Abstract
Purpose of review:
The prevalence of food allergy (FA) is rising globally. This review will discuss recent discoveries regarding the immunologic mechanisms that drive the initial sensitization and allergic response to food antigens, which may inform prevention and treatment strategies.
Recent findings:
Tolerance to food antigens is antigen-specific and promoted by oral exposure early in life and maternal transfer of immune complexes via breast milk. Immunoglobulin G (IgG) can inhibit both the initiation and effector phases of allergic responses to food antigens in mice, and high levels of food-specific IgG4 are associated with acquisition of tolerance in humans. Disruption of the skin barrier provides a route for food sensitization through the actions of mast cells, type 2 innate lymphoid cells (ILC2s) and IL-33 signaling. Regulatory T cells (Tregs) promote acquisition of oral tolerance, although defects in circulating allergen-specific Tregs are not evident in children with established food allergy. Certain microbes can offer protection against the development of IgE and food allergic responses, while dysbiosis increases susceptibility to FA.
Summary:
Tolerance to food antigens is antigen-specific and is promoted by oral exposure early in life, maternal transfer of immune complexes, food-specific IgG, Tregs, an intact skin barrier, and a healthy microbiome.
Keywords: Food allergy, tolerance, IgG4, sensitization, T regulatory cell, skin barrier, antigen specificity, commensal microbiota
INTRODUCTION
Food allergy (FA) affects millions of adults and children around the world. A population-based study in Australia recently found that the prevalence of challenge-confirmed IgE-mediated FA was 11% at 1 year of age and 3.8% at 4 years of age [1]. While food antigens are normally tolerated by the immune system, certain individuals develop food antigen-specific IgE antibodies, thus becoming “sensitized”. Subsequent exposure to the food can result in crosslinking of antigen-specific IgE bound to the high affinity IgE receptor FcεR1 on mast cells and basophils, leading to the release of inflammatory mediators that produce the symptoms of an allergic reaction, which in severe cases can be fatal [2,3].
Recent work in mice and humans has shown that disruption of the skin barrier increases the likelihood of sensitization to food antigens [4–6], while oral exposure results in the differentiation of regulatory T cells (Tregs) that promote tolerance [7–10]. The balance between IgE and other immunoglobulin classes, including IgG, additionally influences the response to food antigens [11–13]. As first suggested by David Strachan with the hygiene hypothesis decades ago, microbiota may also play a central role in food allergy pathogenesis [14]. Several studies have shown that commensal microbiota and their metabolites can protect against sensitization to food antigens [15,16]. Ongoing research focuses on identifying factors that promote dysbiosis as well as specific microbes and microbial products that may be effective in preventing and/or treating FA. This review will focus on recent studies informing the mechanisms underlying susceptibility to food allergy and the pathways that lead to the development of this common disease.
ROLE OF IMMUNOGLOBULINS AND THEIR RECEPTORS IN THE IMMUNE RESPONSE TO FOOD ANTIGENS
Oral exposure to food antigens normally results in antigen-specific immunological tolerance to the food, defined as a failure of the immune system to mount an inflammatory response upon subsequent exposure. Indeed, the Learning Early About Peanut allergy (LEAP) study showed that infants at high risk for developing peanut allergy (due to eczema or egg allergy or both) were protected if they introduced peanut into their diet early in life compared to those who followed strict avoidance [17*,18]. Despite the fact that peanut antigens share homology with other common food allergens including tree nuts and sesame, early introduction and tolerance to peanut had no preventative effect on the development of other food allergies, asthma, or rhinoconjunctivitis, nor did early exposure help resolve eczema or egg allergy [17*].
Recent studies have provided insight into the mechanisms responsible for antigen-specific tolerance induction during infancy. Female mice epicutaneously sensitized to the egg protein ovalbumin (OVA) conferred OVA-specific tolerance to their offspring via immune complexes (IC) transferred to the neonate via breastmilk and, less efficiently, in utero [19**]. This protection required the neonatal crystallizable fragment receptor (FcRn), which is present on neonatal CD11c+ dendritic cells (DCs). Uptake of OVA-IgG immune complexes by these cells induced the formation of OVA-specific Tregs and prevented the development of OVA-specific IgE and anaphylaxis in the offspring [19**]. Importantly, human breastmilk from non-atopic mothers similarly contained OVA-IgG-IC, and feeding this milk to mice expressing humanized FcRn promoted induction of OVA-specific Tregs and tolerance to egg whites [19**]. Soluble FcεR1 may also have a role in negatively regulating IgE responses. Following FcεR1 cross-linking, human monocyte-derived DCs and murine mast cells secrete sFcεR1, which forms an immune complex with IgE [20*]. Soluble FcεR1 prevented IgE binding to cell surface FcεR1 and inhibited human basophil activation in vitro. Furthermore, pretreatment with recombinant sFcεR1 reduced the magnitude of anaphylactic shock in mice [20*]. These studies thus support a role for both FcRn and sFcεR1 in modulating the immune response to food antigens.
Growing evidence suggests that the balance between antigen-specific IgE and IgG antibodies is also important for acquiring natural tolerance to food. A recent study found that IgG, via the inhibitory IgG receptor FcγRIIb, inhibited both the initiation and effector phases of allergic immune responses. Administration of antigen-specific IgG prior to sensitization limited anaphylactic responses in antigen-challenged mice by blocking IgE:FcεR1 activation of mast cells and the production of IL-4 and IL-13, reducing the inflammatory response, and allowing for increased Treg polarization and decreased TH2 effector responses [21*]. Administration of allergen-specific IgG also enhanced the efficacy of oral desensitization in mice with established FA by promoting Treg expansion [21*]. IgG4 in humans has also previously been shown to block allergic responses and promote tolerance [18,22–24]. The epitope diversity of IgE and IgG4 antibodies in children who outgrew cow’s milk allergy was lower than in children with persistent disease, while the affinity of IgG4 was higher in the tolerant group and closer to that of IgE [25]. Similarly, children who were able to tolerate baked egg had a higher egg-specific IgG4/IgE ratio than children who reacted to all forms of egg [26].
ROLE OF THE SKIN BARRIER IN FOOD ALLERGY PATHOGENESIS
The dual exposure hypothesis posits that exposure through the skin promotes sensitization to foods, while oral exposure leads to tolerance [4]. In agreement with this theory, a history of preschool eczema was recently associated with IgE sensitization to food and aeroallergens, as well as polysensitization [27]. However, eczema per se may not be responsible. Children with a mutation in the filaggrin (FLG) gene, which encodes an epidermal protein important in skin barrier function, were also more likely to be sensitized to peanut but not other food or aeroallergens [27]. Although FLG mutations are a risk factor for eczema, the association with peanut allergy remained after controlling for coexistent eczema [28,29]. Interestingly, non-lesional skin of eczema patients with FA exhibited increased transepidermal water loss, decreased filaggrin breakdown products, and keratinocytes that were hyperproliferative and unable to terminally differentiate compared to eczema patients without FA [30*]. A GWAS study also found that challenge proven-FA was associated with FLG mutations, and in this case the association was not only with peanut allergy but with hen’s egg and cow’s milk allergy as well. Although the effect size was largest in children with eczema, a strong association was still present in the absence of eczema [31]. This study further identified a significant association of FA with single nucleotide polymorphishms (SNPs) in SERPINB7, which encodes a protein that is important for epithelial integrity and upper digestive tract function [31]. Moreover, variants in SPINK5, which encodes a protein important in epidermal shedding, have been associated with FA independent of eczema, and preliminary experiments suggested that these variants may cause increased skin barrier permeability [32]. Collectively, these studies support a role for skin barrier integrity in the pathogenesis of FA.
Additional evidence supporting a role for skin exposure in the development of FA comes from mouse models. Tape stripping and epicutaneous sensitization of mice effectively induced antigen-specific IgE and IgG and anaphylaxis after oral challenge [33], while oral exposure before epicutaneous sensitization protected against an anaphylactic reaction to oral challenge [34*]. Moreover, epicutaneous sensitization of mice required fewer antigen challenges to induce an allergic response than intraperitoneal sensitization [35]. Allergic reactions in epicutaneously sensitized mice could be exacerbated by chemical or mechanical damage of the skin barrier, or ameliorated by topical treatment with glucocorticoids [35]. Mice with skin barrier dysfunction as a result of heterozyous mutations in Filaggrin and Matt (Tmem79ma) were also recently shown to be more susceptible to sensitization and anaphylaxis following epicutaneous exposure to food antigens, although concurrent cutaneous exposure to environmental allergens (Alternaria alternata or house dust mite extract) was required in this model [34*].
Recent murine studies have also provided insight into the cellular mechanisms by which a defective skin barrier contributes to FA, highlighting the importance of IL-33, mast cells, and type 2 innate lymphoid cells (ILC2s). Mechanical skin injury via tape stripping induced the expansion of mast cells and ILC2s specifically in the intestine [36*]. Accumulation of intestinal mast cells increased intestinal permeability and drove anaphylaxis to oral challenge after intraperitoneal sensitization [36*]. Following mechanical injury, keratinocytes produced IL-33, which acted on intestinal ILC2s in conjunction with IL-25 to induce IL-4 and IL-13 release and subsequent mast cell expansion [36*]. This process is illustrated in Fig. 1. Congruently, mice sensitized intradermally with TSLP and OVA also required IL-33 signaling in the skin in order for FA to develop. Deletion of IL-33 from epidermal keratinocytes or neutralization of IL-33 signaling systemically lessened the development of OVA-specific IgE, TH2 cytokine production, skin draining lymph node cellularity and allergic diarrhea [37]. Further supporting the important role of IL-33, mice sensitized intradermally with IL-33 and OVA developed oral and GI anaphylaxis symptoms when challenged [37]. Additional signaling for TH2 polarization comes from TSLP-induced basophils which accumulate in the inflamed skin and provide IL-4 [38]. Experimental therapies blocking IL-25, IL-33, or TSLP with monoclonal antibodies in mice effectively prevented the development of food allergy, but a cocktail of all three was required to suppress established food allergy [39*].
Figure 1.
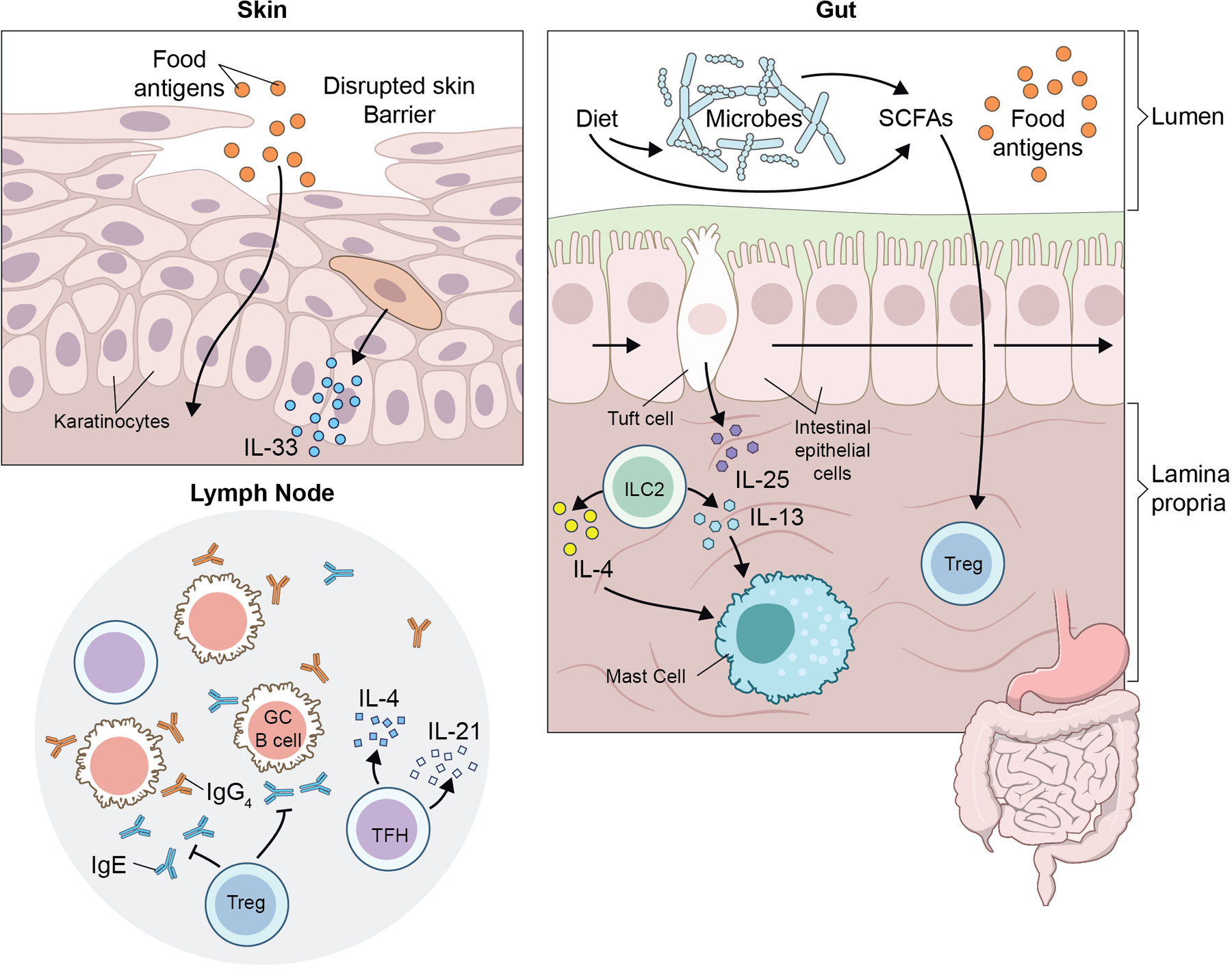
Barrier integrity and environmental factors affect tolerance and sensitization to food antigens. Disruption of the skin barrier leads to increased sensitization to food allergens and keratinocyte production of IL-33. IL-33 can then act in conjuction with Tuft cell derived IL-25 to activate ILC2s in the gut to recruit mast cells, resulting in an increased allergic response. Diet and metabolites produced by commensal microbiota can affect induction of food antigen-specific Treg and Tfh, and thus allergic sensitization. Tfh direct class switching of food-reactive B cells in the germinal center to either protective IgG4 or pro-allergic IgE.
TREGs AND TOLERANCE TO FOOD ANTIGENS
Loss of function mutations in FOXP3, which encodes the Treg lineage-defining transcription factor, result in a failure to produce functional Tregs and consequent widespread lymphoproliferation, autoimmunity, and atopy, including FA, in both humans and mice [40,41]. More recently, patients with Wiskott-Aldrich syndrome (WAS), caused by loss of function mutations in the WAS gene, were found to have elevated total and food antigen-specific IgE levels and an increased prevalence of FA. A mouse model revealed that loss of Was in Tregs alone was sufficient to drive FA and Th2-type inflammation in the small intestine [42]. Impaired Treg activity and high levels of food antigen-specific IgE along with FA and enteropathy were also observed in patients with DOCK8 mutations [43]. Undoubtedly, a failure of Tregs can be a driving force behind the development of FA; however, the extent to which Treg dysfunction contributes to allergy development in the absence of a primary immunodeficiency remains less clear. In support of a role for Treg deficiencies in general FA, a large birth cohort study recently found that infants who developed FA by one year of age had lower percentages of Tregs among CD4+ T cells at birth relative to non-allergic infants [44]. However, this difference was slight (3.75% versus 4.41%) and visible only with exposure to labor, and no correlation between FA status and Treg percentages was found at 6 months or 12 months of age [44]. A small scale study also found a slightly lower Treg count (per μl of blood) in a group of 28 infants within days of an initial confirmed allergic reaction to cow’s milk relative to non-allergic controls [45]. The percentage of Tregs among total CD4+ T cells was not different between the allergic and the control groups, which leaves open the question of whether the difference is in the Tregs themselves or the total CD4+ T cell population [45]. In mice, transfer of complexes of IgG and ovalbumin (OVA) from mothers to offspring via breast milk protected against future OVA sensitization by inducing Foxp3+ OVA-specific Tregs [19**]. While this represents a fascinating mechanism for induction of Tregs to prevent FA, many infants are fed formula that does not contain immune complexes and do not go on to develop food allergies, thus suggesting that this process may promote tolerance but is not required for its establishment.
Several recent studies have focused on identifying and characterizing antigen-specific Tregs in the context of FA and tolerance. Our group identified peanut-specific Tregs in peripheral blood of peanut allergic and non-allergic children based on their upregulation of CD137 after stimulation with crude peanut extract (CPE). We found no difference in the frequency of peanut-specific Tregs in peanut allergic and non-allergic children of school age or at one year of age [46*]. Peanut-specific Tregs from peanut allergic and non-allergic individuals were comparable in percent methylation of the Treg specific demethylated region of FOXP3, a marker of Treg stability, and in their ability to suppress division of CD4+ peanut-specific effector T cells [46*]. Interestingly, we did find significant differences in cytokine production and homing molecule expression by CD4+ peanut-specific effector T cells isolated from allergic and non-allergic individuals, suggesting that differences in the effector T cell response rather than the Treg response may be responsible for driving allergy development. A previous study of aeroantigen-specific T cells similarly concluded that the frequency, gene expression profile, suppressive function, and sequence diversity of the T cell receptor were comparable among birch-specific Tregs from birch allergic and non-allergic individuals, while the birch-specific conventional T cell response differed greatly [47]. Other groups have identified antigen-specific Tregs based on upregulation of CD154 on FOXP3+CD25+CD127- cells after 18 hours of stimulation. Using this method, the frequency of egg-reactive Tregs and peanut-reactive Tregs was not different between individuals reactive or tolerant to these foods, although the researchers noted that IL-2 production by antigen-specific conventional T cells could also drive CD154 expression on Tregs at this time point [26,48]. Removal of Tregs increased the amount of IL-5 and IL-9 in the supernatant of PBMCs cultured with peanut extract in healthy controls but not peanut allergic individuals, which suggests that Tregs may normally prevent release of these cytokines in healthy individuals, although the total amount of these cytokines still appeared higher in the allergic group [48]. One caveat of all these studies is that they were done using peripheral blood, and while food antigen-specific T cells can circulate, a large fraction likely remain in the small intestine [10,49]. Unfortunately, biopsies of the small intestine are much more difficult to obtain, and identification of antigen-specific Tregs using activation markers is technically challenging due to the higher baseline level of T cell activation in the gut.
Finally, several groups have assessed the Treg compartment following treatment for allergy. In mice, epicutaneous immunotherapy of mice sensitized to OVA resulted in the differentiation of OVA-specific CD4+LAP+Foxp3- cells that homed to the gut and protected against mast cell degranulation following oral challenge [50]. In humans, the results have been mixed; while some groups have found an increase in the number and function of food-antigen reactive FOXP3+ Tregs after oral immunotherapy (OIT) [51], others have found no difference, [52,53] or even a decrease in antigen-specific FOXP3+ T cells [54]. Together, these studies suggest that while Tregs play an important role in establishing tolerance to food antigens, there are many other cell types involved in the development and resolution of allergy, and Treg dysfunction may not be the driving force behind the majority of food allergies.
ROLE OF COMMENSAL MICROBIOTA IN THE DEVELOPMENT OF FOOD ALLERGY
The dramatic rise in FA in recent years is hypothesized to result from alterations in the microbiome secondary to frequent antibiotic use in early infancy, an increased rate of Caesarean births, a Westernized diet, and higher rates of formula feeding [55–57]. Studies of germ free (GF) mice have shown that commensal microbiota protect against the development of FA and food antigen-specific IgE; elevation of serum IgE occurs spontaneously in GF mice and increases with age [58**]. The increase in IgE can be attributed to a response to antigens found in mouse chow since feeding GF pups an antigen free diet (AFD) protected against IgE elevation [58**]. The gut associated lymphoid tissue (GALT) of GF mice contained increased numbers of IL-4 producing T follicular helper cells (TFH), a subclass of CD4+ T cells that regulate memory B cell and plasma cell differentiation, that were critical for the elevated IgE phenotype (Fig. 1) [58**]. TFH cell numbers were slow to recover in adult GF mice after TFH depletion, and adult mice produced fewer TFH cells than younger mice when they were switched from an AFD to normal mouse chow. These data suggest that the majority of food antigen-specific TFH cells are generated in early life, rather than replenished with new cells throughout life, which again emphasizes the importance of immune conditioning during early life [58**]. IgE levels remained high in GF mice even after depletion of TFH cells or heavy irradiation, suggesting that IgE levels are maintained by long-lived IgE producing plasma cells and making TFH cells a poor target to reduce IgE levels in FA patients [58**]. Interestingly, while GF mice produced higher levels of OVA specific IgE following sensitization compared to conventionally colonized mice, they were protected from systemic allergic responses [59]. Both at baseline and after sensitization, GF mice had reduced number of mast cells and mast cell protease 1 (MCPT1) levels, and these mast cells were less mature and had reduced functionality compared to conventional mice [59].
Atopic individuals have an altered microbiota signature, raising the question as to whether the composition of the microbiome can be manipulated to protect against allergic disease [15]. Fecal samples taken from cow’s milk allergic (CMA) and healthy volunteer (HV) infants had 58 operational taxonomic units (OTUs) that were differentially abundant, and the HV group contained significantly more OTUs that were potentially protective [60**]. GF mice colonized with microbiota from CMA infants anaphylaxed after oral sensitization and challenge, and had significantly higher levels of serum IgG1, IgE, and mMCPT-1 compared with HV microbiota colonized mice, which were protected from anaphylaxis [60**]. Anaerostipes caccae was enriched in the ileum of HV colonized mice, and monocolization of GF mice with A. caccae protected against anaphylaxis and type two immune responses following sensitization and challenge [60**]. A similar study found that stool from children with egg allergy or sensitization had a significantly different microbiome composition with greater alpha diversity and community richness and increased abundance of phyla Firmucutes and Verrucomicrobia compared to healthy controls [61]. After adjusting for age, eczema severity, breastfeeding, and antibiotic usage, the microbiota of children with egg allergy was enriched for genera Ruminocuccus and Lactococcus, while Leuconostoc was enriched in that of HV [61]. In the egg sensitized group, after adjusting for age and race, the microbiota was enriched for the genera Roseburia and Faecalibacterium [61]. In contrast, a different study found no difference in microbial diversity comparing allergic or sensitized patients to controls when they grouped all food allergies together [62]. Looking at specific genera, Haemophilus, Dialister, and Clostridium were associated with food sensitization, while Citrobacter, Oscillospira, and Lactococcus were associated with FA, and Dorea was associated with both FA and sensitization [62]. Thus, two studies found that egg allergy or FA in general were associated with enrichment of the genera Lactococcus [61,62]. Comparing patients with FA, food sensitization, or controls, 6 OTUs, all within genus Bacteroides, were positively associated with sphingolipid metabolites, food sensitization, and invariant natural killer T cell (iNKT) activation [63]. It was hypothesized that α-galactosylceramide, a sphingolipid that is likely produced by B. fragilis, contributes to the increased iNKT cell activation in food sensitized patients and may have a protective effect [63]. Athough less well-studied, additional evidence suggests that the skin microbiome may also have a modulating effect on FA susceptibility. In the LEAP study, children with S aureus skin colonization were more likely to develop peanut allergy and have persistent egg or peanut allergy independent of eczema severity [64].
Given the growing evidence that dysbiosis contributes to the pathogenesis and potential rise in FA, there has been increasing interest in identifying factors that contribute to alterations in the microbiota. A recent study found that children who received histamine H2 receptor antagonists (H2Ras), proton pump inhibitors (PPI), or antibiotics during infancy, all of which can alter the gut microbiome, had an increased risk for all FA along with specifically cow’s milk, peanut, or egg allergy [65*]. Moreover, PPIs and H2RAs had a dose-dependent effect on the risk of FA, but this was not significant for antibiotic use [65*]. A variety of dietary components can also alter the gut microbiome in a manner that affects susceptibility to FA [66]. Mice fed a high fat diet (HFD) developed obesity and exhibited more severe systemic food allergic reactions, increased mast cell accumulation in the intestine and greater intestinal permeability compared to mice fed conventional chow [67*]. Mice fed a HFD also exhibited an altered microbiota signature that could be transmitted to GF mice and was conserved over time despite switching the recolonized mice to a standard chow diet [67*]. Moreover, the transmitted microbiota conferred increased food allergic responses and susceptibility independent of an obese phenotype [67*]. Additional studies suggest that specific food components and metabolites produced by commensal bacteria in the gut, including non-digestible-oligosaccharides (NDOs) and short-chain-fatty-acids (SCFAs), can impact food allergy development and severity [66,68,69]. Pretreatment with NDOs slightly reduced IgE-mediated basophil degranulation in blood from peanut allergic patients in vitro [70]. SCFAs, including propionate, acetate, and butyrate, are derived from food products such as dairy or fermented from fiber by intestinal bacterial, and their abundance is affected by diet, breastfeeding, and family size [69]. FA and other atopic diseases were less likely to occur in children with the highest levels of butyrate and propionate, whereas high acetate levels were only protective for food sensitization and allergy [69]. Butyrate supplementation of mice during pregnancy and weaning led to an increased percentage of Tregs in the lungs and protection from airway inflammation [69]. Lastly, while general food diversity in early life is protective against allergic diseases, cheese consumption was specifically identified to reduce the risk for food allergy and atopic dermatitis, potentially due to its microbial diversity or relatively high content of SCFAs [71]. Collectively, these studies underscore the importance of environmental factors, including medications and diet, in shaping the microbiome in a manner that influences an infant’s risk of developing FA.
CONCLUSION
Oral exposure to food antigens during infancy promotes antigen-specific tolerance that is further supported by immune complexes present in breast milk and the pro-tolerogenic activities of IgG. In contrast, exposure to food antigens via a disrupted skin barrier leads to food sensitization and allergy as a result of the inflammatory actions of ILC2s, mast cells, and IL-33. While Tregs clearly promote tolerance, children with FA do not exhibit defects in circulating antigen-specific Tregs. Commensal microbiota and their metabolites influence susceptibility to FA and are a likely pathway by which environmental factors influence the risk of FA. In the future, strategies to prevent FA may focus on limiting disruption of the skin barrier, encouraging early colonization with commensals that support a tolerogenic response, and promoting early introduction of allergenic foods. In established FA, targeting allergen-specific T effector cells and long-lived IgE secreting plasma cells may be more effective than attempts to expand antigen-specific Tregs.
KEY POINTS.
Tolerance to food antigens develops early in life through ingestion of food and maternal transfer of immune complexes.
Disruption of the skin barrier either through disease, genetics, or mechanical injury provides a route of sensitization to food antigens through the action of mast cells, ILC2s, and IL-33 signaling.
Deficiencies in Tregs are associated with an increased prevalence of allergic disease, although the frequency and function of food-specific Tregs in children with established FA is similar to healthy controls.
Environmental factors including diet, H2R antagonists, and antibiotics can promote dysbiosis and increase the risk of FA, while healthy infants may harbor microbes that are protective.
Acknowledgements:
We would like to thank Anita Mora and Ryan Kissinger of the National Institutes of Allergy and Infectious Diseases for their assistance with creating our figure.
Financial support and sponsorship:
This research was supported by the Intramural Research Program, National Institute of Allergy and Infectious Diseases, NIH.
Footnotes
Conflicts of interest:
none.
References
- 1.Peters RL, Koplin JJ, Gurrin LC, Dharmage SC, Wake M, Ponsonby AL, Tang MLK, Lowe AJ, Matheson M, Dwyer T, et al. : The prevalence of food allergy and other allergic diseases in early childhood in a population-based study: HealthNuts age 4-year follow-up. J Allergy Clin Immunol 2017, 140:145–153.e8. [DOI] [PubMed] [Google Scholar]
- 2.Kraft S, Kinet J-P: New developments in FcεRI regulation, function and inhibition. Nat Rev 2007, 7:365–378. [DOI] [PubMed] [Google Scholar]
- 3.Reber LL, Hernandez JD, Galli SJ: The pathophysiology of anaphylaxis. J A 2017, 140:335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lack G: Update on risk factors for food allergy. J Allergy Clin Immunol 2012, 129:1187–1197. [DOI] [PubMed] [Google Scholar]
- 5.Strid J, Hourihane J, Kimber I, Callard R, Strobel S: Disruption of the stratum corneum allows potent epicutaneous immunization with protein antigens resulting in a dominant systemic Th2 response. Eur J Immunol 2004, 34:2100–2109. [DOI] [PubMed] [Google Scholar]
- 6.Fallon P, Sasaki T, Sandilands A, Campbell L, Saunders S, Mangan NE, Callanan JJ, Kawasaki H, Shiohama A, Kubo A, et al. : A homozygous frameshift mutation in the murine filaggrin gene facilitates enhanced percutaneous allergen priming. Nat Genet 2009, 41:602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun C-M, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y: Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 2007, 204:1775–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noval Rivas M, Burton OT, Wise P, Charbonnier L-M, Georgiev P, Oettgen HC, Rachid R, Chatila TA: Regulatory T Cell Reprogramming toward a Th2-Cell-like Lineage Impairs Oral Tolerance and Promotes Food Allergy. Immunity 2015, 42:512–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY: Extrathymically generated regulatory T cells control mucosal T H 2 inflammation. Nature 2012, 482:395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim KS, Hong SW, Han D, Yi J, Jung J, Yang BG, Lee JY, Lee M, Surh CD: Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science (80- ) 2016, 351:858–863. [DOI] [PubMed] [Google Scholar]
- 11.Cassard L, Jönsson F, Arnaud S, Daëron M: Fcγ Receptors Inhibit Mouse and Human Basophil Activation. J Immunol 2012, 189:2995–3006. [DOI] [PubMed] [Google Scholar]
- 12.Burton OT, Medina Tamayo J, Stranks AJ, Miller S, Koleoglou KJ, Weinberg EO, Oettgen HC: IgE promotes type 2 innate lymphoid cells in murine food allergy. Clin Exp Allergy 2018, 48:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berin MC: Mucosal antibodies in the regulation of tolerance and allergy to foods. Semin Immunopathol 2012, 34:633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strachan DP: Hay fever, hygiene, and household size. Br Med J 1989, 299:1259–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK, Tjota MY, Seo G-Y, Cao S, Theriault BR, et al. : Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci 2014, 111:13145–13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunyavanich S, Shen N, Grishin A, Wood R, Burks W, Dawson P, Jones SM, Leung DYM, Sampson H, Sicherer S, et al. : Early-life gut microbiome composition and milk allergy resolution. J Allergy Clin Immunol 2016, 138:1122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.du Toit G, Sayre PH, Roberts G3, Lawson K, Sever ML, Bahnson HT, Fisher HR, Feeney M, Radulovic S, Basting M, Plaut M LGINLEAPA study team.: Allergen specificity of early peanut consumption and effect on development of allergic disease in the Learning Early About Peanut Allergy study cohort. J Allergy Clin Immunol 2018, 141:1343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study showed that early introduction of peanut protected against the development of peanut but not other food allergies, suggesting oral tolerance is antigen-specific.
- 18.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, Brough HA, Phippard D, Basting M, Feeney M, et al. : Randomized Trial of Peanut Consumption in Infants at Risk for Peanut Allergy. N Engl J Med 2015, 372:803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohsaki A, Venturelli N, Buccigrosso TM, Osganian SK, Lee J, Blumberg RS, Oyoshi MK: Maternal IgG immune complexes induce food allergen–specific tolerance in offspring. J Exp Med 2018, 215:91–113. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Complexes of allergen-specific IgG and food antigen in breastmilk are transferred to offspring via FcRn in the gut. These complexes induce differentiation of food-specific Tregs, which are protective against allergy.
- 20.Moñino-Romero S, Erkert L, Schmidthaler K, Diesner SC, Sallis BF, Pennington L, Jardetzky T, Oettgen HC, Bohle B, Fiebiger E, et al. : The soluble isoform of human FcɛRI is an endogenous inhibitor of IgE-mediated mast cell responses. Allergy 2019, 74:236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]; *FcɛR1-activated dendritic cells and mast cells release a soluble form of FcɛR1, which can inhibit IgE binding and diminish anaphylaxis.
- 21.Burton OT, Tamayo JM, Stranks AJ, Koleoglou KJ, Oettgen HC: Allergen-specific IgG antibody signaling through FcγRIIb promotes food tolerance. J Allergy Clin Immunol 2018, 141:189–201.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Allergen-specific IgG limits sensitization to food in allergy prone mice by decreasing TH2 responses and promoting Treg induction. In mice with established allergy undergoing oral immunotherapy, IgG facilitates Treg expansion and tolerance in a manner requiring the expression of FcγRIIb on mast cells.
- 22.Strait RT, Morris SC, Finkelman FD: IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and FcγRIIb cross-linking. J Clin Invest 2006, 116:833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burton OT, Logsdon SL, Zhou JS, Medina-Tamayo J, Abdel-Gadir A, Noval Rivas M, Koleoglou KJ, Chatila TA, Schneider LC, Rachid R, et al. : Oral immunotherapy induces IgG antibodies that act through FcγRIIb to suppress IgE-mediated hypersensitivity. J Allergy Clin Immunol 2014, 134:1310–1317.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van de Veen W, Akdis M: Role of IgG4 in IgE-mediated allergic responses. J Allergy Clin Immunol 2016, 138:1434–1435. [DOI] [PubMed] [Google Scholar]
- 25.Caubet JC, Lin J, Ahrens B, Gimenez G, Bardina L, Niggemann B, Sampson HA, Beyer K: Natural tolerance development in cow’s milk allergic children: IgE and IgG4 epitope binding. Allergy 2017, 72:1677–1685. [DOI] [PubMed] [Google Scholar]
- 26.Berin MC, Grishin A, Masilamani M, Leung DYM, Sicherer SH, Jones SM, Burks AW, Henning AK, Dawson P, Grabowska J, et al. : Egg-specific IgE and basophil activation but not egg-specific T-cell counts correlate with phenotypes of clinical egg allergy. J Allergy Clin Immunol 2018, 142:149–158.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johansson EK, Bergström A, Kull I, Lind T, Söderhäll C, van Hage M, Wickman M, Ballardini N, Wahlgren CF: IgE sensitization in relation to preschool eczema and filaggrin mutation. J Allergy Clin Immunol 2017, 140:1572–1579.e5. [DOI] [PubMed] [Google Scholar]
- 28.Palmer CNA, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, Goudie DR, Sandilands A, Campbell LE, Smith FJD, et al. : Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 2006, 38:441–446. [DOI] [PubMed] [Google Scholar]
- 29.Brown SJ, Asai Y, Cordell HJ, Campbell LE, Zhao Y, Liao H, Northstone K, Henderson J, Alizadehfar R, Ben-Shoshan M, et al. : Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol 2011, 127:661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung DYM, Calatroni A, Zaramela LS, LeBeau P, Dyjack NT, Brar KK, David GL, Johnson K, Leung SB, Ramirez-Gama MA, et al. : The nonlesional skin surface distinguishes atopic dermatitis with food allergy as a unique endotype. Sci Tranl Med 2019, 11:2685. [DOI] [PMC free article] [PubMed] [Google Scholar]; *The non-lesional skin of patients with both food allergy and atopic dermatitis exhibited decreased barrier integrity relative to atopic dermatitis patients without food allergy.
- 31.Marenholz I, Grosche S, Kalb B, Rüschendorf F, Blümchen K, Schlags R, Harandi N, Price M, Hansen G, Seidenberg J, et al. : Genome-wide association study identifies the SERPINB gene cluster as a susceptibility locus for food allergy. Nat Commun 2017, 8:1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashley SE, Tan HTT, Vuillermin P, Dharmage SC, Tang MLK, Koplin J, Gurrin LC, Lowe A, Lodge C, Ponsonby AL, et al. : The skin barrier function gene SPINK5 is associated with challenge-proven IgE-mediated food allergy in infants. Allergy 2017, 72:1356–1364. [DOI] [PubMed] [Google Scholar]
- 33.Yu R, Igawa K, Handa Y, Munetsugu T, Satoh T, Yokozeki H: Basophils and mast cells are crucial for reactions due to epicutaneous sensitization to ovalbumin. Exp Dermatol 2017, 26:778–784. [DOI] [PubMed] [Google Scholar]
- 34.Walker M, Green J, Ferrie R, Queener A, Kaplan M, Cook-Mills J: Mechanism for initiation of food allergy: Dependence on skin barrier mutations and environmental allergen costimulation. J Allergy Clin Immunol 2018, 141:1711–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study found that mice with reduced skin barrier function developed allergic sensitization when cutaneously exposed to food and environmental allergens simultaneously. Blockade of ST2 prevented the development of allergy.
- 35.Kawasaki A, Ito N, Murai H, Yasutomi M, Naiki H, Ohshima Y: Skin inflammation exacerbates food allergy symptoms in epicutaneously sensitized mice. Allergy 2018, 73:1313–1321. [DOI] [PubMed] [Google Scholar]
- 36.Leyva-Castillo J-M, Galand C, Kam C, Burton O, Gurish M, Musser MA, Goldsmith JD, Hait E, Nurko S, Brombacher F, et al. : Mechanical Skin Injury Promotes Food Anaphylaxis by Driving Intestinal Mast Cell Expansion. Immunity 2019, 50:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Damage to the skin barrier resulted in keratinocyte production of IL-33, inducing ILC2 and mast cell expansion in the gut, promoting food anaphylaxis.
- 37.Han H, Roan F, Johnston L, Smith D, Bryce P, Ziegler SF: IL-33 promotes gastrointestinal allergy in a TSLP-independent manner. Mucosal Immunol 2018, 11:394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hussain M, Borcard L, Walsh KP, Pena Rodriguez M, Mueller C, Kim BS, Kubo M, Artis D, Noti M: Basophil-derived IL-4 promotes epicutaneous antigen sensitization concomitant with the development of food allergy. J Allergy Clin Immunol 2018, 141:223–234.e5. [DOI] [PubMed] [Google Scholar]
- 39.Khodoun MV, Tomar S, Tocker JE, Wang YH, Finkelman FD: Prevention of food allergy development and suppression of established food allergy by neutralization of thymic stromal lymphopoietin, IL-25, and IL-33. J Allergy Clin Immunol 2018, 141:171–179.e1. [DOI] [PubMed] [Google Scholar]; * Blocking any of the pro-TH2 cytokines (IL-25, IL-33, TSLP) inhibits the development of food allergy. Blockade of all three is necessary to suppress established food allergy.
- 40.Torgerson TR, Linane A, Moes N, Anover S, Mateo V, Rieux-Laucat F, Hermine O, Vijay S, Gambineri E, Cerf-Bensussan N, et al. : Severe Food Allergy as a Variant of IPEX Syndrome Caused by a Deletion in a Noncoding Region of the FOXP3 Gene. Gastroenterology 2007, 132:1705–1717. [DOI] [PubMed] [Google Scholar]
- 41.Lin W, Truong N, Grossman WJ, Haribhai D, Williams CB, Wang J, Martín MG, Chatila TA: Allergic dysregulation and hyperimmunoglobulinemia e in Foxp3 mutant mice. J Allergy Clin Immunol 2005, 116:1106–1115. [DOI] [PubMed] [Google Scholar]
- 42.Lexmond WS, Goettel JA, Lyons JJ, Jacobse J, Deken MM, Lawrence MG, DiMaggio TH, Kotlarz D, Garabedian E, Sackstein P, et al. : FOXP3 + Tregs require WASP to restrain Th2-mediated food allergy. J Clin Invest 2016, 126:4030–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alroqi FJ, Charbonnier LM, Keles S, Ghandour F, Mouawad P, Sabouneh R, Mohammed R, Almutairi A, Chou J, Massaad MJ, et al. : DOCK8 Deficiency Presenting as an IPEX-Like Disorder. J Clin Immunol 2017, 37:811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collier F, Ponsonby AL, O’Hely M, Tang MLK, Saffery R, Molloy J, Gray LE, Ranganathan S, Burgner D, Allen KJ, et al. : Naïve regulatory T cells in infancy: Associations with perinatal factors and development of food allergy. Allergy 2019, 10.1111:all.13822. [DOI] [PubMed] [Google Scholar]
- 45.Perezabad L, López-Abente J, Alonso-Lebrero E, Seoane E, Pion M, Correa-Rocha R: The establishment of cow’s milk protein allergy in infants is related with a deficit of regulatory T cells (Treg) and Vitamin D. Pediatr Res 2017, 81:722–730. [DOI] [PubMed] [Google Scholar]
- 46.Weissler KA, Rasooly M, DiMaggio T, Bolan H, Cantave D, Martino D, Neeland MR, Tang MLK, Dang TD, Allen KJ, et al. : Identification and analysis of peanut-specific effector T and regulatory T cells in children allergic and tolerant to peanut. J Allergy Clin Immunol 2018, 141:1699–1710.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study showed no difference in the quantity or function of circulating peanut-specific Tregs between peanut allergic and non-allergic individuals. Differences in cytokine production and chemokine receptor expression were found in the peanut-specific effector T cell compartment.
- 47.Bacher P, Heinrich F, Stervbo U, Nienen M, Vahldieck M, Iwert C, Vogt K, Kollet J, Babel N, Sawitzki B, et al. : Regulatory T Cell Specificity Directs Tolerance versus Allergy against Aeroantigens in Humans. Cell 2016, 167:1067–1078.e16. [DOI] [PubMed] [Google Scholar]
- 48.Chiang D, Chen X, Jones SM, Wood RA, Sicherer SH, Burks AW, Leung DYM, Agashe C, Grishin A, Dawson P, et al. : Single-cell profiling of peanut-responsive T cells in patients with peanut allergy reveals heterogeneous effector T H 2 subsets. J Allergy Clin Immunol 2018, 141:2107–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson RP, Degano P, Godkin AJ, Jewell DP, Hill AVS: In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat Med 2000, 6:337–342. [DOI] [PubMed] [Google Scholar]
- 50.Tordesillas L, Mondoulet L, Blazquez AB, Benhamou PH, Sampson HA, Berin MC: Epicutaneous immunotherapy induces gastrointestinal LAP+ regulatory T cells and prevents food-induced anaphylaxis. J Allergy Clin Immunol 2017, 139:189–201.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, Berglund JP, Tsai M, Maecker H, O’Riordan G, et al. : Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J Allergy Clin Immunol 2014, 133:500–510.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, Burk C, Hiegel A, Carlisle S, Christie L, et al. : Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol 2014, 133:468–475.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frischmeyer-Guerrerio PA, Masilamani M, Gu W, Brittain E, Wood R, Kim J, Nadeau K, Jarvinen KM, Grishin A, Lindblad R, et al. : Mechanistic correlates of clinical responses to omalizumab in the setting of oral immunotherapy for milk allergy. J Allergy Clin Immunol 2017, 140:1043–1053.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryan JF, Hovde R, Glanville J, Lyu S-C, Ji X, Gupta S, Tibshirani RJ, Jay DC, Boyd SD, Chinthrajah RS, et al. : Successful immunotherapy induces previously unidentified allergen-specific CD4+ T-cell subsets. Proc Natl Acad Sci 2016, 113:E1286–E1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Risnes KR, Belanger K, Murk W, Bracken MB: Antibiotic Exposure by 6 Months and Asthma and Allergy at 6 Years: Findings in a Cohort of 1,401 US Children. Am J Epidemiol 2011, 173:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reynolds LA, Finlay BB: A case for antibiotic perturbation of the microbiota leading to allergy development. Expert Rev Clin Immunol 2013, 9:1019–1030. [DOI] [PubMed] [Google Scholar]
- 57.Sitarik AR, Kasmikha NS, Kim H, Wegienka G, Havstad S, Ownby D, Zoratti E, Johnson CC: Breast-feeding and delivery mode modify the association between maternal atopy and childhood allergic outcomes. J Allergy Clin Immunol 2018, 142:2002–2004.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hong S-W, O E, Lee JY, Lee M, Han D, Ko H-J, Sprent J, Surh CD, Kim KS: Food antigens drive spontaneous IgE elevation in the absence of commensal microbiota. Sci Adv 2019, 5:eaaw1507. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Germ-free mice developed elevated IgE driven by exposure to food antigen. In the absence of commensals, increased numbers of IL-4 producing TFH cells promote the generation of long lived plasma IgE cells, which maintain food sensitization.
- 59.Schwarzer M, Hermanova P, Srutkova D, Golias J, Hudcovic T, Zwicker C, Sinkora M, Akgün J, Wiedermann U, Tuckova L, et al. : Germ-Free Mice Exhibit Mast Cells With Impaired Functionality and Gut Homing and Do Not Develop Food Allergy. Front Immunol 2019, 10:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feehley T, Plunkett CH, Bao R, Choi Hong SM, Culleen E, Belda-Ferre P, Campbell E, Aitoro R, Nocerino R, Paparo L, et al. : Healthy infants harbor intestinal bacteria that protect against food allergy. Nat Med 2019, 25:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Germ-free mice colonized with bacteria from cow’s milk allergic infants developed allergy to cow’s milk, while those colonized with healthy infant bacteria were protected. Specifically, they identified that transfer of Anaerostipes caccae protected against food allergy.
- 61.Fazlollahi M, Chun Y, Grishin A, Wood RA, Burks AW, Dawson P, Jones SM, Leung DYM, Sampson HA, Sicherer SH, et al. : Early-life gut microbiome and egg allergy. Allergy 2018, 73:1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Savage JH, Lee-sarwar KA, Sordillo J, Bunyavanich S, Zhou Y, O’Connor GT, Sandel M, Bacharier LB, Zieger R, Sodergren E, et al. : A prospective microbiome-wide association study of food sensitization and food allergy in early childhood. Allergy 2018, 73:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee-Sarwar K, Kelly RS, Lasky-Su J, Moody DB, Mola AR, Cheng T-Y, Comstock LE, Zeiger RS, O’Connor GT, Sandel MT, et al. : Intestinal microbial-derived sphingolipids are inversely associated with childhood food allergy. J Allergy Clin Immunol 2018, 142:335–338.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsilochristou O, du Toit G, Sayre PH, Roberts G, Lawson K, Sever ML, Bahnson HT, Radulovic S, Basting M, Plaut M, et al. : Association of Staphylococcus aureus colonization with food allergy occurs independently of eczema severity. J Allergy Clin Immunol 2019, doi: 10.1016/j.jaci.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mitre E, Susi A, Kropp LE, Schwartz DJ, Gorman GH, Nylund CM: Association between use of acid-suppressive medications and antibiotics during infancy and allergic diseases in early childhood. JAMA Pediatr 2018, 172:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study found an association between the use of antibiotics or acid-suppressive medications, both of which affect gut bacterial composition, and the development of allergic disease.
- 66.Tan J, Mckenzie C, Vuillermin PJ, Mebius RE, Macia L, Mackay Correspondence CR: Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. CellReports 2016, 15:2809–2824. [DOI] [PubMed] [Google Scholar]
- 67.Hussain M, Bonilla-Rosso G, Kwong Chung CKC, Bäriswyl L, Rodriguez MP, Kim BS, Engel P, Noti M: High dietary fat intake induces a microbiota signature that promotes food allergy. J Allergy Clin Immunol 2019, doi: 10.1016/j.jaci.2019.01.043. [DOI] [PubMed] [Google Scholar]; *Mice fed a high fat diet developed an altered gut microbiome that increased susceptibility to food allergy, independent of obesity.
- 68.Boehm G, Lidestri M, Casetta P, Jelinek J, Negretti F, Stahl B, Marini A: Supplementation of a bovine milk formula with an oligosaccharide mixture increases counts of faecal bifidobacteria in preterm infants. Arch Dis Child Fetal Neonatal Ed 2002, 86:178F–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roduit C, Frei R, Ferstl R, Loeliger S, Westermann P, Rhyner C, Schiavi E, Barcik W, Rodriguez-Perez N, Wawrzyniak M, et al. : High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2019, 74:799–809. [DOI] [PubMed] [Google Scholar]
- 70.Hayen SM, den Hartog Jager CF, Knulst AC, Knol EF, Garssen J, Willemsen LEM, Otten HG: Non-digestible oligosaccharides can suppress basophil degranulation in whole blood of peanut-allergic patients. Front Immunol 2018, 9:1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nicklaus S, Divaret-Chauveau A, Chardon ML, Roduit C, Kaulek V, Ksiazek E, Dalphin ML, Karvonen AM, Kirjavainen P, Pekkanen J, et al. : The protective effect of cheese consumption at 18 months on allergic diseases in the first 6 years. Allergy 2019, 74:788–798. [DOI] [PubMed] [Google Scholar]


