Abstract
Transgender populations are heavily burdened by HIV and other sexually transmissible infections (STIs). However, data on co-infection with HIV and STIs among transgender people are limited. A systematic review was conducted of peer-reviewed articles and conference abstracts between January 2010 and November 2015 that focussed on HIV and STI infections among transgender populations globally. The literature was synthesised and opportunities for improving health research were commented on. Few studies reported HIV–STI co-infection (n = 4), while the majority of studies reported HIV and STI infections separately (n = 23). Most studies were conducted outside of the USA (n = 19), and all but one of these studies reported data on transgender women only. Among USA-based studies (n = 8), several reported data on both transgender men and transgender women (n = 3), whereas other studies reported exclusively on transgender men (n = 1) or transgender women (n = 4). Understanding HIV and STIs among transgender people requires research that simultaneously considers multilevel drivers of vulnerabilities. More data are needed on how the interaction of individual determinants, including biological risks of transmission, programmatic determinants such as service-delivery models and policy-level determinants including institutionalised stigma in healthcare settings, influence the HIV- and STI-related outcomes of transgender populations. Leveraging the knowledge of transgender-specific determinants of HIV and STIs should guide the content and approaches to future HIV and STI prevention and treatment efforts.
Additional keywords: co-infection, STI
Introduction
Mounting evidence indicates that transgender populations are heavily burdened by HIV and other sexually transmissible infections (STIs); in particular, transgender women who have sex with men and transgender sex workers.1–4 The most recent meta-analysis of data from 15 countries estimates that transgender women have a pooled HIV prevalence of 19%, a 49-fold increased odds of HIV infection compared with the general population of reproductive age adults in each country.5 A more recent systematic review inclusive of transgender men found that HIV prevalence varied greatly, with the lowest prevalence among youth and the highest among transgender sex workers and transgender women of colour. HIV data among transgender men were extremely limited and varied by study design, with the highest prevalence in clinical samples and the lowest at HIV testing sites in the USA.1
Many of the same determinants of HIV acquisition and transmission among transgender populations, such as condomless intercourse, also increase risk for other STIs. Further, STIs are known to increase risk for both HIV acquisition and transmission.6 Still, data on HIV and STI co-infection among transgender people remain limited.
Understanding and addressing the burden of HIV, STIs, and their co-infection among transgender people is key to reducing disparities and to improving sexual health among this population. The goal of this systematic review was to identify and synthesise relevant data to determine how future research can better mitigate risks and optimise health and wellbeing outcomes.
Methods
Search strategy and terms
We searched PubMed, EMBASE, SCOPUS, PsycINFO and CINAHL, as well as conference abstracts from the online archives of the International AIDS Conference, the Conference on HIV Pathogenesis, Treatment and Prevention, and the Conference on Retroviruses and Opportunistic Infections. Searches included all manuscripts or abstracts published between 1 January 2010 and 30 November 2015 (inclusive). Search terms for all databases included HIV or AIDS as well as a range of terms for ‘transgender.’ Studies of any design that measured the prevalence or incidence of both HIV/AIDS and STIs among transgender individuals were included. Inclusion criteria were consistently applied across all studies to maintain the methodological rigor of the review. Studies were excluded if it was not possible to disaggregate results for transgender participants from results of cisgender participants. No language or age restrictions were used.
Screening and data abstraction
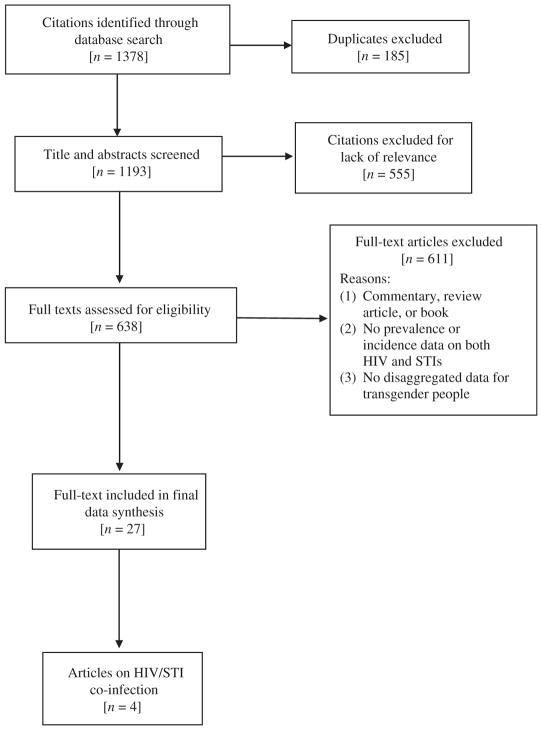
The search was completed on 30 November 2015 (Fig. 1). After duplicates were removed, the titles were screened by two independent coders (ZX and NR) to exclude articles that did not include transgender people. If either screener thought the title was relevant, the abstract was reviewed. The same two reviewers independently assessed the abstracts of the articles that remained after the title review and pulled the full-text of those that met the inclusion criteria or for which the full-text had to be reviewed before a final decision about inclusion could be made. If either reviewer deemed an article relevant, a full-text copy was obtained. A full-text review was completed and data were extracted by two trained coders using standardised data abstraction forms that included details about study design; methods of recruitment; location of the study; sample size; reported HIV prevalence or incidence; STI type and prevalence or incidence; and how HIV and STI diagnoses were obtained. Coders demonstrated greater than 95% agreement, with discrepancies resolved by a third reviewer (AK or TP). At the next level of review (SM), studies were excluded if they included only HIV data without STI data or included only STI data without HIV data. A final additional review of the remaining articles was conducted (SM) to ensure the accurate reporting of all study details.
Fig. 1.
PRISMA flow diagram depicting the flow of information through the different phases of the systematic review.
The results were then organised geographically (global studies in Table 1 and USA-based studies in Table 2) based on data relating either to transgender men (e.g. people who were assigned a female sex at birth and express their gender along a masculine spectrum) or transgender women (e.g. individuals who were assigned a male sex at birth and express their gender along a feminine spectrum). No data were identified that provided estimates of STI or HIV prevalence or incidence among transgender people who do not identify as either transgender men or transgender women. In this study, we report the prevalence of HIV and the three most common STIs: chlamydia, gonorrhoea and syphilis. Other STIs (such as herpes simplex virus and human papillomavirus) measured in the studies are also reported in Tables 1 and 2. The decimals are reported based on how they were published in the original article.
Table 1. HIV and STIs globally among Transgender Men and Transgender Women (n = 19).
STI, sexually transmissible infection; RCT, randomized controlled trail; RDS, respondent driven sample; TLS, time-location sampling
| Authors (year) | Location | Setting and design | Sample size | HIV prevalence | STI type and prevalence | ||
|---|---|---|---|---|---|---|---|
| Self-report (%) | Laboratory test (%) | Self-report | Exam or test | ||||
| Studies including data on transgender men and transgender women (n = 1) | |||||||
| Reisner et al. 20147 | Latin America and the Caribbean, Portugal and Spain | Online/cross-sectional | transgender women = 131 | 6.87 | 9.38% had any STI in the past 12 months; 1.56% chlamydia; 3.91% gonorrhoea; 3.17% syphilis; 4.07% human papillomavirus; 1.59% genital herpes | – | |
| transgender men = 25 | 8 | – | 19.23% had any STI in the past 12 months; 0% chlamydia; 3.85% gonorrhoea; 0% syphilis; 7.69% human papillomavirus; 11.54% genital herpes | – | |||
| Studies including data on transgender women only - Latin and South America (n = 8) | |||||||
| dos Ramos Farías et al. 2011A,8 | Seven cities in Argentina | Non-governmental organization and clinic/cross-sectional | 273 | – | 34.10 | – | Co-infection: 13.8% hepatitis B/syphilis; 9.8% hepatitis B/HIV/syphilis; 9.4% HIV/syphilis other STIs: 72% had at least one STI; 5% chlamydia; 50.4% syphilis; 40.2% hepatitis B; 4.5% hepatitis C; 97.4% human papillomavirus |
| Costa et al. 20159 | Porto Alegre, Brazil | Clinic sample/cross-sectional | 284 | – | 25 | 51.1% had a STI ever | – |
| Solomon et al. 201510 | Guayaquil, Ecuador | Clinic/cross-sectional | 131 | – | 16.80 | – | 75.6% with an STI at screening |
| Lahuerta et al. 201111 | Multiple sites, Guatemala | Clinic/cross-sectional | 37 | – | 4 | – | 0% syphilis |
| Aguayo et al. 2013A,12 | Five sites, Paraguay | Mapped areas/cross-sectional | 237 | – | 27 | – | Co-infection: 6% HIV/syphilis other STIs: 12% syphilis |
| Silva-Santisteban et al. 2012A,13 | Lima, Peru | RDS/cross-sectional | 439 | – | 29.60 | – | Co-infection: 25.8% HIV/herpes simplex virus-2; 10.7% HIV/chronic syphilis; 3.7% HIV/recent syphilis other STIs: 22.9% chronic syphilis; 4.8% recent syphilis; 79.4% herpes simplex virus-2 |
| Verre et al. 201414 | Five cities in Peru | TLS/cross-sectional | 714 | – | 14.29 | 0.11% syphilis | |
| Castillo et al. 201515 | Lima, Peru | Convenience/RCT | 208 | – | 16.9 (baseline) | – | 20.2% rectal chlamydia; 6.8% pharyngeal chlamydia; 12.3% rectal gonorrhoea; 9.7% pharyngeal gonorrhoea; 80% herpes simplex virus-2 |
| Studies including data on transgender women only - Asia (n = 7) | |||||||
| Sahastrabuddhe et al. 201216 | Pune, India | TLS/cross-sectional | 84 | – | 45.20 | 13.4% penile discharges; 2.4% genital warts; 30.1% genital ulcers | 10.3% laboratory confirmed syphilis; 5.4% penile discharge on examination; 15.3% genital ulcers on examination; 10.3% genital warts on examination |
| Shrivastava et al. 2012A,17 | Mumbai, India | TLS/cross-sectional | 40 | – | 25 | – | Co-infection: 2.5% HIV/syphilis other STIs: 12.5% syphilis |
| Subramanian et al. 201318 | Tamil Nadu, India | Clinic/cross-sectional | 807 (404 in Round 1 and 403 in Round 2) | – | Round 1 12% and 9.8% in Round 2 | – | 16.6% syphilis prevalence Round 1 and 4.2% in Round 2 |
| Prabawanti et al. 201119 | Three cities, Indonesia | TLS/cross-sectional | 748 | – | 24.40 | – | 59.10% had one or more STI; 30.4% chlamydia; 29% rectal gonorrhoea; 26.8% syphilis; 47% gonorrhoea and/or chlamydia |
| Kaplan et al. 201520 | Beruit, Lebanon | Convenience/cross-sectional | 10 | 0 | – | 30% had an STI ever | – |
| Shaw et al. 201121 | Eight cities in Pakistan | Convenience/cross-sectional | 1162 | – | 0.60 | 6.5% had an STI in the past 6 months | – |
| Chariyalertsak et al. 201122 | Thailand | Time location/cross-sectional | 140 | 9.30 | – | 2.9% had an STI ever | – |
| Studies including data on transgender women only - Europe (n = 3) | |||||||
| van Veen et al. 201023 | Three cities in the Netherlands | Convenience/cross-sectional | 69 | – | 18.80 | 24% received STI treatment in the past 12 months | – |
| Dias et al. 201524 | Three cities in Portugal | Convenience/cross-sectional | 81 | 17.60% | – | 6.9% had an STI in the past 12 months | – |
| Turner et al. 201325 | London, United Kingdom | Clinic/retrospective | 13 | 0% | – | – | 7.7% chlamydia; 15.4% gonorrhoea; 7.7% genital warts |
Studies with co-infection of HIV and STIs reported.
Table 2. HIV and STIs in the USA among transgender men and transgender women (n = 8).
STI, sexually transmissible infection
| Authors (year) | Location | Setting and design | Sample size | HIV prevalence | STI type and prevalence | ||
|---|---|---|---|---|---|---|---|
| Self-report (%) | Laboratory test (%) | Self-report (%) | Exam or test | ||||
| Studies including data on transgender men and transgender women (n = 3) | |||||||
| Stephens et al. 201126 | San Francisco, CA | Clinic/retrospective | transgender women = 223 | – | 11.20 | In the past 12 months: 2.7 rectal chlamydia; 0 urogenital chlamydia; 4.9 pharyngeal chlamydia; 6.5 rectal gonorrhoea; 1.8 urogenital gonorrhoea; 9.0 pharyngeal gonorrhoea; 1.8 syphilis | Diagnosis at visit: 4.2% rectal chlamydia; 0% urogenital chlamydia; 2.1% pharyngeal chlamydia; 6.3% rectal gonorrhoea; 2% urogenital gonorrhoea; 3.5% pharyngeal gonorrhoea; 2.4% syphilis morbidity; 4.2% syphilis titre |
| transgender men = 69 | – | 10.10 | In past 12 months: 4.4 rectal chlamydia; 4.4 urogenital chlamydia; 1.5 pharyngeal chlamydia; 4.4 rectal gonorrhoea; 2.9 urogenital gonorrhoea; 8.7 pharyngeal gonorrhoea; 1.5 syphilis | Diagnosis at visit: 11.1% rectal chlamydia; 4.2% urogenital chlamydia; 2.44% pharyngeal chlamydia; 3.7% rectal gonorrhoea; 0% urogenital gonorrhoea; 4.9% pharyngeal gonorrhoea; 2.1% syphilis morbidity; 4.2% syphilis titre | |||
| Green et al. 201527 | San Diego, CA | Clinic/retrospective | transgender women = 151 | – | 1.99 | 13.2 any STI; 9.9 chlamydia; 7.3 gonorrhoea; 2.6 syphilis | – |
| transgender men = 30 | – | 3.33 | 13.3 any STI; 0 chlamydia; 13.3 gonorrhoea; 3.33 syphilis | – | |||
| Reisner et al. 201528 | Boston, MA | Clinic/retrospective | transgender women = 63 | – | 7.90 | – | 3.2% chlamydia; 2.1% gonorrhoea; 4.8% syphilis; 3.2% hepatitis C; 3.2% human papillomavirus; 2.1% herpes simplex virus |
| transgender men = 82 | – | 2.40 | – | 1.2% chlamydia; 0.0% gonorrhoea; 1.2% syphilis; 2.4% hepatitis C; 0.0% human papillomavirus; 1.2% herpes simplex virus | |||
| Studies including data on transgender men only (n = 1) | |||||||
| Reisner et al. 201029 | Boston, MA | Venue-based recruitment strategies (including the Internet) and snowball/chain referral sampling/cross-sectional | 16 | 0 | – | 81.3 ever had a STI test; 37.5 reported lifetime history of STI (18.8 herpes, 12.5 trichomonas, 6.3 bacterial vaginosis) | – |
| Studies including data on transgender women only (n = 4) | |||||||
| Taylor et al. 201130 | New York City, NY | Convenience/retrospective | 63 | 51 | – | 14.3 prior STI diagnosis in the past 3 months | – |
| Nuttbrock et al. 201331 | New York City, NY | Convenience/prospective | 591 (baseline); 230 (followed prospectively) | – | 40.1 (baseline) | – | At year 3 follow up: 1.1% chlamydia; 0.0% gonorrhoea; 1.8% syphilis; 5.5% hepatitis B |
| Operario et al. 201132 | San Francisco, CA | Convenience/retrospective | 174 | 41 | – | STI diagnosis or symptoms in the past 12 months 13.2 | – |
| Nemoto et al. 201533 | San Francisco and Oakland, CA | Convenience/cross-sectional | 217 | 46.50 | – | 27.2 history of an STI in the past 12 months | – |
Results
A total of 27 studies were included in our review, and only four reported rates of co-infection.8,12,13,17 All remaining studies reported HIV and STI data separately. Considering the geographic distribution of studies, 19 studies were conducted outside of the USA; all but one7 of these studies reported data on transgender women only. Within the USA, a total of eight studies were identified as reporting HIV and STI data; three studies included both transgender men and transgender women,26,27,28 one study focussed exclusively on transgender men,29 and four studies focussed on transgender women only.30–33
Transgender men
Of the five studies that provided HIV and STI data among transgender men, all except one were USA-based. The international study included data from 25 transgender men in a larger study of men who have sex with men (MSM) in Spain, Portugal, Latin America and the Caribbean.7 In this study, the self-reported HIV prevalence was 8%, and while there was no self-reported cases of chlamydia or syphilis, 3.85% of the transgender men self-reported cases gonorrhoea.7 Among the USA studies,26,27,29,34 sample sizes ranged from as low as 16 to a maximum of 82 participants. The prevalence of HIV ranged from 0 to 10.1%, and varied rates of different STIs were reported. Of note, all of the USA studies were conducted in urban centres of California or Massachusetts.
Transgender women
The only studies to report co-infection were conducted outside of the USA, and focussed exclusively on transgender women. Each of the studies reported co-infection based on laboratory tests for HIV and either physical examination or laboratory tests for STIs. The largest of the studies (n = 439) was conducted in Peru using respondent-driven sampling, and the most frequent co-infection was HIV/herpes simplex virus-2 (25.8%), followed by HIV–chronic syphilis (10.7%), and HIV–recent syphilis (3.7%).13 A cross-sectional study across seven cities in Argentina (n = 273) reported the most frequent co-infection as hepatitis b/syphilis (13.8%), followed by hepatitis B/HIV/syphilis (9.8%) and HIV–syphilis (9.4%).8 Another cross-sectional study conducted in Paraguay spanned five sites (n = 237), and 6% of the sample was co-infected with HIV–syphilis.12 The smallest study was carried out in Mumbai, India (n = 40), and reported that 2.5% of the sample was co-infected with HIV–syphilis.17 Additional rates of HIV and other STIs are included in Table 1. All remaining studies reported rates of HIV and STIs separately.
By region, the largest studies were conducted in Asia, with studies from Pakistan,21 India18 and Indonesia.19 The largest of the studies (n = 1162) was carried out across eight cities in Pakistan whereby 0.5% laboratory-tested HIV positive and 6.5% self-reported an STI in the past 6 months.21 The study in Tamil Nadu, India, consisted of two cross-sectional studies: the laboratory-tested HIV prevalence reduced from 12% in Study 1 (n = 404) to 9.8% in Study 2 (n = 403), and the syphilis prevalence reduced from 16.6% to 4.2%.18 The study from Indonesia covered three cities (n = 748) and 24.4% laboratory-tested HIV positive and exam or test confirmed the following rates of STIs: 30.4% for chlamydia, 29% for rectal gonorrhoea and 26.8% for syphilis.19 Additional studies from Asia included relatively small sample sizes (between 10 and 140 participants), and the HIV prevalence ranged from 0% (n = 10 participants) to 45.24% (n = 84).16,20,22 Two of the studies included only self-reported STIs,20,22 whereas the third included both self-reported and exam- or test-confirmed STIs.16
There were also several large studies (defined as a sample size of 200 or more) conducted in Latin and South America,8,12,9 with most data from Peru.13,14,15 The largest study included five cities in Peru (n = 714), where 14.29% tested HIV positive and 0.11% of the sample self-reported having syphilis.14 In addition to the rates of co-infection reported above, the study in Lima, Peru, found 29.6% of the sample laboratory-tested HIV positive, and either an exam or a test confirmed 22.9% of the sample had chronic syphilis and 4.8% had recent syphilis.13 The third study from Peru (n = 208) showed 17% of the sample was laboratory-tested HIV positive, and either an exam or a test confirmed the following rates of STIs: 20.2% rectal chlamydia, 6.8% pharyngeal chlamydia, 12.3% rectal gonorrhoea and 9.7% pharyngeal gonorrhoea.15 Two other large studies were conducted in Latin and South America. One study in Porto Alegre, Brazil (n = 284) showed that 25% of the sample was laboratory-tested HIV positive, and more than half (51.1%) self-reported a history of STIs.9 The other study reported that 34.1% of the sample laboratory-tested HIV positive and an exam or a test confirmed a prevalence of 5% for chlamydia and 50.4% for syphilis.8 Additional studies from Ecuador10 and Guatemala11 reported 4% and 16.8% of the samples laboratory-tested positive for HIV (respectively). The study from Ecuador reported 75.6% of the sample tested positive for an STI at screening, and the study from Guatemala reported no cases of syphilis. Finally, a multi-country study, including an online sample (n = 131) from Latin America and the Caribbean, Portugal and Spain, gave a self-reported prevalence of HIV (6.87%) and other STIs (1.56% chlamydia, 3.91% gonorrhoea, and 3.17% syphilis).7
All studies from Europe were small (between 13 and 81 participants) and used a mix of both self-report and laboratory-tested data to report HIV prevalence estimates ranging between 0%25 and 18.8%,23 an overall lower prevalence compared with the studies from Asia as well as Latin and South America. Only one study included STI rates based on laboratory or clinical examination,25 while the remaining three studies included self-reported STI prevalence.23,24
All of the USA-based studies were conducted in cities within California, New York or Massachusetts, and all but one study35 were carried out in a single city. The largest study (n = 591) was conducted in New York City (NYC) and at baseline, 40.1% tested positive for HIV and 40 new cases (17.4%) of HIV or STI were observed during the 3-year follow up.36 Two larger studies from San Francisco (n = 223)26 and San Diego (n = 151)27 reported 11.2% and 1.99% of the sample lab-tested positive for HIV respectively. The San Francisco-based study extensively documented both self-report and exam- or test-confirmed STIs,26 whereas the San Diego-based study recorded the following self-reported rates of STIs: 13.2% reporting having any STI, 9.9% chlamydia, 7.3% gonorrhoea and 26% syphilis.27 Additionally, two studies were conducted in one32 or two cities33 of California. The self-reported HIV prevalence was 41% and 46.5%, while 13.2% and 27.2% said either an exam or a test was used to confirm STI diagnosis in the past 12 months. Finally, two smaller studies (both samples were n = 63) were carried out in NYC30 and Boston.28 In the NYC-based study, self-reported HIV was 51%, and 14.3% of the sample reported prior STI diagnosis in the past 3 months.30 In the Boston-based study, 7.9% of the sample laboratory-tested HIV positive, and the following STIs were confirmed by an exam or a test: 3.2% chlamydia, 2.1% gonorrhoea and 4.8% syphilis.28
Discussion
In summary, this review found that studies across the world indicate a high prevalence of HIV and STIs in transgender people globally. In the few studies that describe HIV and STI co-infections, the prevalence ranged between 2.5%18 and 13.8%.8 Where comparative data were available, transgender populations faced higher rates of HIV and STIs compared with other groups, a finding consistent with the most recent systematic reviews and a meta-analyses of transgender health.1–4 For example, the latest systematic review1 reported HIV rates in the USA ranging from 2.0% and 40.1% among transgender women, whereas we found studies reporting rates between 1.99% and 46.50% among the same group. Further, the same systematic review reported that the HIV prevalence in the USA among transgender men was between 0.5% and 4.3% compared with 0% and 10.1% of transgender men in the present study. Similar to these studies, the substantial variation by location including high- versus low- and middle-income settings, subpopulation including transgender women versus transgender men, and reporting mechanisms such as self-report versus laboratory confirmed can complicate efforts to accurately contextualise these results. Until more studies investigate HIV, STIs and their co-infection, the understanding of determinants of these infections among transgender populations will remain limited.
Additionally, there were several methodological issues that undermine the data quality of this review. For example, there was limited data on the anatomy of transgender people included in these studies. Generally, studies were largely drawn from adult clinic samples based in urban communities. Even studies using techniques to limit the bias presented by clinic-based samples, including respondent-driven sampling, depended heavily on recruitment from non-governmental organisations (NGOs) providing HIV- and STI-related services. Further, sometimes there was limited data on structural drivers or a focus on behavioural drivers only, or both.
Ultimately, the review of existing studies highlights critical gaps in knowledge on HIV–STI co-infection prevalence, as well as limited data on their drivers. To address this, we draw on the studies reviewed, in addition to our collective years of research experience and implementation, to discuss how research can address the multilevel drivers of HIV and STI co-infection as they relate to individuals, programs and services, and laws and policies. Further, we discuss how a syndemic perspective is critical to understand the interaction between these levels. In so doing, we aim to inform how future research can more adequately and accurately assess and address the factors contributing to HIV and STI co-infection globally.
Areas for future research
Attention to transgender health has dramatically increased, especially with respect to HIV and STIs among transgender women globally. However, we did find that rates of HIV and STI co-infection were rarely reported in the research literature. Understanding the prevalence and distribution of STIs among people living with HIV is important, given increased HIV transmissibility with an active STI and the potential effect of STIs on viral load. Further, given the increased susceptibility to HIV acquisition as a result of having an STI, more research on co-infection, including STIs that are both symptomatic and asymptomatic, will inform efforts to more effectively intervene on HIV transmission risk dynamics. As momentum increasingly builds for dedicated studies on transgender individuals, there has also been a call to exclude transgender women from existing studies of cisgender MSM. While it is important to have more research focussed exclusively on transgender health, it is important also to highlight that doing so may inadvertently contribute to less data in the interim.
Individual-level drivers
Our understanding of HIV and STI co-infection would be greatly improved if the anatomical configuration of the transgender sample were specified in research. For example, physiologically, the different risks for HIV and STIs among transgender men who retain a vagina compared with those who do not remain unclear. Moreover, the different biological risks for HIV and STIs among transgender women who have had penile-inversion or sigmoid colon vaginoplasty also warrant further attention. Asking participants about their anatomical configuration inevitably raises a range of challenges. Specifically, thoughtful consideration must be given regarding how best to ask a transgender person questions about anatomy that may be inconsistent with their gender identity. This presents a key opportunity for meaningful participation of transgender individuals,37 from the conceptualisation through the implementation of research. Doing so helps ensure questions are framed appropriately, are gender-affirming, and gather empirically relevant and accurate information on HIV and STI transmissibility.
Further, there is growing recognition that transgender populations do not fit any simple characterisation and the desire for universal nomenclature may not capture the realities of local culture. While terms such as ‘transvestite’ and ‘transsexual’ may have negative connotations in some areas, the local context should inform the range of terms that participants themselves use to define a diversity of preferences and experiences. For example, in Brazil, the terms ‘transvestite’ and ‘transsexual’ are commonly used by the communities themselves and though fluid and dependent on context, ‘transvestite’ can relay differing degrees of presentation as a woman whereas ‘transsexual’ can relay different degrees of transitioning.38–40 Again, the participation of transgender individuals in the research process is critical,37 and only in so doing will research be capable of addressing how the risk of HIV and STIs differs or is the same across different gender identities and anatomical configurations.
Of note, including a broader range of gender identity terms will also complicate efforts to systematically review published data on the prevalence of HIV and STIs. For example, the use of MeSH terms acts as a thesaurus for related terms while searching biomedical literature. Currently, 31 terms are included under the MeSH term ‘transgender’, most of which reflect outdated terminology to help catalogue historical research. Looking forward, terminology around transgender health will likely expand to acknowledge and study a broader spectrum of gender identities, in addition to individuals who do not identify with a masculine or feminine binary. This means that research tools will also need to adapt and include a broader range of terms. Doing so will prove critical to fully understanding the scale of co-infection among gender diverse people globally.
Programmatic and service-level drivers
There are several ways research can better address programmatic and service-level drivers of HIV and STI risk. For example, it is important that data are representative of transgender individuals more broadly, not simply adults seeking services for ill health that may result in overestimating HIV and STI prevalence estimates. Making sure that studies are not limited to adult populations is necessary to inform early prevention and intervention efforts. Given existing data demonstrating that transgender adolescents experience additional health-related vulnerabilities due to bullying and other forms of enacted stigma,41,42 research is needed to understand the unique needs of young transgender populations. Efforts to collect more representative data need to expand beyond a limited number of major metropolitan areas and communities outside of frequently mapped venues where transgender people have been shown to congregate.43 While doing this can present challenges to reaching desired sample sizes, new technological approaches such as web-based or electronic data capture warrant integration and consideration to better reach a broader range of transgender people.44 Diversifying the transgender communities that are sampled in research studies, including those engaged and not engaged in care, will contribute to a more accurate understanding of HIV and STIs among transgender communities more broadly.
Additionally, the constellation of services provided should also be considered.4 For example, in Peru, given the exceptionally high prevalence of HIV among transgender women, almost all services are co-located with other infectious disease services such as tuberculosis (TB). This presents barriers to transgender individuals seeking care, fearing the potential to also contract TB, especially among those who are immunocompromised due to the stage of their HIV infection.45 So, while the integration of services may be beneficial in some circumstances, careful attention to (un) intended consequences should be considered.
Both HIV and STIs present a unique set of considerations for the provision of prevention and treatment services. In particular, the emergence of pre-exposure prophylaxis (PrEP), whereby adherence to a daily pill can significantly reduce the risk of HIV infection, has led to several new avenues of research. For example, studies report contrasting results regarding whether and how the potential biomedical protection from HIV affects behavioural prevention such as condom use and subsequent risk of STIs.46 Considering the unique needs of transgender populations, studies have raised issues regarding additional barriers to the uptake and adherence of PrEP.47,48 Specifically, the lack of trans-inclusive marketing of PrEP, prioritisation of hormone use, and mistrust of providers due to past healthcare experiences have been highlighted.48 Public health campaigns and providers have an ongoing responsibility to provide accurate messaging around the importance of continued condom use to prevent a range of STIs, even as the risk of HIV infection may be significantly reduced as a result of taking PrEP. Similar concerns persist about the potential for behavioural disinhibition following viral suppression, underscoring the need to continually revisit how biomedical strategies can be complemented by behavioural risk-reduction efforts. Specific to transgender populations, efforts are warranted to address unique concerns related to potential drug interactions between PrEP and transition-related hormones, alongside medical distrust based on a long history of negative interactions with providers and stigmatisation by the health system more broadly.
With respect to the high prevalence of STIs reported across studies, the use of periodic presumptive treatment (PPT) (e.g. intermittent antibiotic treatment without screening for STIs and often without any symptoms) as well as syndromic treatment (e.g. antibiotic treatment based on symptoms of STIs but without screening to confirm a specific infection) raise a host of issues. For example, many of the studies reviewed included a high proportion of sex workers;8,21,25,24 therefore, both PPT and syndromic treatment may be useful in places where testing for STIs is limited and/or may cost less than STI screening. However, there are also concerns that, in addition to the negative health consequences of long-term antibiotic use, PPT and syndromic treatment may contribute to the stereotype that all sex workers have STIs.49 Further, studies on the potential interaction between PPT and syndromic treatment with the use of antiretroviral therapy are needed,50 and the unique issues pertinent to transgender populations (e.g. potential pill burden with hormonal therapy) remain poorly understood.
Legal and policy-level drivers
Increasingly, there is attention to how laws, policies and practices, as well as cultural norms, can influence the health of transgender people.51 For instance, one study showed how a conservative social climate affected the ability of transgender people to access health services.52 The authors reported that healthcare providers living in a predominantly Republican voting state were more likely to refuse giving care to transgender individuals. Another study found that transgender people living in states with more gender protections reported fewer lifetime suicide attempts.53 In the USA, there has been varied success with passing laws and policies that protect the legal rights of transgender individuals with respect to their employment, housing and educational opportunities. Additionally, the resurgence of Religious Freedom Restoration Acts, which would make it legal for clinicians to deny healthcare services based on their religious beliefs, is of particular concern. Of note, despite challenging legal and policy environments (e.g. contexts with few protective laws for transgender individuals), our review highlights robust research on the health of transgender women in some parts of Asia,21,19 as well as in Latin and South America.8,9,12–15 This underscores the need for more south-to-south collaborations to learn how research can be successfully carried out in challenging legal and policy environments.
Across settings, due to the pervasive stigma and discrimination that transgender individuals face,54–56 there continues to be a range of structural barriers that specifically contribute to the risk for HIV and STIs.51,57 For example, even where protective laws and policies are in place, it can be extremely difficult for transgender people to secure employment. This economic instability can then increase the likelihood of engaging in sex work. Additional structural barriers, such as the inability to obtain legal documents reflecting one’s current gender identity, also limits transgender people’s ability to access available health services to prevent and/or treat STIs. For example, gender identity on national identity documents cannot be legally changed in Peru.45 Thus, transgender women often face discrimination when trying to access healthcare services, including HIV and STI screening and treatment; they may even be denied care altogether if the sex stated on their national identity document does not correspond to their current gender presentation.58 In summary, research continues to document the deleterious effect of stigma and discrimination,a and if not addressed, any progress towards improving access 6to much-needed HIV and STI prevention and treatment services may be negated for transgender people.
Syndemic effects among multilevel drivers
Increasing research is exploring the syndemic effects of negative health outcomes among transgender people, with particular attention to the disproportionately high rates of poor mental health and substance use and abuse.1 While data are emerging on the ways in which these factors affect sexual risk taking more broadly, more attention is needed to understand their specific effect on HIV/STI testing, diagnosis, and adherence to treatment. Further, although syndemics are often conceptualised at the individual-level, there is a need to integrate a multilevel framework in research to understand the syndemic dynamics driving HIV, STIs and their co-infection. For example, individual- (e.g. biological risk of HIV and STI transmission), programmatic- (e.g. models of HIV and STI service delivery) and policy-level (e.g. institutionalised stigma in healthcare settings) drivers synergistically interact to increase vulnerability of transgender people to poor HIV- and STI-related outcomes. Future epidemiological and interventional research will benefit from considering whether those syndemics that increase HIV, STI and co-infection risk differ for transgender people relative to other at-risk groups.
Conclusion
Where studied, HIV and STI prevalence is high among transgender individuals, particularly transgender women. Despite synergistic effects of co-infection, few studies have examined the prevalence of HIV–STI co-infections. Very little has been published on the multilevel drivers of HIV and STI infections in the transgender population globally. Understanding HIV, STIs and their co-infection in transgender people necessitates research that considers individual, programmatic and policy-level drivers simultaneously. Identifying transgender-specific determinants of HIV and STIs will guide future prevention, intervention and treatment efforts that are responsive to the diverse needs of transgender populations everywhere.
Footnotes
MacCarthy S, Reisner SL, Nunn A, Perez-Brumer A, Operario D. The Time Is Now: Attention Increases to Transgender Health in the United States but Scientific Knowledge Gaps Remain. LGBT Heath 2015. doi:10.1089/lgbt.2014.0073
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Poteat T, Scheim A, Xavier J, Reisner S, Baral S. Global epidemiology of HIV infection and related syndemics affecting transgender people. J Acquir Immune Defic Syndr. 2016;72(Suppl 3):S210–9. doi: 10.1097/QAI.0000000000001087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poteat T, Wirtz AL, Radix A, Borquez A, Silva-Santisteban A, Deutsch MB, et al. HIV risk and preventive interventions in transgender women sex workers. Lancet. 2015;385:274–86. doi: 10.1016/S0140-6736(14)60833-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baral S, Poteat T, Wirtz A, Stromdahl S, Beyrer C. Global burden of HIV infection among transgender persons: a systematic review and meta-analysis. J Int AIDS Soc. 2012;15:98–9. [Google Scholar]
- 4.Reisner SL, Poteat T, Keatley J, Cabral M, Mothopeng T, Dunham E, et al. Global health burden and needs of transgender populations: a review. Lancet. 2016;388:412–36. doi: 10.1016/S0140-6736(16)00684-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baral SD, Poteat T, Stromdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(3):214–22. doi: 10.1016/S1473-3099(12)70315-8. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. HIV/AIDS & STDs. 2016 Available online at: https://http://www.cdc.gov/std/hiv/default.htm [verified May 2017]
- 7.Reisner SL, Biello K, Rosenberger JG, Austin SB, Haneuse S, Perez-Brumer A, et al. Using a two-step method to measure transgender identity in Latin America/the Caribbean, Portugal, and Spain. Arch Sex Behav. 2014;43(8):1503–14. doi: 10.1007/s10508-014-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.dos Ramos Farías MS, Garcia MN, Reynaga E, Romero M, Vaulet ML, Fermepin MR, et al. First report on sexually transmitted infections among trans (male to female transvestites, transsexuals, or transgender) and male sex workers in Argentina: high HIV, HPV, HBV, and syphilis prevalence. Int J Infect Dis. 2011;15(9):e635–40. doi: 10.1016/j.ijid.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Costa AB, Fontanari AM, Jacinto MM, da Silva DC, Lorencetti EK, da Rosa Filho HT, et al. Population-based HIV prevalence and associated factors in male-to-female transsexuals from Southern Brazil. Arch Sex Behav. 2015;44(2):521–4. doi: 10.1007/s10508-014-0386-z. [DOI] [PubMed] [Google Scholar]
- 10.Solomon MM, Nureña CR, Tanur JM, Montoya O, Grant RM, McConnell J. Transactional sex and prevalence of STIs: a cross-sectional study of MSM and transwomen screened for an HIV prevention trial. Int J STD AIDS. 2015;26(12):879–86. doi: 10.1177/0956462414562049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lahuerta M, Sabido M, Giardina F, Hernandez G, Palacios JF, Ortiz R, et al. Comparison of users of an HIV/syphilis screening community-based mobile van and traditional voluntary counselling and testing sites in Guatemala. Sex Transm Infect. 2011;87(2):136–40. doi: 10.1136/sti.2010.043067. [DOI] [PubMed] [Google Scholar]
- 12.Aguayo N, Munoz SR, Aguilar G. P3. 35 HIV and SYPHILIS prevalence and behaviour, practises and attitudes of the TRANS population in Paraguay, 2011. Sex Transm Infect. 2013;89:A254. doi: 10.1136/sextrans-2013-051184.0788. [DOI] [Google Scholar]
- 13.Silva-Santisteban A, Raymond HF, Salazar X, Villayzan J, Leon S, McFarland W, et al. Understanding the HIV/AIDS epidemic in transgender women of Lima, Peru: results from a sero-epidemiologic study using respondent driven sampling. AIDS and Behavior. 2012;16(4):872–81. doi: 10.1007/s10461-011-0053-5. [DOI] [PubMed] [Google Scholar]
- 14.Verre MC, Peinado J, Segura ER, Clark J, Gonzales P, Benites C, et al. Socialization patterns and their associations with unprotected anal intercourse, HIV, and syphilis among high-risk men who have sex with men and transgender women in Peru. AIDS Behav. 2014;18(10):2030–9. doi: 10.1007/s10461-014-0787-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castillo R, Konda KA, Leon SR, Silva-Santisteban A, Salazar X, Klausner JD, et al. HIV and sexually transmitted infection incidence and associated risk factors among high-risk MSM and male-to-female transgender women in Lima, Peru. J Acquir Immune Defic Syndr. 2015;69(5):567–75. doi: 10.1097/QAI.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahastrabuddhe S, Gupta A, Stuart E, Godbole S, Ghate M, Sahay S, et al. Sexually transmitted infections and risk behaviors among transgender persons (Hijras) of Pune, India. J Acquir Immune Defic Syndr. 2012;59(1):72–8. doi: 10.1097/QAI.0b013e318236bd6f. [DOI] [PubMed] [Google Scholar]
- 17.Shrivastava SR, Bobhate PS. Prevalence of HIV and syphilis in patients attending sexually transmitted infections (STI) clinic in an Urban Slum. J Res Health Sci. 2012;12(1):7–14. [PubMed] [Google Scholar]
- 18.Subramanian T, Ramakrishnan L, Aridoss S, Goswami P, Kanguswami B, Shajan M, et al. Increasing condom use and declining STI prevalence in high-risk MSM and TGs: evaluation of a large-scale prevention program in Tamil Nadu, India. BMC Public Health. 2013;13:857. doi: 10.1186/1471-2458-13-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prabawanti C, Bollen L, Palupy R, Morineau G, Girault P, Mustikawati DE, et al. HIV, sexually transmitted infections, and sexual risk behavior among transgenders in Indonesia. AIDS and Behavior. 2011;15(3):663–73. doi: 10.1007/s10461-010-9790-0. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan RL, Wagner GJ, Nehme S, Aunon F, Khouri D, Mokhbat J. Forms of safety and their impact on health: an exploration of HIV/AIDS-related risk and resilience among trans women in Lebanon. Health Care Women Int. 2015;36(8):917–35. doi: 10.1080/07399332.2014.896012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw SY, Emmanuel F, Adrien A, Holte-Mckenzie M, Archibald CP, Sandstrom P, et al. The descriptive epidemiology of male sex workers in Pakistan: a biological and behavioural examination. Sex Transm Infect. 2011;87(1):73–80. doi: 10.1136/sti.2009.041335. [DOI] [PubMed] [Google Scholar]
- 22.Chariyalertsak S, Kosachunhanan N, Saokhieo P, Songsupa R, Wongthanee A, Chariyalertsak C, et al. HIV incidence, risk factors, and motivation for biomedical intervention among gay, bisexual men, and transgender persons in Northern Thailand. PLoS One. 2011;6(9):e24295. doi: 10.1371/journal.pone.0024295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Veen MG, Gotz HM, van Leeuwen PA, Prins M, van de Laar MJ. HIV and sexual risk behavior among commercial sex workers in the Netherlands. Arch Sex Behav. 2010;39(3):714–23. doi: 10.1007/s10508-008-9396-z. [DOI] [PubMed] [Google Scholar]
- 24.Dias S, Gama A, Fuertes R, Mendão L, Barros H. Risk-taking behaviours and HIV infection among sex workers in Portugal: Results from a cross-sectional survey. Sex Transm Infect. 2015;91(5):346–52. doi: 10.1136/sextrans-2014-051697. [DOI] [PubMed] [Google Scholar]
- 25.Turner R, Campbell M, Day S, Sullivan A. P2. 164 High STI rates in a nurse delivered outreach service for sex workers-SWISH clinic. Sex Transm Infect. 2013;89:A138. doi: 10.1136/sextrans-2013-051184.0428. [DOI] [Google Scholar]
- 26.Stephens SC, Bernstein KT, Philip SS. Male to female and female to male transgender persons have different sexual risk behaviors yet similar rates of STDs and HIV. AIDS and Behavior. 2011;15(3):683–6. doi: 10.1007/s10461-010-9773-1. [DOI] [PubMed] [Google Scholar]
- 27.Green N, Hoenigl M, Morris S, Little SJ. Risk behavior and sexually transmitted infections among transgender women and men undergoing community-based screening for acute and early HIV infection in San Diego. Medicine (Baltimore) 2015;94(41):e1830. doi: 10.1097/MD.0000000000001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reisner SL, Vetters R, White JM, Cohen EL, LeClerc M, Zaslow S, et al. Laboratory-confirmed HIV and sexually transmitted infection seropositivity and risk behavior among sexually active transgender patients at an adolescent and young adult urban community health center. AIDS Care. 2015;27(8):1031–6. doi: 10.1080/09540121.2015.1020750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reisner SL, Perkovich B, Mimiaga MJ. A mixed methods study of the sexual health needs of New England transmen who have sex with nontransgender men. AIDS Patient Care STDS. 2010;24(8):501–13. doi: 10.1089/apc.2010.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor RD, Bimbi DS, Joseph HA, Margolis AD, Parsons JT. Girlfriends: evaluation of an HIV-risk reduction intervention for adult transgender women. AIDS Education and Prevention. 2011;23(5):369–78. doi: 10.1521/aeap.2011.23.5.469. [DOI] [PubMed] [Google Scholar]
- 31.Nuttbrock L, Bockting W, Rosenblum A, Hwahng S, Mason M, Macri M, et al. Gender abuse, depressive symptoms, and HIV and other sexually transmitted infections among male-to-female transgender persons: a three-year prospective study. Am J Public Health. 2013;103(2):300–7. doi: 10.2105/AJPH.2011.300568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Operario D, Nemoto T, Iwamoto M, Moore T. Unprotected sexual behavior and HIV risk in the context of primary partnerships for transgender women. AIDS and Behavior. 2011;15(3):674–82. doi: 10.1007/s10461-010-9795-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemoto T, Cruz TM, Iwamoto M, Sakata M. A tale of two cities: access to care and services among African-American transgender women in Oakland and San Francisco. LGBT Health. 2015;2(3):235–42. doi: 10.1089/lgbt.2014.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reisner SL, White Hughto JM, Pardee D, Sevelius J. Syndemics and gender affirmation: HIV sexual risk in female-to-male trans masculine adults reporting sexual contact with cisgender males. International Journal of STD & AIDS. 2016;27(11):955–66. doi: 10.1177/0956462415602418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nemoto T, Operario D, Keatley J. Health and social services for male-to-female transgender persons of color in San Francisco. International Journal of Transgenderism. 2005;8(2/3):5–19. doi: 10.1300/J485v08n02_02. [DOI] [Google Scholar]
- 36.Nuttbrock L, Bockting W, Rosenblum A, Hwahng S, Mason M, Macri M, et al. Gender abuse and incident HIV/STI among transgender women in New York City: buffering effect of involvement in a transgender community. AIDS & Behavior. 2015;19(8):1446–53. doi: 10.1007/s10461-014-0977-7. [DOI] [PubMed] [Google Scholar]
- 37.Reisner S, Keatley J, Baral S. Transgender community voices: a participatory population perspective. Lancet. 2016;388(10042):327–30. doi: 10.1016/S0140-6736(16)30709-7. [DOI] [PubMed] [Google Scholar]
- 38.Dourado I, Silva LA, Magno L, Lopes M, Cerqueira C, Prates A, et al. Construindo pontes: a pratica da interdisciplinaridade. Estudo PopTrans: um estudo com travestis e mulheres transexuais em Salvador, Bahia, Brasil [Building bridges: interdisciplinarity in practice. PopTrans Study: a study with transvestites and transsexual women in Salvador, Bahia State, Brazil] Cad Saude Publica. 2016;32(9):e00180415. doi: 10.1590/0102-311x00181415. [DOI] [PubMed] [Google Scholar]
- 39.MacCarthy S, Reisner S, Hoffmann M, Perez-Brumer A, Silva-Santisteban A, Nunn A, et al. Mind the gap: implementation challenges break the link between HIV/AIDS research and practice. Cad Saude Publica. 2016;32(10):e00047715. doi: 10.1590/0102-311X00047715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grinsztejn B, Jalil EM, Monteiro L, Velasque L, Moreira RI, Garcia AC, et al. Unveiling of HIV dynamics among transgender women: a respondent-driven sampling study in Rio de Janeiro, Brazil. Lancet HIV. 2017;4(4):e169–76. doi: 10.1016/S2352-3018(17)30015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hwahng SJ, Nuttbrock L. Adolescent gender-related abuse, androphilia, and HIV risk among transfeminine people of color in New York City. J Homosex. 2014;61(5):691–713. doi: 10.1080/00918369.2014.870439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reisner SL, Greytak EA, Parsons JT, Ybarra ML. Gender minority social stress in adolescence: disparities in adolescent bullying and substance use by gender identity. Journal of Sex Research. 2015;52(3):243–56. doi: 10.1080/00224499.2014.886321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reisner SL, Deutsch MB, Bhasin S, Bockting W, Brown GR, Feldman J, et al. Advancing methods for US transgender health research. Curr Opin Endocrinol Diabetes Obes. 2016;23(2):198–207. doi: 10.1097/MED.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feldman J, Romine RS, Bockting WO. HIV risk behaviors in the U.S. transgender population: prevalence and predictors in a large internet sample. J Homosex. 2014;61(11):1558–88. doi: 10.1080/00918369.2014.944048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reisner SL, Perez-Brumer AG, McLean SA, Lama JR, Silva-Santisteban A, Huerta L, et al. Perceived barriers and facilitators to integrating HIV prevention and treatment with cross-sex hormone therapy for transgender women in Lima, Peru. AIDS Behav. 2017 doi: 10.1007/s10461-017-1768-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calabrese SK, Magnus M, Mayer K, Krakower D, Eldahan A, Hawkins L, et al. “Support Your Client at the Space That They’re in”: HIV pre-exposure prophylaxis (PrEP) prescribers’ perspectives on PrEP-related risk compensation. AIDS Patient Care STDS. 2017;31(4):196–204. doi: 10.1089/apc.2017.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deutsch MB, Glidden DV, Sevelius J, Keatley J, McMahan V, Guanira J, et al. HIV pre-exposure prophylaxis in transgender women: a subgroup analysis of the iPrEx trial. Lancet HIV. 2015;2(12):e512–9. doi: 10.1016/S2352-3018(15)00206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sevelius JM, Keatley J, Calma N, Arnold E. ‘I am not a man’: trans-specific barriers and facilitators to PrEP acceptability among transgender women. Glob Public Health. 2016;11(7–8):1060–75. doi: 10.1080/17441692.2016.1154085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Global Network of Sex Works Projects. Community guide: HIV and STI testing and treatment policies. Edinburgh, Scotland: NSWP; 2016. [Google Scholar]
- 50.Champredon D, Bellan S, Delva W, Hunt S, Shi C, Smieja M, et al. The effect of sexually transmitted co-infections on HIV viral load amongst individuals on antiretroviral therapy: a systematic review and meta-analysis. BMC Infect Dis. 2015;15:249. doi: 10.1186/s12879-015-0961-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winter S, Diamond M, Green J, Karasic D, Reed T, Whittle S, et al. Transgender people: health at the margins of society. Lancet. 2016;388(10042):390–400. doi: 10.1016/S0140-6736(16)00683-8. [DOI] [PubMed] [Google Scholar]
- 52.White Hughto JM, Murchison GR, Clark K, Pachankis JE, Reisner SL. Geographic and individual differences in healthcare access for U.S. transgender adults: a multilevel analysis. LGBT Health. 2016;3:424–433. doi: 10.1089/lgbt.2016.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perez-Brumer A, Hatzenbuehler ML, Oldenburg CE, Bockting W. Individual- and structural-level risk factors for suicide attempts among transgender adults. Behav Med. 2015;41(3):164–71. doi: 10.1080/08964289.2015.1028322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.James SE, Herman JL, Rankin S, Keisling M, Mottet L, Anafi M. The report of the 2015 U.S. transgender survey. Washington, DC: National Center for Transgender Equality; 2016. [Google Scholar]
- 55.Ghoshal N, Knight K. Rights in transition: making legal recognition for transgender people a global priority. USA: Human Rights Watch; 2016. [Google Scholar]
- 56.United Nations Human Rights Office of the High Commissioner. Annual report. Geneva: Human Rights Council; 2011. Discriminatory laws and practices and acts of violence against individuals based on their sexual orientation and gender identity. Report of the United Nations High Commissioner for Human Rights. Available online at: http://www.ohchr.org/Documents/Issues/Discrimination/A.HRC.19.41_English.pdf [verified 7 September 2017] [Google Scholar]
- 57.Winter S, Settle E, Wylie K, Reisner S, Cabral M, Knudson G, et al. Synergies in health and human rights: a call to action to improve transgender health. Lancet. 2016;388(10042):318–21. doi: 10.1016/S0140-6736(16)30653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perez-Brumer AG, Reisner SL, McLean SA, Silva-Santisteban A, Huerta L, Mayer KH, et al. Leveraging social capital: multilevel stigma, associated HIV vulnerabilities, and social resilience strategies among transgender women in Lima, Peru. J Int AIDS Soc. 2017;20(1):1–8. doi: 10.7448/IAS.20.1.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]