Abstract
Monitoring serum immunoglobulin G (IgG) levels in pediatric oncology patients and treating subtherapeutic levels with intravenous immunoglobulin (IVIG) may prevent infections; however, evidence is limited. This retrospective study assessed pediatric acute lymphoblastic leukemia (ALL) patients diagnosed 2006–2011 to evaluate if monitoring/supplementing IgG would reduce febrile illnesses during maintenance chemotherapy. A subject was categorized as “ever IgG monitored” if they had one or more IgG levels checked and their risk days were stratified into not IgG monitored days and IgG monitored days. IgG monitored days were further stratified into IgG monitored with IVIG supplementation, monitored with no IVIG supplementation (IgG level >500mg/dl) and monitored with no IVIG supplementation days (IgG level <500 mg/dl). Generalized linear mixed effects poisson models were used to compare events (febrile episode, positive blood culture, and febrile upper respiratory infection (URI) rates among these groups. In 136 patients, the febrile episode rate was higher in the ever IgG monitored cohort than the never monitored cohort (5.26 vs. 3.78 episodes/1000 days). Among monitored patients, IVIG monitoring and supplementation did not significantly impact the febrile episode, febrile URI, or the positive blood culture rates. These data suggest that monitoring/supplementing low IgG is not indicated for infection prophylaxis in ALL patients during maintenance chemotherapy.
Keywords: pediatric hematology/oncology, IVIG, support care, immunology, ALL, infections in immunocompromised hosts
Introduction:
Infection remains a leading cause of morbidity and mortality in pediatric acute lymphoblastic leukemia (ALL) patients1,2. One potential mechanism contributing to infection morbidity is secondary hypogammaglobinemia as a result of chemotherapy treatment. Immunoglobulin supplementation with intravenous immunoglobulin (IVIG) for primary immunodeficiencies is a well-established practice known to reduce the risk of serious infection3–6. The evidence for prophylactic IVIG supplementation in patients with secondary hypogammaglobinemia, however, is much less clear. Studies have shown a benefit of immunoglobulin replacement for lowering the rate of infectious complications in adult patients with chronic lymphocytic leukemia (CLL) and multiple myeloma7. A 2009 meta-analysis of immunoglobulin replacement in hematopoietic stem cell transplant (HCT) patients concluded that there is no advantage in terms of survival or infection prevention; however, many of the studies included in this review were old and the majority of the subjects were not pediatric patients 8. Recently, a study of 150 pediatric transplant patients showed no added benefit to giving IVIG regardless of counts versus only giving it those with levels <400 mg/dl9. Specifically in the pediatric ALL population, one study showed a significant reduction in systemic viral, fungal and bacterial infections with the administration of prophylactic IVIG to children with refractory ALL after allogeneic stem cell transplantation; however, this study was over 30 years ago and contained only 50 patients 10. Recent guidelines jointly sponsored by the Centers for Disease Control and Prevention, Infectious Diseases Society of America (IDSA), American Society for Blood and Marrow Transplantation and the European Group for Blood and Marrow Transplantation limit recommendations for the use of immunoglobulin prophylaxis to the first 100 days after HCT for pediatric and adult patients with severe hypogammaglobulinemia11.
Outside of the stem cell transplant population, there are no published studies specifically looking at the role of immunoglobulin monitoring and supplementation in pediatric ALL patients. Yet, IVIG supplementation is still a clinical practice with significant practice variation across treatment centers and individual providers with respect to which (if any) children should receive IVIG supplementation during chemotherapy treatment. Individual practice differences amongst pediatric oncologists at our institution from 2006 – 2011 offered the opportunity to conduct a retrospective study to determine the impact of IVIG treatment on the incidence of febrile episodes in pediatric patients treated for ALL.
Methods:
Study Population and Selection Criteria:
With Institutional Review Board approval, we conducted a retrospective medical record review of all pediatric oncology patients diagnosed and treated for ALL at Monroe Carell Jr. Children’s Hospital at Vanderbilt between 2006 and 2011. All patients were treated on or according to Children’s Oncology Group protocols. Evaluation of maintenance therapy was selected as it was the only time period of sufficient length where all patients were treated similarly (daily oral 6-mercaptopurine, weekly oral methotrexate, monthly 5 days steroid pulses and monthly vincristine injection). Patients were excluded from the study if they died or transferred care to another hospital prior to initiation of maintenance chemotherapy. Patients were also excluded if they received a HCT as part of their therapy as all HCT patients at our institution receive IgG monitoring and supplementation. Additionally, patients with infant ALL were excluded from our study as the treatment regimen for infant ALL differs significantly from protocols for patients with pre-B ALL and T ALL.
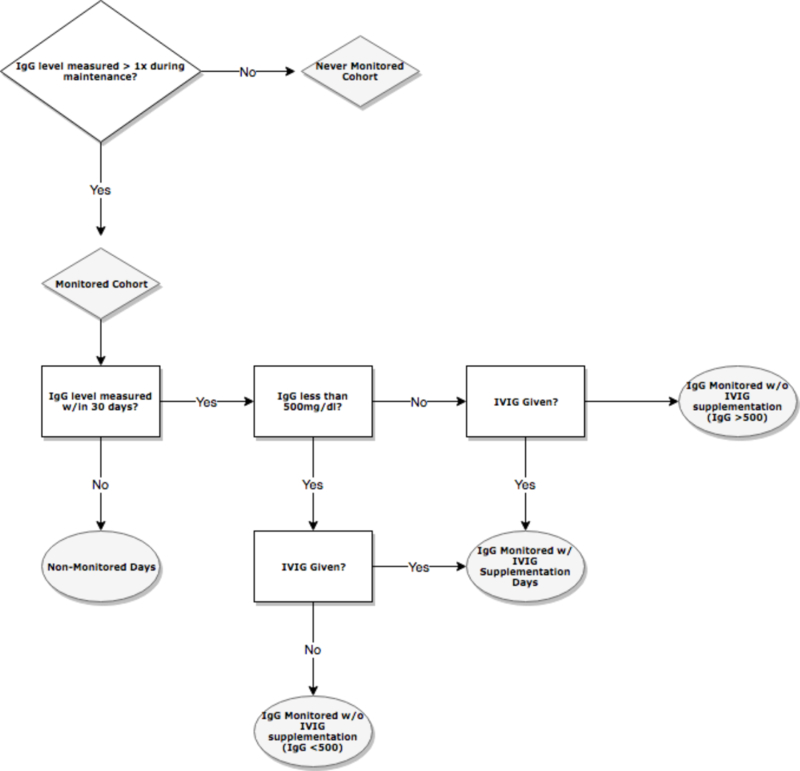
All subject records were reviewed to determine if they received immunoglobulin monitoring and/or IVIG supplementation as evidenced by documented IgG levels in the medical record. Our institution had no standard guideline for IgG monitoring in ALL patients and the decision to measure serum IgG and administer IVIG was dependent on the clinical practice preference of the attending physician. Among reviewed subjects, risk days were categorized into monitoring periods. A subject was considered monitored for 30 days after an IgG level was checked or for 30 days after supplementation with IVIG. The indicated IgG threshold for recommended IVIG intervention was set at 500 mg/dl as it was a threshold used by many providers at this institution and is a common standard guideline for IgG trough levels12. The 30 day interval was chosen based on the pharmacokinetics of IVIG which has a typical half-life of 25–35 days in patients with primary immunodeficiencies13. Thus, the risk days among monitored patients were divided into the following categories: 1) not IgG monitored, 2) ever IgG monitored with IVIG supplementation, 3) ever IgG monitored with no IVIG supplementation (IgG level >500mg/dl) and 4) ever IgG monitored with no IVIG supplementation (IgG level <500 mg/dl) (Figure 1). As such, a single ever monitored patient may have had risk days categorized into each of the four above categories. No patients in the cohort received alternative sources of IgG including subcutaneous administration or prophylactic antibiotics other than for Pneuomocystis jirovecii prevention. Patients received annual influenza vaccine, but other routine vaccines were delayed while the patient was on treatment.
Figure 1.
Risk Days Stratification
Data Collection:
The primary outcome was defined a priori as the number of febrile episodes (defined as a temperature ≥38°C for >1 hour or temperature ≥38.3°C for any duration in accordance with the 2010 IDSA guidelines)14. Fevers occurring within 7 days of a previous febrile episode were excluded as were fevers occurring while a subject was receiving antibiotics for a previous infection. Each febrile episode was also further evaluated for upper respiratory symptoms (URI) symptoms (defined as cough, runny nose, or congestion as documented in the medical record) and presence of confirmed bloodstream infection. All febrile episodes were captured from the start of maintenance chemotherapy through the end of 2012. Subjects were censored at time of central line removal, end of therapy, death, lost to follow-up, 30 days prior to stem cell transplantation, or at the end of the study period. Medical record review was completed using a web-based data collection application and included demographic information, timing and complications of IVIG infusions, and treatment regimen information in addition to the data described above.
Statistical Analysis:
Patient characteristics were summarized by median with interquartile range for continuous variables and frequency with percentages for categorical variables. The rates of febrile episodes, positive blood cultures, and febrile URI were expressed as episodes per 1000 subject-days. To evaluate the differences in these rates among management groups and to take into account the multiple management periods obtained from one patient, generalized mixed effect poisson models were used. All statistical analyses were performed using R software version 3.3 and p-values <0.05 were considered statistically significant.
Results:
Cohort Characteristics:
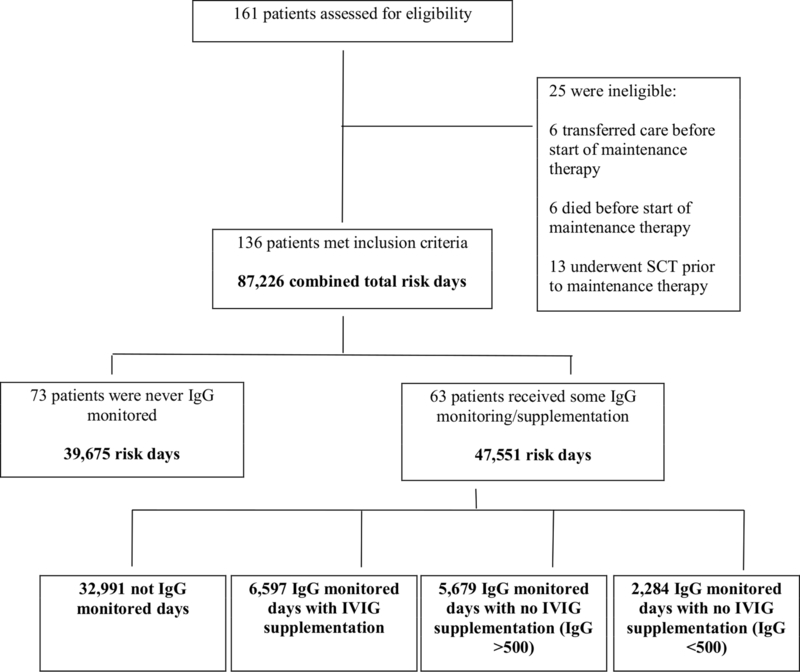
Of the 136 patients included in the study, 73 never had IgG levels monitored at any point during maintenance therapy and 63 had at least one period in which IgG levels were monitored. There were 87226 total risk days with 39675 from the never monitored cohort and 47551 from the ever monitored cohort. Of the risk days in the ever monitored cohort, 32,991 were classified as not IgG monitored days, 6597 were classified as IgG monitored with IVIG supplementation, 5679 were classified as IgG monitored with no IVIG supplementation (IgG level >500mg/dl), and 2,284 were classified as IgG monitored with no IVIG supplementation (IgG level <500 mg/dl) (Figure 2).
Figure 2.
Patient Flow Diagram
Baseline characteristics for the subjects in each cohort are given in Table 1. The median age of diagnosis for the subjects was five years and 58.8% were male. There were more male subjects in the IgG monitored group (66.7%) than the never IgG monitored group (52.1%). Per COG protocols, the maintenance phase is longer for male patients than for female patients thus the overall number of risk days for the monitored patients is longer despite there being fewer patients. Pre-B ALL was the predominant ALL subtype (86%). There were no statistically significant differences in demographic characteristics between monitored and non-monitored subjects. There was no statistically significant difference in ALL subtype between monitored and non-monitored subjects.
Table 1:
Patient Characteristics
| Overall (n = 136 ) | Never IgG Monitored (n = 73) | IgG Monitored (n = 63) | |
|---|---|---|---|
| Age at diagnosis in years, median (25–75%) | 5 (3–9) | 5 (3–9) | 5 (3–8) |
| Gender | |||
| Male | 80 (58.8%) | 38 (52.1%) | 42 (66.7%) |
| Female | 56 (41.2%) | 35 (47.9%) | 21 (33.3%) |
| ALL Subtype | |||
| Pre B-Cell | 117 (86.0%) | 63 (86.3%) | 54 (85.7%) |
| T-Cell | 19 (14.0%) | 10 (13.7%) | 9 (14.3%) |
| Race | |||
| White | 112 (82.4%) | 58 (79.5%) | 54 (85.7%) |
| Black | 19 (14.0%) | 10 (13.7%) | 9 (14.3%) |
| Hawaiian/Pacific Islander | 0 (0%) | 0 (0%) | 0 (0%) |
| Asian | 2 (1.5%) | 2 (2.7%) | 0 (0%) |
| American Indian | 0 (0%) | 0 (0%) | 0 (0%) |
| Mixed Race | 2 (1.5%) | 2 (2.7%) | 0 (0%) |
| Unknown | 1 (0.7%) | 1 (1.4%) | 0 (0%) |
| Ethnicity | |||
| Hispanic | 11 (8.1%) | 6 (8.2%) | 5 (7.9%) |
| Not Hispanic | 100 (73.5%) | 56 (76.7%) | 44 (69.8%) |
| Unknown | 25 (18.4%) | 11 (15.1%) | 14 (22.2%) |
Abbreviations: ALL = Acute Lymphoblastic Leukemia
IgG levels were measured a total of 468 times with a median value of 535mg/dl (25th Q = 438mg/dl, 75th Q = 656mg/dl). The median number of IgG levels checked in each subject was 3 (25th Q = 2, 75th Q = 12.5). IgG levels were measured more frequently during RSV season (as defined by CDC reports of RSV activity during the study years) with a mean of 7.50 IgG levels checked each month during RSV season (October-March) and a mean of 5.50 IgG levels checked each month during non-RSV season (April-September) (p = 0.088) 15. IgG <500 mg/dl was detected in 39 subjects on 192/468 (41%) of the occasions and 63% of the time was supplemented with IVIG. IgG <400 mg/dl was detected 72 times in 25 subjects and IgG <300 mg/dl was detected only 13 times in 10 subjets. IVIG was also given 58/276 (21%) of the times the level was >500 mg/dl. The median absolute neutrophil count at time of IgG levels checks was 1900/µl (25th Q = 1275/µl, 75th Q = 2733 µl) and in only 3¾68 (7.1%) of the cases was the subject found to be severely neutropenic (ANC <500/ µl).
Febrile Episodes:
Episode rates for overall febrile episodes, febrile upper respiratory infections and confirmed blood stream infections are provided in Table 2. Four hundred febrile episodes were captured during the risk period, with 150 occurred in never IgG monitored subjects and 250 occurred in ever IgG monitored subjects. The febrile episode rate for never IgG monitored subjects (3.78/1000 days) was lower than the febrile episode rate for the ever IgG monitored subjects (5.26/1000 days) (p = 0.05). Among ever IgG monitored patients we did not observe a statistically significant difference in the rates of measured febrile events between not IgG monitored days (5.43/1000 days), IgG monitored with IVIG supplementation (5.61/1000 days), IgG monitored with no IVIG supplementation (IgG level >500mg/dl), (4.93/1000 days) and IgG monitored with no IVIG supplementation (IgG level <500 mg/dl) (2.63/1000 days) (p=0.44). The rate of febrile URIs was also higher among ever IgG monitored patients as compared to the never IgG monitored patients; however, as with overall febrile episodes there was not a statistically significant difference between not IgG monitored days, IgG monitored with IVIG supplementation, IgG monitored with no IVIG supplementation (IgG level >500mg/dl), and IgG monitored with no IVIG supplementation (IgG level <500 mg/dl). There was no statistically significant difference in the rate of positive blood cultures between any of the subgroups analyzed.
Table 2:
Febrile episode rates
| Risk Days | Episodes | Febrile Episode Rate (/1000 days) | Positive Blood Cultures | Positive Blood Culture Rate (/1000 days) | Febrile URI Episodes | Febrile URI Rate (/1000 days) | |
|---|---|---|---|---|---|---|---|
| Entire Cohort | 87226 | 400 | 4.56 | 12 | 0.14 | 265 | 3.04 |
| Never IgG Monitored | 39675 | 150 | 3.78+ | 6 | 0.15+ | 104 | 2.62+ |
| Ever IgG Monitored | 47551 | 250 | 5.26+ | 6 | 0.13+ | 161 | 3.39* |
| Not IgG monitored Days | 32991 | 179 | 5.43* | 4 | 0.12* | 115 | 3.49* |
| IgG monitored with IVIG supplementation | 6597 | 37 | 5.61* | 1 | 0.15* | 26 | 3.94* |
| IgG monitored with no IVIG supplementation (IgG level >500mg/dl) | 5679 | 28 | 4.93* | 1 | 0.18* | 17 | 2.99* |
| IgG monitored with no IVIG supplementation (IgG level <500 mg/dl) | 2284 | 6 | 2.63* | 0 | 0* | 3 | 1.31* |
| +p=0.05, *p=0.44 | +p=1, | +p=0.23, *p=0.59 |
: Comparing Never monitored subjects with Ever Monitored subjects
: Comparing among not IgG monitored, IgG monitored with IVIG supplementation, IgG monitored with no IVIG supplementation (IgG level >500mg/dl), and IgG monitored with no IVIG supplementation (IgG level <500 mg/dl). Note: for positive blood culture rate, no comparison among Ever monitored subjects was attempted due to the limited number of events.
IVIG Complications:
IVIG was given 215 times to 38 subjects during the study. The dose of IVIG ranged from 0.5gm/kg – 1gm/kg with 81% receiving the 0.5gm/kg dose. There were 9 reported complications (1 fever, 1 nausea/vomiting, 5 headaches, 1 allergy (non-anaphylaxis), and 1 nausea/fever) giving a total complication rate of 4.2%. No life threatening adverse events occurred due to IVIG administration.
Discussion:
The aim of this retrospective cohort study was to determine the impact of immunoglobulin monitoring and supplementation during maintenance chemotherapy (per COG protocols) on the incidence of febrile episodes in children with ALL. To our knowledge, no previous study has looked at the efficacy of IVIG for this indication in this patient population. Our data suggest that there is not a clear role for IgG monitoring and IVIG supplementation in infection prophylaxis for ALL patients during maintenance therapy as there is no statistically significant reduction in febrile episodes between when monitoring/supplementation did and did not occur. This finding was consistent across all episode subgroups studied (overall number of febrile episodes, URI symptoms, and positive blood culture). The overall rate of infectious complications during maintenance chemotherapy for ALL is relatively low, so it is possible that among higher risk patient populations a benefit could be observed, but further study is needed. This overall low rate of infectious complications could, in part, be due to higher vaccination rates of both the patient population and their household contacts as compared to earlier years. Particularly, flu vaccination of the patients and household contacts could have played a role in overall reduction of infection in this population.
This study is limited by its retrospective design and by the inconsistent nature of how patients were monitored and supplemented. Since there is no standard of care for IgG monitoring and IVIG supplementation among pediatric ALL patients, there was significant variability between providers as to which patients (if any) were monitored, the duration and timing of monitoring during therapy, and also the threshold for IVIG supplementation. Additionally, although we chose a commonly used supplementation threshold of 500mg/dl for our study, there is not clear evidence as to which minimum IgG level is protective, so it is possible that an effect could be seen with a higher IgG trough goal as has been suggested by the B-cell deficiency literature16,17.
The higher rate of febrile episodes among the ever IgG monitored cohort as compared to the never IgG monitored cohort signifies that the choice of patients for monitoring/supplementation was not entirely random and that some factor made these patients higher risk. It is possible that some providers chose to monitor certain patients only after they had had repeated infections as they saw these patients as needing a higher level of infection prophylaxis, however low neutrophil count did not appear to be a deciding factor. It is unclear as to exactly what factor led to the IgG monitored cohort having a higher incidence of febrile episodes as the demographic characteristics were similar between the two. We attempted to better explain this by a dividing the risk days of the ever IgG monitored patients into IgG monitored with IVIG supplementation, IgG monitored with no IVIG supplementation (IgG level >500mg/dl) and IgG monitored with no IVIG supplementation (IgG level <500 mg/dl) in order to precisely evaluate during which periods the ever-monitored patients had the elevated febrile episode incidence. It is also possible that intrapatient variability of IgG monitoring occurred in our cohort. Some patients may only have been monitored during seasons at high risk of infection such as the cold and flu season. This is supported by our finding that the mean number of IgG levels checked in our study population was higher during peak RSV months. This practice would potentially increase the febrile episode rate during the monitored time period.
Despite these limitations, this study represents the only published analysis of the effectiveness of IVIG prophylaxis in the pediatric ALL population. Based on the findings herein, there is no clear evidence to support the routine use of IgG monitoring and IVIG prophylaxis in pediatric ALL patients during maintenance therapy. Further study is warranted to investigate this therapy in higher risk patient populations including patients undergoing higher risk phases of therapy and those at greater risk for infection.
Acknowledgments
Funding sources: NCRR/NIH; Grant number: CA090625 and KL2TR000446; NCI/NIH2P30CA068485–19
Abbreviations key:
- ALL
Acute Lymphoblastic Leukemia
- HCT
Hematopoietic Stem Cell Transplant
- ICU
Intensive Care Unit
- IgG
Immunoglobulin G
- IVIG
Intravenous Immunoglobulin
- URI
Upper Respiratory Infection
Footnotes
Conflicts of Interest: None to report.
References:
- 1.Licciardello M, Pegoraro A, Cesaro S. Prophylaxis and therapy of viral infections in pediatric patients treated for malignancy. Pediatric reports 2011;3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lex C, Körholz D, Kohlmüller B, et al. Infectious complications in children with acute lymphoblastic leukemia and T-cell lymphoma-a rationale for tailored supportive care. Supportive care in cancer 2001;9(7):514–521. [DOI] [PubMed] [Google Scholar]
- 3.Quartier P, Debré M, De Blic J, et al. Early and prolonged intravenous immunoglobulin replacement therapy in childhood agammaglobulinemia: a retrospective survey of 31 patients. The Journal of pediatrics 1999;134(5):589–596. [DOI] [PubMed] [Google Scholar]
- 4.Busse PJ, Razvi S, Cunningham-Rundles C. Efficacy of intravenous immunoglobulin in the prevention of pneumonia in patients with common variable immunodeficiency. Journal of Allergy and Clinical Immunology 2002;109(6):1001–1004. [DOI] [PubMed] [Google Scholar]
- 5.Ammann AJ, Ashman RF, Buckley RH, et al. Use of intravenous γ-globulin in antibody immunodeficiency: results of a multicenter controlled trial. Clinical immunology and immunopathology 1982;22(1):60–67. [DOI] [PubMed] [Google Scholar]
- 6.Buckley RH, Schiff RI. The use of intravenous immune globulin in immunodeficiency diseases. New England Journal of Medicine 1991;325(2):110–117. [DOI] [PubMed] [Google Scholar]
- 7.Raanani P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O. Immunoglobulin prophylaxis in chronic lymphocytic leukemia and multiple myeloma: systematic review and meta-analysis. Leukemia & lymphoma 2009;50(5):764–772. [DOI] [PubMed] [Google Scholar]
- 8.Raanani P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O. Immunoglobulin prophylaxis in hematopoietic stem cell transplantation: systematic review and meta-analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009;27(5):770–781. [DOI] [PubMed] [Google Scholar]
- 9.Foster JH, Cheng WS, Nguyen NY, Krance R, Martinez C. Immunoglobulin prophylaxis in pediatric hematopoietic stem cell transplant. Pediatr Blood Cancer 2018:e27348. [DOI] [PubMed] [Google Scholar]
- 10.Graham-Pole J, Camitta B, Casper J, et al. Intravenous immunoglobulin may lessen all forms of infection in patients receiving allogeneic bone marrow transplantation for acute lymphoblastic leukemia: a pediatric oncology group study. Bone marrow transplantation 1988;3(6):559–566. [PubMed] [Google Scholar]
- 11.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009;15(10):1143–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darabi K, Abdel-Wahab O, Dzik WH. Current usage of intravenous immune globulin and the rationale behind it: the Massachusetts General Hospital data and a review of the literature. Transfusion 2006;46(5):741–753. [DOI] [PubMed] [Google Scholar]
- 13.Koleba T, Ensom MH. Pharmacokinetics of intravenous immunoglobulin: a systematic review. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 2006;26(6):813–827. [DOI] [PubMed] [Google Scholar]
- 14.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clinical infectious diseases 2011;52(4):e56–e93. [DOI] [PubMed] [Google Scholar]
- 15.Prevention CfDCa. Respiratory Syncytial Virus — United States, July 2007—June 2011. . Morbidity and Mortality Weekly Reports 2011; https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6035a4.htm#fig [PubMed]
- 16.Orange JS, Grossman WJ, Navickis RJ, Wilkes MM. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: A meta-analysis of clinical studies. Clin Immunol 2010;137(1):21–30. [DOI] [PubMed] [Google Scholar]
- 17.Quinti I, Soresina A, Guerra A, et al. Effectiveness of immunoglobulin replacement therapy on clinical outcome in patients with primary antibody deficiencies: results from a multicenter prospective cohort study. J Clin Immunol 2011;31(3):315–322. [DOI] [PubMed] [Google Scholar]