Abstract
Non-mammalian models of CIPN remain relatively sparse, but the knowledge gained from the few published studies suggest that these species have great potential to serve as a discovery platform for new pathways and underlying genetic mechanisms of CIPN. These models permit large-scale genetic and pharmacological screening, and they are highly suitable for in vivo imaging. CIPN phenotypes described in rodents have been confirmed in those models, and conversely, genetic players leading to axon de- and regeneration under conditions of chemotherapy treatment identified in these non-mammalian species have been validated in rodents. Given the need for non-traditional approaches with which to identify new CIPN mechanisms, these models bear a strong potential due to the conservation of basic mechanisms by which chemotherapeutic agents induce neurotoxicity.
Keywords: CIPN, non-mammalian, Drosophila, zebrafish C. elegans, axon degeneration, chemotherapy-induced peripheral neuropathy, in vivo imaging, review
Non-mammalian CIPN studies provide new insight into axon degeneration mechanisms
Chemotherapy-induced peripheral neuropathy (CIPN) affects 30–70% of chemotherapy patients, depending on the administered drug. Intriguingly, intense research efforts point to remarkably similar causes of axon degeneration, regardless of the type of drug that is administered. For instance, reactive oxygen species formation and mitochondrial damage are two mechanisms that have been suggested to be involved in CIPN (Areti et al., 2014; Bobylev et al., 2015; Flatters and Bennett, 2006; Lisse et al., 2016; Podratz et al., 2011a; Podratz et al., 2017; Zheng et al., 2011, 2012), as well as in diabetic (Barrière et al., 2012; Waldron et al., 2018), hereditary (Barneo-Muñoz et al., 2015; Niemann et al., 2009; Palau et al., 2009), and other types of neuropathy (Flatters, 2015; Kanda et al., 2016). Nevertheless, there are still no therapeutic agents available that can mitigate neuropathies in patients, and thus, more research is needed to identify molecular factors that can be effectively targeted.
The vast majority of studies that contribute to our knowledge of CIPN are derived from in vitro and in vivo studies using rodents. A disadvantage of mammalian models is that rodents are less amenable for genetic and pharmacological screens compared to non-mammalian models. Thus, the identification of new genes or pathways that underlie CIPN has been relatively sparse and depended on a trial-and-error system. To use a more unbiased approach, several labs have developed non-mammalian models, which are ideally suitable for large genetic and chemical screens and also for in vivo imaging. The combination of screening technologies and in vivo imaging is powerful in that it permits the manipulation of processes induced by chemotherapeutic agents and the direct observation of the effects in the living animal. Although cell culture is also suitable for live imaging, cell culture experiments do not recapitulate the in vivo environment and the interactions that occur between the various cell types and tissues present in a living organism. Although rodent models are more suitable in this respect, the need to section tissues for non-behavioral analyses does not reveal more than a snapshot of what is really happening inside the body. Interpretations from these experiments can be difficult and may lead to incorrect conclusions. Imagine a chess game in which the observer can only see the positions of the figures at several time-points throughout the match and must infer the moves made in-between that lead to the end outcome. It will be necessary to observe the full game to understand how individual players and moves lead to the ultimate checkmate. Similarly, a biological context can be understood best if observed in the living animal.
CIPN studies benefit especially from in vivo imaging approaches due to a number of reasons: (1) Experimental approaches using rodent models or cell culture have not revealed sufficient mechanisms that are useful for therapeutic development, and using in vivo imaging may help to observe axons during the degenerative process, which can reveal additional information. (2) Various non-mammalian CIPN models have shown striking similarity to mammals. It is therefore likely that the mechanisms leading to axon de- and regeneration are conserved. (3) Genetic tools, rapid generation time, and large animal numbers can be obtained from non-mammalian species, which allows for the analysis of chemotherapy-induced neurotoxicity with high throughput, which increases overall statistical power and the potential identification of molecular pathways implicated in CIPN.
Although non-mammalian models are permissive for screening and in vivo studies, the question arises whether these species are comparable to rodent models and human CIPN conditions. In this article, we will review studies that have been performed in Drosophila melanogaster, C. elegans, and zebrafish (Danio rerio) and compare the findings in these models to human CIPN mechanisms. We hope that this review will facilitate an understanding by clinicians about the pros and cons of these models and help researchers improve these models where necessary to make them more comparable to human CIPN. Each of the non-mammalian species has its advantages, and because some of the results have been already validated in mammals, it appears that the mechanisms are, at least in part, conserved. These animal models possess peripheral neurons that send unmyelinated or myelinated axons into the skin and muscle, and thus the effects of chemotherapeutic agents on these peripheral axons can be studied. Intriguingly, the peripheral nervous system is the most ancient part of the nervous system in terms of its evolution, and also the cytoskeleton and replication machinery, which are primarily targeted by chemotherapeutic agents, are highly conserved. Thus, many of the mechanisms should in principle hold true with respect to human CIPN. It is also worth noting that rodent models are not always the best models to study human disease conditions. In fact, less than 8% of the findings in rodent models can be ultimately translated into humans (Mak et al., 2014). The best approach, therefore, would be to study the conservation of CIPN mechanisms in multiple species, as this may be the strongest indicator of their conservation in humans. Thus, identifying conserved genes or pathways in non-mammalian and mammalian species will most likely represent the best strategy to successfully develop therapeutic targets with which to treat human CIPN.
We will begin this review with Drosophila melanogaster, an invertebrate model in which Dual Leucine Zipper Kinase (DLK) and Nicotinamide Mononucleotide Adenylyltransferase (NMNAT) were discovered to play essential roles in paclitaxel-induced axon de- and regeneration (Bhattacharya et al., 2012; Miller et al., 2009). We will then highlight a Drosophila model with which to study cisplatin-induced neurotoxicity, and in particular climbing behavior (Podratz et al., 2011a; Podratz et al., 2013; Podratz et al., 2011b). Besides Drosophila, we will further discuss a study in which mechanisms of paclitaxel neurotoxicity were analyzed in C. elegans using mouse NMNAT, which was found to be protective when overexpressed in worms (Mao et al., 2016), emphasizing the conservation of axon de- and regeneration mechanisms. Finally, we will explore zebrafish as a model for vincristine (Khan et al., 2012) and paclitaxel (Lisse et al., 2016) neurotoxicity and emphasize the role that epidermal keratinocytes play in paclitaxel-induced sensory axon degeneration. Zebrafish somatosensory neurons are most similar to those present in mammals, and the larvae are highly suitable for in vivo imaging. Zebrafish have become increasingly popular as a pre-clinical drug discovery model because of this and other advantages including large egg numbers, rapid development, and the conservation of ~80% of human disease-causing genes. (Gibert et al., 2013). In summary, non-mammalian models are highly suitable to gain a mechanistic understanding of CIPN (Figure 1). Moreover, they have specific advantages over mammalian models that can be exploited to facilitate drug development.
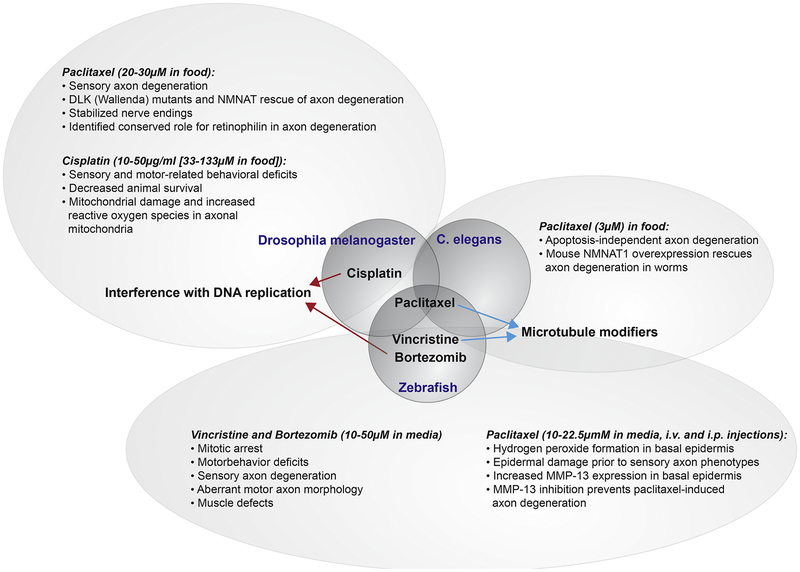
Figure 1. Overview of identified mechanisms in non-mammalian models leading to chemotherapy-induced neurotoxicity.
Three model species shown in the middle bubbles (Drosophila, C. elegans, and zebrafish) have been assessed for neurotoxic effects derived from various chemotherapeutic agents known to cause CIPN in humans. All species were studied for their response to paclitaxel treatment (microtubule stabilizer), whereas cisplatin (DNA-replication interfering) was uniquely studied in Drosophila and vincristine (microtubule destabilizer) and bortezomib (proteasome inhibitor) were uniquely studied in zebrafish. The outcomes of each of these studies are shown in the outer bubbles.
Drosophila
Similar to other insects, Drosophila has two primary sensory neuron types that are positioned in segmental sensory organs along the body wall (Singhania and Grueber, 2014). These neurons project peripheral axons to the basal (inner) surface of the epidermis and along internal scaffold structures. Their central axons bundle together and project into the antero-ventral CNS. Unlike human peripheral nerves, the peripheral axons of Drosophila are ensheathed by glial processes, which do not contain myelin (Coutinho-Budd and Freeman, 2013). In addition, myelin-related genes such as orthologs for PMP22 and MPZ do not exist (Bussmann and Storkebaum, 2017). Given these differences, Drosophila is less suitable with respect to modeling CIPN conditions affecting Schwann cell functions. However, the architectural properties of the dendritic arbors that innervate the epidermis are comparable to mammalian sensory neurons; both extend finely branched free nerve endings into the epidermis (Im and Galko, 2012). Each Drosophila sensory organ contains different subtypes of peripheral neurons, consistent with mammalian peripheral sensory ganglia. Also, the various sensory modalities are conserved and can, therefore, be assessed in the context of chemotherapy treatment. Segmental motor neurons that project peripheral axons into muscle can be utilized to study chemotherapeutic agents that cause motor axon degeneration. An advantage of Drosophila larvae is the segmentation of neurons along the body, which allows drug effects to be examined in different neurons along the various segments (Podratz et al., 2017). Another advantage of Drosophila is its high progeny numbers, which enable large genetic screens to identify modifiers of CIPN.
Paclitaxel-induced neurotoxicity in Drosophila melanogaster
Bhattacharya and colleagues established a Drosophila model to study paclitaxel neurotoxicity (Bhattacharya et al., 2012). Paclitaxel, a taxane, was originally isolated from the Pacific yew tree and valued for its antimitotic properties (McLaughlin et al., 1981). Molecular studies have shown that paclitaxel binds to β-tubulin in α and β-tubulin dimers, which prevents the depolymerization of microtubules, leading to their stabilization (Amos and Löwe, 1999; Arnal and Wade, 1995; Hari et al., 2006; Nogales et al., 1995; Parness and Horwitz, 1981). This effect is beneficial during cancer treatment because it prevents cancer cells from undergoing mitosis. During cell division, the microtubules attached to the kinetochores need to pull the chromosomes into each daughter cell, but with paclitaxel bound to the microtubules, they can no longer depolymerize and thus mitosis is inhibited (Jordan et al., 1996). Paclitaxel also induces apoptosis in cancer cells, presumably by its effects on mitochondria (Ren et al., 2018). Although neurons are differentiated and do not divide, paclitaxel has severe side effects in the neurons, leading to sensory axon degeneration. The precise mechanisms remain largely unknown, but microtubule aggregation and mitochondrial damage have been shown to occur in rodents using in vitro (Melli et al., 2008) and in vivo approaches (Bobylev et al., 2015; Flatters and Bennett, 2006).
To identify molecular players of paclitaxel neurotoxicity and identify possible therapeutic targets, Bhattacharya and colleagues treated Drosophila larvae up to the third instar larval stage with 30μM paclitaxel. The effects on sensory axon degeneration were examined after 3 and 4 days of treatment using GFP detection and immunofluorescence staining of sensory nerves between segments A3 and A4. In addition, the authors performed transmission electron microscopy (TEM) to analyze axonal loss at the ultrastructural level. To be comparable to clinical features, the treatments were given with respect to the completion of neuronal differentiation to avoid perturbations of neurogenesis and axonal pathfinding. However, it remains unclear from this study whether the dose of 30μM paclitaxel was the maximal dose tolerated by flies or chosen due to the visible effects on axons. It is therefore difficult to assess whether similar effects occur in humans. It is also unclear whether the treatment regimen led to persistent paclitaxel dosing and its uptake into cells was clinically relevant. Variables like food intake, time until the food was consumed, and the metabolic rate of each fly must be considered when assessing the outcomes. Nevertheless, these parameters caused phenotypes that are observed in mammals and hallmarks of paclitaxel-induced peripheral neuropathy, including axonal swelling, fragmentation and debris formation, and axon loss (Gornstein and Schwarz, 2014). TEM further showed a ~2-fold decrease in axon number and large holes in peripheral nerves, corresponding to the loss of axons. Intriguingly, the remaining axons appeared relatively normal, suggesting that only a subset of neurons might be affected. This finding is especially interesting considering a similar subset-specific accumulation of paclitaxel was also observed in our studies using zebrafish time-lapse imaging (Lisse et al., 2016). Whether specific neuronal subtypes are targeted in humans has not been definitively assessed.
Similar to what has been reported in patients, the effects of paclitaxel-induced axonal loss were caused independent of cell death. Activation of the anti-apoptotic protein p35, which blocks Caspase-3, could not rescue paclitaxel’s neurotoxic effects (Bhattacharya et al., 2012), suggesting that apoptosis does not play a role in paclitaxel-induced axon degeneration. Thus, overall, the phenotypes induced by paclitaxel in Drosophila sensory neurons are comparable to mammalian paclitaxel-induced peripheral neuropathy (PIPN). Importantly, Bhattacharya and colleagues indicate that paclitaxel-induced axon degeneration is a highly regulated process in which Wallerian degeneration plays a role. Wlds (Wallerian Degeneration Slow, Wlds) mice show a delay in Wallerian degeneration due to the presence of a chimeric protein containing the NAD synthase NMNAT (Lyon et al., 1993; Mack et al., 2001). Subsequent studies have shown similar effects of NMANT in Drosophila (Zhai et al., 2006) and zebrafish (Martin et al., 2010), suggesting its conserved functions and importance in this regulated process. Bhattacharya et al. examined whether overexpression of NMNAT also prevented paclitaxel-induced axon degeneration and indeed found a protective effect. Given the strong conservation of NMNAT function in protecting axons from degenerating, there could be potential to prevent PIPN in patients by treating with Nicotineamide Adenine Dinucleotide (NAD) or NMNAT.
Furthermore, another molecular player implicated in promoting axon degeneration in Drosophila is DLK (Miller et al., 2009). DLK also plays a role in paclitaxel-induced neurotoxicity since mutants of the Drosophila DLK homolog, wallenda, show protection against axon degeneration induced by paclitaxel treatment (Bhattacharya et al., 2012). The effect is sensory neuron-specific since RNAi knockdown of wallenda specifically in sensory neurons was sufficient to inhibit axon degeneration. Intriguingly, DLK functions in axon degeneration are also conserved in C. elegans (Ghosh-Roy et al.) and mice (Miller et al., 2009), suggesting that its role in paclitaxel neurotoxicity is likely also conserved in humans.
Bhattacharya and colleagues were interested in identifying additional factors that can protect axons from degenerating under conditions of paclitaxel treatment and performed an RNAi screen, which identified retinophilin, a MORN family member, as a novel player in axon degeneration. Intriguingly, RNAi-mediated knockdown of the mammalian homolog MORN4 in mouse DRG neurons also stabilizes degenerating axons. This further highlights the conservation of molecular factors implicated in axon degeneration and the potential to target these proteins in patients using pharmacological approaches.
In addition to the findings from Bhattacharya and colleagues, another group used Drosophila to examine paclitaxel-induced neurotoxicity, specifically in nociceptive C4da neurons using behavioral assays and in vivo imaging (Brazill et al., 2018a) (Figure 2). C4da neurons innervate the epidermis where they mediate pain sensation with their elaborate dendritic arbors. Nociceptive signals are conveyed to the ventral nerve cord via synapse formation between the long central axons of C4da neurons and CNS neurons (Im and Galko, 2012). C4da neuron stimulation normally leads to stereotypic fly behaviors, which were assessed in the context of paclitaxel treatment. Gentle touch of Drosophila larvae at sub-threshold temperatures typically induces a peristaltic crawling behavior. However, when noxious temperatures are used to stimulate the animals, they display corkscrew-like rolling withdrawal behaviors (Tracey et al., 2003). In the study by Brazill and colleagues, third instar larvae (72–10h post egg laying) were exposed to temperatures of 42°C and above, which led to a dose-dependent (10–30μM paclitaxel) decrease in withdrawal latency (Brazill et al., 2018a). The strongest phenotype was observed using 42°C in combination with 20μM paclitaxel when all tested flies withdrew faster from the noxious stimulus than their controls. While 20μM paclitaxel caused a decrease in withdrawal latency in 100% of the animals, 30μM paclitaxel caused the flies to either respond faster than the controls or not respond at all. In addition, the authors showed that 20μM paclitaxel had stabilizing effects on dendritic branches. Taking in consideration studies in zebrafish (shown below) and mammals, their finding suggests a possible hormetic function of paclitaxel, whereby low concentrations exert a stabilizing phenotype on axons and high concentrations promote axon degeneration, consistent with the findings by Bhattacharya et al. (Bhattacharya et al., 2012). This idea correlates with observations in the mouse where injections of low paclitaxel concentrations after spinal cord injury led to the stabilization of axons and axon regeneration (Hellal et al., 2011), whereas higher concentrations induce axon degeneration, as shown in cell culture (Yang et al., 2009) and seen in individuals with paclitaxel-induced peripheral neuropathy.
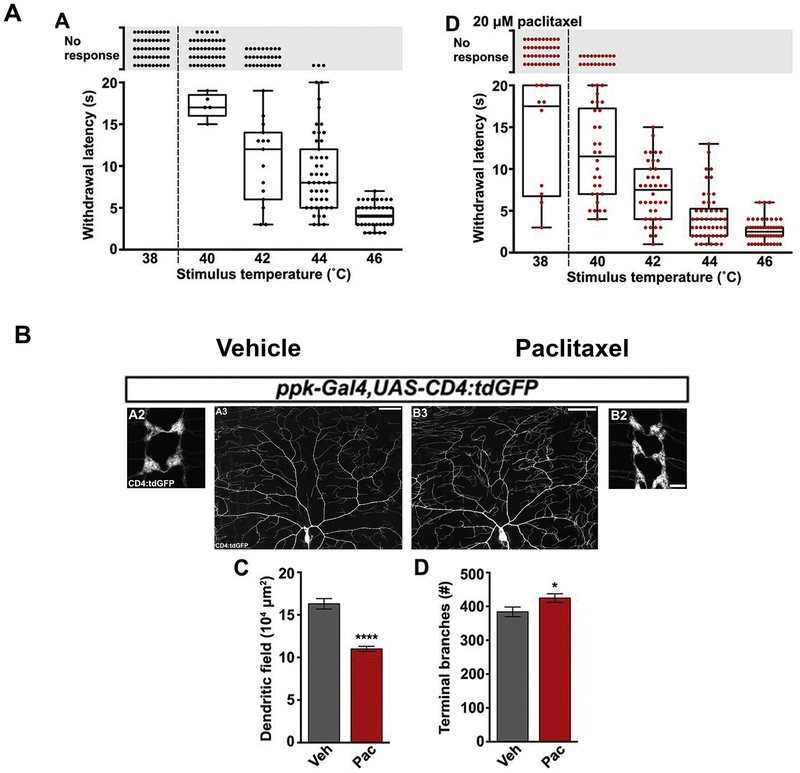
Figure 2. In vivo analysis of paclitaxel-induced axon damage in Drosophila.
This figure was reprinted with permission from Figures 1 and 2 (Brazill et al., 2018b). (A) A: Thermal nociceptive profile of third instar larvae (120 hours after egg laying) Larvae were stimulated with indicated temperatures following vehicle-containing food exposure for 24 hours. Points indicate individual larvae. No response was counted if larvae did not withdraw from heat stimulus after 20 seconds. D: Profile in larvae treated with paclitaxel under same conditions. 42C showed a robust withdrawal response in all larvae tested. (B) A/B: Confocal Projections of fixed, filet-dissected vehicle or paclitaxel-treated larvae showing class IV dendritic arborization (C4da) neuronal compartments labeled by CD4:tdGFP. A2/B2: C4da axon terminals project in a ladder-like pattern in the ventral nerve cord. scale bar: 10μm A3/B3: Dorsal dendrite projections of C4da ddaC neurons shown with cell body near the center at the bottom of the frame. Scale bar: 50μm. C: Quantification of area of dendritic field shows a reduced field upon paclitaxel treatment. D: Quantification of number of terminal branches shows an increase upon paclitaxel treatment suggestive of dendrite-stabilizing effects of paclitaxel.
Surprisingly, the decreased withdrawal latency of paclitaxel-treated flies was not due to the degeneration of epidermal nerve endings as expected. Rather, axons were also stabilized, yet a nociceptive response was observed. This finding is consistent with observations in rats in which axons did not degenerate upon paclitaxel treatment with doses up to 2mg/kg despite the development of heat-hyperalgesia, mechano-allodynia, mechano-hyperalgesia, and cold-allodynia (Polomano et al., 2001). Dendritic arbors of C4da neurons normally undergo a dynamic growth and retraction behavior, but retraction appeared to be affected by paclitaxel treatment, leading to axon stabilization and increased dendritic branch density following treatment for at least 24 hours. Thus, interference with axonal function regardless of stabilization or degeneration appears to impact sensory function. Intriguingly, similar to Bhattacharya et al., NMNAT, was able to partially protect C4da neurons from thermal hypersensitivity.
One unexplained finding is that the dendritic peripheral branches in paclitaxel-treated larvae did not contain an intact microtubule network despite the apparent stabilization of the dendritic branch tips. One explanation was that the neuron-specific microtubule marker used in this study, Futsch, does not detect the microtubules in the branch tips. However, other cytoskeletal markers were not assessed. It, therefore, remains unclear whether paclitaxel exerted its dendritic branch stabilizing effects on microtubules or other cytoskeletal or cellular components, such as F-actin.
Cisplatin-induced neurotoxicity in Drosophila
Cisplatin belongs to the platinum class of DNA replication-interfering drugs. In mammals and humans, cisplatin treatment causes crosslinking of guanines, thereby triggering activation of apoptotic cascades and cancer cell death (Kelman and Peresie, 1979). Side effects are neurotoxicity, nausea, nephrotoxicity, ototoxicity, hair loss, and visual problems. Noncompetitive inhibition of the membrane-bound, mechanosensitive sodium-hydrogen ion transporter, NHE-1, by cisplatin treatment was identified as a possible cause of neurotoxic effects in the inner ear and peripheral neurons (Milosavljevic et al., 2010). However, cisplatin also caused mitochondrial damage by binding to mitochondrial DNA (Podratz et al., 2011a), and it remains unclear whether mitochondrial damage contributes to the observed neurotoxic effects. Cisplatin causes DRG neuron-specific peripheral neuropathy in about 36%–38% of patients when administered at low cumulative doses (<500 mg/m2) (Dolan et al., 2017; Seretny et al., 2014). Strikingly, almost 100% of the patients display cisplatin-induced peripheral neuropathy symptoms when treated with cumulative doses between 500–600 mg/m2.
Because of the lack of understanding about cisplatin’s neurotoxic mechanisms, a Drosophila model was developed due to its amenability for genetic studies and the ease with which to assess stereotypical behaviors. Adult flies were fed for 3 days with varying doses of cisplatin (10–400μg/ml), which was followed by either a 3 or 6-day recovery period depending on the analyses. Using Inductively Coupled Plasma Mass Spectrometry, it was determined that cisplatin accumulated at 1 molecule per 2500 base pairs. This accumulation was similar to what the authors previously found in a rat model (Podratz et al., 2011b), indicating the conservation of cisplatin-DNA binding interactions. Similar to humans (Baan et al., 1985), cisplatin caused DNA adduct formation. High doses (>50μg/ml) caused decreased animal survival and apoptosis in brain neurons and oocytes. Flies showed concentration-dependent abnormal geotactic climbing behavior when treated with cisplatin for at least 3 days. These motor deficits were attributed to caspase-3 dependent neuronal apoptosis in the brain. Interestingly, cisplatin has also been found to cause the death of neurons in the cerebral and cerebellar cortices, caudo-putamen, and hippocampus of rats treated with this chemotherapeutic agent, also leading to behavioral abnormalities (Owoeye et al., 2018). Thus, there appears to be some conservation of cisplatin-induced neurotoxic effects within the central nervous system. Whether these observations can be ultimately linked to CIPN remains to be shown given that these effects are brain-specific. Possibly this model could be useful to study cisplatin transport and metabolism into neurons, as the blood-brain barrier in this insect appears to be comprised of specific glial cells rather than polarized, tightly packed endothelial cells as seen in humans, which could show certain similarities with the peripheral nervous system.
Subsequent studies also investigated the effects of cisplatin treatment on the peripheral motor neurons (Podratz et al., 2017). Two cisplatin concentrations (10 and 25μg/ml) were compared to assess both behavioral responses and effects on motor axon mitochondria in Drosophila larvae. These findings indicate that cisplatin induces changes in sensory and motor functions, mitochondrial membrane potential, and reactive oxygen species formation in motor axons for both concentrations.
Another study tested the role of genetic background in cisplatin neurotoxicity given that humans as well as rodents respond to cisplatin in varying degrees (Groen et al., 2018). Using a negative geotactic climbing assay, the authors found a significantly different background-specific response to cisplatin treatment in various wild type Drosophila strains tested. However, this response was not due to differences in cisplatin-dependent DNA adducts. Also, the decreased survival of flies at higher doses was not strain-dependent. Nevertheless, the delivery method of cisplatin, either with or without 10% sucrose, influenced the rate of cisplatin-induced lethality. The authors noted a conserved function for glutathione peroxidase (PHGPx) in flies that influenced cisplatin sensitivity, which is also known to play a similar role in humans. Glutathione peroxidases function to protect cells from oxidative damage. Knockdown of PHGPx significantly prevented climbing abnormalities but did not affect survival. These findings support the idea that oxidative stress is a critical player in the neurotoxic effects of chemotherapeutic agents. Given the conserved function of PHGPx, flies may provide a platform for additional genetic screens that could reveal novel conserved players involved in cisplatin neurotoxicity. Whether this model can be valuable to study CIPN remains to be investigated.
Paclitaxel-induced neurotoxicity in C. elegans
C. elegans are highly amenable to genetic manipulations and this model has been well-characterized anatomically and genetically. Because of their optical clarity, worms are excellent models for in vivo imaging. Many of their genes are conserved in humans and these animals possess peripheral sensory neurons that innervate the skin and mediate responses to external stimulation (Goodman, 2006). For instance, the sensory neurons convey painful stimuli that can lead to noxious thermal avoidance behaviors (Husson et al., 2012; Wittenburg and Baumeister, 1999).
C. elegans possesses six touch receptor neurons (anterior neurons: ALML, ALMR, and AVM; and posterior neurons: PLMR and PVM), and these send out long projections into the cuticle (the skin of worms). These axons cover nearly half of the body and within the cuticle are engulfed by hypodermal cells (Chalfie and Sulston, 1981). C. elegans mechanosensory neurons mediate sensory stimuli from internal and external forces, such as those generated during locomotive behavior (Goodman, 2006). Their axons have been well characterized in terms of cytoskeletal composition. Studies by Chalfie and colleagues showed that C. elegans mechanosensory axons contain 15-protofilament microtubules, unlike mammalian peripheral axons that possess 13-protofilament microtubules. These additional filaments appear to be unique to C. elegans and arise from the tubulin genes, mec-7 and mec-12. Each protofilament is crosslinked to another protofilament and these are arrayed with their ends pointing toward the plasma membrane (Bounoutas et al., 2009; Chalfie and Sulston, 1981; Chalfie and Thomson, 1979; Savage et al., 1989).
Previous studies in mutant worms for the spectrin unc-70 gene showed that the axons of these mechanosensory neurons degenerate (Hammarlund et al., 2007), and a similar observation was made in a C. elegans necrosis model (Zhang et al., 2008). Thus C. elegans may be amenable to studies of axon degeneration induced by chemotherapy drugs. Mao and colleagues previously showed in the mouse CNS that NMNAT protects axons from hypoxic injury-induced degeneration in vitro and in vivo (Araki et al., 2004; Conforti et al., 2000; Mack et al., 2001). They speculated whether NMNAT may also be able to rescue paclitaxel neurotoxicity in C. elegans mechanosensory neurons, which would further emphasize the conserved function of this enzyme. In this study, worms from the egg laying stage to adulthood (Day 15) were treated with 3μM paclitaxel by placing the worms onto paclitaxel-containing plates. Paclitaxel feeding led to significant degeneration of peripheral axons of mechanosensory neurons examined using a GFP reporter line (mec-4::GFP) (Figure 3). Axon degeneration was evident due to the presence of axonal fragmentation and beading. It was noted that paclitaxel significantly retarded the growth of hatched larvae and thus, paclitaxel could have induced early developmental defects leading to axon degeneration through indirect effects. Performing further assays to assess microtubule and mitochondria function, as well as axonal transport to analyze the direct effects of paclitaxel may shed light on the mechanisms.
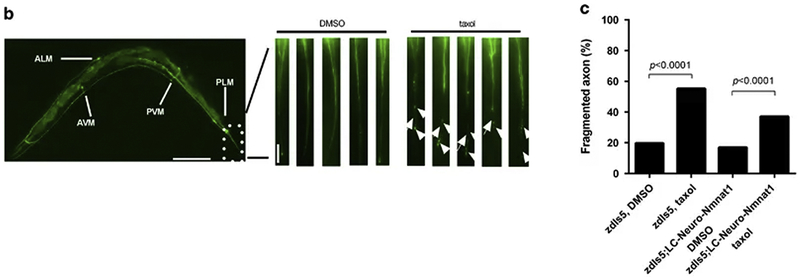
Figure 3.
This figure was with permission reprinted from Figure 5 (Mao et al., 2016). In (b) worm mechanosensory neurons and axons are labeled by zdIs5[mec-4::GFP] (scale bar: 100 μm; scale bar in inset: 10 μm). Representative images from the tail region comparing DMSO buffer versus taxol. Note fragmented (arrow) and beaded (arrowhead) axons in the taxol condition. (c) Taxol-induced axon degeneration was partially rescued by Neuro-Nmnat1[gcIs35(neuro-m-nonN-Nmnat1]).
Interestingly, axon degeneration was especially pronounced in the PLM neurons that innervate the tail regions, which may be most comparable to the distal extremities. Why this region was more affected remains to be shown but it may be dependent on neuronal subtype specificity for paclitaxel that predominantly affects the tail neurons. Alternatively, external factors in the tail region could have influenced the axons. This is especially interesting given our findings in zebrafish in which also the distal tail fin axons are the most prominent to degenerate with paclitaxel treatment. Our findings suggest adhesion defects in the skin play a role. Because C. elegans skin is very different, it remains questionable whether the findings are related. Intriguingly, axon degeneration was rescued when overexpressing low levels of a mouse non-nuclear localized gain-of-function NMNAT1 gene. It was previously shown that NMNAT1 overexpression rescued mouse DRG axon degeneration in vitro and in vivo when induced by hypoxia (Verghese et al. 2011). In the study from Mao and colleagues, a similar cytoprotective effect for NMNAT1, besides in C. elegans sensory neurons, was shown in mouse hippocampal neurons. Thus, the cytoprotective functions of NMNAT are not only conserved across species but also independent of the type of insult leading to axon degeneration. Thus, NMNAT must function downstream of earlier defects, also indicated by its role in the actual fragmentation and degeneration process in Wlds mutants (Lyon et al., 1993; Martin et al., 2010; Zhai et al., 2006). The protective effects of NMNAT1 have been specifically attributed to functions in the mitochondria given that Nmnat3 localization to mitochondria was able to phenocopy Wlds mutants and protect axons from degeneration. Similarly, the protective effects of NMNAT1 stemmed from activation of the mitochondrial unfolded protein response under conditions of hypoxia (Mao et al., 2016).
Follow-up studies should include shorter incubation times with paclitaxel at later stages to identify whether similar axon degeneration phenotypes can be induced that are more clinically relevant following differentiation of the neurons. Another aspect to consider is the difference in the microtubule composition of C. elegans mechanosensory axons. Unlike the common 13-protofilament microtubules, the touch receptor neurons have 15 protofilaments, which may affect paclitaxel binding or its microtubule-stabilizing properties. Thus, studies pertaining to microtubule binding mechanisms might not be suitable in this model. Given the amenability of C. elegans for time-lapse imaging, further studies could investigate paclitaxel mechanisms in vivo.
Zebrafish (Danio rerio) models of CIPN
In recent years, zebrafish has become a premier model to study human diseases. About 80% of human disease-causing genes are conserved in zebrafish and thus, the likelihood of identifying molecular factors with similar functions in humans is relatively high. Zebrafish are also suitable for in vivo imaging due to their optical clarity and propensity for long-term maintenance in anesthetic and mounting medium as embryos and larvae. Thus, they are ideal for live imaging of axon degeneration. The rapid development of the peripheral sensory neurons and their differentiation within 48 hours allows for short waiting time until CIPN can be analyzed. Zebrafish possess two populations of somatosensory neurons that are formed around 18 hours post fertilization in the head and body, the trigeminal neurons and Rohon-Beard (RB) neurons, respectively. RB neurons are special in that they are transient and die between 2–4 weeks of age. They are then replaced by DRG neurons and thus juvenile and adult zebrafish have trigeminal and DRG neurons that are comparable to mammals. Despite the early developmental difference, RB neurons are molecularly equivalent to trigeminal and DRG neurons in that they share molecular markers (Norton et al., 2000; Palanca et al., 2013) that are also found in mammalian DRG and trigeminal neurons (Palanca et al., 2013). Similarly, their axons arborize within the skin similar to mammalian somatosensory neurons (Maklad et al., 2009; Sagasti et al., 2005). RB neurons coincide in their existence with the rather rudimentary skin in embryonic and larval zebrafish, which is made up of two layers, the outer enveloping (or periderm) layer, and the inner basal keratinocyte layer. Similar to mammals, the basal keratinocyte layer is innervated by unmyelinated sensory neurons. Around 4 weeks, the skin starts to differentiate into a stratified epithelium, at which time DRG neurons are fully functional (McGraw et al., 2012).
While zebrafish are useful models for CIPN research, RB neurons are unmyelinated and thus, in vivo imaging during larval stages can only be informative of the effects of chemotherapeutic agents on unmyelinated axons. Nevertheless, studies in the adults may also clarify the effects of those agents on myelinating Schwann cells. Another positive aspect of CIPN studies in zebrafish is the ease to perform chemical screens whereby chemicals can be added to the water. A drawback might be that the drugs are being taken up by the skin during such screens, which may not fully represent the human conditions. However, we have shown that, for instance, similar effects of paclitaxel on axons can be achieved regardless of the administration route, whether intravenous or in the water.
Vincristine and Bortezomib-induced neurotoxicity in zebrafish
Vincristine belongs to the vinca alkaloid class of chemotherapeutic agents, which disrupt microtubule assembly by binding with high affinity to the α-tubulin subunit of α/ß-tubulin dimers that assemble at the microtubule ends (Addington and Freimer, 2016; Lobert et al., 1996; Sertel et al., 2011). During cell division, disruption of the mitotic spindle apparatus leads to cell cycle arrest and a block in mitosis. Bortezomib, in contrast, is a 26S proteasome complex inhibitor and was found to inhibit various cell signaling pathways, among them NF-κB , leading to cell cycle arrest, apoptosis, and inhibition of angiogenesis (Jackson et al., 2005). Similar to other chemotherapeutic agents, vincristine and bortezomib cause peripheral neuropathy as a side effect. A recent meta-analysis showed that vincristine causes neuropathy in about 20% of patients (Seretny et al., 2014), leading to progressive sensorimotor neuropathy and, occasionally, to autonomic neuropathy (Lavoie Smith et al., 2015). Symptom onset can be rapid but is usually delayed and cumulative. Age also plays a role in the severity of symptoms whereby younger individuals are affected to a lesser extent compared with older patients. Bortezomib was shown to damage mitochondria and the endoplasmic reticulum, leading to disruption of Ca2+ homeostasis in cancer cells. Dysregulation of Ca2+ homeostasis has also been implicated in painful bortezomib-induced peripheral neuropathy (Argyriou et al., 2008; Landowski et al., 2005; Tomita et al., 2019).
Khan et al. developed a zebrafish model (Khan et al., 2012) to study the effects of both vincristine and bortezomib on axon degeneration using in vivo imaging and behavioral outcome measures. Zebrafish embryos were treated as early as 24 hours post fertilization for 24 hours with either vincristine or bortezomib at a time when axons are actively innervating the skin and have not yet fully differentiated. The results are therefore difficult to interpret because it is unclear how these chemotherapeutic agents affected sensory neuron development. As expected, however, treatment caused a dose-dependent increase in phospho-histone 3 labeling, which indicated cell cycle arrest at the mitotic phase. Treatment with either drug also reduced sensory axon branch density in the upper trunk, but these effects were highly variable between animals, as noted by the authors. In humans, CIPN usually progresses from the distal to proximal extremities. If the effects were expected to be comparable in zebrafish, this may have contributed to the lack of a consistent phenotype, since the upper trunk region is comparable to the torso in humans. Moreover, bortezomib treatment has been shown to cause only mild axonal degeneration, and neuronal death has been rarely observed in humans (Cavaletti et al., 2007). Other outcome measures, at least for bortezomib treatment, might be more informative and relevant to the clinic, such as nerve conduction velocity measurements.
Consistent with clinical findings, the authors found sensory and motor abnormalities when zebrafish embryos were analyzed for behavioral deficits upon treatment with either vincristine or bortezomib. Normally, zebrafish larvae escape from a stimulus with a stereotypical c-start movement (Eaton et al., 2001). This response was altered in a dose-dependent manner by both chemotherapeutic agents and indicated sensory and motor deficits in the animals. This and other well-defined behavioral responses in zebrafish can be useful to define motor deficits or sensory abnormalities.
Paclitaxel-induced neurotoxicity in zebrafish
We showed that zebrafish are excellent to study the mechanisms of paclitaxel-induced peripheral neurotoxicity (Lisse et al., 2016) (Figure 4). In our studies, paclitaxel was administered to the fish at 22μM (the highest dose that we found to be tolerated by larval zebrafish) using various routes. This chemotherapeutic agent was either supplied into the water of post-hatching larval zebrafish starting at 2 days post fertilization (dpf) for 4 days or injected once daily into the cardinal vein of larvae at 2, 3 and 4dpf, whereas adult animals received four intraperitoneal injections. The caudal fin was chosen as a region in which sensory axon degeneration was analyzed since this region is most comparable to the distal extremities in humans. The animals developed signs of paclitaxel neurotoxicity regardless of the method of administration, suggesting that drug delivery does not cause significant differences in the symptoms. Consistent with observations in humans, our studies showed that larval and adult zebrafish treated with paclitaxel displayed degeneration of unmyelinated axons of RB sensory neurons within the epidermis. A similar phenotype was also reported in rat models of paclitaxel neurotoxicity (Bennett et al., 2011; Boyette-Davis et al., 2011; Jin et al., 2008; Siau et al., 2006). The most prominent effects were typically observed in the distal region of the caudal fin, similar to the onset of human chemotherapy-induced peripheral neuropathy in the fingers and toes. In humans, high doses and prolonged treatment have been associated with motor deficits, but our zebrafish model did not develop any motor deficits, consistent with paclitaxel’s primary effect on sensory neurons (Gornstein and Schwarz, 2014).
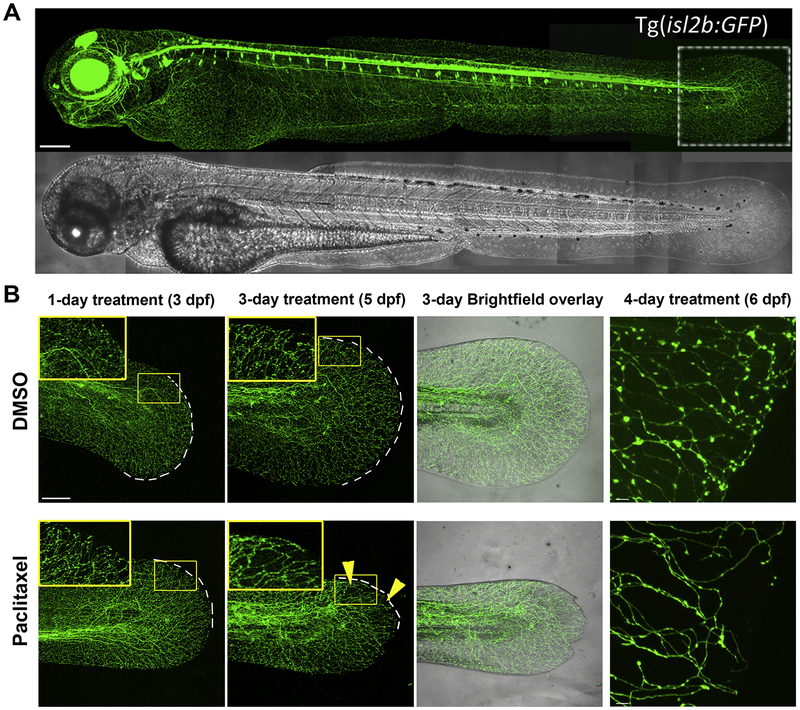
Figure 4. In vivo analysis of paclitaxel-induced neurotoxicity in zebrafish.
This figure is in part re-used from (Lisse et al., 2016). (A) Tg(isl2b:GFP) zebrafish larva with fluorescent sensory neurons in the head and body, which innervate the epidermis. (B) Larval zebrafish treated with either DMSO control vehicle (top) or 22μM paclitaxel (bottom) after 1, 3, and 4 days following treatment begin. Sensory axons selectively degenerate in the distal tail fin of zebrafish around 3–4 days when treated with paclitaxel but not DMSO.
Because larval zebrafish harbor only two layers of skin, paclitaxel treatment revealed a striking phenotype particularly in the basal keratinocyte layer of the epidermis. Since this layer is buried deep inside the mammalian epidermis, this phenotype may have been overlooked. Our studies showed that paclitaxel treatment induced an increased sensitivity of zebrafish larvae to mechanical stress, leading to injury formation and shedding of keratinocytes, assessed with scanning electron microscopy. Further analysis revealed the formation of increased hydrogen peroxide levels in keratinocytes, and upregulation of the matrix-metalloproteinase, MMP-13 specifically in the basal keratinocyte layer. Strikingly, pharmacological inhibition of MMP-13 prevented paclitaxel-induced sensory axon degeneration and injury formation and also rescued wound healing defects induced by paclitaxel. We recently showed that this mechanism is also conserved in diabetic peripheral neuropathy in zebrafish and mice, where MMP-13 inhibition was able to reverse neuropathy induced by a high fat/high sugar diet (Waldron et al., 2018). Unpublished evidence also suggests similar conservation in paclitaxel-treated rats. Thus, zebrafish can reveal novel conserved mechanisms of paclitaxel-induced neurotoxicity, which may lead to human therapies. One interesting finding in this study was that paclitaxel conjugated to fluorescein accumulated primarily in the skin, and we were unable to detect paclitaxel in the peripheral nerve endings in the skin for at least 12 hours after injection. In contrast, the epidermis was damaged within less than 3 hours of treatment. Intriguingly, paclitaxel accumulated in the soma of some sensory neurons but not all, suggesting that (1) peripheral axons may receive extremely low paclitaxel concentrations below detection level, and (2) different subtypes have specific mechanisms that either promote or prevent paclitaxel accumulation. This also explains why some axons degenerate while others remain unaffected.
Conclusions and future perspectives
CIPN studies using non-mammalian species indicate that these models are useful to identify genetic mechanisms underlying chemotherapy-induced neurotoxicity. These models have provided new candidate genes that have conserved functions in rodents; thus, the likelihood of being conserved in humans is relatively high. Non-mammalian CIPN models are limited to few chemotherapeutic agents and there is potential for further expansion of these studies and the establishment of additional CIPN models. Non-rodent models have various advantages over rodents, such as the ability to perform large-scale genetic and pharmacological screens and the amenability for in vivo imaging. However, a few thoughts should be considered when analyzing CIPN in these species. For instance, conclusions about phenotypes observed in non-mammalian species are difficult to relate to humans because the anatomical differences make it more difficult to compare. Although, most models can be useful to study unmyelinated axon phenotypes. There is further difficulty determining whether a given dose leading to neurotoxicity is comparable to human CIPN. It is especially critical that standardized methods for determining the dose leading to CIPN are considered because the evidence in flies and mice shows that lower concentrations of paclitaxel can have stabilizing, and even positive effects on nerve endings. A useful readout when establishing a CIPN model could be to determine the effects of a given chemotherapeutic agent on axonal mitochondria. Mitochondrial damage in peripheral axons appears to be a commonly observed phenotype under various neuropathy conditions (Areti et al., 2014; Barneo-Muñoz et al., 2015; Cassereau et al., 2014; Dai et al., 2019; Flatters, 2015; Sifuentes-Franco et al., 2017; Stacpoole et al., 2019; Zheng et al., 2012), and our data also shows that a similar phenotype is induced in zebrafish keratinocytes and sensory axons following paclitaxel treatment. Because rodent studies have shown that painful CIPN does not necessarily correlate with axon degeneration (Bennett et al., 2011), assessment of mitochondria function might be more reliable in establishing CIPN presence. Mitochondrial damage in axons examined at the ultrastructure level using transmission electron microscopy could, in principle, be achieved with minimal effort regardless of the size of the animal. It also does not rely on the use of electrophysiology to assess CIPN, which requires expertise. However, such studies are difficult to perform in smaller animals, like invertebrates and zebrafish.
As an alternative to examining mitochondrial damage, one could establish CIPN models by using the maximum tolerated dose of a given chemotherapeutic agent, as this would be consistent with doses administered to cancer patients. The Drosophila cisplatin model included survival data (Podratz et al., 2011b), but none of the other studies revealed why a particular concentration of the chemotherapeutic agent was chosen. Our zebrafish paclitaxel model was established based on the following parameters. We initially determined the LD50 (~10–22μM depending on the administration in larval or adult fish) (Lisse et al., 2016) and used these doses to analyze CIPN phenotypes. Although our calculations determined that these doses are ~100-fold below the concentrations administered in humans, it reflects a comparable dose in terms of survival. The tolerated dose likely reflects variations in drug metabolism, and thus, concentrations cannot be used similar to humans in these animals. Because of this, studies aimed at drug uptake and bioavailability or assessing the onset of CIPN are less useful given these differences. The route by which a chemotherapeutic agent is administered appears to be less important for the induction of CIPN. For instance, CIPN phenotypes comparable to other neuropathies have been observed in all species regardless of how the chemotherapeutic agent was administered and at which dose (Cavaletti et al., 2000; Cavaletti et al., 1995). Differences in the severity of phenotypes arose, however, these were mostly background-dependent, as shown in mice (Smith et al., 2004) and Drosophila treated with paclitaxel and cisplatin (Groen et al., 2018), respectively.
In conclusion, non-mammalian models have shown to be useful in identifying CIPN mechanisms and further validation studies revealed their conservation in rodents. In the future, it will be important to carefully design CIPN studies using non-mammalian species, in terms of dosage and phenotype analysis, to be able to better compare observed mechanisms with the human condition. The ability to perform large-scale genetic and drug screening assays in combination with in vivo imaging of axon de- and regeneration has the potential to identify additional genetic players and pharmacological candidates that could be useful in treating CIPN in humans. Identified genes will have to be validated in rodents but their successful validation increases the likelihood that the mechanisms are also conserved in humans.
Highlights.
C. elegans, Drosophila and zebrafish as chemotherapy-induced neurotoxicity models
In vivo imaging revealed new CIPN mechanisms with conserved functions in mammals
NMNAT, DLK, and MORN are major players in the control of paclitaxel-induced axon degeneration
ROS and altered microtubule function are aberrant in paclitaxel-induced peripheral neuropathy
Epidermal damage contributes to paclitaxel neurotoxicity through activation of MMP-13
Acknowledgments
We thank Antonio Cadiz, Marisa Benjamin, and Natalie Schmidt for critically reading the manuscript and providing constructive feedback. We thank the Peripheral Nerve Society and Toxic Neuropathy Consortium for their support.
Funding sources
This work was supported by grants from the National Institutes of Health [grant 5RO1CA215973] and a CTSI-Pilot Award (University of Miami).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflicts of interest.
References
- Addington J, Freimer M, 2016. Chemotherapy-induced peripheral neuropathy: an update on the current understanding. F1000Res 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos LA, Löwe J, 1999. How Taxol stabilises microtubule structure. Chem Biol 6, R65–69. [DOI] [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J, 2004. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 305, 1010–1013. [DOI] [PubMed] [Google Scholar]
- Areti A, Yerra VG, Naidu V, Kumar A, 2014. Oxidative stress and nerve damage: role in chemotherapy induced peripheral neuropathy. Redox Biol 2, 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyriou AA, Iconomou G, Kalofonos HP, 2008. Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood 112, 1593–1599. [DOI] [PubMed] [Google Scholar]
- Arnal I, Wade RH, 1995. How does taxol stabilize microtubules? Curr Biol 5, 900–908. [DOI] [PubMed] [Google Scholar]
- Baan RA, Zaalberg OB, Fichtinger-Schepman AM, Muysken-Schoen MA, Lansbergen MJ, Lohman PH, 1985. Use of monoclonal and polyclonal antibodies against DNA adducts for the detection of DNA lesions in isolated DNA and in single cells. Environ Health Perspect 62, 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barneo-Muñoz M, Juárez P, Civera-Tregón A, Yndriago L, Pla-Martin D, Zenker J, Cuevas-Martín C, Estela A, Sánchez-Aragó M, Forteza-Vila J, Cuezva JM, Chrast R, Palau F, 2015. Lack of GDAP1 induces neuronal calcium and mitochondrial defects in a knockout mouse model of charcot-marie-tooth neuropathy. PLoS Genet 11, e1005115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrière DA, Rieusset J, Chanteranne D, Busserolles J, Chauvin MA, Chapuis L, Salles J, Dubray C, Morio B, 2012. Paclitaxel therapy potentiates cold hyperalgesia in streptozotocin-induced diabetic rats through enhanced mitochondrial reactive oxygen species production and TRPA1 sensitization. Pain 153, 553–561. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Liu GK, Xiao WH, Jin HW, Siau C, 2011. Terminal arbor degeneration--a novel lesion produced by the antineoplastic agent paclitaxel. Eur J Neurosci 33, 1667–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya MR, Gerdts J, Naylor SA, Royse EX, Ebstein SY, Sasaki Y, Milbrandt J, DiAntonio A, 2012. A model of toxic neuropathy in Drosophila reveals a role for MORN4 in promoting axonal degeneration. J Neurosci 32, 5054–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobylev I, Joshi AR, Barham M, Ritter C, Neiss WF, Höke A, Lehmann HC, 2015. Paclitaxel inhibits mRNA transport in axons. Neurobiol Dis 82, 321–331. [DOI] [PubMed] [Google Scholar]
- Bounoutas A, O’Hagan R, Chalfie M, 2009. The multipurpose 15-protofilament microtubules in C. elegans have specific roles in mechanosensation. Curr Biol 19, 1362–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyette-Davis J, Xin W, Zhang H, Dougherty PM, 2011. Intraepidermal nerve fiber loss corresponds to the development of taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain 152, 308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazill JM, Cruz B, Zhu Y, Zhai RG, 2018a. Nmnat mitigates sensory dysfunction in a. Dis Model Mech 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazill JM, Cruz B, Zhu Y, Zhai RG, 2018b. Nmnat mitigates sensory dysfunction in a Drosophila model of paclitaxel-induced peripheral neuropathy. Dis Model Mech 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussmann J, Storkebaum E, 2017. Molecular pathogenesis of peripheral neuropathies: insights from Drosophila models. Curr Opin Genet Dev 44, 61–73. [DOI] [PubMed] [Google Scholar]
- Cassereau J, Codron P, Funalot B, 2014. Inherited peripheral neuropathies due to mitochondrial disorders. Rev Neurol (Paris) 170, 366–374. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Cavalletti E, Oggioni N, Sottani C, Minoia C, D’Incalci M, Zucchetti M, Marmiroli P, Tredici G, 2000. Distribution of paclitaxel within the nervous system of the rat after repeated intravenous administration. Neurotoxicology 21, 389–393. [PubMed] [Google Scholar]
- Cavaletti G, Gilardini A, Canta A, Rigamonti L, Rodriguez-Menendez V, Ceresa C, Marmiroli P, Bossi M, Oggioni N, D’Incalci M, De Coster R, 2007. Bortezomib-induced peripheral neurotoxicity: a neurophysiological and pathological study in the rat. Exp Neurol 204, 317–325. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Tredici G, Braga M, Tazzari S, 1995. Experimental peripheral neuropathy induced in adult rats by repeated intraperitoneal administration of taxol. Exp Neurol 133, 64–72. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Sulston J, 1981. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev Biol 82, 358–370. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Thomson JN, 1979. Organization of neuronal microtubules in the nematode Caenorhabditis elegans. J Cell Biol 82, 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L, Tarlton A, Mack TG, Mi W, Buckmaster EA, Wagner D, Perry VH, Coleman MP, 2000. A Ufd2/D4Cole1e chimeric protein and overexpression of Rbp7 in the slow Wallerian degeneration (WldS) mouse. Proc Natl Acad Sci U S A 97, 11377–11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho-Budd J, Freeman MR, 2013. Probing the enigma: unraveling glial cell biology in invertebrates. Curr Opin Neurobiol 23, 1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Tang S, Biao X, Xiao X, Chen C, Li J, 2019. Colistin induced peripheral neurotoxicity involves mitochondrial dysfunction and oxidative stress in mice. Mol Biol Rep 46, 1963–1972. [DOI] [PubMed] [Google Scholar]
- Dolan ME, El Charif O, Wheeler HE, Gamazon ER, Ardeshir-Rouhani-Fard S, Monahan P, Feldman DR, Hamilton RJ, Vaughn DJ, Beard CJ, Fung C, Kim J, Fossa SD, Hertz DL, Mushiroda T, Kubo M, Einhorn LH, Cox NJ, Travis LB, Group PS, 2017. Clinical and Genome-Wide Analysis of Cisplatin-Induced Peripheral Neuropathy in Survivors of Adult-Onset Cancer. Clin Cancer Res 23, 5757–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton RC, Lee RK, Foreman MB, 2001. The Mauthner cell and other identified neurons of the brainstem escape network of fish. Prog Neurobiol 63, 467–485. [DOI] [PubMed] [Google Scholar]
- Flatters SJ, 2015. The contribution of mitochondria to sensory processing and pain. Prog Mol Biol Transl Sci 131, 119–146. [DOI] [PubMed] [Google Scholar]
- Flatters SJ, Bennett GJ, 2006. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain 122, 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh-Roy A, Wu Z, Goncharov A, Jin Y, Chisholm AD, Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J Neurosci 30, 3175–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibert Y, Trengove MC, Ward AC, 2013. Zebrafish as a genetic model in pre-clinical drug testing and screening. Curr Med Chem 20, 2458–2466. [DOI] [PubMed] [Google Scholar]
- Goodman MB, 2006. Mechanosensation WormBook, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gornstein E, Schwarz TL, 2014. The paradox of paclitaxel neurotoxicity: Mechanisms and unanswered questions. Neuropharmacology 76 Pt A, 175–183. [DOI] [PubMed] [Google Scholar]
- Groen CM, Podratz JL, Treb K, Windebank AJ, 2018. Drosophila strain specific response to cisplatin neurotoxicity. Fly (Austin) 12, 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund M, Jorgensen EM, Bastiani MJ, 2007. Axons break in animals lacking beta-spectrin. J Cell Biol 176, 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari M, Loganzo F, Annable T, Tan X, Musto S, Morilla DB, Nettles JH, Snyder JP, Greenberger LM, 2006. Paclitaxel-resistant cells have a mutation in the paclitaxel-binding region of beta-tubulin (Asp26Glu) and less stable microtubules. Mol Cancer Ther 5, 270–278. [DOI] [PubMed] [Google Scholar]
- Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, Kapitein LC, Strikis D, Lemmon V, Bixby J, Hoogenraad CC, Bradke F, 2011. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science 331, 928–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson SJ, Costa WS, Wabnig S, Stirman JN, Watson JD, Spencer WC, Akerboom J, Looger LL, Treinin M, Miller DM, Lu H, Gottschalk A, 2012. Optogenetic analysis of a nociceptor neuron and network reveals ion channels acting downstream of primary sensors. Curr Biol 22, 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SH, Galko MJ, 2012. Pokes, sunburn, and hot sauce: Drosophila as an emerging model for the biology of nociception. Dev Dyn 241, 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson G, Einsele H, Moreau P, Miguel JS, 2005. Bortezomib, a novel proteasome inhibitor, in the treatment of hematologic malignancies. Cancer Treat Rev 31, 591–602. [DOI] [PubMed] [Google Scholar]
- Jin HW, Flatters SJ, Xiao WH, Mulhern HL, Bennett GJ, 2008. Prevention of paclitaxel-evoked painful peripheral neuropathy by acetyl-L-carnitine: effects on axonal mitochondria, sensory nerve fiber terminal arbors, and cutaneous Langerhans cells. Exp Neurol 210, 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L, 1996. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res 56, 816–825. [PubMed] [Google Scholar]
- Kanda H, Liu S, Iida T, Yi H, Huang W, Levitt RC, Lubarsky DA, Candiotti KA, Hao S, 2016. Inhibition of Mitochondrial Fission Protein Reduced Mechanical Allodynia and Suppressed Spinal Mitochondrial Superoxide Induced by Perineural Human Immunodeficiency Virus gp120 in Rats. Anesth Analg 122, 264–272. [DOI] [PubMed] [Google Scholar]
- Kelman AD, Peresie HJ, 1979. Mode of DNA binding of cis-platinum(II) antitumor drugs: a base sequence-dependent mechanism is proposed. Cancer Treat Rep 63, 1445–1452. [PubMed] [Google Scholar]
- Khan TM, Benaich N, Malone CF, Bernardos RL, Russell AR, Downes GB, Barresi MJ, Hutson LD, 2012. Vincristine and bortezomib cause axon outgrowth and behavioral defects in larval zebrafish. J Peripher Nerv Syst 17, 76–89. [DOI] [PubMed] [Google Scholar]
- Landowski TH, Megli CJ, Nullmeyer KD, Lynch RM, Dorr RT, 2005. Mitochondrial-mediated disregulation of Ca2+ is a critical determinant of Velcade (PS-341/bortezomib) cytotoxicity in myeloma cell lines. Cancer Res 65, 3828–3836. [DOI] [PubMed] [Google Scholar]
- Lavoie Smith EM, Li L, Chiang C, Thomas K, Hutchinson RJ, Wells EM, Ho RH, Skiles J, Chakraborty A, Bridges CM, Renbarger J, 2015. Patterns and severity of vincristine-induced peripheral neuropathy in children with acute lymphoblastic leukemia. J Peripher Nerv Syst 20, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisse TS, Middleton LJ, Pellegrini AD, Martin PB, Spaulding EL, Lopes O, Brochu EA, Carter EV, Waldron A, Rieger S, 2016. Paclitaxel-induced epithelial damage and ectopic MMP-13 expression promotes neurotoxicity in zebrafish. Proc Natl Acad Sci U S A 113, E2189–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobert S, Vulevic B, Correia JJ, 1996. Interaction of vinca alkaloids with tubulin: a comparison of vinblastine, vincristine, and vinorelbine. Biochemistry 35, 6806–6814. [DOI] [PubMed] [Google Scholar]
- Lyon MF, Ogunkolade BW, Brown MC, Atherton DJ, Perry VH, 1993. A gene affecting Wallerian nerve degeneration maps distally on mouse chromosome 4. Proc Natl Acad Sci U S A 90, 9717–9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack TG, Reiner M, Beirowski B, Mi W, Emanuelli M, Wagner D, Thomson D, Gillingwater T, Court F, Conforti L, Fernando FS, Tarlton A, Andressen C, Addicks K, Magni G, Ribchester RR, Perry VH, Coleman MP, 2001. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci 4, 1199–1206. [DOI] [PubMed] [Google Scholar]
- Mak IW, Evaniew N, Ghert M, 2014. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res 6, 114–118. [PMC free article] [PubMed] [Google Scholar]
- Maklad A, Nicolai JR, Bichsel KJ, Evenson JE, Lee TC, Threadgill DW, Hansen LA, 2009. The EGFR is required for proper innervation to the skin. J Invest Dermatol 129, 690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao XR, Kaufman DM, Crowder CM, 2016. Nicotinamide mononucleotide adenylyltransferase promotes hypoxic survival by activating the mitochondrial unfolded protein response. Cell Death Dis 7, e2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SM, O’Brien GS, Portera-Cailliau C, Sagasti A, 2010. Wallerian degeneration of zebrafish trigeminal axons in the skin is required for regeneration and developmental pruning. Development 137, 3985–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw HF, Snelson CD, Prendergast A, Suli A, Raible DW, 2012. Postembryonic neuronal addition in zebrafish dorsal root ganglia is regulated by Notch signaling. Neural Dev 7, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JL, Miller RW, Powell RG, Smith CR, 1981. 19-Hydroxybaccatin III, 10-deacetylcephalomannine, and 10-deacetyltaxol: new antitumor taxanes from Taxus wallichiana. J Nat Prod 44, 312–319. [DOI] [PubMed] [Google Scholar]
- Melli G, Taiana M, Camozzi F, Triolo D, Podini P, Quattrini A, Taroni F, Lauria G, 2008. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Exp Neurol 214, 276–284. [DOI] [PubMed] [Google Scholar]
- Miller BR, Press C, Daniels RW, Sasaki Y, Milbrandt J, DiAntonio A, 2009. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat Neurosci 12, 387–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosavljevic N, Duranton C, Djerbi N, Puech PH, Gounon P, Lagadic-Gossmann D, Dimanche-Boitrel MT, Rauch C, Tauc M, Counillon L, Poët M, 2010. Nongenomic effects of cisplatin: acute inhibition of mechanosensitive transporters and channels without actin remodeling. Cancer Res 70, 7514–7522. [DOI] [PubMed] [Google Scholar]
- Niemann A, Wagner KM, Ruegg M, Suter U, 2009. GDAP1 mutations differ in their effects on mitochondrial dynamics and apoptosis depending on the mode of inheritance. Neurobiol Dis 36, 509–520. [DOI] [PubMed] [Google Scholar]
- Nogales E, Wolf SG, Khan IA, Ludueña RF, Downing KH, 1995. Structure of tubulin at 6.5 A and location of the taxol-binding site. Nature 375, 424–427. [DOI] [PubMed] [Google Scholar]
- Norton WH, Rohr KB, Burnstock G, 2000. Embryonic expression of a P2X(3) receptor encoding gene in zebrafish. Mech Dev 99, 149–152. [DOI] [PubMed] [Google Scholar]
- Owoeye O, Adedara IA, Farombi EO, 2018. Pretreatment with taurine prevented brain injury and exploratory behaviour associated with administration of anticancer drug cisplatin in rats. Biomed Pharmacother 102, 375–384. [DOI] [PubMed] [Google Scholar]
- Palanca AM, Lee SL, Yee LE, Joe-Wong C, Trinh le A, Hiroyasu E, Husain M, Fraser SE, Pellegrini M, Sagasti A, 2013. New transgenic reporters identify somatosensory neuron subtypes in larval zebrafish. Dev Neurobiol 73, 152–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palau F, Estela A, Pla-Martín D, Sánchez-Piris M, 2009. The role of mitochondrial network dynamics in the pathogenesis of Charcot-Marie-Tooth disease. Adv Exp Med Biol 652, 129–137. [DOI] [PubMed] [Google Scholar]
- Parness J, Horwitz SB, 1981. Taxol binds to polymerized tubulin in vitro. J Cell Biol 91, 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podratz JL, Knight AM, Ta LE, Staff NP, Gass JM, Genelin K, Schlattau A, Lathroum L, Windebank AJ, 2011a. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol Dis 41, 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podratz JL, Lee H, Knorr P, Koehler S, Forsythe S, Lambrecht K, Arias S, Schmidt K, Steinhoff G, Yudintsev G, Yang A, Trushina E, Windebank A, 2017. Cisplatin induces mitochondrial deficits in Drosophila larval segmental nerve. Neurobiol Dis 97, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podratz JL, Staff NP, Boesche JB, Giorno NJ, Hainy ME, Herring SA, Klennert MT, Milaster C, Nowakowski SE, Krug RG, Peng Y, Windebank AJ, 2013. An automated climbing apparatus to measure chemotherapy-induced neurotoxicity in Drosophila melanogaster. Fly (Austin) 7, 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podratz JL, Staff NP, Froemel D, Wallner A, Wabnig F, Bieber AJ, Tang A, Windebank AJ, 2011b. Drosophila melanogaster: a new model to study cisplatin-induced neurotoxicity. Neurobiol Dis 43, 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polomano RC, Mannes AJ, Clark US, Bennett GJ, 2001. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain 94, 293–304. [DOI] [PubMed] [Google Scholar]
- Ren X, Zhao B, Chang H, Xiao M, Wu Y, Liu Y, 2018. Paclitaxel suppresses proliferation and induces apoptosis through regulation of ROS and the AKT/MAPK signaling pathway in canine mammary gland tumor cells. Mol Med Rep 17, 8289–8299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagasti A, Guido MR, Raible DW, Schier AF, 2005. Repulsive interactions shape the morphologies and functional arrangement of zebrafish peripheral sensory arbors. Curr Biol 15, 804–814. [DOI] [PubMed] [Google Scholar]
- Savage C, Hamelin M, Culotti JG, Coulson A, Albertson DG, Chalfie M, 1989. mec-7 is a beta-tubulin gene required for the production of 15-protofilament microtubules in Caenorhabditis elegans. Genes Dev 3, 870–881. [DOI] [PubMed] [Google Scholar]
- Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, Colvin LA, Fallon M, 2014. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 155, 2461–2470. [DOI] [PubMed] [Google Scholar]
- Sertel S, Fu Y, Zu Y, Rebacz B, Konkimalla B, Plinkert PK, Krämer A, Gertsch J, Efferth T, 2011. Molecular docking and pharmacogenomics of vinca alkaloids and their monomeric precursors, vindoline and catharanthine. Biochem Pharmacol 81, 723–735. [DOI] [PubMed] [Google Scholar]
- Siau C, Xiao W, Bennett GJ, 2006. Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Exp Neurol 201, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifuentes-Franco S, Pacheco-Moisés FP, Rodríguez-Carrizalez AD, Miranda-Díaz AG, 2017. The Role of Oxidative Stress, Mitochondrial Function, and Autophagy in Diabetic Polyneuropathy. J Diabetes Res 2017, 1673081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhania A, Grueber WB, 2014. Development of the embryonic and larval peripheral nervous system of Drosophila. Wiley Interdiscip Rev Dev Biol 3, 193–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SB, Crager SE, Mogil JS, 2004. Paclitaxel-induced neuropathic hypersensitivity in mice: responses in 10 inbred mouse strains. Life Sci 74, 2593–2604. [DOI] [PubMed] [Google Scholar]
- Stacpoole PW, Martyniuk CJ, James MO, Calcutt NA, 2019. Dichloroacetate-induced peripheral neuropathy. Int Rev Neurobiol 145, 211–238. [DOI] [PubMed] [Google Scholar]
- Tomita S, Sekiguchi F, Deguchi T, Miyazaki T, Ikeda Y, Tsubota M, Yoshida S, Nguyen HD, Okada T, Toyooka N, Kawabata A, 2019. Critical role of Ca. Toxicology 413, 33–39. [DOI] [PubMed] [Google Scholar]
- Tracey WD, Wilson RI, Laurent G, Benzer S, 2003. painless, a Drosophila gene essential for nociception. Cell 113, 261–273. [DOI] [PubMed] [Google Scholar]
- Waldron AL, Schroder PA, Bourgon KL, Bolduc JK, Miller JL, Pellegrini AD, Dubois AL, Blaszkiewicz M, Townsend KL, Rieger S, 2018. Oxidative stress-dependent MMP-13 activity underlies glucose neurotoxicity. J Diabetes Complications 32, 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenburg N, Baumeister R, 1999. Thermal avoidance in Caenorhabditis elegans: an approach to the study of nociception. Proc Natl Acad Sci U S A 96, 10477–10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang IH, Siddique R, Hosmane S, Thakor N, Höke A, 2009. Compartmentalized microfluidic culture platform to study mechanism of paclitaxel-induced axonal degeneration. Exp Neurol 218, 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai RG, Cao Y, Hiesinger PR, Zhou Y, Mehta SQ, Schulze KL, Verstreken P, Bellen HJ, 2006. Drosophila NMNAT maintains neural integrity independent of its NAD synthesis activity. PLoS Biol 4, e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Bianchi L, Lee WH, Wang Y, Israel S, Driscoll M, 2008. Intersubunit interactions between mutant DEG/ENaCs induce synthetic neurotoxicity. Cell Death Differ 15, 1794–1803. [DOI] [PubMed] [Google Scholar]
- Zheng H, Xiao WH, Bennett GJ, 2011. Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Exp Neurol 232, 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Xiao WH, Bennett GJ, 2012. Mitotoxicity and bortezomib-induced chronic painful peripheral neuropathy. Exp Neurol 238, 225–234. [DOI] [PubMed] [Google Scholar]