave reflection in individuals with prehypertension: A pilot study. American Journal of Hypertension, 24: 40-44. 2011. [DOI] [PubMed] [Google Scholar]
- Food and Agricultural Materials Inspection Center. 1953. FAMIC, Japan; Act No. 35. [Google Scholar]
- Furudate F, Meguro T. Content and composition of free amino acids in cabbage grown under various cultivation conditions[in Japanese]. Journal of Home Economics of Japan, 53: 199-203. 2002. [Google Scholar]
- Ito K, Erwan E, Nagasawa M, Furuse M, Chowdhury VS. Changes in free amino acid concentrations in the blood, brain and muscle of heat-exposed chicks. British Poultry Science, 55: 644-652. 2014. [DOI] [PubMed] [Google Scholar]
- Janssen WMMA. European table of energy values for poultry feedstuffs. 3rd ed. Spelderholt center for poultry research and information services, Beekbergen, the Netherlands: 1989. [Google Scholar]
- Koga Y, Ohtake R. Study report on the constituents of squeezed watermelon. Journal of the Chemical Society of Tokyo, 35: 519-528. 1914. [Google Scholar]
- Mandel H, Levy N, Izkovitch S, Korman SH. Elevated plasma citrulline and arginine due to consumption of Citrullus vulgaris (watermelon). Journal of Inherited Metabolic Disease, 28: 467-472. 2005. [DOI] [PubMed] [Google Scholar]
- Marder J, Arad Z. Panting and acid-base regulation in heat stressed birds. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 94: 395-400. 1989. [DOI] [PubMed] [Google Scholar]
- Ohmori T, Mutaguchi Y, Yoshikawa Y, Doi K, Ohshima T. Amino acid components of lees in salmon fish sauce are tyrosine and phenylalanine. Journal of Bioscience and Bioengineering, 112: 256-258. 2011. [DOI] [PubMed] [Google Scholar]
- Rimando AM, Perkins-Veazie P. Determination of citrulline in watermelon rind. Journal of Chromatography A, 1078: 196-200. 2005. [DOI] [PubMed] [Google Scholar]
- Savory JC. Influence of ambient temperature on feeding activity parameters and digestive function in domestic fowls. Physiology and Behavior, 38: 353-357. 1986. [DOI] [PubMed] [Google Scholar]
- Suenaga R, Yamane H, Tomonaga S, Asechi M, Adachi N, Tsuneyoshi Y, Kurauchi I, Sato H, Denbow DM, Furuse M. Central L-arginine reduced stress responses are mediated by Lornithine in neonatal chicks. Amino Acids, 35: 107-113. 2008. [DOI] [PubMed] [Google Scholar]
- Tamir H, Ratner S. Enzymes of arginine metabolism in chicks. Archives of Biochemistry and Biophysics, 102: 249-258. 1963. [DOI] [PubMed] [Google Scholar]
- Tarazona-Díaz MP, Viegas J, Moldao-Martins M, Aguayo E. Bioactive compounds from flesh and by-product of fresh-cut watermelon cultivars. Journal of the Science of Food and Agriculture, 91: 805-812. 2011. [DOI] [PubMed] [Google Scholar]
- Wada M. On the occurrence of a new amino acid in watermelon. Proceedings of the Imperial Academy, 6: 15-17. 1930. [Google Scholar]


