Abstract
BACKGROUND:
Red meat allergy has historically been understood as a rare disease of atopic children, but the discovery of the “α-Gal syndrome,” which relates to IgE to the oligosaccharide galactose-α-1,3-galactose (α-Gal), has challenged that notion.
OBJECTIVE:
To describe the clinical and immunologic characteristics of a large group of subjects with self-reported allergy to mammalian meat.
METHODS:
This was an observational study of 261 children and adults (range, 5-82 years) who presented for evaluation for allergic reactions to mammalian meat. Results were based on serum assays and a detailed questionnaire.
RESULTS:
α-Gal specific IgE ≥ 0.35 IU/mL was detected in 245 subjects and symptom onset occurred ≥2 hours after eating mammalian meat in 211 (81%). Component testing supported a diagnosis of α-Gal syndrome in 95%, pork-cat syndrome in 1.9%, and primary beef allergy in 1.1%. Urticaria was reported by 93%, anaphylaxis by 60%, and gastrointestinal symptoms by 64%. Levels of IgE and IgG specific to α-Gal were similar in subjects who reported early- or delayed-onset symptoms, and in those with and without anaphylaxis. Levels of α-Gal specific IgE and severity of reactions were similar among those with and without traditional atopy, and among children (n = 35) and adults (n = 226). Blood group B trended toward being under-represented among α-Gal-sensitized subjects; however, α-Gal specific IgE titers were high in symptomatic cases with B-antigen.
CONCLUSIONS:
The α-Gal syndrome is a regionally common form of food allergy that has a characteristic but not universal delay in symptom onset, includes gastrointestinal symptoms, can develop at any time in life, and is equally common in otherwise nonatopic individuals.
Keywords: Alpha-gal; Galactose-α-1,3-galactose; Food allergy; Red meat; Anaphylaxis
Allergy to red meat (ie, meat derived from mammals) has historically been considered to be rare, and primarily a disease of young atopic children.1 In 2009, we reported that IgE to the oligosaccharide galactose-α-1,3-galactose (α-Gal), a glycan of nonprimate mammals that is homologous to the B-group blood-antigen, was associated with episodes of delayed anaphylaxis to red meat in subjects in the Southeastern United States.2,3 Since that time the connection between IgE to α-Gal and red meat allergy has been strengthened and patients with compatible clinical stories who are also sensitized to α-Gal have been described on 6 continents.4 Subjects who produce IgE to α-Gal can react to red meat, internal organs, and other products derived from mammals (such as milk, cheese, gelatin, some biologic drugs, and gelatin-containing vaccines), and thus, the allergy is now commonly referred to as the “α-Gal syndrome.”5,6 This syndrome can be difficult to recognize on account of several factors. Most patients develop this syndrome after many years of safely eating beef, pork, or lamb and, unlike most forms of food allergy associated with IgE, there is often a delay of 3 to 6 hours between eating mammalian meat and the appearance of hives or other symptoms.7
To date the immunologic mechanisms that contribute to sensitization and the delay in symptom onset have not been well characterized. Recent reports have suggested that subjects with B-group blood-antigen are protected from developing the syndrome, although this has not been observed in all cohorts.8–10 The development of specific IgE (sIgE) to α-Gal in the United States has consistently been associated with bites of the Lone Star tick (Amblyomma americanum), although it is clear that other ticks are causally related in other parts of the world.4,11 In some regions, in particular the Southeastern United States, there is now evidence that α-Gal is a major contributor to food allergy and is an important cause of anaphylaxis.12,13 Nonetheless, to date most reports on the α-Gal syndrome have been limited to relatively small observational studies and case series, and there have been few studies that directly compared the syndrome in adults and children. Here we systematically investigated the clinical and immunologic features of the α-Gal syndrome, as well as other etiologies that can contribute to red meat allergy, among 261 adults and children who presented for evaluation of allergic reactions to mammalian meat.
METHODS
Human subjects
The studies reported here were approved by the University of Virginia Human Investigation Committee (HIC No. 13298) and involved enrollment from June 2009 to August 2015. Subjects presenting to the University of Virginia Allergy Clinic with recurrent urticaria, anaphylaxis, or other self-reported allergic symptoms attributed to mammalian meat were recruited. A total of 261 adults (aged 19-82 years) and children (aged 5-18 years) completed the consent form, filled out a detailed questionnaire, and provided serum for serologic analyses. In addition, 111 subjects who reported urticaria or anaphylaxis of unclear etiology and 36 subjects who denied any history of urticaria or anaphylaxis were also investigated. All subjects filled out a questionnaire that assessed a history of “hives” (red, itchy welts), anaphylaxis (allergic reaction that included low blood pressure and/or light headedness and/or trouble breathing), and gastrointestinal (GI) symptoms, the perceived association with foods, and the timing of symptom onset. In cases where a range of times was provided (reflecting multiple allergic events), the earliest time was used for analysis. Questions about the ABO blood group, allergic history including asthma and rhinitis, exposure history, and emergency presentation were also asked. Urticaria (ie, hives) and anaphylaxis were defined by self-report on the questionnaire. Seasonal or perennial rhinitis was defined by self-report and asthma by a history of physician diagnosis. Atopy was determined by the presence of sIgE ≥ 0.7 IU/mL to 1 or more of 3 inhalant allergens: dust mite, Timothy grass, or ragweed. Subjects were considered to be sensitized to α-Gal if they had sIgE to α-Gal ≥ 0.35 IU/mL.
IgE and IgG assays
Total and sIgE antibodies were measured using commercially available ImmunoCAP assays (Thermo Fisher/Phadia, US, Portage, Mich) performed with the ImmunoCAP 250 instrument, and the results were expressed as IU/mL (1 IU ≈ 2.4 ng of IgE).14 Sera were assayed for sIgE to α-Gal, beef, pork, milk, cat, dog, common ragweed, Timothy grass, German cockroach, dust mite (Dermatophagoides pteronyssinus), Alternaria, honeybee, and wasp (Vespula species, which includes yellow jackets) as well as egg, peanut, wheat, chicken, and shrimp. The cutoff used for a positive test in these assays was 0.35 IU/mL. In subjects that tested negative for sIgE to α-Gal, we evaluated the results for α-Gal, beef, pork, and cat using a threshold of 0.1 IU/mL and additionally assayed sIgE to cat serum albumin (Fel d 2) and pork serum albumin (Sus s 1). The streptavidin CAP technique was used to measure IgE to α-Gal using cetuximab on the solid phase.15,16 Specific IgG (sIgG) to α-Gal was measured by using α-Gal conjugated to human serum albumin (V-Labs, Inc., Covington, La) radiolabeled by the chloramine T method for use in solid-phase radioimmunoassays with results reported in arbitrary units (U)/mL, as previously reported.17
ABO blood type investigation
Sera from 192 subjects were reverse typed for ABO blood group status using commercially available reference cells (Immucor, Norcross, Ga) according to the manufacturer’s instructions. A total of 212 subjects answered positively on questionnaire to knowing their blood group. Together, 299 subjects had ABO data available from reverse typing and/or from the questionnaire. A total of 105 subjects had blood testing and questionnaire results available; of these, 102 had matching results, for a 97% agreement rate. For the 3 cases with disagreement between testing and questionnaire, the testing result was used to designate ABO status.
Statistical analysis
Comparisons between 2 groups were made using the Mann-Whitney U test. Positive antibody titers were log-transformed and values were expressed as geometric means ± the 95% confidence interval. Categorical variables were compared with Pearson’s χ2 or Fisher’s exact test as indicated. Correlation analysis was conducted with Spearman’s rank coefficient (rs). A 2-tailed P value <.05 was considered to indicate statistical significance. Statistical analysis was performed with GraphPad Prism (version 7) software (La Jolla, Calif).
RESULTS
Demographics, clinical, and immunological characteristics of the cohort
Allergic symptoms related to mammalian meat were reported by 261 subjects, and 245 of these had detectable α-Gal sIgE (≥0.35 IU/mL) (Table I). The titers of α-Gal sIgE exceeded those of sIgE to beef and pork extracts in 243 cases, which is consistent with α-Gal being the primary epitope in the red meat extracts for these subjects.2 Most cases presented during adulthood with disease onset reported after age 40 in 58% of subjects and after age 60 in 16%. Urticaria was reported in 93%, GI symptoms in 64%, and anaphylaxis in 60%. Isolated GI symptoms were reported by 9 subjects (3%). Symptom onset was delayed by at least 2 hours in 211 subjects (81%). Urgent or emergent care had been sought by 64% for their allergic reactions, and 98% reported histories of tick or chigger bites. Of the 261 subjects, 24 reported allergic symptoms related to ingestion of dairy (9.2%) and 2 subjects reported reactions to gelatin (0.8%).
TABLE I.
Demographic, clinical, and immunologic characteristics of children and adults reporting reactions to red meat
| Characteristics | Total (n = 261) | Children (n = 35) | Adults (n = 226) | P value (comparison of children versus adults) |
|---|---|---|---|---|
| Age at enrollment, y, median (range) | 49 (5-82) | 13 (5-18) | 51 (19-82) | – |
| Sex, male (%) | 120 (46%) | 26 (74%) | 94 (42%) | <.001* |
| Race | ||||
| Caucasian | 247 (95%) | 30 (86%) | 217 (96%) | .03* |
| African American | 5 (2%) | 1 (3%) | 4 (2%) | .52* |
| Other | 9 (3%) | 4 (11%) | 5 (2%) | .02* |
| Symptom | ||||
| Hives | 242 (93%) | 31 (89%) | 211 (93%) | .30* |
| Anaphylaxis | 157 (60%) | 17 (49%) | 140 (62%) | .14* |
| GI | 167 (64%) | 23 (66%) | 144 (64%) | .85* |
| Only GI | 9 (3%) | 0 (0%) | 9 (4%) | .61* |
| Timing | ||||
| 0 to <1 h | 27 (10%) | 3 (9%) | 24 (11%) | >.99* |
| 1 to <2 h | 14 (5%) | 1 (3%) | 13 (6%) | .70* |
| ≥2 h | 211 (81%) | 31 (89%) | 180 (80%) | .26* |
| Not specified | 9 (3%) | 0 (0%) | 9 (4%) | .61* |
| History of asthma, n (%) | 43 (16%) | 10 (29%) | 33 (15%) | .05* |
| Urgent or emergency care | 166 (64%) | 18 (51%) | 148 (66%) | .13* |
| Tick or chigger bite in last 10 y | 255 (98%) | 35 (100%) | 220 (97%) | >.99* |
| Total IgE (IU/mL), GM (95% CI) | 144 (125-166) | 170 (120-239) | 141 (121-164) | .42† |
| α-Gal | ||||
| sIgE, n > 0.35 IU/mL (%) | 245 (94%) | 34 (97%) | 211 (93%) | .70* |
| sIgE (IU/mL), GM (95% CI) | 17.7 (15-21) | 8.2 (5-13) | 20.1 (16-25) | .009† |
| sIgE as % of total, GM (95% CI) | 11.9 (10-14) | 5.3 (4-8) | 13.5 (12-16) | <.001† |
| sIgG (U/mL), GM (95% CI) | 157 (129-191) | 79 (37-169) | 174 (143-211) | .05† |
α-Gal, Galactose-α-1,3-galactose; CI, confidence interval; GI, gastrointestinal; GM, geometric mean.
Fisher’s exact test.
Mann-Whitney U test.
Compared with adults there was a higher frequency of males among the children; however, the nature and timing of clinical symptoms was very similar in children and adults (Table I). The prevalence of GI symptoms was also similar in children (66%) and adults (64%). The frequency of positive α-Gal sIgE titers among subjects reporting reactions to mammalian meat was similar between children (97%) and adults (93%), although the levels of α-Gal sIgE were higher in adults than children (Figure 1, A). sIgE to beef, pork, and milk were detectable in the majority of children and adults. Levels of α-Gal sIgE correlated with total IgE in both children (rs = 0.48, P = .004) and adults (rs = 0.70, P < .001) (Figure 1, B).
FIGURE 1.
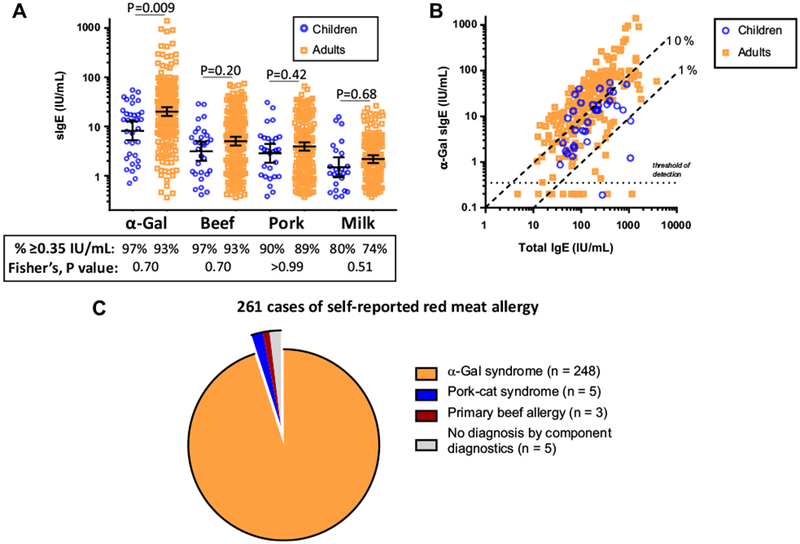
Comparison of specific IgE (sIgE) results in children and adults: A, sIgE to galactose-α-1,3-galactose (α-Gal) and mammalian extracts; B, relationship between total IgE and α-Gal sIgE; C, distribution of component-based diagnoses of 261 cases of self-reported mammalian meat allergy.
Among the 261 cases reporting reactions to red meat, there were 16 cases with negative α-Gal sIgE testing that we sought to further characterize with additional sIgE assays. These included α-Gal and beef sIgE assays at a lower threshold (cutoff of 0.1 IU/mL) and sIgE to a panel of markers for pork-cat syndrome (cat extract, Fel d 2 [feline serum albumin] and Sus s 1 [pork serum albumin]).1 The results suggested a diagnosis for 11 of the 16 cases—5 subjects had low-titer sIgE to α-Gal (ie, 0.1-0.35 IU/mL), 3 subjects had a sIgE signature suggestive of pork-cat syndrome, and 3 subjects had sIgE to beef but not α-Gal (or sIgE to beef greater than sIgE to α-Gal in 1 case) (Table IIA). Of note, 2 subjects with positive α-Gal sIgE may have had pork-cat syndrome on the basis of a higher sIgE to pork, cat, and Fel d 2 than to α-Gal (Table IIB). Taken together, of the 261 cases seen in evaluation of red meat allergy, at least 248 (95%) were likely related to α-Gal (Figure 1, C).
TABLE II.
(A) Component analysis of subjects reporting allergic reactions to red meat but not having α-Gal sIgE ≥ 0.35 IU/mL. (B) Component analysis of 2 subjects reporting allergic reactions to red meat with α-Gal sIgE ≥ 0.35 IU/mL, but component signature suggestive of pork-cat syndrome
| Demographic | Timing | Total IgE | α-Gal | Beef | Pork | Milk | Cat | Fel d 2 | Sus s 1 |
|---|---|---|---|---|---|---|---|---|---|
| (A) Component analysis of subjects reporting allergic reactions to red meat but not having α-Gal sIgE ≥ 0.35 IU/mL | |||||||||
| Low titer α-Gal | |||||||||
| 42 M | 6 h | 113 | 0.26 | 0.22 | 0.13 | 0.19 | <0.10 | 0.13 | nd |
| 49 F | 4-8 h | 25 | 0.18 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | nd |
| 58 F | 0:20-0:30 | 62 | 0.16 | nd | nd | Nd | nd | nd | nd |
| 52 F | 0:30 | 13 | 0.16 | <0.10 | <0.35 | <0.10 | <0.10 | <0.10 | nd |
| 30 M | 1:30 | 356 | 0.19 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | nd |
| Suggestive of primary beef allergy | |||||||||
| 68 F | 2 h | 85 | 0.2 | 0.42 | 0.11 | 0.24 | <0.10 | <0.10 | nd |
| 47 F | 4 h | 5 | <0.10 | 1.30 | <0.10 | <0.35 | <0.35 | <0.35 | nd |
| 42 F | 3 h | 1157 | <0.10 | 0.18 | 0.11 | <0.35 | <0.35 | <0.10 | nd |
| Suggestive of pork-cat syndrome | |||||||||
| 51 M | 1 h | 83 | <0.10 | <0.10 | 0.65 | <0.35 | 3.74 | 2.28 | 0.62 |
| 16 M | 6-8 h | 277 | <0.10 | <0.10 | 0.28 | 0.11 | 2.83 | 0.10 | 0.19 |
| 43 F | 0:15-5 h | 42 | <0.35 | <0.35 | 0.37 | <0.35 | 3.87 | 4.18 | nd |
| No component diagnosis | |||||||||
| 32 F | 2 h | 71 | <0.10 | <0.10 | <0.10 | <0.10 | <0.35 | <0.35 | nd |
| 50 F | 6 h | 88 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | nd |
| 45 F | 0:30-1 h | 22 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | nd |
| 20 M | 4 h | 74 | <0.10 | <0.10 | <0.10 | <0.10 | 0.13 | <0.10 | nd |
| 28 M | 6 h | 63 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | nd |
| (B) Component analysis of 2 subjects reporting allergic reactions to red meat with α-Gal sIgE ≥ 0.35 IU/mL but component signature suggestive of pork-cat syndrome | |||||||||
| 47 F | 8-12h | 197 | 0.62 | 0.11 | 3.40 | <0.10 | 17.70 | 26.80 | 0.28 |
| 21 F | 0:05 | 328 | 1.41 | 1.10 | 7.10 | 5.08 | 5.71 | 12.30 | nd |
α-Gal, Galactose-α-1,3-galactose; sIgE, specific IgE.
Key for demographics and symptom onset: shown are age (y), sex (M-male, F-female), and time (min) between mammalian meat ingestion and self-reported symptom onset (in cases with 2 values a range of times were reported, reflecting onset during different allergic reactions). All values expressed in IU/mL. Limit of detection varied as either 0.10 IU/mL or 0.35 IU/mL, as indicated; nd denotes serum was not available for assay; values in bold type indicate labs that are consistent with the relevant syndrome.
Comparison of reactions to mammalian meat: symptom onset and presentation
Although the majority of subjects reported delays of 3 to 6 hours, 41 subjects (16%) reported reactions in under 2 hours (Figure 2, A). The cutoff of 2 hours was used in keeping with consensus guidelines on the timing of immediate reactions.18 Among the 41 early responders, the prevalence of females was higher (68% vs 52%; χ2, P = .04) (Table E1, available in this article’s Online Repository at www.jaci-inpractice.org). Although 6 of the early responders were negative for α-Gal sIgE at ≥0.35 IU/mL, 3 of those had evidence of a diagnosis using component assays (Table II). Overall, 93% of the early responders had evidence that α-Gal was the relevant epitope. Of the 41 early responders, 14 reported that some of their reactions occurred with a delay of at least 2 hours. Using self-report of anaphylaxis as a surrogate for reaction severity, there was no difference in severity in subjects reporting early and delayed reactions (Figure 2, B). Moreover, there were no significant differences in levels of sIgE or sIgG to α-Gal between early and delayed responders (Figure 2, C). We also assessed α-Gal sIgE and sIgG titers in subjects who reported anaphylaxis and in those who reported urticaria alone, and the levels were similar in both groups (Figure 2, D). Titers of α-Gal sIgE were lower in subjects with isolated GI symptoms; nonetheless, there were 5 subjects with isolated GI symptoms who had α-Gal sIgE exceeding 3% of total IgE, including 2 with α-Gal sIgE titers >10 IU/mL.
FIGURE 2.
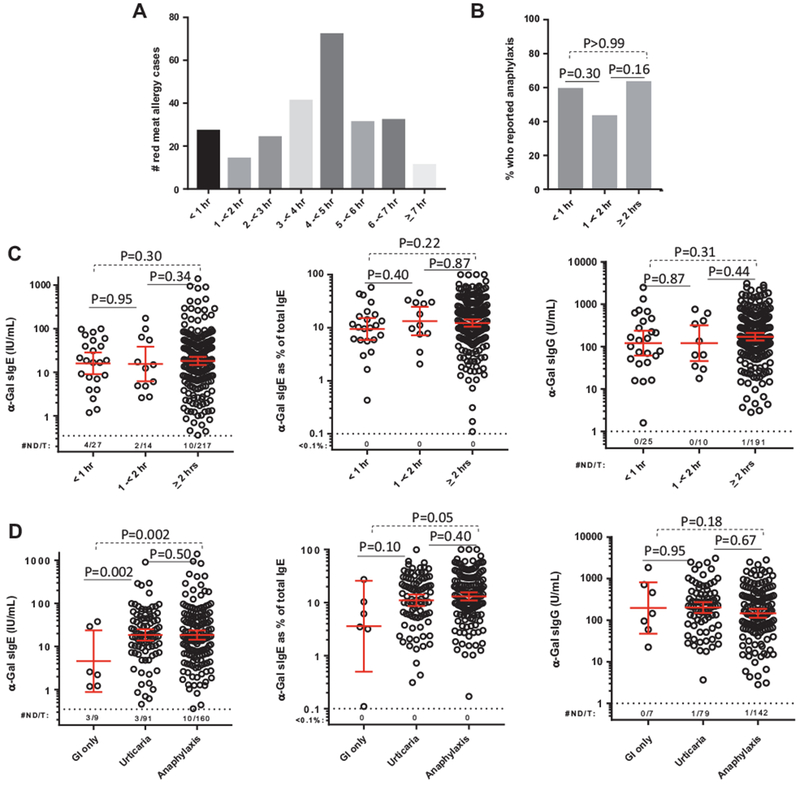
A, Histogram demonstrating time to reported initial symptom onset after mammalian meat ingestion. B, Frequency of subjects who reported anaphylaxis in relation to the timing of initial symptom onset with comparison using χ2. C, Levels of galactose-α-1,3-galactose (α-Gal) specific IgE (sIgE), percentage of α-Gal sIgE of total IgE, and α-Gal specific IgG (sIgG) stratified according to when symptoms began. Three subjects who did not provide a specific time were excluded from analysis. For subjects who reported multiple times (reflecting a history of multiple reactions) the earliest time indicated was used for stratification. D, Levels of α-Gal sIgE, percentage of α-Gal sIgE of total IgE, and α-Gal sIgG stratified according to symptoms reported. One subject who reported only constitutional symptoms was excluded from analysis. Number of samples not detected/total samples tested (#ND/T). Comparisons with the Mann-Whitney U test and positive values expressed as geometric mean with 95% confidence interval.
Sensitization to α-Gal and the α-Gal syndrome in relation to traditional atopy
Sensitization to α-Gal is known to relate to tick bites and cases have been reported who had no past history of atopy.19 To address this in the present cohort, we characterized sIgE to a panel of common inhalant, food, and venom extracts. We also assayed sIgE in a reference population (n = 147) that included 36 controls without any history of urticaria or anaphylaxis and 111 individuals who were enrolled from the allergy clinic with histories of urticaria or anaphylaxis but no perceived association with red meat. Taken together the total group included 408 subjects, of whom sIgE to α-Gal was positive in 311 individuals and negative in 97 (Table E2, available in this article’s Online Repository at www.jaci-inpractice.org). Whether classified as α-Gal sIgE positive (n = 311) or as red meat allergic (n = 261), the prevalence of sensitization to allergens unrelated to α-Gal was similar (Table III). The prevalence of sIgE to inhalants was similar among α-Gal sIgE positive and α-Gal sIgE negative subjects. The frequency of sensitization to nonmammalian foods trended higher in α-Gal sIgE positive subjects, and was statistically significant for wheat (19.6% vs 8.0%, Fisher’s exact test, P = .01). The α-Gal sIgE positive subjects also had a higher frequency of sensitization to bee (21.6 vs 4.8%, Fisher’s exact test, P < .001) and wasp (37.8% vs 9.5%, Fisher’s exact test, P < .001) venom (Table III).
TABLE III.
Specific IgE (sIgE) results in subjects reporting reactions associated to red meat (n = 261) and among the whole cohort stratified as α-Gal sIgE negative (n = 97) or positive (n = 311)
| Specific antibodies* | Red meat allergic (n = 261) | Red meat allergic + reference controls | ||
|---|---|---|---|---|
| α-Gal sIgE < 0.35 (n = 97) | α-Gal sIgE ≥ 0.35 (n = 311) | P value† | ||
| Mammal | ||||
| sIgE to beef | 225/247 (91.1%) | 8/90 (8.9%) | 269/293 (91.8%) | <.001 |
| sIgE to pork | 213/246 (86.6%) | 7/87 (8.0%) | 255/293 (87.0%) | <.001 |
| sIgE to milk | 169/242 (69.8%) | 5/90 (5.6%) | 203/288 (70.5%) | <.001 |
| sIgE to cat | 156/234 (66.7%) | 18/91 (19.8%) | 186/282 (66.0%) | <.001 |
| sIgE to dog | 167/235 (71.1%) | 14/89 (15.7%) | 198/285 (69.5%) | <.001 |
| sIgE to gelatin | 11/232 (4.7%) | 0/85 (0%) | 12/280 (4.3%) | .08 |
| Inhalants | ||||
| sIgE to dust mite | 68/232 (29.3%) | 30/89 (33.7%) | 80/282 (28.4%) | .18 |
| sIgE to cockroach | 41/229 (17.9%) | 8/88 (9.1%) | 47/276 (17.0%) | .07 |
| sIgE to Timothy | 60/227 (26.4%) | 25/88 (28.4%) | 70/275 (25.5%) | .58 |
| sIgE to ragweed | 53/228 (23.2%) | 15/88 (17.0%) | 63/276 (22.8%) | .25 |
| sIgE to birch tree | 33/227 (14.5%) | 15/88 (17.0%) | 42/275 (15.3%) | .69 |
| sIgE to Alternaria | 23/228 (10.1%) | 9/86 (10.5%) | 28/276 (10.1%) | .93 |
| Food | ||||
| sIgE to egg | 15/231 (6.5%) | 3/86 (3.5%) | 21/280 (7.5%) | .22 |
| sIgE to wheat | 42/231 (18.2%) | 7/88 (8.0%) | 55/281 (19.6%) | .01 |
| sIgE to peanut | 29/230 (12.6%) | 6/86 (7.0%) | 38/281 (13.5%) | .10 |
| sIgE to shrimp | 33/223 (14.8%) | 7/85 (8.2%) | 39/273 (14.3%) | .15 |
| sIgE to chicken | 0/39 (0%) | 0/7 (0%) | 0/48 (0%) | – |
| Venom | ||||
| sIgE to bee | 53/222 (23.9%) | 4/84 (4.8%) | 58/269 (21.6%) | <.001 |
| sIgE to wasp | 82/222 (36.9%) | 8/84 (9.5%) | 102/270 (37.8%) | <.001 |
α-Gal, Galactose-α-1,3-galactose.
Cutoff for positive sIgE was 0.35 IU/mL.
Fisher’s exact test.
To further explore the relationship between α-Gal sIgE and atopy, we stratified among subjects reporting red meat allergy where atopy was defined by the presence of sIgE ≥ 0.7 IU/mL to at least 1 of 3 common inhalant allergens (dust mite, Timothy grass, and ragweed). Of the 261 subjects, sera from 229 had been assayed for all 3 inhalants, of whom 86 (38%) were positive to at least 1 inhalant and 143 (62%) had <0.7 IU/mL to each of the 3 inhalants. The titers and prevalence of α-Gal sIgE were similar in atopics and nonatopics (Figure 3, A). The relationship between α-Gal sIgE and total IgE was strong in those with (rs = 0.57, P < .001) and without atopy (rs = 0.76, P < .001); thus sIgE to α-Gal can be an important contributor to total IgE regardless of traditional atopic sensitization (Figure 3, B). Among subjects with red meat allergy there was no difference in the timing of reactions after eating red meat or the frequency of different symptoms in relation to atopy (Figure 3, C). Sensitization to nonmammalian foods such as wheat, peanut, and shrimp, but not mammalian foods, was predominantly observed in atopic subjects, and the prevalence of sIgE to wasp venom was high in both atopic (54%) and nonatopic (27%) subjects (Figure 3, D).
FIGURE 3.
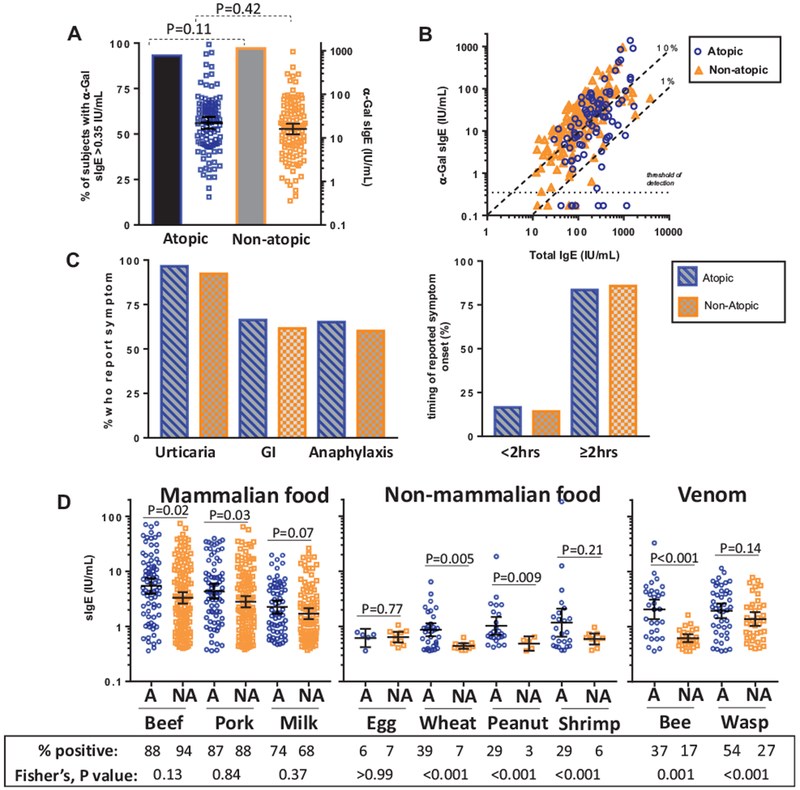
Relationship between characteristics of galactose-α-1,3-galactose (α-Gal) syndrome and atopy among subjects reporting reactions to red meat. A, Prevalence (bar graph, left y-axis) and titers (scatter plot, right y-axis) of specific IgE (sIgE) to α-Gal in atopics and nonatopics (where atopy defined as sIgE ≥ 0.7 IU/mL to dust mite, Timothy grass, and/or ragweed). B, Correlation of α-Gal sIgE and total IgE in atopic (n = 83) and nonatopic subjects (n = 143) who reported allergic reactions to mammalian meat. C, Symptoms reported by red meat allergic subjects in relation to atopy status. D, Prevalence (shown in table format under the graph) and titers of sIgE to mammalian and nonmammalian foods, and venom, among atopic and nonatopic subjects reporting reactions to red meat where A denotes atopic and NA denotes nonatopic. Data expressed as geometric mean with 95% confidence interval; levels of detected sIgE compared by the Mann-Whitney U test and prevalence compared by Fisher’s exact test. GI, Gastrointestinal.
ABO blood groups, α-Gal specific antibodies, and red meat allergy
Subjects with B-antigen blood groups (B or AB) have been reported to be protected from developing the α-Gal syndrome.8,9 Among the overall cohort of 408, ABO blood type information was available for 299 subjects. The frequency of subjects with B-antigen was less than the national average and was not explained by the racial make-up of the subjects (Table E3A, available in this article’s Online Repository at www.jaci-inpractice.org).20 Blood group B/AB was present in 14.3% of α-Gal sIgE negative subjects, 9.0% of α-Gal sensitized subjects (χ2, P = .20 for comparison with α-Gal sIgE negative), and 8.0% of the subjects who were both sensitized to α-Gal and reported mammalian meat allergy (χ2, P = .13 for comparison with α-Gal sIgE negative) (Table E3B, available in this article’s Online Repository at www.jaci-inpractice.org). A sensitivity analysis restricted to subjects who underwent reverse typing showed similar results to the analysis that included information from both blood typing and questionnaire (Table E3C, available in this article’s Online Repository at www.jaci-inpractice.org). Regardless of IgE sensitization status, the levels of sIgG to α-Gal among subjects with B/AB were lower as compared with those with blood group A or O (Figure 4, A). Surprisingly, α-Gal sIgE titers were often high among subjects with mammalian meat allergy who had blood group B/AB, and these subjects also trended toward an increased ratio of α-Gal sIgE to sIgG (Figure 4, B).
FIGURE 4.
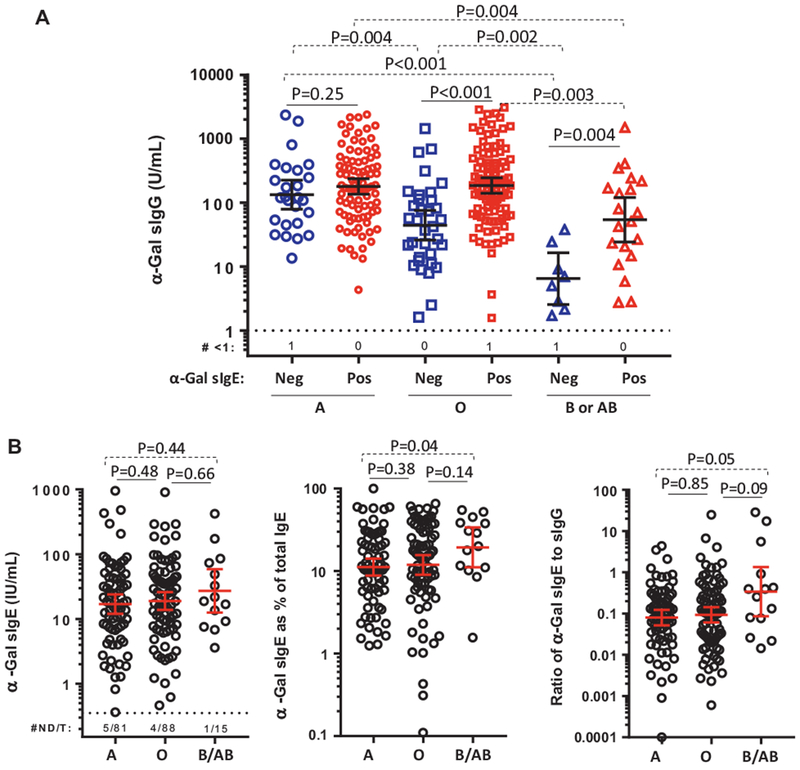
A, Levels of galactose-α-1,3-galactose (α-Gal) specific IgG (sIgG) stratified by α-Gal specific IgE (sIgE) sensitization status in subjects reporting reactions to mammalian meat and the reference cohort. B, Levels of α-Gal sIgE, α-Gal sIgE as percentage of total IgE, and ratio of α-Gal sIgE to α-Gal sIgG stratified by ABO status in subjects reporting reactions to mammalian meat. Data expressed as geometric mean with 95% confidence interval; number of samples not detected/total samples tested (#ND/T); comparisons with the Mann-Whitney U test. GI, Gastrointestinal.
DISCUSSION
Here we report that the component analysis of 261 children and adults reporting allergic reactions to red meat supported a diagnosis of α-Gal syndrome in 248 (95%) of the subjects, results which confirm that the α-Gal syndrome is a dominant cause of red meat allergy in the Southeastern United States. An important element of the current report is the head-to-head comparison of adults and children. Although other publications have described features of the α-Gal syndrome in children, here we demonstrate that there is no difference in the timing or nature of symptoms, or in tick exposure history, in children and adults.21,22 Titers of α-Gal sIgE were lower in children than adults, but the titers nonetheless often approached or exceeded 10% of total IgE. Although a major aim of the study was to investigate the clinical and immunologic features of the α-Gal syndrome, the use of component diagnostics also enabled us to identify other causes of red meat allergy. In this cohort, 5 subjects had serologic markers consistent with pork-cat syndrome, that is, positive sIgE titers to cat, pork, and Fel d 2.23 Three subjects had sIgE to beef greater than the value of sIgE to α-Gal (of whom 2 had sIgE to α-Gal < 0.1 IU/mL), suggesting primary beef allergy as the diagnosis. All 3 of these subjects were well into adulthood, which is interesting given that primary beef allergy is generally considered a disease of childhood;24 on the other hand, Bos d 6 (bovine serum albumin) has been reported as a dominant cause of beef allergy in children and sIgE to Bos d 6 (bovine serum albumin) was negative in the 2 current cases where serum was available for measurement.25,26 Although not part of the current cohort, we have seen cases of red meat allergic adults who had high titers to components such as Bos d 6 and gelatin.27
Allergic reactions to red meat commonly included anaphylaxis, hives, and GI distress, and those reactions often led to presentation for emergency medical care. With the exception of subjects presenting with isolated GI symptoms, who had relatively lower titers, neither absolute nor relative α-Gal sIgE levels were associated with reaction severity. Although it was not formally part of our original questionnaire, it has been our experience that GI symptoms related to α-Gal syndrome most commonly involve cramping abdominal pain. Oral food challenge studies have demonstrated that mammalian meat allergy that relates to α-Gal sensitization often occurs with a delay of 3-6 hours;7 however, in the current report, the immune profile was similar between subjects who reported reactions to mammalian meat that were delayed by over 2 hours as well as the 16% of subjects who reported symptom onset occurring in less than 2 hours. This finding shows that not all reactions that relate to α-Gal and mammalian meat ingestion occur with delayed kinetics, a finding that has been reported in other countries.22,28 One notable difference between subjects who reported early versus delayed symptoms was the increased proportion of females in the early-onset group. The explanation for why a minority of subjects experience reactions without the characteristic delay is unclear, but possible explanations include: (1) the quantity or quality (eg, glycoprotein vs glycolipid forms of α-Gal) of α-Gal-containing epitopes that are consumed;29 (2) presence of cofactors such as alcohol, nonsteroidal anti-inflammatory drugs, or exercise;6,28 (3) a role for sex hormones or other biological factors that vary between males and females;30 (4) heightened sensitivity in subjects with a lower threshold for activation of cells and pathways that mediate allergic responses (eg, mast cell disorders),31 and/or (5) sensitization to epitopes in addition to α-Gal that are present in mammalian meat, such as albumin or immunoglobulins, which have been associated with immediate hypersensitivity.1
Most subjects who had sIgE to α-Gal also had sIgE to extracts derived from mammalian sources, which, based on the strength of correlations between the sIgE assays (Figure E1, available in this article’s Online Repository at www.jaci-inpractice.org) and earlier work on inhibition and dilution studies, is explained by the presence of α-Gal epitopes in the extracts.2,15,32 Consistent with our clinical experience, there was not a positive association between traditional atopy (ie, inhalant sensitization) and α-Gal; however, sIgE to bee and wasp venom were both positively associated with sensitization to α-Gal. This association could reflect a shared risk relating to outdoor exposures. Another possibility is that α-Gal or other similar epitopes could be present within the venom of stinging insects; however, the large number of sera with sIgE to α-Gal that were negative for venom makes this unlikely. A third possibility is that shared responses to α-Gal and venom could occur because some subjects are predisposed to generate IgE after epicutaneous exposures. This latter suggestion is intriguing in view of the fact that sIgE to nonmammalian food allergens was more prevalent (wheat), or trended toward being more prevalent (egg, peanut, and shrimp), in subjects who were sensitized to α-Gal. Thus, differences in cutaneous immunity, as described in the dual-allergen exposure hypothesis, could be a risk modifier for sensitization not only to some food allergens, but also to α-Gal and insect venom.33,34 On the other hand, many subjects without traditional atopy were sensitized to α-Gal and the levels of sIgE to α-Gal were similar in those with or without atopy. We are also unaware of any association between atopic dermatitis and the α-Gal syndrome. Taken together, the favored explanation is that ticks represent venomous ectoparasites with salivary factors that have potent adjuvant activity and favor type 2 immune responses even in subjects who are not predisposed to atopy.19,35,36 Differences in host immunity, such as skin barrier defects, are less likely to be critical in sensitization because lone star tick “mouthparts,” even those of larvae or nymphs, are sufficiently long to penetrate the epidermis during the feeding process.37,38
In the current report, the frequency of blood group B (including B or AB blood types) trended lower in subjects who had symptomatic α-Gal syndrome than in nonsensitized control subjects. This finding was similar to the results of 2 recent reports that concluded that blood group B confers protection against the development of α-Gal sensitization and α-Gal syndrome.8,9 For example, in the Swedish cohort reported by Apostolovic et al (which has similar expected B blood group representation in the population), the frequency of B-antigen in subjects with α-Gal syndrome was similar to the result in our cohort at 5.9% versus 8.0%, respectively.8,9 The explanation for the negative association between blood group B and α-Gal syndrome likely relates to the fact that α-Gal is structurally similar to the B-antigen and that a significant fraction of naturally occurring anti-B antibodies also recognize α-Gal.9,39,40 Consistent with this, our data confirmed other reports that α-Gal sIgG levels are lower in subjects with B-antigen (Figure 4, A).9,41,42 An additional observation was that sIgE to α-Gal was often high titer in subjects with B-antigen and symptomatic mammalian meat allergy. This finding differs from the recent report from Brestoff et al,8 which reported that α-Gal sIgE titers were low in subjects with B-antigen who had α-Gal syndrome. The explanation for this discrepancy is unclear, but we note in the report of Apostolovic et al9 that of the 3 B-antigen positive subjects with α-Gal syndrome, 2 had α-Gal sIgE titers of >40 IU/mL. Collectively, our conclusion is that subjects with B-antigen are partially protected from developing α-Gal syndrome, but that the syndrome can develop if α-Gal sIgE titers are high.
There are several limitations to consider. Although our results strongly suggest that α-Gal syndrome is a major cause of allergic reactions to red meat in our region, the study design does not provide an estimate of the population prevalence of the syndrome. However, a recent report by Pattanaik et al12 from Memphis, Tennessee, supports our view that α-Gal syndrome is the most common cause of adult-onset anaphylaxis in the Southeastern United States. In the current report, α-Gal syndrome was defined by clinical history and the presence of a positive α-Gal sIgE level. Although we did not conduct oral challenges, we do not routinely carry out, or recommend, oral challenges to mammalian meat. In our experience, such challenges are difficult to perform safely in an outpatient setting. We also did not monitor the response to a diet of red meat avoidance, but in our clinic and an ongoing survey of allergists in areas where the syndrome is common, 80% to 90% of subjects respond to a red meat elimination diet. Another limitation is that anaphylaxis was defined based on self-report in questionnaires at enrollment. We are aware that this definition is not specific, and may not in all cases correspond to the formal definition of anaphylaxis. However, anaphylaxis has historically been challenging to define, and many cases of anaphylaxis relating to α-Gal, including cases requiring hospital admission, have been seen in the emergency departments at the University of Virginia and the University of North Carolina. The definition of atopy as sIgE ≥ 0.7 IU/mL to dust mite, Timothy, or ragweed has limitations; however, using a more conservative definition of atopy with a panel of 6 aeroallergens at a lower titer (ie, cutoff of 0.35 IU/mL) yielded similar results (see Figure E2 in this article’s Online Repository at www.jaci-inpractice.org). Another limitation is that assays for isotype-specific antibodies other than IgE and IgG were not reported here; however, prior reports have consistently shown that sIgG1 and sIgG2, but not sIgG4, are the dominant contributors to the specific IgG titer in subjects with α-Gal syndrome.9,41,43
In summary, we report here the analysis of a large cohort who experienced allergic reactions to mammalian meat, of whom 95% were sensitized to an oligosaccharide that is present in blood and tissues of nonprimate mammals. Consistent with the premise that tick bites are the major cause of α-Gal sensitization, there was no association between sensitization to α-Gal and inhalant allergens. The data are in general agreement with prior reports that have suggested that the presence of blood group B is protective against the development of α-Gal syndrome; however, the current analysis makes it clear that subjects with B-antigen can still acquire the syndrome. Taken together, this large observational cohort supports the connection between sIgE to α-Gal and a syndrome of anaphylaxis to mammalian meat that (1) commonly emerges during adulthood but can also present in children, (2) has a characteristic but not universal delay of at least 2 hours, (3) responds to a diet of red meat avoidance, and (4) relates to a preceding bite from lone star ticks, and is not associated with traditional atopic disease.
Supplementary Material
What is already known about this topic?
Over the last 10 years, the syndrome of delayed anaphylaxis to mammalian meat in patients with IgE to galactose-α-1,3-galactose (α-Gal) has been confirmed in large areas of the United States and in many other countries.
What does this article add to our knowledge?
This analysis of 261 subjects provides evidence that titers of IgE and IgG specific for α-Gal are not a predictor of the symptoms, the severity of reactions, or the time taken to respond after eating meat.
How does this study impact current management guidelines?
The α-Gal syndrome should be considered as an explanation for allergic symptoms, including abdominal pain, related to red meat at any age, regardless of atopic history, ABO blood group, symptom severity, and timing of symptom onset.
Acknowledgments
We appreciate Clay Ford, MS, at the University of Virginia StatLab, and Gina Petroni, PhD, and Mark Conaway, PhD, at the University of Virginia School of Medicine Department of Public Health Sciences for assistance with statistical analysis.
This work was supported by the National Institutes of Health: grants T32 AI-007496 (JMW), KO8 AI-085190 (SPC), and R37 AI-20565 (TAEP-M).
Abbreviations used
- α-Gal
Galactose-α-1,3-galactose
- α-Gal sIgE
α-Gal specific IgE
- GI
Gastrointestinal
- sIgE
Specific IgE
- sIgG
Specific IgG
Footnotes
Conflicts of interest: S. P. Commins has been on the speaker’s bureau for Genentech. T. A. E. Platts-Mills has a patent on an IgE assay to α-Gal and has received assay support from Thermo-Fisher/Phadia. The rest of the authors declare that they have no conflicts of interest.
REFERENCES
- 1.Wilson JM, Platts-Mills TAE. Meat allergy and allergens. Mol Immunol 2018; 100:107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol 2009;123:426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galili U The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol 2005;83:674–86. [DOI] [PubMed] [Google Scholar]
- 4.van Nunen SA. Tick-induced allergies: mammalian meat allergy and tick anaphylaxis. Med J Aust 2018;208:316–21. [DOI] [PubMed] [Google Scholar]
- 5.Platts-Mills TA, Commins SP. Emerging antigens involved in allergic responses. Curr Opin Immunol 2013;25:769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer J, Yazdi AS, Biedermann T. Clinical spectrum of alpha-Gal syndrome: from immediate-type to delayed immediate-type reactions to mammalian innards and meat. Allergo J Int 2016;25:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Commins SP, James HR, Stevens W, Pochan SL, Land MH, King C, et al. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose-alpha-1,3-galactose. J Allergy Clin Immunol 2014;134:108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brestoff JR, Tesfazghi MT, Zaydman MA, Jackups R Jr, Kim BS, Scott MG, et al. The B antigen protects against the development of red meat allergy. J Allergy Clin Immunol Pract 2018;6:1790–1791.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apostolovic D, Rodrigues R, Thomas P, Starkhammar M, Hamsten C, van Hage M. Immunoprofile of alpha-Gal- and B-antigen-specific responses differentiates red meat-allergic patients from healthy individuals. Allergy 2018;73:1525–31. [DOI] [PubMed] [Google Scholar]
- 10.Cabezas-Cruz A, de la Fuente J, Fischer J, Hebsaker J, Lupberger E, Blumenstock G, et al. Prevalence of type I sensitization to alpha-gal in forest service employees and hunters: is the blood type an overlooked risk factor in epidemiological studies of the alpha-Gal syndrome? Allergy 2017;72:2044–7. [DOI] [PubMed] [Google Scholar]
- 11.Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol 2011;127:1286–1293.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pattanaik D, Lieberman P, Lieberman J, Pongdee T, Keene AT. The changing face of anaphylaxis in adults and adolescents. Ann Allergy Asthma Immunol 2018;121:594–7. [DOI] [PubMed] [Google Scholar]
- 13.Commins SP, Kelly LA, Ronmark E, James HR, Pochan SL, Peters EJ, et al. Galactose-alpha-1,3-galactose-specific IgE is associated with anaphylaxis but not asthma. Am J Respir Crit Care Med 2012;185:723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seagroatt V, Anderson SG. The second international reference preparation for human serum immunoglobulin E and the first British standard for human serum immunoglobulin E. J Biol Stand 1981;9:431–7. [DOI] [PubMed] [Google Scholar]
- 15.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med 2008;358:1109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erwin EA, Custis NJ, Satinover SM, Perzanowski MS, Woodfolk JA, Crane J, et al. Quantitative measurement of IgE antibodies to purified allergens using streptavidin linked to a high-capacity solid phase. J Allergy Clin Immunol 2005;115:1029–35. [DOI] [PubMed] [Google Scholar]
- 17.Schuyler AJ, Wilson JM, Tripathi A, Commins SP, Ogbogu PU, Kruzsewski PG, et al. Specific IgG4 antibodies to cow’s milk proteins in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol 2018;142:139–148.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burks AW, Tang M, Sicherer S, Muraro A, Eigenmann PA, Ebisawa M, et al. ICON: food allergy. J Allergy Clin Immunol 2012;129:906–20. [DOI] [PubMed] [Google Scholar]
- 19.Wilson JM, Schuyler AJ, Schroeder N, Platts-Mills TA. Galactose-alpha-1,3-galactose: atypical food allergen or model IgE hypersensitivity? Curr Allergy Asthma Rep 2017;17:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garratty G, Glynn SA, McEntire R, Retrovirus Epidemiology Donor Study. ABO and Rh(D) phenotype frequencies of different racial/ethnic groups in the United States. Transfusion 2004;44:703–6. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy JL, Stallings AP, Platts-Mills TA, Oliveira WM, Workman L, James HR, et al. Galactose-alpha-1,3-galactose and delayed anaphylaxis, angioedema, and urticaria in children. Pediatrics 2013;131:e1545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mabelane T, Basera W, Botha M, Facey Thomas H, Ramjith J, Levin ME. Predictive values of alpha-gal IgE levels and alpha-gal IgE:total IgE ratio and oral food challenge proven meat allergy in a population with a high prevalence of reported red meat allergy. Pediatr Allergy Immunol 2018;29:841–9. [DOI] [PubMed] [Google Scholar]
- 23.Posthumus J, James HR, Lane CJ, Matos LA, Platts-Mills TA, Commins SP. Initial description of pork-cat syndrome in the United States. J Allergy Clin Immunol 2013:131923–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiocchi A, Restani P, Riva E. Beef allergy in children. Nutrition 2000;16:454–7. [DOI] [PubMed] [Google Scholar]
- 25.Werfel SJ, Cooke SK, Sampson HA. Clinical reactivity to beef in children allergic to cow’s milk. J Allergy Clin Immunol 1997;99:293–300. [DOI] [PubMed] [Google Scholar]
- 26.Restani P, Fiocchi A, Beretta B, Velona T, Giovannini M, Galli CL. Meat allergy: III—proteins involved and cross-reactivity between different animal species. J Am Coll Nutr 1997;16:383–9. [DOI] [PubMed] [Google Scholar]
- 27.Retterer MKC, Workman LJ, Bacon JR, Platts-Mills TAE. Specific IgE to gelatin as a cause of anaphylaxis to zoster vaccine. J Allergy Clin Immunol 2018;141:1956–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer J, Hebsaker J, Caponetto P, Platts-Mills TA, Biedermann T. Galactose-alpha-1,3-galactose sensitization is a prerequisite for pork-kidney allergy and cofactor-related mammalian meat anaphylaxis. J Allergy Clin Immunol 2014; 134:755–759.e1. [DOI] [PubMed] [Google Scholar]
- 29.Wilson JM, Platts-Mills TAE. The oligosaccharide galactose-α-1,3-galactose and the α -Gal syndrome: insights from an epitope that is causal in IgE-mediated immediate and delayed anaphylaxis. EMJ Allergy Immunol 2018;3:89–98. [Google Scholar]
- 30.Chen W, Mempel M, Schober W, Behrendt H, Ring J. Gender difference, sex hormones, and immediate type hypersensitivity reactions. Allergy 2008;63: 1418–27. [DOI] [PubMed] [Google Scholar]
- 31.Carter MC, Ruiz-Esteves KN, Workman L, Lieberman P, Platts-Mills TAE, Metcalfe DD. Identification of alpha-gal sensitivity in patients with a diagnosis of idiopathic anaphylaxis. Allergy 2018;73:1131–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuyler AJ, Tripathi A, Workman LJ, Wilson JM, Erwin EA, Lawrence MG, et al. Underestimation of specific IgE measurements using extract-based assays on undiluted sera revealed through dilution. J Allergy Clin Immunol Pract 2018; 6:1070–1072.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox AT, Sasieni P, du Toit G, Syed H, Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. J Allergy Clin Immunol 2009;123:417–23. [DOI] [PubMed] [Google Scholar]
- 34.Lack G Epidemiologic risks for food allergy. J Allergy Clin Immunol 2008;121:1331–6. [DOI] [PubMed] [Google Scholar]
- 35.Kotal J, Langhansova H, Lieskovska J, Andersen JF, Francischetti IM, Chavakis T, et al. Modulation of host immunity by tick saliva. J Proteomics 2015;128:58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cabezas-Cruz A, Valdes JJ. Are ticks venomous animals? Front Zool 2014;11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown SJ, Knapp FW. Amblyomma americanum: sequential histological analysis of adult feeding sites on guinea pigs. Exp Parasitol 1980;49:303–18. [DOI] [PubMed] [Google Scholar]
- 38.Brown SJ, Knapp FW. Amblyomma americanum: sequential histological analysis of larval and nymphal feeding sites on guinea pigs. Exp Parasitol 1980;49:188–205. [DOI] [PubMed] [Google Scholar]
- 39.Galili U, Buehler J, Shohet SB, Macher BA. The human natural anti-Gal IgG. III. The subtlety of immune tolerance in man as demonstrated by crossreactivity between natural anti-Gal and anti-B antibodies. J Exp Med 1987;165:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galili U The Natural Anti-Gal Antibody as Foe Turned Friend in Medicine. 1st ed Cambridge, MA: Elsevier; 2017. [Google Scholar]
- 41.Rispens T, Derksen NI, Commins SP, Platts-Mills TA, Aalberse RC. IgE production to alpha-gal is accompanied by elevated levels of specific IgG1 antibodies and low amounts of IgE to blood group B. PLoS One 2013;8:e55566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oostingh GJ, Davies HF, Arch BN, Bradley JA, Taylor CJ. Potential implications of ABO blood group for vascular rejection in pig to human kidney xenotransplantation. Xenotransplantation 2003;10:278–84. [DOI] [PubMed] [Google Scholar]
- 43.Kollmann D, Nagl B, Ebner C, Emminger W, Wohrl S, Kitzmuller C, et al. The quantity and quality of alpha-gal-specific antibodies differ in individuals with and without delayed red meat allergy. Allergy 2017;72:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


