Abstract
The spinal cord dorsal horn is the first relay station of the neural network for processing somatosensory information. High-throughput recording methods facilitate the study of sensory coding in the cortex but have not been successfully applied to study spinal cord circuitry until recently. Here, we review the development of the in vivo two-photon spinal calcium imaging preparation and biological findings from the first systematic characterization of the spinal response to cutaneous thermal stimuli, focusing on the difference between the coding of heat and cold, and the contribution of different peripheral inputs to thermosensory response in the spinal cord. Future work combining this technology with genetic tools and animal models of chronic pain will further elucidate the role of each neuronal type in the spinal thermosensory coding and their plasticity under pathological condition.
Introduction
Neural basis of thermosensation
The thermosensory system safeguards our survival by detecting and interpreting a wide range of temperature. In mammals, environmental temperature is first converted into electrical impulses that are transmitted in the nervous system. Then, this information needs to be computed in the central nervous system, and this process ultimately generates the perception of temperature and evokes appropriate physiological and behavioral responses.
The first step of thermosensation occurs in the primary sensory neurons, whose cell bodies are located in the dorsal root ganglia (DRG). These neurons send their distal axons to the skin, where temperature is first detected. The proximal axons of these temperature-detecting DRG neurons target the dorsal horn of the spinal cord, where information is further processed. The past two decades have witnessed major breakthroughs in understanding this first step. Various molecules have been identified to convert thermal energy into membrane depolarization in sensory neurons. These molecules, mostly belongs to the Transient Receptor Potential (TRP) channel family, detect heat (TRPV1, Anoctamin 1, TRPV2, TRPM3, TRPM5, TRPA1), warmth (TRPV3, TRPV4, TRPM2) or cold (TRPM8, TRPA1, TRPC5) (Bautista et al., 2007; Caterina et al., 2000; Caterina et al., 1999; Caterina et al., 1997; Cho et al., 2012; Colburn et al., 2007; Dhaka et al., 2007; Guler et al., 2002; McKemy et al., 2002; Moqrich et al., 2005; Peier et al., 2002a; Peier et al., 2002b; Smith et al., 2002; Story et al., 2003; Talavera et al., 2005; Tan and McNaughton, 2016; Vandewauw et al., 2018; Vriens et al., 2011; Watanabe et al., 2002; Xu et al., 2002; Zimmermann et al., 2011). When characterized in heterologous expression systems, each TRP channel features a distinct activation threshold temperature and responds when temperature reaches above (for a heat detector) or below (for a cold detector) this point. These various receptors thus mediate the detection of a wide range of environmental temperature.
The characterization of molecular detectors of temperature has also advanced our understanding of the coding of cutaneous temperature by primary sensory neurons. Using electrophysiological recording, in vitro/in vivo calcium imaging, and analysis of expression profiles of TRP channels, accumulating data demonstrate that heat and cold, the two general temperature modalities, are detected by largely segregated populations of primary sensory neurons (as labeled lines) (Basbaum et al., 2009; Bautista et al., 2007; Emery et al., 2016; Julius, 2013; Knowlton et al., 2013; Ma, 2010; Peier et al., 2002a; Pogorzala et al., 2013; Yarmolinsky et al., 2016). Within the hot or cold ranges, there are evidences support that separated subsets of primary sensory neurons detect innocuous and noxious temperatures (Darian-Smith et al., 1979; Hallin et al., 1982; Handwerker and Kobal, 1993; Knowlton et al., 2013; Ma, 2010, 2012; Peier et al., 2002a; Pogorzala et al., 2013; Story et al., 2003; Torebjork et al., 1984).
In contrast to the relatively well-understood logic of temperature coding at periphery, how temperature is processed by the central nervous system remains elusive. One scenario is that the central nervous system simply inherits the labeled line coding at periphery. However, emerging evidence suggests that although specificity exists to some extent, neurons processing different thermosensory information crosstalk intensively (Craig et al., 2001; Duan et al., 2014; Hachisuka et al., 2016; McCoy et al., 2013; Ross et al., 2010; Zheng et al., 2010). The best known example is the thermal grill illusion, reported by Thunberg and Alrutz more than 120 years ago: when stimulating the skin with interlaced warm and cold tubing, the subject experiences burning pain (Alrutz, 1898; Craig and Bushnell, 1994; Thunberg, 1896). In this case, no hot stimulus exists in the physical world, but the perception of burning hot is “synthesized” in the nervous system by the cold and warm inputs. This phenomenon demonstrates that incoming primary sensory afferents must have interacted in the central nervous system, so that a new type of sensation could be formed without direct activation of the peripheral sensory fibers that normally mediate that type of sensation.
The dorsal horn of the spinal cord is the secondary relay station of cutaneous temperature, where initial processing of temperature information occurs. Neurons activated by temperature (as well as itch and pain) are concentrated in the most superficial laminae (laminae I – II) of the spinal dorsal horn but are also scattered in laminae V of the deep dorsal horn. The spinal dorsal horn has a complex circuit architecture (Abraira et al., 2017; Bourane et al., 2015; Cui et al., 2016; Duan et al., 2014; Hachisuka et al., 2016; Spike et al., 2003; Todd, 2010). In the spinal dorsal horn, less than 1% of neurons are projection neurons that innervate the brain; whereas vast majority of neurons are local interneurons (Spike et al., 2003; Todd, 2010). This composition indicates sophisticated local computation within the spinal dorsal horn. Unlike projection neurons that can be characterized and targeted based on the regions they innervate, the abundance of interneurons cannot be easily categorized and targeted by classical retrograde tracing strategy. Systematic efforts in classifying dorsal horn neurons using high throughput transcriptome analysis have not been done until very recently, which shows the dorsal horn neurons can be divided to as many as 30 distinct clusters, including 15 excitatory neuronal clusters and 15 inhibitory neuronal clusters (Haring et al., 2018). However, each cluster has been defined by the expression of a combination of multiple genes, which makes it challenging to further functional characterization of each cluster. Other criteria, such as neuron morphology and cellular electrophysiological property have also revealed the high diversity of dorsal horn neurons (Abraira et al., 2017; Cui et al., 2016; Duan et al., 2014; Todd, 2010). Moreover, classifying neurons using one criterion only show limited correlation with results using a different criterion, further indicating the great extent of neuronal diversity in the spinal dorsal horn. The complexity of the spinal dorsal horn poses significant challenges for dissecting the spinal circuitry for sensing temperature as well as other somatosensory modalities.
Methodological considerations for studying sensory coding in the spinal dorsal horn
Traditionally, temperature-responsive spinal neurons have been investigated by in vivo electrophysiology. These studies reveal the impressive functional diversity of dorsal horn neurons. Hellon and Misra report spinal neurons that respond to cold, heat, or both (Hellon and Misra, 1973). Among each of these subsets, some of the neurons also respond to mechanical stimuli. Adding to the complexity, neurons respond to temperature stimuli with distinct kinetics, including neurons only respond to the change in temperature, neurons respond statically to steady temperature, and neurons that respond to both. Many of their findings were later confirmed by other studies, some of which further characterized the distributions of neurons’ thermal activation thresholds and temperature-response relationships (Burton 1975; Bester et al., 2000). More recently, Craig and colleagues provided a relatively comprehensive examination of response to cutaneous temperature of spinothalamic neurons, a major output of the spinal dorsal horn in cats (Andrew and Craig, 2001; Craig et al., 2001). They functionally classify temperature-responsive spinothalamic neurons in the superficial laminae into four major classes, based on their response to (1) noxious mechanical and heat stimuli, (2) heat, cold, and mechanical stimuli, (3) cold only, and (4) warm only. Neurons within each subclass greatly differ by their activation threshold, temperature-response relationship, and response kinetics (Andrew and Craig, 2001; Craig et al., 2001).
Although results from in vivo electrophysiological recording provided the first glance of the how spinal neurons respond to cutaneous temperature change, the high extent of transcriptomic, projectomic, morphological, and physiological diversity of spinal neurons necessitates large numbers of neurons to be functionally sampled before sensory processing could be understood at the network level. In this regard, in vivo electrophysiology is significantly limited by its throughput. For example, out of 474 neurons Craig and colleagues recorded, only 10 neurons are warm-responsive (Andrew and Craig, 2001), making it infeasible to further analyzing their response properties in detail or to characterize them after loss-of-function manipulations. Besides the low throughput of in vivo electrophysiology, further dissection of the thermosensory circuitry requires one to understand the roles of distinct cell types in processing temperature information (Luo et al., 2008, 2018). In this regard, in vivo electrophysiology is limited in its capacity to correlate the response of a group of neurons with their gene expression and connectivity profiles, such that this group of neurons can be reliably targeted using genetic or circuit tracing tools.
Recently-developed in vivo calcium imaging tools allow neuronal activity to be probed by measuring intracellular calcium level, which can be detected by the emerging variety of calcium indicators. The calcium imaging technology has complemented electrophysiology and immediate early gene expression analysis in understanding the neural coding in many systems. Compared to electrophysiology and immediate early gene expression analysis, calcium imaging is advantageous in several ways. 1) Calcium imaging allows activities of many neurons to be recorded simultaneously. In this way, response to mild sensory stimuli, such as lukewarm temperature which normally does not activate many neurons (Andrew and Craig, 2001), can now be effectively captured and correctly classified (Ran et al., 2016). Similarly, when characterizing response in mutants with reduced responsiveness, calcium imaging allows responses from sufficient number of neurons to be recorded and compared with responses from wild type animals. This is particularly important for studying the spinal dorsal horn, given the high degree of heterogeneity of neurons (Abraira et al., 2017; Cui et al., 2016; Todd, 2010). 2) Calcium imaging also allows specific populations of defined neuronal cell types to be directly visualized and recorded. This includes both genetically-defined cell types using cell type-specific driver lines, as well as projection- or inputs-defined cell types using retrograde tracer and anterograde/retrograde trans-synaptic tracing tools (see Genetic dissection of spinal circuits), and functionally-defined cell types using genetic tools (Callaway and Luo, 2015; DeNardo and Luo, 2017; Lo and Anderson, 2011; Luo et al., 2018; Schwarz et al., 2015; Tasaka et al., 2018; Wertz et al., 2015; Wickersham et al., 2007; Zingg et al., 2017). 3) Calcium imaging allows correlating neurons response properties with their precise spatial locations, as neurons can be directly visualized in space. The high spatial resolution makes calcium imaging particularly suitable for investigating possible sensory maps (Chen et al., 2011; Ohki et al., 2005; Rothschild et al., 2010; Stettler and Axel, 2009). 4) Calcium imaging offers exquisite spatial resolution, which is essential when specifically analyzing the activity of dendrites and axons (Broussard et al., 2018; Jia et al., 2010; Petreanu et al., 2012; Sun et al., 2016; Vrontou et al., 2013). 5) Calcium imaging allows the same population of neurons to be recorded over multiple days to examine how chronic forms of plasticity, such as chronic pain, changes sensory processing.
While calcium imaging is advantageous in many respects, it is important to keep in mind the limitations of calcium imaging. 1) Light scattering limits the imaging depth in highly scattering tissue like the spinal cord. Using two-photon microscopy, structures ~1 mm below the cortical surface would be imaged (Theer et al., 2003). However, deep tissue imaging requires high excitation power, which in turn results in significant tissue heating (Podgorski and Ranganathan, 2016). This limits the practical imaging depth to the most superficial ~300 um, when a bright fluorophore (tdTomato) is used (Ran et al., Unpublished data). In vivo electrophysiology should be the method of choice when recording from neurons in the deep dorsal horn / ventral horn. 2) Calcium signal is an indirect measurement of neuronal activity and is slow in nature. While the temporal resolution of calcium signal usually does not limit the analysis of response kinetics to slow stimuli, such as temperature and chemicals, deconvolution methods may be necessary in other situations, such as characterizing the adaptation for mechanosensitive neurons (Pnevmatikakis et al., 2016; Vogelstein et al., 2010; Yaksi and Friedrich, 2006).
In vivo calcium imaging of temperature-evoked response in the spinal cord
We pioneered the development and use of in vivo calcium imaging to study the coding of cutaneous temperature in the spinal cord (Ran et al., 2016). We surgically exposed the dorsal surface of the lumbar spinal cord in anesthetized mouse via laminectomy and bulk-loaded the calcium dye Oregon Green BAPTA-1 AM (OGB) into the superficial laminae of the spinal cord. A pair of spinal clamps that fix the vertebrae were used to stabilize the preparation for imaging (Fig. 2A) (Johannssen and Helmchen, 2010; Ran et al., 2016). In order to visualize neurons that are located in the spinal cord of an intact mouse, we chose two-photon excitation microscopy, which uses two photons for each excitation of fluorophores. As a result, the microscopy allows infrared laser to be used for excitation. When compared to traditional laser scanning fluorescence microscopy, the longer wavelength of the infrared laser decreases scattering and background absorption, leading to an exponential increase in penetration depth in tissues. Besides the increased light penetration, two-photon microscopy also benefits from decreased background fluorescence, reduced phototoxicity, and reduced photobleaching. Using this platform, activities of hundreds of spinal neurons, located in the most superficial two to three laminae, could be simultaneously recorded. In a typical experimental setting, approximately 500 neurons can be identified in a field-of-view of 438 × 438 μm. Neurons located in deeper laminae can be visualized when labeled with brighter fluorophores, such as tdTomato, although these deeper dorsal horn neurons, which are presumably not temperature-sensitive, were not focused in the study (Ran et al., 2016).
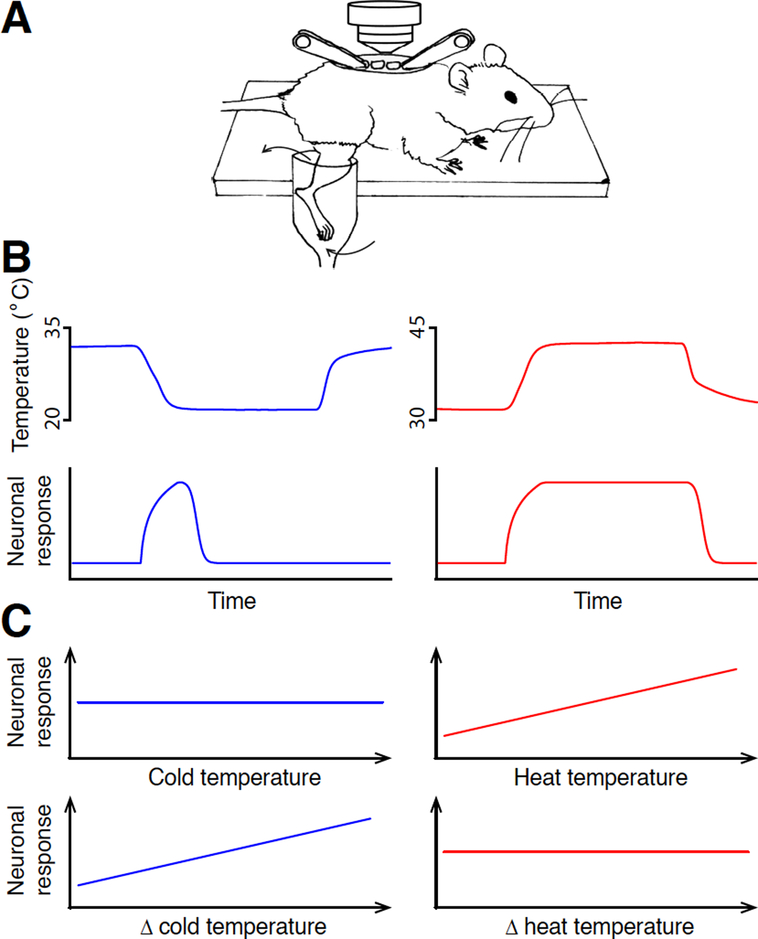
Figure 2 |. Different coding of heat and cold by spinal neurons.
A. A schematic illustrating the in vivo spinal cord calcium imaging preparation, adapted from Ran et al., 2016.
B. Cold-responsive spinal neurons signal change of temperature, whereas heat-responsive spinal neurons signal absolute temperature.
C. The response amplitude of a cold response correlates with the amplitude of temperature change, whereas the response amplitude of a heat response correlates with the absolute value of target temperature.
The high throughput offered by calcium imaging also possesses a technical challenge for delivering sensory stimuli that activate large number of spinal neurons: as the collective receptive field of these many neurons covers a large area of the skin, it necessitates a thermal stimulation system that could evenly and rapidly change the temperature of this large skin area. Previous in vivo electrophysiological experiments often first identify the exact receptive field of the recorded neuron, usually by electric stimulation (Andrew and Craig, 2001; Craig et al., 2001). Once the receptive field is identified, thermal stimulation is usually delivered by a Peltier device, a solid-state device that transfer heat from one side to the other when electric current flows through the device. The solid-state unit is not compatible with calcium imaging in mice, as it does not allow temperature to be evenly distributed across a large and curved body surface area. Thus, we engineered a stimulation delivery apparatus, which perfuses water at a high flow rate into a container that contains the mouse hind limb. The rate of temperature change can be precisely controlled by rapidly adjusting the temperature of the perfusate. Combining this stimulation delivery apparatus and our spinal calcium imaging platform, robust calcium responses to strong and mild cold, lukewarm and hot temperatures can be reliably recorded.
When we recorded neuronal responses to heat and cold, we made an unexpected finding that (1) spinal neurons use distinct logic for coding heat and cold stimuli. The initial observation is that when the stimulation temperature ramps up or down during the heat/cold stimulation, the responses to heat usually correlate with the absolute temperature of the stimulation. By contrast, although cold responses are only elicited after temperature drop passes a threshold, most responses to cold peak quickly after stimulation onset, well before the stimulation temperature reaches the target cold temperature. The peak time coincides with the period when temperature drops most rapidly. While similar rapid adaptive responses to thermal stimuli have been documented by in vivo electrophysiological recordings previously, the limited throughput of in vivo electrophysiology precluded the kinetics of large numbers of neurons from being analyzed quantitatively. More importantly, a fundamental question regarding the fast response to cold persists: is the rapid adaption a function of the cellular physiological property of the neuron, or is it a faithful code for temperature change? Namely, if one slowly drops the temperature at a constant rate, would neurons fire persistently? To temporally dissociate the change of temperature from the absolute temperature, we used a two-stage temperature stimulus. During Stage One. The stimuli were delivered at different temperature changing rates before reaching the target temperature, then the temperature was maintained at its target for an extended period of time during Stage Two. We discovered that heat responses behave like simple thermometers and correlate with the absolute temperature. By contrast, cold responses usually peak within the temperature change stage and rapidly desensitized when the absolute temperature is cold but is not changing (Fig. 2B). This conclusion is further supported by the fact that the amplitudes of cold responses are similar when temperature drops a certain amount of degrees, regardless of the absolute temperature. By comparison, heat responses are similar when temperature reaches the same target value, regardless of the extent of temperature change (Fig. 2C). Together, these results indicate that neurons signal temperature change for cold and absolute temperature for heat.
How do the spinal neurons use these two different coding strategies for heat and cold? This difference may either be inherited from the DRG neurons or be generated at the spinal level. While a definitive answer to this question is yet to be provided, recent calcium imaging studies of the primary thermosensory neurons in the DRG and the trigeminal ganglia have examined the response kinetics to temperature stimuli in detail (Wang et al., 2018a; Yarmolinsky et al., 2016). The temperature-evoked calcium responses in primary sensory neurons somewhat resemble our spinal imaging data: heat responses largely reflect the absolute temperature, and most cold responses adapt rapidly. These similarities in response kinetics between the spinal neurons and primary afferents likely suggest that the spinal neurons adopt their coding from their inputs. Nevertheless, when comparing the response kinetics between temperature-responsive spinal neurons and primary sensory neurons, we noticed considerable differences. For example, as target stimulation temperature decreases, responses of the same trigeminal neuron become drastically more transient, whereas response kinetics of a given spinal neuron is not significantly changed by target temperature (Yarmolinsky et al., 2016). A small subset of trigeminal neurons showed bimodal response to both the onset of temperature drop and to the absolute cold temperature as temperature decreases during stimulation (Yarmolinsky et al., 2016). These data indicate that additional signal transformation at the spinal level does exist, although differences in stimulation methods and calcium indicators may explain some of the discrepancies (Ran et al., 2016; Yarmolinsky et al., 2016). Further investigation will shed light on these intriguing questions.
Besides the unexpected findings of the different coding strategy for heat and cold, our novel spinal imaging system allowed us to conduct the first systematic examination regarding neural coding of cutaneous temperature in the spinal cord and to make a series of other discoveries.
(2). Broadly- and narrowly-tuned neurons in the spinal cord.
We confirmed previous findings that the superficial dorsal horn contains heat-only, cold-only neurons, as well as dually-tuned neurons that respond to both cold and heat. The proportion of dually-tuned neurons increases to stronger temperature stimulation (Ran et al., 2016). The substantial number of dually-tuned neurons at the spinal level is in sharp contrast with the largely segregated ensembles primary sensory neurons that are dedicated to detect heat and cold (Emery et al., 2016; Ran et al., 2016; Wang et al., 2018a; Yarmolinsky et al., 2016). For example, only less than 5% of all the thermosensory neurons in the trigeminal ganglion respond to both strong heat (51 °C) and cold temperatures (4 °C) (Yarmolinsky et al., 2016). By contrast, roughly half of the thermosensory spinal neurons are dually-tuned to respond to similar temperatures (50 °C and 5 °C, respectively) (Ran et al., 2016). It is likely that most of the dually-tuned spinal neurons receive convergence inputs from heat- and cold-sensitive primary sensory neurons (Ran et al., 2016). The heat-only, cold-only and dually-tuned neurons are generally intermingled in the superficial dorsal horn, although the proportion of heat-responsive neurons increases when going deeper into the dorsal horn (Ran et al., 2016), consistent with the innervation pattern of TRPM8- and TRPV1-expressing DRG neurons (Bautista et al., 2007).
(3). Population-intensity codes of temperature gradient.
The activation threshold temperature of both heat- and cold-responsive neurons are fairly evenly distributed across a wide temperature range, rather than being clustered around the activation thresholds of thermosensitive TRP channels. This implies that the sensation of temperature modalities that has distinct motivational valences (such as warmth and heat) may be encoded by the population of spinal neurons as a whole, rather than recruiting distinct populations of neurons as dedicated labeled lines for noxious and innocuous temperatures. Note that the temperature-response relationships on the heat and cold ranges are different. The steep increase in the number of neurons activated as heat temperature increases may be significant in differentiating the lukewarm temperature that is attractive to most animals from noxious heat (Ran, 2017).
(4). Different input-contribution to heat and cold responses.
Most of the heat-responsive spinal neurons, regardless of their activation threshold temperature, predominantly receive inputs from TRPV1-expressing DRG neurons. This is consistent with previous data that demonstrate the essential role of TRPV1-expressing neuron in sensing noxious heat (Mishra et al., 2011), but also suggest a novel role of these neurons in detecting warmth (see below) (Caterina et al., 2000; Yarmolinsky et al., 2016). By contrast, cold-responsive spinal neurons receive inputs from two populations of DRG neurons. Those neurons that are activated at mild cold temperatures (higher than 26 °C) almost solely receive inputs from TRPM8-expressing DRG neurons, whereas neurons get activated by strong cold temperatures (lower than 26 °C) largely receive cold information from TRPV1-expressing DRG neurons (Knowlton et al., 2013; Pogorzala et al., 2013). Together, these results suggest TRPV1-expressing DRG neurons may serve as a common pathway for thermal nociception. Meanwhile, the TRPV1- and TRPM8-expressing neurons appear to have lower activation thresholds in vivo than those characterized in heterologous systems or dissociated DRG neurons (Bautista et al., 2007; Caterina et al., 1997). It is worth noticing that the sensitivity of TRPV1-expressing DRG neurons to strong cold cannot be attributed to TRPA1, a channel previously indicated to detect noxious cold that is exclusively expressed by TRPV1-expressing neurons (Karashima et al., 2009; Story et al., 2003), as TRPA1 knockout animals show normal cold sensitivity (Ran et al., 2016). This is further supported by evidence that TRPA1-expressing primary afferents do not respond to cold (Yarmolinsky et al., 2016). What molecular transducer confers cold sensitivity in TRPV1-expressing neurons is an intriguing question that requires further investigation.
(5). The sensation of warmth.
Compared to our understanding of the molecular mechanisms of sensing heat and cold, the neural basis of warmth sensation is relatively unclear. Several candidate receptors, such as TRPV1, TRPV3, TRPV4, TRPM2, TRPM4, TRPM5, have been implied in warmth sensation (Chung et al., 2004; Moqrich et al., 2005; Talavera et al., 2005; Tominaga and Caterina, 2004; Xu et al., 2002; Yarmolinsky et al., 2016). However, the role of these receptors is still a matter of debate in the field (Huang et al., 2011). This is largely due to the lack of experimental assay in assessing the contributions of loss-of-function mutants: a lukewarm temperature does not trigger nocifensive behavior, the two-plate temperature preference behavioral test involves a second confounding temperature, and warmth-responsive neurons are rare and cannot be reliably captured by in vivo electrophysiology (Andrew and Craig, 2001). We previously reported that after genetic ablation of TRPV1-expressing DRG neurons, spinal response to warmth is impaired (Ran et al., 2016). The finding indicates that TRPV1-expressing DRG neurons, while previously considered as heat nociceptors, are also necessary for the sensation of warmth.
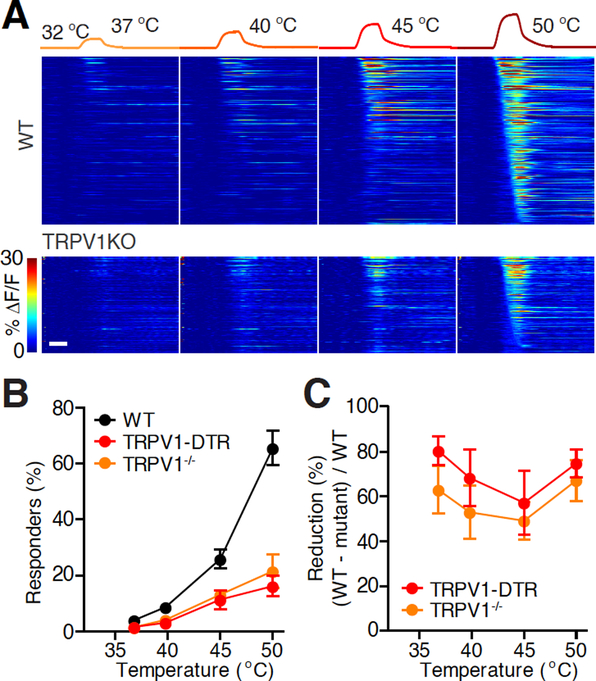
Importantly, is the warmth sensitivity of TRPV1-expressing DRG neurons conferred by TRPV1 channel itself or other receptors in these neurons? Here we show that knocking out TRPV1 receptor significantly impaired spinal responses to warmth (Fig. 3). Response to warm temperatures (37 °C and 40 °C) was reduced by 63% and 53% in TRPV1−/− mice (Caterina et al., 2000), respectively, compared to WT. In fact, spinal response to warm and hot temperatures in TRPV1−/− mice largely resembled response in mice in which TRPV1-expressing DRG neurons were genetically ablated (Ran et al., 2016). This similarity indicates that heat sensitivity of TRPV1-expressive neurons is predominantly contributed by TRPV1 channel. Our current result, together with another recent imaging study of primary sensory neurons (Yarmolinsky et al., 2016), established the essential role of TRPV1 receptor in sensing warmth.
Figure 3 |. Role of TRPV1 channel and TRPV1-expressing DRG neurons in sensing warmth.
A. Heat maps of the activities of all heat-responding neurons in representative fields-of-view (FOVs) in an example wild type (WT, 396 neurons) and TRPV1−/− (227 neurons) mouse. Neurons were considered responders when the maximum ΔF/F of each individual trial exceeded 5% and 2.5 times of σ0 (s.d. of the prestimulus baseline) above F0 of each individual trial, and the maximum ΔF/F of the averaged and smoothed response exceeded 5% and 3 times σ0 above F0 of the response averaged from all the trials of the same stimulus. From detailed methods, see Ran et al., 2016. Scale bar, 10 s.
B. Temperature-response relationship in WT and TRPV1−/− mice. (Black: WT, n = 10 mice; orange: TRPV1−/−, n = 4 mice; red: TRPV1-DTR, n = 9 mice. WT and TRPV1-DTR data are from Ran et al., 2016. Mann-Whitney test between TRPV1−/− and WT, P = 0.053, P = 0.024, P = 0.008, P = 0.004 for the four temperatures, respectively.)
C. The percentage of reduction (calculate from B) of heat-responding neurons in TRPV1−/− mice compared to WT.
Interestingly, ablating TRPM8-expressing cold-sensitive DRG neurons actually increases the response to a warm temperature, to the extent that the response to warmth resembles response to a hot temperature (Ran et al., 2016). These data indicate that normal sensation of warmth requires coordinated activities from the cold- and heat-sensitive inputs. It is interesting to note that genetic ablation of peptidergic nociceptors, which partially overlaps with heat-sensitive DRG neurons, enhances the sensitivity of some spinal neuron to cold (McCoy et al., 2013). Together, these results may suggest common crossover antagonism between the heat and cold circuits, a logic that is shared by the thermosensory circuit in Drosophila (Liu et al., 2015).
Future perspective
Our previous research is among the first in using in vivo spinal cord calcium imaging to study coding of somatosensory stimuli. While it provides a comprehensive analysis of the coding of cutaneous temperature of the spinal cord, it also illustrates the power of the functional imaging system in studying sensory coding in the spinal cord.
Technical considerations
In vivo calcium imaging is a rapidly-evolving field. New fluorescent calcium indicators and new imaging instrumentation emerge constantly. In our previous research, the calcium dye OGB was chosen over genetically-encoded calcium indicators to avoid tissue inflammation that develops after local viral injection (which likely alters the properties of sensory neurons) (Ran et al., 2016). Meanwhile, the relatively bright baseline fluorescence of OGB (compared to GCaMP6) and the fact that GCaMP imaging is more prone to neuropil contamination are additional motivations for the choice. Other researchers chose the fluorescence resonance energy transfer (FRET)-based calcium indicator protein, Yellow Cameleon, for its insensitivity to motion artifacts (Nishida et al., 2014). Labeling was achieved by in utero electroporation, which avoids tissue inflammation after local injection in adult animals (Nishida et al., 2014).
Over the past two years since our first study was published, novel tools and methods have emerged, which provide additional options to analyze spinal calcium signals in response to somatic stimuli. Using even longer wavelength, three-photon imaging further increases achievable imaging depth (Ouzounov et al., 2017; Wang et al., 2018b). Adaptive optics corrects optical aberration and improves imaging performance in deep tissue (Wang et al., 2015). Miniature two-photon microscope allows deep tissue imaging in freely behaving animals (Zong et al., 2017). Newer versions of GCaMP such as jGCaMP7b, offers brighter baseline fluorescence (Dana et al., 2018). Red-shifted calcium indicator with high signal-to-noise ratio permits calcium imaging of neurons located in deeper spinal laminae (Dana et al., 2016). Soma-targeting GCaMP variants constrain the localization of GCaMP to the soma and proximal dendrites, whereas axon-targeting GCaMP is optimized for measuring afferent activity (Broussard et al., 2018). Newly-engineered adeno-associated viruses that allow efficient delivery of GCaMP to the spinal cord by systemic injection can avoid local inflammation that occurs after spinal injection (Chan et al., 2017; Deverman et al., 2016). Recently-developed data analysis pipelines allow better signal segregation and neuropil decontamination (Keemink et al., 2018; Pachitariu et al., 2017; Pnevmatikakis et al., 2016). These novel imaging tools and methods can be integrated into the current imaging system to examine biological processes that we were unable to investigate before.
Genetic dissection of spinal circuits
How will in vivo spinal imaging further advance our understanding of somatosensory circuitry of the spinal cord? Modern neural circuit dissection often first utilizes genetic methods to target individual neuronal types, and additional tools can then be brought in to trace the anatomical connections of these neuronal types or to manipulate their activities to establish their roles in circuit function. Over the past few years, research in the field has characterized the roles of a number of neuronal types in somatosensation (Abraira et al., 2017; Bourane et al., 2015; Christensen et al., 2016; Cui et al., 2016; Duan et al., 2014; Foster et al., 2015; Francois et al., 2017; Lu et al., 2013; Peirs et al., 2015; Petitjean et al., 2015). All of these studies use mouse genetics to target specific populations of neuronal types defined by their endogenous gene expression. Recent single-cell RNA sequencing studies have established a comprehensive atlas of dorsal horn neuronal types (Haring et al., 2018; Sathyamurthy et al., 2018). One study tried to correlate the cell types identified by single-cell RNA sequencing with immediate early gene (IEG) expression induced by noxious cold and heat stimuli using in situ hybridization (Haring et al., 2018). Functional manipulation of those neuronal types has provided important understanding into the organization of spinal somatosensory circuits. In vivo calcium imaging, compared to IEG expression analysis, offers much better throughput, multiplexity, and temporal resolution (the high temporal resolution of calcium imaging is particularly critical in differentiating IEG expression induced by heating and the cooling stage when temperature returns to baseline after heating) (Ran et al., 2016; Wang et al., 2018a). The arsenal of cell type-specific reporter transgenic lines, the ever-growing collection of cell type-specific driver mouse lines, and their corresponding reporter mouse lines (including intersectional reporters) offer a great tool box to genetically dissect each individual component in the spinal circuitry (Daigle et al., 2018; Luo et al., 2008, 2018; Madisen et al., 2015; Madisen et al., 2010). Besides genetic access to neuronal cell types defined by endogenous gene expression, recent tools that target functionally-, projection- or inputs-defined cell types have emerged. Retrograde tracers and the recently-developed anterograde trans-synaptic tracing tools can label specific populations of neurons defined by their projection targets and their inputs, respectively (Callaway and Luo, 2015; Lo and Anderson, 2011; Luo et al., 2018; Miyamichi et al., 2013; Schwarz et al., 2015; Zingg et al., 2017). Activity-driven targeting tools, mostly based on immediate early gene, allow genetic access of functionally-defined cell types (DeNardo and Luo, 2017; Luo et al., 2018). These mouse genetic and viral tracing tools can be readily combined with the spinal cord calcium imaging system to examine the responsive properties of each genetically- or anatomically-defined neuronal subtype. Moreover, optogenetic or pharmacogenetic activation/silencing of specific components of the spinal circuits can be combined with calcium imaging to establish the causal role of these spinal cell types in sensory processing.
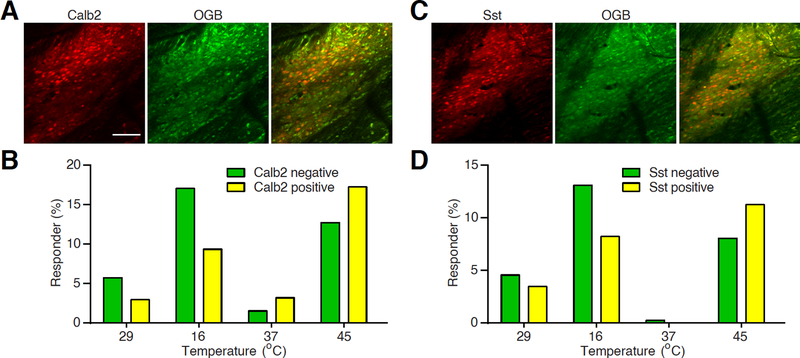
Somatostatin (Sst) and calbindin 2/calretinin (Calb2) lineages neurons are two major populations of excitatory neurons in the spinal dorsal horn (Duan et al., 2014). Together, these neurons compose 11 of 15 dorsal horn excitatory subtypes classified in the recent single cell RNA sequencing study (Haring et al., 2018). Genetic ablation of Sst lineage neurons results in profound loss of mechanical sensitivity, whereas ablation of Calb2+ neurons also impairs heat nociception, measured by affective licking behavior at high heat (54 °C) but not intermediate heat (50 °C) (Duan et al., 2014). The role of Sst+ resembles the nociceptive transmission neurons in the gate control theory of mechanical pain (Duan et al., 2014; Melzack and Wall, 1965). However, controversy arises as acute inhibition of Sst+ neurons using pharmacogenetic approach leads to deficits in both mechano- and thermosensitivity (Christensen et al., 2016), arguing possible long-term compensatory mechanism in mediating thermal nociception after neuronal ablation. To investigate the role of Sst+ and Calb2+ neurons in processing thermal stimuli under a natural, unperturbed condition, we set out to examine the responses of these neurons to thermal stimuli using our spinal imaging platform. We cross Sst-cre and Calb2-cre with a Cre-dependent tdTomato allele and compared the response profiles of tdTomato+ and tdTomato− neurons within the same mice (Fig. 4A and 4C) (Madisen et al., 2010; Taniguchi et al., 2011). Both Sst+ and Calb2+ neurons preferentially responded to heat stimuli. Interestingly, significantly lower proportions of Sst+ and Calb2+ neurons responded to cold stimuli (Fig. 4B and 4D). Our current data are consistent with recent studies showing the involvement of this population of neurons in mediating thermal stimuli, while the superior temporal and single cell resolution offered by calcium imaging provides quantitative information regarding thermal responsiveness of each cell type (Christensen et al., 2016; Haring et al., 2018). It is worth noticing that both Sst and Calb2 lineages consist of heterogeneous sub-populations of neurons (Haring et al., 2018). Imaging the response of each neuronal subtype or activating/silencing each type while examining its effect on other neurons’ responses to temperature change will establish the functional roles of each neuronal type in processing thermosensory information.
Figure 4 |. Responses of spinal Sst+ and Calb2+ neurons to thermal stimuli.
A. Example two-photon images of OGB-labeled Calb2+ and Calb2− neurons. Scale bar, 100 μm.
B. A histogram showing the percentage of Calb2+ and Calb2− neurons that respond to corresponding temperature stimuli.
C. Example two-photon images of OGB-labeled Sst+ and Sst− neurons.
D. A histogram showing the percentage of Sst+ and Sst− neurons that respond to corresponding temperature stimuli.
Thermosensory coding under pathological conditions
The thermosensory circuit is plastic. After injury, the hypersensitive thermosensory circuit protects animals from further tissue damage. Various types of injury may alter the circuit in distinct ways, so that the animal is alerted that a particular type of damaging stimulus is imminent. For example, inflammatory pain is characterized by elevated sensitivity to heat, whereas neuropathic pain and ciguatera intoxication are usually accompanied by cold allodynia (Bagnis et al., 1979; Huang et al., 2006; Jorum et al., 2003). The spinal cord calcium imaging system can be combined with animal models of pain to investigate the change in patterned neuronal activities, with or without somatic stimuli (Prescott et al., 2014). The ability to image the same populations of neurons before and after injury enables researchers to ask if neuronal populations change their response to temperature stimuli in a universal manner, or if distinct functionally-defined neuronal ensembles change their responses differently (Ran et al., unpublished data). Genetic and pharmacological manipulations can be combined with chronic pain models and calcium imaging, such that changes in response of each genetically-defined neuronal type can be characterized, and mechanisms underlying the plasticity under chronic pain can be investigated (Cichon et al., 2017). Such efforts will greatly advance our understanding of the thermosensory circuit and the mechanisms of pain.
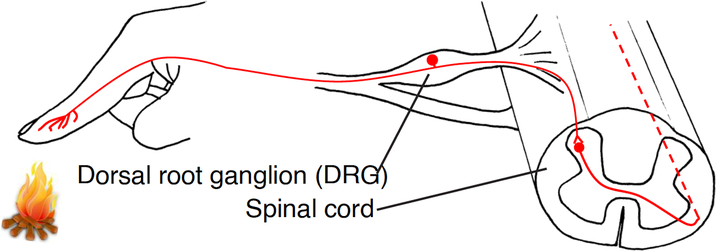
Figure 1 |. The neural circuitry for sensing cutaneous temperature.
Cutaneous temperature is first detected by the free nerve endings in the skin. The cell bodies of these neurons are located in the dorsal root ganglia. These neurons synapse onto the dorsal horn neurons in the spinal cord. Thermal information is processed in the dorsal horn of the spinal cord before being transmitted into the brain.
Highlights.
Ca2+ imaging is a novel platform to study sensory coding in the spinal cord.
Spinal Ca2+ imaging reveals the coding logic of temperature sensation.
Warmth sensation requires TRPV1 channel and TRPM8-expressing DRG neurons.
Temperature stimuli-evoked responses in genetically-defined spinal cell types.
Ca2+ imaging is suited for genetic neural circuit dissection and chronic pain study.
Acknowledgements
This work was supported by grants from Firmenich Next Generation Fund, Terman Fellowship, NIH grant NS101407, and start-up funding from Stanford University (to X.K.C).
References
- Abraira VE, Kuehn ED, Chirila AM, Springel MW, Toliver AA, Zimmerman AL, Orefice LL, Boyle KA, Bai L, Song BJ, et al. (2017). The Cellular and Synaptic Architecture of the Mechanosensory Dorsal Horn. Cell 168, 295–310 e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alrutz S (1898). On the temperature senses: II. The sensation of ‘hot’. Mind 7, 140–144. [Google Scholar]
- Andrew D, and Craig AD (2001). Spinothalamic lamina I neurones selectively responsive to cutaneous warming in cats. J Physiol 537, 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnis R, Kuberski T, and Laugier S (1979). Clinical observations on 3,009 cases of ciguatera (fish poisoning) in the South Pacific. The American journal of tropical medicine and hygiene 28, 1067–1073. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, and Julius D (2009). Cellular and molecular mechanisms of pain. Cell 139, 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, and Julius D (2007). The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, 204–208. [DOI] [PubMed] [Google Scholar]
- Bourane S, Duan B, Koch SC, Dalet A, Britz O, Garcia-Campmany L, Kim E, Cheng L, Ghosh A, Ma Q, et al. (2015). Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science 350, 550–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard GJ, Liang Y, Fridman M, Unger EK, Meng G, Xiao X, Ji N, Petreanu L, and Tian L (2018). In vivo measurement of afferent activity with axon-specific calcium imaging. Nature neuroscience 21, 1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM, and Luo L (2015). Monosynaptic Circuit Tracing with Glycoprotein-Deleted Rabies Viruses. The Journal of neuroscience : the official journal of the Society for Neuroscience 35, 8979–8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, and Julius D (2000). Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306–313. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, and Julius D (1999). A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398, 436–441. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, and Julius D (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824. [DOI] [PubMed] [Google Scholar]
- Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu WL, Sanchez-Guardado L, Lois C, Mazmanian SK, Deverman BE, et al. (2017). Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nature neuroscience 20, 1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gabitto M, Peng Y, Ryba NJ, and Zuker CS (2011). A gustotopic map of taste qualities in the mammalian brain. Science 333, 1262–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Yang YD, Lee J, Lee B, Kim T, Jang Y, Back SK, Na HS, Harfe BD, Wang F, et al. (2012). The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nature neuroscience 15, 1015–1021. [DOI] [PubMed] [Google Scholar]
- Christensen AJ, Iyer SM, Francois A, Vyas S, Ramakrishnan C, Vesuna S, Deisseroth K, Scherrer G, and Delp SL (2016). In Vivo Interrogation of Spinal Mechanosensory Circuits. Cell reports 17, 1699–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MK, Lee H, Mizuno A, Suzuki M, and Caterina MJ (2004). TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. The Journal of biological chemistry 279, 21569–21575. [DOI] [PubMed] [Google Scholar]
- Cichon J, Blanck TJJ, Gan WB, and Yang G (2017). Activation of cortical somatostatin interneurons prevents the development of neuropathic pain. Nature neuroscience 20, 1122–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn RW, Lubin ML, Stone DJ Jr., Wang Y, Lawrence D, D’Andrea MR, Brandt MR, Liu Y, Flores CM, and Qin N (2007). Attenuated cold sensitivity in TRPM8 null mice. Neuron 54, 379–386. [DOI] [PubMed] [Google Scholar]
- Craig AD, and Bushnell MC (1994). The thermal grill illusion: unmasking the burn of cold pain. Science 265, 252–255. [DOI] [PubMed] [Google Scholar]
- Craig AD, Krout K, and Andrew D (2001). Quantitative response characteristics of thermoreceptive and nociceptive lamina I spinothalamic neurons in the cat. J Neurophysiol 86, 1459–1480. [DOI] [PubMed] [Google Scholar]
- Cui L, Miao X, Liang L, Abdus-Saboor I, Olson W, Fleming MS, Ma M, Tao YX, and Luo W (2016). Identification of Early RET+ Deep Dorsal Spinal Cord Interneurons in Gating Pain. Neuron 91, 1137–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle TL, Madisen L, Hage TA, Valley MT, Knoblich U, Larsen RS, Takeno MM, Huang L, Gu H, Larsen R, et al. (2018). A Suite of Transgenic Driver and Reporter Mouse Lines with Enhanced Brain-Cell-Type Targeting and Functionality. Cell 174, 465–480 e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana H, Mohar B, Sun Y, Narayan S, Gordus A, Hasseman JP, Tsegaye G, Holt GT, Hu A, Walpita D, et al. (2016). Sensitive red protein calcium indicators for imaging neural activity. eLife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana H, Sun Y, Mohar B, Hulse B, Hasseman JP, Tsegaye G, Tsang A, Wong A, Patel R, Macklin JJ, et al. (2018). High-performance GFP-based calcium indicators for imaging activity in neuronal populations and microcompartments. bioRxiv, 434589. [DOI] [PubMed] [Google Scholar]
- Darian-Smith I, Johnson KO, LaMotte C, Shigenaga Y, Kenins P, and Champness P (1979). Warm fibers innervating palmar and digital skin of the monkey: responses to thermal stimuli. J Neurophysiol 42, 1297–1315. [DOI] [PubMed] [Google Scholar]
- DeNardo L, and Luo L (2017). Genetic strategies to access activated neurons. Current opinion in neurobiology 45, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverman BE, Pravdo PL, Simpson BP, Kumar SR, Chan KY, Banerjee A, Wu WL, Yang B, Huber N, Pasca SP, et al. (2016). Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nature biotechnology 34, 204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, and Patapoutian A (2007). TRPM8 is required for cold sensation in mice. Neuron 54, 371–378. [DOI] [PubMed] [Google Scholar]
- Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, et al. (2014). Identification of spinal circuits transmitting and gating mechanical pain. Cell 159, 1417–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery EC, Luiz AP, Sikandar S, Magnusdottir R, Dong X, and Wood JN (2016). In vivo characterization of distinct modality-specific subsets of somatosensory neurons using GCaMP. Science advances 2, e1600990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster E, Wildner H, Tudeau L, Haueter S, Ralvenius WT, Jegen M, Johannssen H, Hosli L, Haenraets K, Ghanem A, et al. (2015). Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron 85, 1289–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois A, Low SA, Sypek EI, Christensen AJ, Sotoudeh C, Beier KT, Ramakrishnan C, Ritola KD, Sharif-Naeini R, Deisseroth K, et al. (2017). A Brainstem-Spinal Cord Inhibitory Circuit for Mechanical Pain Modulation by GABA and Enkephalins. Neuron 93, 822–839 e826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, and Caterina M (2002). Heat-evoked activation of the ion channel, TRPV4. The Journal of neuroscience : the official journal of the Society for Neuroscience 22, 6408–6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachisuka J, Baumbauer KM, Omori Y, Snyder LM, Koerber HR, and Ross SE (2016). Semi-intact ex vivo approach to investigate spinal somatosensory circuits. eLife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallin RG, Torebjork HE, and Wiesenfeld Z (1982). Nociceptors and warm receptors innervated by C fibres in human skin. Journal of neurology, neurosurgery, and psychiatry 45, 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker HO, and Kobal G (1993). Psychophysiology of experimentally induced pain. Physiological reviews 73, 639–671. [DOI] [PubMed] [Google Scholar]
- Haring M, Zeisel A, Hochgerner H, Rinwa P, Jakobsson JET, Lonnerberg P, La Manno G, Sharma N, Borgius L, Kiehn O, et al. (2018). Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nature neuroscience 21, 869–880. [DOI] [PubMed] [Google Scholar]
- Hellon RF, and Misra NK (1973). Neurones in the dorsal horn of the rat responding to scrotal skin temperature changes. J Physiol 232, 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhang X, and McNaughton PA (2006). Inflammatory pain: the cellular basis of heat hyperalgesia. Current neuropharmacology 4, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Li X, Yu Y, Wang J, and Caterina MJ (2011). TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Molecular pain 7, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Rochefort NL, Chen X, and Konnerth A (2010). Dendritic organization of sensory input to cortical neurons in vivo. Nature 464, 1307–1312. [DOI] [PubMed] [Google Scholar]
- Johannssen HC, and Helmchen F (2010). In vivo Ca2+ imaging of dorsal horn neuronal populations in mouse spinal cord. J Physiol 588, 3397–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorum E, Warncke T, and Stubhaug A (2003). Cold allodynia and hyperalgesia in neuropathic pain: the effect of N-methyl-D-aspartate (NMDA) receptor antagonist ketamine--a double-blind, cross-over comparison with alfentanil and placebo. Pain 101, 229–235. [DOI] [PubMed] [Google Scholar]
- Julius D (2013). TRP channels and pain. Annu Rev Cell Dev Biol 29, 355–384. [DOI] [PubMed] [Google Scholar]
- Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, and Voets T (2009). TRPA1 acts as a cold sensor in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America 106, 1273–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keemink SW, Lowe SC, Pakan JMP, Dylda E, van Rossum MCW, and Rochefort NL (2018). FISSA: A neuropil decontamination toolbox for calcium imaging signals. Scientific reports 8, 3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, and McKemy DD (2013). A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 2837–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WW, Mazor O, and Wilson RI (2015). Thermosensory processing in the Drosophila brain. Nature 519, 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo L, and Anderson DJ (2011). A Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron 72, 938–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Dong H, Gao Y, Gong Y, Ren Y, Gu N, Zhou S, Xia N, Sun YY, Ji RR, et al. (2013). A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. The Journal of clinical investigation 123, 4050–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Callaway EM, and Svoboda K (2008). Genetic dissection of neural circuits. Neuron 57, 634–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Callaway EM, and Svoboda K (2018). Genetic Dissection of Neural Circuits: A Decade of Progress. Neuron 98, 256–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q (2010). Labeled lines meet and talk: population coding of somatic sensations. The Journal of clinical investigation 120, 3773–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q (2012). Population coding of somatic sensations. Neuroscience bulletin 28, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Garner AR, Shimaoka D, Chuong AS, Klapoetke NC, Li L, van der Bourg A, Niino Y, Egolf L, Monetti C, et al. (2015). Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 85, 942–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature neuroscience 13, 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy ES, Taylor-Blake B, Street SE, Pribisko AL, Zheng J, and Zylka MJ (2013). Peptidergic CGRPalpha primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold. Neuron 78, 138–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, and Julius D (2002). Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58. [DOI] [PubMed] [Google Scholar]
- Melzack R, and Wall PD (1965). Pain mechanisms: a new theory. Science 150, 971–979. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Tisel SM, Orestes P, Bhangoo SK, and Hoon MA (2011). TRPV1-lineage neurons are required for thermal sensation. The EMBO journal 30, 582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamichi K, Shlomai-Fuchs Y, Shu M, Weissbourd BC, Luo L, and Mizrahi A (2013). Dissecting local circuits: parvalbumin interneurons underlie broad feedback control of olfactory bulb output. Neuron 80, 1232–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, and Patapoutian A (2005). Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 307, 1468–1472. [DOI] [PubMed] [Google Scholar]
- Nishida K, Matsumura S, Taniguchi W, Uta D, Furue H, and Ito S (2014). Three-dimensional distribution of sensory stimulation-evoked neuronal activity of spinal dorsal horn neurons analyzed by in vivo calcium imaging. PloS one 9, e103321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki K, Chung S, Ch’ng YH, Kara P, and Reid RC (2005). Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433, 597–603. [DOI] [PubMed] [Google Scholar]
- Ouzounov DG, Wang T, Wang M, Feng DD, Horton NG, Cruz-Hernandez JC, Cheng YT, Reimer J, Tolias AS, Nishimura N, et al. (2017). In vivo three-photon imaging of activity of GCaMP6-labeled neurons deep in intact mouse brain. Nature methods 14, 388–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachitariu M, Stringer C, Dipoppa M, Schröder S, Rossi LF, Dalgleish H, Carandini M, and Harris KD (2017). Suite2p: beyond 10,000 neurons with standard two-photon microscopy. bioRxiv, 061507. [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, et al. (2002a). A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715. [DOI] [PubMed] [Google Scholar]
- Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, et al. (2002b). A heat-sensitive TRP channel expressed in keratinocytes. Science 296, 2046–2049. [DOI] [PubMed] [Google Scholar]
- Peirs C, Williams SP, Zhao X, Walsh CE, Gedeon JY, Cagle NE, Goldring AC, Hioki H, Liu Z, Marell PS, et al. (2015). Dorsal Horn Circuits for Persistent Mechanical Pain. Neuron 87, 797–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitjean H, Pawlowski SA, Fraine SL, Sharif B, Hamad D, Fatima T, Berg J, Brown CM, Jan LY, Ribeiro-da-Silva A, et al. (2015). Dorsal Horn Parvalbumin Neurons Are Gate-Keepers of Touch-Evoked Pain after Nerve Injury. Cell reports 13, 1246–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Gutnisky DA, Huber D, Xu NL, O’Connor DH, Tian L, Looger L, and Svoboda K (2012). Activity in motor-sensory projections reveals distributed coding in somatosensation. Nature 489, 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pnevmatikakis EA, Soudry D, Gao Y, Machado TA, Merel J, Pfau D, Reardon T, Mu Y, Lacefield C, Yang W, et al. (2016). Simultaneous Denoising, Deconvolution, and Demixing of Calcium Imaging Data. Neuron 89, 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgorski K, and Ranganathan G (2016). Brain heating induced by near-infrared lasers during multiphoton microscopy. J Neurophysiol 116, 1012–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogorzala LA, Mishra SK, and Hoon MA (2013). The cellular code for mammalian thermosensation. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 5533–5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SA, Ma Q, and De Koninck Y (2014). Normal and abnormal coding of somatosensory stimuli causing pain. Nature neuroscience 17, 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran C (2017). Representations of temperature in the spinal cord. Temperature 4, 214–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran C, Hoon MA, and Chen X (2016). The coding of cutaneous temperature in the spinal cord. Nature neuroscience 19, 1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, et al. (2010). Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron 65, 886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild G, Nelken I, and Mizrahi A (2010). Functional organization and population dynamics in the mouse primary auditory cortex. Nature neuroscience 13, 353–360. [DOI] [PubMed] [Google Scholar]
- Sathyamurthy A, Johnson KR, Matson KJE, Dobrott CI, Li L, Ryba AR, Bergman TB, Kelly MC, Kelley MW, and Levine AJ (2018). Massively Parallel Single Nucleus Transcriptional Profiling Defines Spinal Cord Neurons and Their Activity during Behavior. Cell reports 22, 2216–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ, et al. (2015). Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature 524, 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, et al. (2002). TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418, 186–190. [DOI] [PubMed] [Google Scholar]
- Spike RC, Puskar Z, Andrew D, and Todd AJ (2003). A quantitative and morphological study of projection neurons in lamina I of the rat lumbar spinal cord. The European journal of neuroscience 18, 2433–2448. [DOI] [PubMed] [Google Scholar]
- Stettler DD, and Axel R (2009). Representations of odor in the piriform cortex. Neuron 63, 854–864. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, et al. (2003). ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829. [DOI] [PubMed] [Google Scholar]
- Sun W, Tan Z, Mensh BD, and Ji N (2016). Thalamus provides layer 4 of primary visual cortex with orientation- and direction-tuned inputs. Nature neuroscience 19, 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, and Nilius B (2005). Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438, 1022–1025. [DOI] [PubMed] [Google Scholar]
- Tan CH, and McNaughton PA (2016). The TRPM2 ion channel is required for sensitivity to warmth. Nature 536, 460–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, et al. (2011). A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaka GI, Guenthner CJ, Shalev A, Gilday O, Luo L, and Mizrahi A (2018). Genetic tagging of active neurons in auditory cortex reveals maternal plasticity of coding ultrasonic vocalizations. Nature communications 9, 871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theer P, Hasan MT, and Denk W (2003). Two-photon imaging to a depth of 1000 microm in living brains by use of a Ti:Al2O3 regenerative amplifier. Optics letters 28, 1022–1024. [DOI] [PubMed] [Google Scholar]
- Thunberg T (1896). Uppsala Lakforen. Fdrh 2, 489. [Google Scholar]
- Todd AJ (2010). Neuronal circuitry for pain processing in the dorsal horn. Nature reviews Neuroscience 11, 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, and Caterina MJ (2004). Thermosensation and pain. Journal of neurobiology 61, 3–12. [DOI] [PubMed] [Google Scholar]
- Torebjork HE, Schady W, and Ochoa J (1984). Sensory correlates of somatic afferent fibre activation. Human neurobiology 3, 15–20. [PubMed] [Google Scholar]
- Vandewauw I, De Clercq K, Mulier M, Held K, Pinto S, Van Ranst N, Segal A, Voet T, Vennekens R, Zimmermann K, et al. (2018). Publisher Correction: A TRP channel trio mediates acute noxious heat sensing. Nature 559, E7. [DOI] [PubMed] [Google Scholar]
- Vogelstein JT, Packer AM, Machado TA, Sippy T, Babadi B, Yuste R, and Paninski L (2010). Fast nonnegative deconvolution for spike train inference from population calcium imaging. J Neurophysiol 104, 3691–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, Benoit M, Xue F, Janssens A, Kerselaers S, et al. (2011). TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 70, 482–494. [DOI] [PubMed] [Google Scholar]
- Vrontou S, Wong AM, Rau KK, Koerber HR, and Anderson DJ (2013). Genetic identification of C fibres that detect massage-like stroking of hairy skin in vivo. Nature 493, 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Belanger E, Cote SL, Desrosiers P, Prescott SA, Cote DC, and De Koninck Y (2018a). Sensory Afferents Use Different Coding Strategies for Heat and Cold. Cell reports 23, 2001–2013. [DOI] [PubMed] [Google Scholar]
- Wang K, Sun W, Richie CT, Harvey BK, Betzig E, and Ji N (2015). Direct wavefront sensing for high-resolution in vivo imaging in scattering tissue. Nature communications 6, 7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Ouzounov DG, Wu C, Horton NG, Zhang B, Wu CH, Zhang Y, Schnitzer MJ, and Xu C (2018b). Three-photon imaging of mouse brain structure and function through the intact skull. Nature methods 15, 789–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, and Nilius B (2002). Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. The Journal of biological chemistry 277, 47044–47051. [DOI] [PubMed] [Google Scholar]
- Wertz A, Trenholm S, Yonehara K, Hillier D, Raics Z, Leinweber M, Szalay G, Ghanem A, Keller G, Rozsa B, et al. (2015). PRESYNAPTIC NETWORKS. Single-cell-initiated monosynaptic tracing reveals layer-specific cortical network modules. Science 349, 70–74. [DOI] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJ, Mori T, Finke S, Conzelmann KK, Young JA, and Callaway EM (2007). Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53, 639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, et al. (2002). TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418, 181–186. [DOI] [PubMed] [Google Scholar]
- Yaksi E, and Friedrich RW (2006). Reconstruction of firing rate changes across neuronal populations by temporally deconvolved Ca2+ imaging. Nature methods 3, 377–383. [DOI] [PubMed] [Google Scholar]
- Yarmolinsky DA, Peng Y, Pogorzala LA, Rutlin M, Hoon MA, and Zuker CS (2016). Coding and Plasticity in the Mammalian Thermosensory System. Neuron 92, 1079–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Lu Y, and Perl ER (2010). Inhibitory neurones of the spinal substantia gelatinosa mediate interaction of signals from primary afferents. J Physiol 588, 2065–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K, Lennerz JK, Hein A, Link AS, Kaczmarek JS, Delling M, Uysal S, Pfeifer JD, Riccio A, and Clapham DE (2011). Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. Proceedings of the National Academy of Sciences of the United States of America 108, 18114–18119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg B, Chou XL, Zhang ZG, Mesik L, Liang F, Tao HW, and Zhang LI (2017). AAV-Mediated Anterograde Transsynaptic Tagging: Mapping Corticocollicular Input-Defined Neural Pathways for Defense Behaviors. Neuron 93, 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong W, Wu R, Li M, Hu Y, Li Y, Li J, Rong H, Wu H, Xu Y, Lu Y, et al. (2017). Fast high-resolution miniature two-photon microscopy for brain imaging in freely behaving mice. Nature methods 14, 713–719. [DOI] [PubMed] [Google Scholar]