Abstract
Purpose of Review:
Eosinophils are short-lived granulocytes that contain a variety of proteins and lipids traditionally associated with host defense against parasites. The primary goal of this review is to examine more recent evidence that challenged this outdated role of eosinophils in the context of pulmonary infections with helminths, viruses, and bacteria.
Recent Findings:
While eosinophil mechanisms that counter parasites, viruses and bacteria are similar, the kinetics and impact may differ by pathogen type. Major antiparasitic responses include direct killing, immunoregulation, as well as some mechanisms by which parasite survival/growth is supported. Antiviral defenses may be as unembellished as granule protein-induced direct killing or more urbane as serving as a conduit for better adaptive immune responses to the invading virus. Although sacrificial, eosinophil DNA emitted in response to bacteria help trap bacteria to limit dissemination. Herein, we discuss the current research redefining eosinophils as multifunctional cells that are active participants in the host defense against lung pathogens.
Summary:
Eosinophils recognize and differentially respond to invading pathogens, allowing them to deploy innate defense mechanisms to contain and clear the infection, or modulate the immune response. Modern technology and animal models have unraveled hitherto unknown capabilities of this surreptitious cell that indubitably has more functions awaiting discovery.
Keywords: helminth, virus, bacteria
Introduction
Eosinophils are granulocytes derived from CD34+ pluripotent hematopoietic stem cells in the bone marrow and belong to the innate branch of the immune system. While sharing some phenotypic and functional similarities with other members of the granulocyte family, eosinophils maintain their individualilty by their granule structure and contents, and the sophisticated means by which they are released. Although previously described by other investigators, Paul Ehrlich is credited for the identification and appellation of eosinophils in 1879 based on their unique staining properties from eosin uptake by granules [1]. Eosinophils have been evolutionarily preserved across organisms; invertebrates like annelids, insects, and crabs, contain eosinophil-like cells, while vertebrates including fish, reptiles and even lampreys have cells that are morphologically similar to the classical mammalian eosinophils [2, 3].
Eosinophils are regarded as terminally differentiated cells that reside in mucosal tissues. Pluripotent CD34+ stem cells commit to the myeloid lineage through expression of GATA-1, PU.1, and c/EBP transcription factors and develop into mature eosinophils when stimulated with IL-3, GM-CSF, and IL-5 [4]. Their presence in the bone marrow may help sustain plasma B cell populations long-term, thereby contributing to overall humoral memory especially to vaccines [5, 6]. Once released into the blood stream, eosinophils migrate to the thymus, mammary glands, gastrointestinal tract, and the uterus, where they may function in organ development and remodeling during homeostasis and disease [7, 8]. Resting eosinophils are maintained in the bone marrow, blood, and spleen [9]. Chemokines (primarily eotaxins) as well as IL-5 released in response to stimuli, signal eosinopoiesis and emigration to the trigger site where their survival depends on microenvironmental availability of growth factors, especially IL-5 [4, 10].
Despite the wealth of information garnered over the past 140 years resulting in 46,250 articles (at the time of writing) on PubMed dating back to 1911, the exact biophysical function of eosinophils and the reason for their evolutionary conservation remains unclear. Eosinophil deficiency in humans and mice appears to have no impact [8, 11], and yet, eosinophils and their products are generally considered injurious agents during allergic asthma [12, 13]. Their presence in stimulated tissues appears to be the focus point for investigation into their functions and contribution to disease. The structural composition of eosinophil granules is unique with an electron dense core mainly consisting of major basic protein (MBP) and an electron lucent matrix, containing other eosinophil-specific cationic proteins (high affinity for acidic dyes) in addition to a variety of cytokines, demarcated by a trilaminar membrane [14, 15]. Functions of eosinophil cationic proteins range from affecting mitogenic and motogenic properties of other cells, to inducing tissue injury and promoting repair [7, 16]. Lipid bodies in the cytoplasm permit additional functions for eosinophils as regulators of lipid metabolism and eicosanoid production sites [17, 18]. The number of lipid bodies contained in eosinophils increases with inflammation [18] further emphasizing the dynamic and responsive nature of these cells.
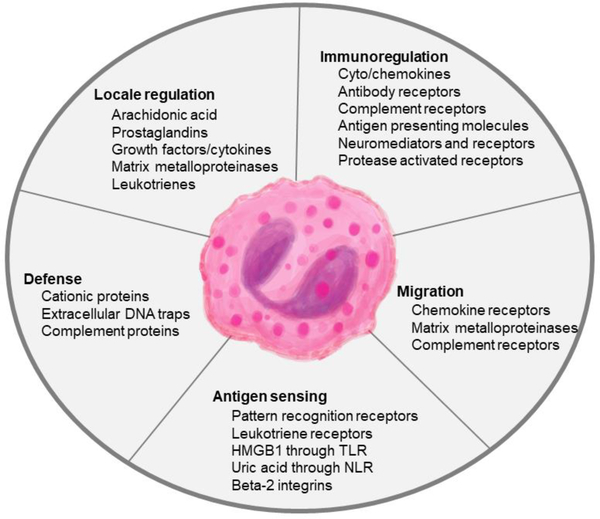
Largely owing to their presence in the targeted tissues, eosinophils are deemed to be detrimental in diseases like allergic asthma. Reducing the number of circulating eosinophils through anti-IL-5/IL-5R antibodies (mepolizumab, reslizumab, and benralizumab) in patients with severe asthma correlates with reduced asthma exacerbations, however, this effect is also noted in non-eosinophilic asthmatics [19]. Given that asthma inflammation is rich in leukocytes both in variety and abundance, it is difficult to ascertain cells that are causative from those that are recruited but have spectator impacts. Since a number of other leukocytes are responsive to IL-5 (B cells [20], mast cells [21], basophils [22]), alleviation of symptoms with anti-IL-5 therapies may be due to a variety of other factors that influence asthma inflammation and a definite tissue-destructive role for eosinophils may not be assigned. Similarly, their abundance in tissues hosting parasites led to the notion that anti-helminth immunity was the raison d’être for eosinophils. The LIAR (local immunity and/or remodeling/repair) hypothesis [3] suggests instead that eosinophils are recruited to locales of tissue damage where they engage in contributory effector functions at the inflammatory foci together with other cells to regain tissue homeostasis. The multitude of mediators contained within [23] basically makes this cell a miniature immune system capable of a broad range of functions [7, 8, 10, 23] which underscores their importance to host immunity (Figure 1).
Figure 1. Functions of eosinophils during lung infections.
Eosinophil granule contents including granule proteins and cytokines promote direct defense strategies to counter pathogens in addition to enhancing the functions of surrounding immune cells. The expression of a variety of receptors on the eosinophil surface allows the cells to sense and respond to the environment in real-time.
Akin to other organ systems, the respiratory system performs secondary functions such as host defense, acid balance, optimizing cardiac output, and filtration [24–26]. Mainly owing to the large surface area and vasculature for their primary function of gas exchange, the lungs are incessantly at risk for pathogen infections. In addition to mucosal defenses that include antimicrobial peptides, muco-ciliary escalator, surfactants, and the physical barrier that the airway lining provides, resident cells of the pulmonary system like macrophages engage in routine antigen clearance to prevent infection and maintain lung homeostasis. Recently, lung resident eosinophils were identified to perform a similar function [27]. While best known functions of eosinophils have been delineated in pulmonary disease triggered by allergens and parasites, eosinophil responses to respiratory viruses and bacteria are now being rapidly elucidated. Readers are referred to a number of excellent reviews that discuss eosinophil responses to allergens [28, 29] and their potential role in asthma [13, 30, 31]. The purpose of this article is to provide a compendium of the literature that focuses on the functions of eosinophils during pulmonary stages of parasite infections as well as during viral and bacterial infections of the lungs.
Eosinophils and Worms: In Sickness and in Health
Helminths are now considered “old world” pathogens because morbidities associated with them only affect small fraction (albeit hundreds of millions) of the world’s population at present [32]. However, helminths are likely an important aspect of the “old friends” hypothesis suggesting that their elimination in the human host may have led to an increase in aberrant diseases like asthma. Nematodes, trematodes, and cestodes have lung migration and/or dwelling phases in their life cycles that can trigger inflammation with an eosinophil predominance in the blood and tissue that occurs within hours to days after infection [33, 34].
Defense against extracellular multicellular organisms generally involves humoral and cellular components of innate and adaptive immunity, as phagocytosis may not be feasible due to size. Eosinophil granule proteins, MBP, eosinophil peroxidase (EPO), and eosinophil cationic protein (ECP) are all cytotoxic to parasites [16], and eosinophils rapidly aggregate around helminths killing them within minutes [35] as a display of their lethality. The immunoregulatory roles played by MBP and EPO during parasite infection is evident in studies that have utilized mice deficient in these proteins [36]. Both MBP and EPO deficient mice have increased worm burden and size, and altered macrophage and T cell functions in response to nematode infection [37]. Additionally, eosinophil derived neurotoxin (EDN) is an alarmin that promotes dendritic cell (DC) polarization toward TH2 [38] thereby setting the stage for a TH2 immune response during helminth infections. Other eosinophil secretory products such as IL-4, IL-5, TGF-β may accompany granule proteins during eosinophil degranulation to regulate the landscape surrounding helminths during lung infection [7, 39, 40].
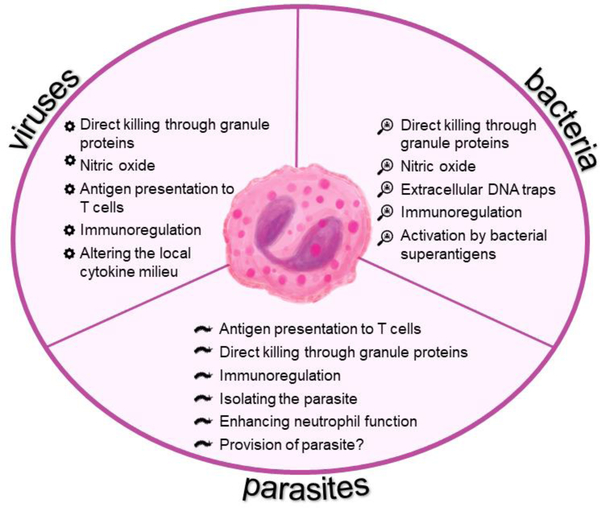
While fully capable of killing helminths directly, it is unclear whether eosinophils perform this action in vivo [39]. Studies utilizing eosinophil deficient mice show little [36, 41] to no [39, 42] benefit during helminth infection suggesting that eosinophil responses to parasites may be situational and redundant [43]. Furthermore, eosinophils are necessary for the survival of Trichinella spiralis in mouse skeletal muscles [42], with enhanced recruitment of neutrophils observed in their absence resulting in nitric oxide mediated killing of the parasite, and reduced numbers of IL-4 producing CD4+ T cells [44]. Since T cells are incapable of directly killing these large extracellular multicellular organisms, it is speculated that T cells orchestrate parasite damage through disabling, degrading, and dislodging effects [40] of which eosinophils are particularly useful for degrading effects which destroy parasite integrity. Anti-helminth properties of eosinophils may be redefined as more sophisticated functions of eosinophils are identified. It is possible that eosinophils are recruited to the lungs during parasite infections as an effector cell to directly harm the parasite as well as an immunomodulatory cell to enhance cell-cell crosstalk at the infection site to expel the parasite and promote wound repair mechanisms or temporally safeguard the parasite to maintain a ‘healthy’ antigen burden in the tissue to help reduce allergies (Figure 2).
Figure 2. Anti-pathogen responses of eosinophils during lung infections.
Broad anti-pathogen functions of eosinophils are applicable to respiratory viruses and bacteria as well as the lung-phase of parasites.
When Eosinophils Meet Viruses
Common respiratory viruses such as respiratory syncytial virus (RSV), rhinovirus (RV), and influenza A virus (IAV) annually infect millions worldwide and cause severe morbidity and mortality incurring a significant economic burden to society. Infection of the respiratory epithelia triggers the release of cytokines and chemokines that may inadvertently incur eosinophil recruitment to the lungs. Additionally, eosinophils can be recruited to the airways during chronic lung conditions, where they may encounter invading respiratory pathogens. Allergic asthma is often characterized by the presence of eosinophils in the peribronchovascular areas of the lungs, in sputum, and in the blood, although it should be noted that not all asthmatics have eosinophilia [45] and severe asthma can develop even in eosinophil deficient patients [11]. Virus-induced wheezing seems to predispose children to asthma later in life [46–48], and although correlation does not necessarily intimate causation, this observation led to the hypothesis that early virus exposure preconditions the lungs to subsequent reactions to environmental agents. Conversely, the presence of eosinophils in the allergic airways may alter host responses to virus infections. As such, investigating the function of eosinophils during respiratory infections is of benefit. Eosinophils undergo piecemeal degranulation in response to IAV [49], and RSV [50], although the kinetics and dynamics of granule protein release has not been determined. Once released, granule proteins may directly impact viral infectivity/load, or influence resident leukocytes (dendritic cells, neutrophils [51] and macrophages [52]) and the epithelial cells [53] to indirectly hinder virus dissemination. While it is accepted that eosinophil granule proteins are released in response to viruses, the mechanisms by which they reduce viral infectivity or impact viral pathogenesis is still unclear.
Perhaps the most attention drawn to eosinophils in the context of viruses arose during the unfortunate first RSV vaccine trial in which infants that received the formalin inactivated virus had more severe disease when naturally infected and vaccinated children that died had heightened eosinophilia in the lungs [54]. Herein, eosinophil recruitment and degranulation were thought to have contributed to sustained inflammation and tissue damage that aggravated virus-associated pathophysiology [55]. Formalin-inactivated RSV vaccinated monkeys were demonstrated to have elevated TH2 responses with eosinophilia and severe disease after virus challenge [56]. Although similar findings have been reported in murine models of RSV, the function of eosinophils in the tissue during the infection is unclear [57]. Domachowske et al. demonstrated in vitro that eosinophils reduce RSV infectivity in a dose-dependent manner [58]. Since then, many others have reported the antiviral role of eosinophils against respiratory virus infection, although it appears that the mechanisms employed by eosinophils for antiviral immunity varies by virus family.
Eosinophils have been showcased as active contributors to innate immunity against virus infection rather than bystanders [55, 59, 60]. Their recruitment kinetics into the virus-infected tissue suggests that they may be required for tissue healing [61, 62], although it has not been specifically investigated. Indeed, eosinophils are capable of responding to rhinovirus [63], RSV [64], pneumonia virus of mice [65], IAV [49], and parainfluenza virus [66]. The arsenal of immune regulators within eosinophils allow these cells to act directly or indirectly in response to respiratory viruses. Eosinophil granule proteins have clear antiviral functions wherein virus infectivity is reduced in the presence of ECP, eosinophil derived neurotoxin (EDN), and EPO [58, 67]. While MBP has been demonstrated to induce cytopathology in epithelial cells [68], it is unclear whether MBP plays a direct antiviral role against respiratory viruses. In our hands, recombinant MBP does not affect IAV infectivity (unpublished data). Eosinophil degranulation and products thereof have been reported in RSV patients [55, 69] thereby providing a clinical justification to investigate pathways of eosinophil recruitment, activation, and functions during respiratory virus infections.
Eosinophils also use other components in their arsenal in defense against viruses. Nitric oxide (NO), a free radical gas produced by nitric oxide synthase (NOS) using L-arginine, is a mediator of numerous biological functions including neurotransmission, vasodilation, inflammation, immune regulation and host defense [70]. Eosinophils generate NO [71] that reacts with superoxide anion (O2-) to form peroxynitrite (ONOO-) which functions as a cytotoxic compound. Eosinophil-derived NO can mediate antiviral responses to parainfluenza virus [66] and RSV [72] through viral load reduction. Interestingly, NO synthesis by eosinophils is dependent on interferon responses during virus infection [66]. As producers of a variety of cytokines, eosinophils themselves may contribute to the local cytokine milieu during virus infections as we have found them to release IFNγ in response to IAV [49]. Therefore, maintaining the intricate balance between TH1 and TH2 immune responses supports antiviral immunity.
More targeted immune responses through cellular and humoral immunity are required to fully clear virus, stop ongoing inflammation, and regain homeostasis. Specific antigen presentation and co-stimulation are required to activate T cells to initiate the adaptive immune cascade. Ovalbumin-pulsed eosinophils transferred intratracheally, migrate to the draining lymph nodes and present peptide to trigger ovalbumin-specific T cell activation in mice [73–76] showcasing their ability to moonlight as antigen presenting cells. In the context of IAV and other virus infections, effective viral clearance largely depends on CD8+ T cell activation [77–79]. Our studies established that eosinophils upregulate MHCI and CD86 in response to IAV, are found in the T cell zones when transferred into infected mice, engage in direct interaction with CD8+ T cells, and promote the recruitment of virus-specific CD8+ T cells into the lungs to enhance antiviral immunity in the host [49]. It is still unclear how eosinophils obtain viral peptides for presentation, and while our work suggests that antigen availability may be due to susceptibility to infection [49], it may also be possible that eosinophils obtain viral antigen from the environment through phagocytosis, a function which they are capable of [80]. Rather than being activated directly by virus binding/sensing, eosinophil responses to respiratory viruses may be governed by other leukocytes. It has been reported that human eosinophils degranulate in response to RSV, RV or parainfluenza virus only in the presence of CD4+ T cells and DCs [81].
Eosinophils may tailor their antiviral responses to virus type, and multiple mechanisms may partake independently, or in combination (Figure 2). Granule proteins are clearly virucidal in vitro against a variety of viruses, however, it is still unclear if such a function occurs or is important in vivo during an active infection. Since piecemeal degranulation occurs in response to viruses, it is necessary to determine the kinetics and sequence of granule contents that are released in response to each type of virus. If eosinophils do indeed function as putative antigen presenters to elevate T cell responses, it is important to determine the antigen processing and presentation processes within these cells.
Eosinophils Trap Bacteria
Although eosinophils have been historically implicated in allergy and helminth infection, they also possess the ability to recognize, ingest and kill bacteria [82, 83]. The importance of eosinophils in host defense against bacteria has been demonstrated in vivo. Transgenic mice overexpressing IL-5 subjected to cecal ligation puncture or infected intraperitoneally with Pseudomonas aeruginosa, show prolonged survival compared to control mice without eosinophilia [84, 85]. Conversely, there is higher outgrowth of P. aeruginosa following intraperitoneal infection of eosinophil-deficient PHIL mice than wild-type mice, a phenotype that is rescued by the adoptive transfer of eosinophils prior to infection [84]. Although eosinophils are phagocytic, both uptake and intracellular killing of bacteria are significantly lower than for other phagocytes [82, 86, 87]. Therefore, it is likely that the contribution of eosinophils during bacterial infection is related to their potent killing of extracellular bacteria by mechanisms such as released antimicrobial granule proteins and generation of extracellular DNA traps.
Eosinophil granule proteins, in particular the cationic proteins, have been ascribed antimicrobial properties since the 1970s, and treatment of mice with purified granule proteins significantly reduces P. aeruginosa burden in vivo [84]. Following interaction with specific triggers, eosinophils can generate an extracellular web of either mitochondrial [85] or nuclear [88] DNA that can physically trap pathogens and act as a scaffold for granule proteins such as MBP and ECP [85, 89]. Of significance, the generation of nuclear DNA nets occurs during a specific cytolytic process termed ‘eosinophil extracellular trap cell death’ (EETosis) [88], whereas cells that extrude mitochondrial DNA retain viability [85, 90]. In addition to granule proteins, intact eosinophil granules can also be enmeshed in the extracellular DNA traps, some of which are able to respond to cytokines and secrete their contents within the tissue [88]. It is likely that by facilitating the co-localization of bacteria and antimicrobial granule proteins, eosinophil extracellular DNA traps provide a mechanism to optimize bacterial killing while limiting non-specific damage to surrounding tissue.
Many cationic proteins and peptides have been implicated in bacterial defense due to their innate affinity to negatively charged lipid membranes, and similarly EPO, MBP and ECP have defined antibacterial functions. In 1978, Migler et al. reported that a lysate of eosinophils enriched from a patient with eosinophilia possessed potent bactericidal activity against Staphylococcus aureus and Escherichia coli when combined with hydrogen peroxide and a halide, which they attributed to the heme peroxidase EPO [91]. Jong et al. (1980) later confirmed this assumption by reproducing the E. coli killing using purified guinea pig EPO [92]. Human EPO is also bactericidal against Mycobacterium tuberculosis; interestingly, killing can occur in the absence of exogenous hydrogen peroxide, albeit at a slower rate than when hydrogen peroxide is supplemented [93]. Although rapid bacterial killing is observed in vitro, Epx null mice with Alternaria alternata-induced eosinophilia cleared Haemophilus influenzae from the airways equally as well as wildtype mice, despite having fewer recruited eosinophils, and eosinophils that were present were less likely to express TLR4 [94]. This disparity between the in vitro and in vivo findings needs to be clarified with further studies. Following its release in response to pathogen sensing, MBP, which exists as an inert nanocrystal within mature eosinophil granules, is converted to a cytotoxic entity by granule acidification and the subsequent formation of extracellular amyloids [16]. Protein aggregation is crucial to the bactericidal activity of MBP, and disruption of amyloid formation significantly reduces its ability to kill E. coli [95]. Similarly, ECP also forms amyloid-like aggregates [96, 97], and has bactericidal activity against both Gram-positive and -negative bacteria [98]. Human ECP has a high affinity for both peptidoglycan, highly expressed in the cell wall of Gram-positive bacteria, and lipopolysaccharide (LPS), a constituent of the outer membrane of Gram-negative bacteria [99]. While ECP binding to peptidoglycan does not trigger autolysis or result in visible cell damage of Gram-positive bacteria, ECP causes considerable depolarization of the outer membrane and promotes bacteria agglutination in Gram-negative bacteria [99]. Both non-aggregating ECP mutants and E. coli LPS truncation mutants reduce bacterial agglutination and killing [97, 100] suggesting that, following the recognition of LPS by ECP, the formation of amyloid-like aggregates on the surface of Gram-negative bacteria cause the disruption of the lipid bilayer. Although EDN shares 89% cDNA sequence homology with ECP [101] and has reported antiviral activity [102], it has not been reported to directly kill bacteria. Instead, EDN augments bacterial clearance by recruiting and activating dendritic cells [103, 104].
The release of granule proteins in response to bacterial stimuli is surprisingly discriminatory, with some bacteria stimulating global degranulation, while others promote the selective release of granule components by piecemeal degranulation [105]. In vitro studies indicate that ECP is secreted by human eosinophils predominantly in response to Gram-negative bacteria, whereas EPO and MBP are released following exposure to a selection of both Gram-negative and -positive bacteria [106]. EDN is released upon stimulation with heat-killed Clostridium difficile and Staphylococcus aureus, but not with H. influenzae, Prevotella sp, or Bifidobacterium bifidum [107, 108]. The mechanisms dictating these discrete eosinophil responses to bacteria can be attributed to distinct pathogen sensing mechanisms. Human eosinophils express complement receptors [109, 110], Fc receptors [111, 112], and a wide repertoire of pattern-recognition receptors, including all toll-like receptors (except TLR-8), nucleotide-binding oligomerization domain (NOD)-like receptors (NLR) 1 and 2, and the C-type lectin receptor Dectin-1 [113–120]. The expression of these receptors on eosinophils can vary during disease conditions and upon stimulation by bacterial components during infection [115]. For example, Driss et al. reported that eosinophils from individuals with eosinophilia express TLR-2 and TLR-4 on their surface, whereas eosinophils from healthy patients did not [115]. The release of EPO, which has antimicrobial activity against mycobacteria, occurs following TLR-2-mediated activation of human eosinophils by M. bovis bacillus Calmette-Guerin, along with the generation of α-defensin and reactive oxygen species [115]. Furthermore peptidoglycan, another TLR-2 ligand, causes eosinophils to selectively release intracellular stores of proinflammatory cytokines IL-1β, IL-6, IL-8 and GRO-α, a process that is abolished by a TLR-2 neutralizing antibody [120]. The release of ECP from granules can be stimulated by LPS sensing through TLR-4 and CD14 on eosinophils [113]. The ability of eosinophils to selectively release granule content upon recognition of pathogen motifs is an elegant mechanism to prevent the excessive inflammation and damage to the surrounding host cells, while promoting the killing of local bacteria.
Cell-to-cell crosstalk may play a crucial role in inflammatory responses triggered by eosinophils during bacterial infections. Activated eosinophils can express MHCII on their surface, as evident in eosinophils recovered from the blood, sputum and airways of asthmatics [121–123] and can be induced in vitro by incubation with GM-CSF [124]. S. aureus superantigens SEA and SEB (staphylococcal enterotoxins A and B) and TSST-1 (toxic shock syndrome toxin) bind to MHCII on eosinophils inhibiting apoptosis [125] and promoting MHCII-TCR crosslinking [126] to activate CD4+ T cells. However, although GM-CSF-activated eosinophils stimulate resting CD4+ T cells after incubation with SEA and SEB [127], studies defining eosinophils as antigen presenting cells capable of processing and presenting bacterial antigens are limited and conflicting. Weller and colleagues reported that HLA-DR+ eosinophils incubated with tetanus toxoid then fixed with paraformaldehyde were able to promote T cell proliferation whereas cells fixed prior to toxoid pulsing were not, indicating that antigen processing was required for the proliferative effect [128]. Conversely, Mawhorter et al. did not observe proliferation of M. tuberculosis purified protein derivative (PPD)-specific CD4+ T cells during co-culture with PPD-pulsed eosinophils [127].
While eosinophils are indeed capable of responding to pathogenic bacteria (Figure 2), their exact functions during an active infection in vivo are yet to be elucidated. Since eosinophil recruitment in macrophage CD14 deficient allergic mice infected with IAV is stunted [129], it is possible that similar intercellular crosstalk predominates during bacterial infections in the lungs where eosinophils may help modulate immune responses and help in the reparative processes that follow neutrophilic inflammation.
Conclusion
Eosinophils are multifunctional cells of the immune system equipped with an arsenal of specialized proteins with clear antihelminthic, antiviral, and antibacterial properties (Figure 2). In addition, the storage of a plethora of cytokines and chemokines, neuropeptides, and growth factors provide them with the necessary tools to regulate the microenvironment including the leukocytes that may surround them in an inflamed tissue. Recent evidence strongly negates the antediluvian notion that the sole purpose of the eosinophil is to counter parasites. In contrast, the evolutionary conservation of these cells certainly suggests that they serve an important, albeit redundant, role in the pulmonary immune response both in sickness and in health.
Acknowledgements
AES wishes to thank all those eosinophil aficionados (Drs. Helene Rosenberg, Praveen Akuthota, Rossana Melo, Hirohito Kita, Elizabeth Jacobsen, and especially, the late Dr. James Lee) that have guided her viewpoint through conversation on all ‘things’ eosinophil! We thank John Snyder for the artistic rendering of the eosinophil. This work was supported in part by the National Institutes of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI125481 to AES. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Kay AB, The early history of the eosinophil. Clin Exp Allergy, 2015. 45(3): p. 575–82. [DOI] [PubMed] [Google Scholar]
- 2.McGarry MP, The Evolutionary Origins and Presence of Eosinophils in Extant Species, in Eosinophils in Health and Disease, Rosenberg HF and Lee JJ, Editors. 2013, Elsevier; p. 13–17. [Google Scholar]
- 3.Lee JJ, et al. , Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy, 2010. 40(4): p. 563–75.••This article provides an in depth argument for why eosinophils should not be inferred as terminally differentiated cells that are harmful to the host when activated, but instead, why they should be considered component of the innate immune system that performs a more sophisticated role in maintaining tissue homeostasis aiding in healing and repair.
- 4.Park YM and Bochner BS, Eosinophil survival and apoptosis in health and disease. Allergy Asthma Immunol Res, 2010. 2(2): p. 87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu VT and Berek C, Immunization induces activation of bone marrow eosinophils required for plasma cell survival. Eur J Immunol, 2012. 42(1): p. 130–7. [DOI] [PubMed] [Google Scholar]
- 6.Chu VT, et al. , Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol, 2011. 12(2): p. 151–9. [DOI] [PubMed] [Google Scholar]
- 7.Shamri R, Xenakis JJ, and Spencer LA, Eosinophils in innate immunity: an evolving story. Cell Tissue Res, 2011. 343(1): p. 57–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobsen EA, et al. , The expanding role(s) of eosinophils in health and disease. Blood, 2012. 120(19): p. 3882–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voehringer D, van Rooijen N, and Locksley RM, Eosinophils develop in distinct stages and are recruited to peripheral sites by alternatively activated macrophages. J Leukoc Biol, 2007. 81(6): p. 1434–44. [DOI] [PubMed] [Google Scholar]
- 10.Davoine F. and Lacy P, Eosinophil cytokines, chemokines, and growth factors: emerging roles in immunity. Front Immunol, 2014. 5: p. 570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gleich GJ, et al. , The Consequences of not having Eosinophils. Allergy, 2013. 68: p. 829–835.••This paper discusses patient subsets that do not have eosinophils and their responses to stimuli.
- 12.Kay AB, The role of eosinophils in the pathogenesis of asthma. Trends Mol Med, 2005. 11(4): p. 148–52. [DOI] [PubMed] [Google Scholar]
- 13.Fahy JV, Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc, 2009. 6(3): p. 256–9. [DOI] [PubMed] [Google Scholar]
- 14.Muniz VS, Weller PF, and Neves JS, Eosinophil crystalloid granules: structure, function, and beyond. J Leukoc Biol, 2012. 92(2): p. 281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spencer LA, et al. , Eosinophil secretion of granule-derived cytokines. Front Immunol, 2014. 5: p. 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acharya KR and Ackerman SJ, Eosinophil granule proteins: form and function. J Biol Chem, 2014. 289(25): p. 17406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bozza PT, Yu W, and Weller PF, Mechanisms of formation and function of eosinophil lipid bodies: inducible intracellular sites involved in arachidonic acid metabolism. Mem Inst Oswaldo Cruz, 1997. 92 Suppl 2: p. 135–40. [DOI] [PubMed] [Google Scholar]
- 18.Melo RC and Weller PF, Unraveling the complexity of lipid body organelles in human eosinophils. J Leukoc Biol, 2014. 96(5): p. 703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farne HA, et al. , Anti-Il-5 Therapies for Asthma, in Cochrane Database of Systematic Reviews. 2017, John Wiley & Sons, Ltd; p. 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim ES, Sohn YW, and Moon A, TGF-beta-induced transcriptional activation of MMP-2 is mediated by activating transcription factor (ATF)2 in human breast epithelial cells. Cancer Lett, 2007. 252(1): p. 147–56. [DOI] [PubMed] [Google Scholar]
- 21.Dahl C, et al. , Human mast cells express receptors for IL-3, IL-5 and GM-CSF; a partial map of receptors on human mast cells cultured in vitro. Allergy, 2004. 59(10): p. 1087–96. [DOI] [PubMed] [Google Scholar]
- 22.Rios FG, et al. , Lung function and organ dysfunctions in 178 patients requiring mechanical ventilation during the 2009 influenza A (H1N1) pandemic. Crit Care, 2011. 15(4): p. R201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg HF, Dyer KD, and Foster PS, Eosinophils: changing perspectives in health and disease. Nat Rev Immunol, 2013. 13(1): p. 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joseph D, Puttaswamy RK, and Krovvidi H, Non-respiratory functions of the lung. Continuing Education in Anaesthesia Critical Care and Pain, 2013. 13(3): p. 98–102. [Google Scholar]
- 25.Leuenberger P, [Clinical importance of non-respiratory functions of the lung]. Schweiz Med Wochenschr, 1983. 113(29): p. 1006–10. [PubMed] [Google Scholar]
- 26.Marshall BE, Editorial views: Non-respiratory functions of the lung. Anesthesiology, 1973. 39(6): p. 573–4. [DOI] [PubMed] [Google Scholar]
- 27.Mesnil C, et al. , Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest, 2016. 126(9): p. 3279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fulkerson PC and Rothenberg ME, Targeting eosinophils in allergy, inflammation and beyond. Nat Rev Drug Discov, 2013. 12(2): p. 117–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobsen EA, Lee NA, and Lee JJ, Re-defining the unique roles for eosinophils in allergic respiratory inflammation. Clin Exp Allergy, 2014. 44(9): p. 1119–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calhoun WJ, Sedgwick J, and Busse WW, The role of eosinophils in the pathophysiology of asthma. Ann N Y Acad Sci, 1991. 629: p. 62–72. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg HF, Phipps S, and Foster PS, Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol, 2007. 119(6): p. 1303–10; quiz 1311–2. [DOI] [PubMed] [Google Scholar]
- 32.Gazzinelli-Guimaraes PH and Nutman TB, Helminth parasites and immune regulation. F1000Res, 2018. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunst H, et al. , Parasite infections of the lung: a guide for the respiratory physician. Thorax, 2011. 66: p. 528–536. [DOI] [PubMed] [Google Scholar]
- 34.Craig JM and Scott AL, Helminths in the lungs. Parasite Immunol, 2014. 36(9): p. 463–74. [DOI] [PubMed] [Google Scholar]
- 35.Patnode ML, et al. , Leukotriene B4 amplifies eosinophil accumulation in response to nematodes. J Exp Med, 2014. 211(7): p. 1281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Connell AE, et al. , Major basic protein from eosinophils and myeloperoxidase from neutrophils are required for protective immunity to Strongyloides stercoralis in mice. Infect Immun, 2011. 79(7): p. 2770–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Specht S, et al. , Lack of eosinophil peroxidase or major basic protein impairs defense against murine filarial infection. Infect Immun, 2006. 74(9): p. 5236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang D, et al. , Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med, 2008. 205: p. 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klion AD and Nutman TB, The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol, 2004. 113(1): p. 30–7. [DOI] [PubMed] [Google Scholar]
- 40.Maizels RM, Hewitson JP, and Smith KA, Susceptibility and immunity to helminth parasites. Curr Opin Immunol, 2012. 24(4): p. 459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gurish MF, et al. , CCR3 is required for tissue eosinophilia and larval cytotoxicity after infection with Trichinella spiralis. J Immunol, 2002. 168(11): p. 5730–6. [DOI] [PubMed] [Google Scholar]
- 42.Fabre V, et al. , Eosinophil deficiency compromises parasite survival in chronic nematode infection. J Immunol, 2009. 182(3): p. 1577–83.••This paper provides evidence for the alternative role of eosinophils as supporters of parasite growth and survival in hosts.
- 43.Huang L. and Appleton JA, Eosinophils in Helminth Infection: Defenders and Dupes. Trends Parasitol, 2016. 32(10): p. 798–807.•This paper provides a balanced review of the role of eosinophils during worm infections focusing on both the beneficial and detrimental aspects to the parasite.
- 44.Gebreselassie NG, et al. , Eosinophils preserve parasitic nematode larvae by regulating local immunity. J Immunol, 2012. 188(1): p. 417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carr TF, Zeki AA, and Kraft M, Eosinophilic and Noneosinophilic Asthma. Am J Respir Crit Care Med, 2018. 197(1): p. 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Busse WW, Lemanske RF Jr., and Gern JE, Role of viral respiratory infections in asthma and asthma exacerbations. Lancet, 2010. 376(9743): p. 826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gern JE, The ABCs of rhinoviruses, wheezing, and asthma. J Virol, 2010. 84(15): p. 7418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim CK, Callaway Z, and Gern JE, Viral Infections and Associated Factors That Promote Acute Exacerbations of Asthma. Allergy Asthma Immunol Res, 2018. 10(1): p. 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samarasinghe AE, et al. , Eosinophils Promote Antiviral Immunity in Mice Infected with Influenza A Virus. J Immunol, 2017. 198(8): p. 3214–3226.••This paper reports that eosinophils can be infected by Influenza A Virus, selectively release granule contents by piecemeal degranulation during influenza, and act as antigen presenting cells to CD8+ T cells.
- 50.Percopo CM, et al. , Activated mouse eosinophils protect against lethal respiratory virus infection. Blood, 2014. 123(5): p. 743–52.••Here, the authors provide in vivo evidence that eosinophils can be activated by pneumonia virus infection of mice, resulting in degranulation and reduced viral load.
- 51.Akuthota P, et al. , Immunoregulatory roles of eosinophils: a new look at a familiar cell. Clin Exp Allergy, 2008. 38(8): p. 1254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rankin JA, Harris P, and Ackerman SJ, The effects of eosinophil-granule major basic protein on lung-macrophage superoxide anion generation. J Allergy Clin Immunol, 1992. 89(3): p. 746–52. [DOI] [PubMed] [Google Scholar]
- 53.O’Reilly MA, et al. , Neonatal oxygen increases sensitivity to influenza A virus infection in adult mice by suppressing epithelial expression of Ear1. Am J Pathol, 2012. 181(2): p. 441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim HW, et al. , Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol, 1969. 89(4): p. 422–34. [DOI] [PubMed] [Google Scholar]
- 55.Garofalo R, et al. , Eosinophil degranulation in the respiratory tract during naturally acquired respiratory syncytial virus infection. J Pediatr, 1992. 120(1): p. 28–32. [DOI] [PubMed] [Google Scholar]
- 56.De Swart RL, et al. , Immunization of macaques with formalin-inactivated respiratory syncytial virus (RSV) induces interleukin-13-associated hypersensitivity to subsequent RSV infection. J Virol, 2002. 76(22): p. 11561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knudson CJ, et al. , RSV vaccine-enhanced disease is orchestrated by the combined actions of distinct CD4 T cell subsets. PLoS Pathog, 2015. 11(3): p. e1004757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Domachowske JB, et al. , Eosinophil cationic protein/RNase 3 is another RNase A-family ribonuclease with direct antiviral activity. Nucleic Acids Res, 1998. 26(14): p. 3358–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenberg HF, Dyer KD, and Domachowske JB, Respiratory viruses and eosinophils: exploring the connections. Antiviral Res, 2009. 83(1): p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenberg HF, Dyer KD, and Domachowske JB, Eosinophils and their interactions with respiratory virus pathogens. Immunol Res, 2009. 43(1–3): p. 128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samarasinghe AE, et al. , The immune profile associated with acute allergic asthma accelerates clearance of influenza virus. Immunol Cell Biol, 2014. 92: p. 449–459.•This paper reports the unexpected observation that acute allergic asthma, which is associated with lung eosinophilia is a protective co-morbidity during Influenza A Infection with increased virus-specific CD8+ T cells. These in vivo data support a role for eosinophils in the anti-viral response.
- 62.To EE, et al. , Intranasal and epicutaneous administration of Toll-like receptor 7 (TLR7) agonists provides protection against influenza A virus-induced morbidity in mice. Sci Rep, 2019. 9(1): p. 2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Handzel ZT, et al. , Eosinophils bind rhinovirus and activate virus-specific T cells. J Immunol, 1998. 160(3): p. 1279–84. [PubMed] [Google Scholar]
- 64.Phipps S, et al. , Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood, 2007. 110(5): p. 1578–86. [DOI] [PubMed] [Google Scholar]
- 65.Dyer KD, et al. , Pneumoviruses infect eosinophils and elicit MyD88-dependent release of chemoattractant cytokines and interleukin-6. Blood, 2009. 114(13): p. 2649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drake MG, et al. , Human and Mouse Eosinophils Have Antiviral Activity against Parainfluenza Virus. Am J Respir Cell Mol Biol, 2016. 55(3): p. 387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Domachowske JB, et al. , Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis, 1998. 177(6): p. 1458–64.••This paper provides the first indication that eosinophil granule proteins have antiviral potential.
- 68.Ishioka T, et al. , Effects of respiratory syncytial virus infection and major basic protein derived from eosinophils in pulmonary alveolar epithelial cells (A549). Cell Biol Int, 2011. 35(5): p. 467–74. [DOI] [PubMed] [Google Scholar]
- 69.Colocho Zelaya EA, Orvell C, and Strannegard O, Eosinophil cationic protein in nasopharyngeal secretions and serum of infants infected with respiratory syncytial virus. Pediatr Allergy Immunol, 1994. 5(2): p. 100–6. [DOI] [PubMed] [Google Scholar]
- 70.Gryglewski RJ, et al. , Protective role of pulmonary nitric oxide in the acute phase of endotoxemia in rats. Circ Res, 1998. 82(7): p. 819–27. [DOI] [PubMed] [Google Scholar]
- 71.MacPherson JC, et al. , Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. J Immunol, 2001. 166(9): p. 5763–72. [DOI] [PubMed] [Google Scholar]
- 72.Su YC, et al. , Dual proinflammatory and antiviral properties of pulmonary eosinophils in respiratory syncytial virus vaccine-enhanced disease. J Virol, 2015. 89(3): p. 1564–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shi HZ, et al. , Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest, 2000. 105(7): p. 945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Rijt LS, et al. , Airway eosinophils accumulate in the mediastinal lymph nodes but lack antigen-presenting potential for naive T cells. J Immunol, 2003. 171(7): p. 3372–8. [DOI] [PubMed] [Google Scholar]
- 75.MacKenzie JR, et al. , Eosinophils promote allergic disease of the lung by regulating CD4(+) Th2 lymphocyte function. J Immunol, 2001. 167(6): p. 3146–55. [DOI] [PubMed] [Google Scholar]
- 76.Wang HB, et al. , Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J Immunol, 2007. 179(11): p. 7585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bender BS, et al. , Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med, 1992. 175(4): p. 1143–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Topham DJ, Tripp RA, and Doherty PC, CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol, 1997. 159(11): p. 5197–200. [PubMed] [Google Scholar]
- 79.Duan S. and Thomas PG, Balancing Immune Protection and Immune Pathology by CD8(+) T-Cell Responses to Influenza Infection. Front Immunol, 2016. 7: p. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cline MJ, Hanifin J, and Lehrer RI, Phagocytosis by human eosinophils. Blood, 1968. 32(6): p. 922–34.•This paper provides seminal evidence for the phagocytotic potential of eosinophils.
- 81.Davoine F, et al. , Virus-induced eosinophil mediator release requires antigen-presenting and CD4+ T cells. J Allergy Clin Immunol, 2008. 122(1): p. 69–77, 77.e1–2. [DOI] [PubMed] [Google Scholar]
- 82.DeChatelet LR, et al. , Comparison of intracellular bactericidal activities of human neutrophils and eosinophils. Blood, 1978. 52(3): p. 609–17. [PubMed] [Google Scholar]
- 83.Mickenberg ID, Root RK, and Wolff SM, Bactericidal and metabolic properties of human eosinophils. Blood, 1972. 39(1): p. 67–80. [PubMed] [Google Scholar]
- 84.Linch SN, et al. , Mouse eosinophils possess potent antibacterial properties in vivo. Infect Immun, 2009. 77(11): p. 4976–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yousefi S, et al. , Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med, 2008. 14(9): p. 949–53.••This paper provides evidence that eosinophils release mitocondrial DNA in defense against Gram-negative bacteria without compromising its viability.
- 86.Hatano Y, et al. , Phagocytosis of heat-killed Staphylococcus aureus by eosinophils: comparison with neutrophils. Apmis, 2009. 117(2): p. 115–23. [DOI] [PubMed] [Google Scholar]
- 87.Takeuchi O. and Akira S, Pattern recognition receptors and inflammation. Cell, 2010. 140(6): p. 805–20. [DOI] [PubMed] [Google Scholar]
- 88.Ueki S, et al. , Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood, 2013. 121(11): p. 2074–83.•This paper provides evidence for extracellular trap formation by eosinophils when activated through immunoglobulins, PAF, calcium ionophore, and PMA in a process termed EETosis.
- 89.Dworski R, et al. , Eosinophil and neutrophil extracellular DNA traps in human allergic asthmatic airways. J Allergy Clin Immunol, 2011. 127(5): p. 1260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morshed M, et al. , Thymic stromal lymphopoietin stimulates the formation of eosinophil extracellular traps. Allergy, 2012. 67(9): p. 1127–37. [DOI] [PubMed] [Google Scholar]
- 91.Migler R, DeChatelet LR, and Bass DA, Human eosinophilic peroxidase: role in bactericidal activity. Blood, 1978. 51(3): p. 445–56. [PubMed] [Google Scholar]
- 92.Jong EC, Henderson WR, and Klebanoff SJ, Bactericidal activity of eosinophil peroxidase. J Immunol, 1980. 124(3): p. 1378–82. [PubMed] [Google Scholar]
- 93.Borelli V, et al. , Human eosinophil peroxidase induces surface alteration, killing, and lysis of Mycobacterium tuberculosis. Infect Immun, 2003. 71(2): p. 605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Percopo CM, et al. , Impact of eosinophil-peroxidase (EPX) deficiency on eosinophil structure and function in mouse airways. J Leukoc Biol, 2019. 105(1): p. 151–161.••This paper describes one of the few studies that translates in vitro bacteriocidal properties of eosinophil granule proteins to in vivo models. Despite reports that EPX is bactericial in vitro., the authors found that mice lacking EPX were able to clear Haemophilus influenzae equally as well as EPX-sufficient mice.
- 95.Soragni A, et al. , Toxicity of eosinophil MBP is repressed by intracellular crystallization and promoted by extracellular aggregation. Mol Cell, 2015. 57(6): p. 1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Torrent M, et al. , Eosinophil cationic protein aggregation: identification of an N-terminus amyloid prone region. Biomacromolecules, 2010. 11(8): p. 1983–90. [DOI] [PubMed] [Google Scholar]
- 97.Torrent M, et al. , Exploring new biological functions of amyloids: bacteria cell agglutination mediated by host protein aggregation. PLoS Pathog, 2012. 8(11): p. e1003005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lehrer RI, et al. , Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. J Immunol, 1989. 142(12): p. 4428–34. [PubMed] [Google Scholar]
- 99.Torrent M, et al. , Eosinophil cationic protein high-affinity binding to bacteria-wall lipopolysaccharides and peptidoglycans. Biochemistry, 2008. 47(11): p. 3544–55. [DOI] [PubMed] [Google Scholar]
- 100.Pulido D, et al. , Antimicrobial action and cell agglutination by the eosinophil cationic protein are modulated by the cell wall lipopolysaccharide structure. Antimicrob Agents Chemother, 2012. 56(5): p. 2378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rosenberg HF, Ackerman SJ, and Tenen DG, Human eosinophil cationic protein. Molecular cloning of a cytotoxin and helminthotoxin with ribonuclease activity. J Exp Med, 1989. 170(1): p. 163–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Domachowske JB, et al. , Evolution of antiviral activity in the ribonuclease A gene superfamily: evidence for a specific interaction between eosinophil-derived neurotoxin (EDN/RNase 2) and respiratory syncytial virus. Nucleic Acids Res, 1998. 26(23): p. 5327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang D, et al. , Human ribonuclease A superfamily members, eosinophil-derived neurotoxin and pancreatic ribonuclease, induce dendritic cell maturation and activation. J Immunol, 2004. 173(10): p. 6134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang D, et al. , Eosinophil-derived neurotoxin (EDN), an antimicrobial protein with chemotactic activities for dendritic cells. Blood, 2003. 102(9): p. 3396–403. [DOI] [PubMed] [Google Scholar]
- 105.Melo RC and Weller PF, Piecemeal degranulation in human eosinophils: a distinct secretion mechanism underlying inflammatory responses. Histol Histopathol, 2010. 25(10): p. 1341–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Svensson L. and Wenneras C, Human eosinophils selectively recognize and become activated by bacteria belonging to different taxonomic groups. Microbes Infect, 2005. 7(4): p. 720–8. [DOI] [PubMed] [Google Scholar]
- 107.Hosoki K, et al. , Differential activation of eosinophils by bacteria associated with asthma. Int Arch Allergy Immunol, 2013. 161 Suppl 2: p. 16–22. [DOI] [PubMed] [Google Scholar]
- 108.Hosoki K, et al. , Differential activation of eosinophils by ‘probiotic’ Bifidobacterium bifidum and ‘pathogenic’ Clostridium difficile. Int Arch Allergy Immunol, 2010. 152 Suppl 1: p. 83–9. [DOI] [PubMed] [Google Scholar]
- 109.Fischer E, et al. , Human eosinophils express CR1 and CR3 complement receptors for cleavage fragments of C3. Cell Immunol, 1986. 97(2): p. 297–306. [DOI] [PubMed] [Google Scholar]
- 110.Gupta S, et al. , Surface markers of human eosinophils. Blood, 1976. 48(5): p. 755–63. [PubMed] [Google Scholar]
- 111.Lopez AF, Battye FL, and Vadas MA, Fc receptors on mouse neutrophils and eosinophils: antigenic characteristics, isotype specificity and relative cell membrane density measured by flow cytometry. Immunology, 1985. 55(1): p. 125–33. [PMC free article] [PubMed] [Google Scholar]
- 112.Mantovani B, Different roles of IgG and complement receptors in phagocytosis by polymorphonuclear leukocytes. J Immunol, 1975. 115(1): p. 15–7. [PubMed] [Google Scholar]
- 113.Plotz SG, et al. , The interaction of human peripheral blood eosinophils with bacterial lipopolysaccharide is CD14 dependent. Blood, 2001. 97(1): p. 235–41. [DOI] [PubMed] [Google Scholar]
- 114.Nagase H, et al. , Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J Immunol, 2003. 171(8): p. 3977–82. [DOI] [PubMed] [Google Scholar]
- 115.Driss V, et al. , TLR2-dependent eosinophil interactions with mycobacteria: role of alpha-defensins. Blood, 2009. 113(14): p. 3235–44. [DOI] [PubMed] [Google Scholar]
- 116.Kvarnhammar AM, Petterson T, and Cardell LO, NOD-like receptors and RIG-I-like receptors in human eosinophils: activation by NOD1 and NOD2 agonists. Immunology, 2011. 134(3): p. 314–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wong CK, et al. , NOD-like receptors mediated activation of eosinophils interacting with bronchial epithelial cells: a link between innate immunity and allergic asthma. Cell Mol Immunol, 2013. 10(4): p. 317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ahren IL, et al. , Nontypeable Haemophilus influenzae activates human eosinophils through beta-glucan receptors. Am J Respir Cell Mol Biol, 2003. 29(5): p. 598–605. [DOI] [PubMed] [Google Scholar]
- 119.Willment JA, et al. , The human beta-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. Eur J Immunol, 2005. 35(5): p. 1539–47. [DOI] [PubMed] [Google Scholar]
- 120.Wong CK, et al. , Intracellular signaling mechanisms regulating toll-like receptor-mediated activation of eosinophils. Am J Respir Cell Mol Biol, 2007. 37(1): p. 85–96. [DOI] [PubMed] [Google Scholar]
- 121.Hansel TT, et al. , Sputum eosinophils from asthmatics express ICAM-1 and HLA-DR. Clin Exp Immunol, 1991. 86(2): p. 271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sedgwick JB, et al. , Comparison of airway and blood eosinophil function after in vivo antigen challenge. J Immunol, 1992. 149(11): p. 3710–8. [PubMed] [Google Scholar]
- 123.Mengelers HJ, et al. , Immunophenotyping of eosinophils recovered from blood and BAL of allergic asthmatics. Am J Respir Crit Care Med, 1994. 149(2 Pt 1): p. 345–51. [DOI] [PubMed] [Google Scholar]
- 124.Lucey DR, Nicholson-Weller A, and Weller PF, Mature human eosinophils have the capacity to express HLA-DR. Proc Natl Acad Sci U S A, 1989. 86(4): p. 1348–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wedi B, et al. , Staphylococcal exotoxins exert proinflammatory effects through inhibition of eosinophil apoptosis, increased surface antigen expression (CD11b, CD45, CD54, and CD69), and enhanced cytokine-activated oxidative burst, thereby triggering allergic inflammatory reactions. J Allergy Clin Immunol, 2002. 109(3): p. 477–84. [DOI] [PubMed] [Google Scholar]
- 126.Fraser JD, Clarifying the mechanism of superantigen toxicity. PLoS Biol, 2011. 9(9): p. e1001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mawhorter SD, Kazura JW, and Boom WH, Human eosinophils as antigen-presenting cells: relative efficiency for superantigen- and antigen-induced CD4+ T-cell proliferation. Immunology, 1994. 81(4): p. 584–91. [PMC free article] [PubMed] [Google Scholar]
- 128.Weller PF, et al. , Accessory cell function of human eosinophils. HLA-DR-dependent, MHC-restricted antigen-presentation and IL-1 alpha expression. J Immunol, 1993. 150(6): p. 2554–62. [PubMed] [Google Scholar]
- 129.Palipane M, et al. , Macrophage CD14 impacts immune defenses against influenza virus in allergic hosts. Microb Pathog, 2019. 127: p. 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]