Abstract
Objectives
In March 2018, the Food and Drug Administration (FDA) announced its authorization of a direct-to-consumer (DTC) genetic test for three pathogenic BRCA1/2 variants. We sought to determine to whether social media discussion increased following the authorization, who was driving social media conversations, and what topics were discussed.
Methods
Using Crimson Hexagon, we described tweets before, during, and after the FDA announcement authorizing 23andMe to return BRCA1/2 results (3/4/18–3/10/18). We conducted qualitative coding of a subset of 605 tweets to better understand Twitter communication.
Results
We identified 11 055 twitter posts across the week of FDA’s announcement. Twitter discourse about 23andMe and the FDA authorization peaked the day following the FDA’s press release. Most tweets (48.6%) were informational and 26.3% were either expressing opinions (about 23andMe and/or FDA authorization, 14.9%) or testimonials (personal experiences with genetic testing, 11.4%). The types of tweets varied over the week-long period (P < .001).
Discussion
Twitter discussion about the FDA’s authorization of DTC for three pathogenic BRCA1/2 variants increased immediately following the announcement. As more genetic technologies are brought to the DTC market, social media sites, like Twitter, will play a role in disseminating this information, providing a platform for information exchange, consumer testimonials, opinion pieces, and research.
Keywords: breast cancer, genetic testing, social media, FDA, direct-to-consumer testing
BACKGROUND AND SIGNIFICANCE
Despite a previous warning letter to 23andMe, Inc.1 to stop the marketing of their product for the diagnosis of disease in 2013,2 the Food and Drug Administration (FDA) authorized the first direct-to-consumer (DTC) test by 23andMe, Inc. for pathogenic BRCA1/2 variants in March 6, 2018. While there are over a thousand pathogenic variants associated with elevated risk of hereditary breast and ovarian cancer, this test provides results for three pathogenic variants that are most commonly found among those with Ashkenazi Jewish decent.3 This FDA authorization marks the first time consumers can use DTC testing for pathogenic variants related to cancer risk in the United States without a doctor’s order.
While public uptake of DTC genetic testing has increased over time,4 understanding of these tests remains low; in 2014, only 38% of people in the United States were aware of DTC testing.5 Recent studies show that people learn about potential harms and benefits of health applications, such as DTC testing, from social media and the internet.6 Events in the news, such as the FDA announcement or a celebrity health disclosure, may increase awareness about health applications. As an example, in May 2013, Angelina Jolie described her experiences with BRCA1/2 testing and subsequent risk management in the New York Times, which prompted a 112% increase in internet searches about BRCA1/2.7 It is unknown to what extent the FDA announcement may have impacted online discussion about DTC testing, and what type of information was shared.
OBJECTIVES
Thus, the objectives of this study were to learn more about (1) who engaged in social media discourse via Twitter surrounding the FDA announcement about 23andMe, (2) whether discussions of this testing increased over the period, and (3) what topics were discussed.
MATERIALS AND METHODS
Data source
We used the social media analysis tool Crimson Hexagon (Boston, MA, USA),7,8 to examine public engagement in Twitter discussions about 23andMe spanning before, during, and after the FDA’s announcement of its authorization for the company to offer select BRCA1/2 test results (3/4/2018–3/10/2018). Our search criteria included related hashtags (#FDA AND (#BRCA OR #BRCA1 OR #BRCA2)). We also included keywords: “23&me” OR “23 & me” OR “23andme” OR “23 and me.” The selection of hashtags and keywords was based on relevancy to our topic of focus. Generalizable terms such as “cancer” or “variant” were excluded to better ensure tweets would be directly related to the FDA’s announcement.
Measures and analyses
Outcomes of interest included the number of tweets overtime and the top influencers (i.e., users who drive conversations around topics and have high post engagement rates, such as retweets) who engaged in discourse about BRCA1/2 and the FDA announcement. To obtain data on the content of tweets, we conducted qualitative coding for a random subset of tweets (n = 605) until we reached saturation of themes. Two authors double-coded 10% of the qualitative subset using a preidentified set of codes, compared coding, adjusted the codebook, and then independently coded remaining tweets after consistency of coding was reached. The final codebook included codes for whether the tweet was informational, a testimonial (i.e., personal story about using 23andMe), an opinion piece (i.e., opinions about 23andMe, excluding testimonials), research-related, other, or “colloquial” (i.e., 23andMe used in everyday lexicon). We also coded the sentiment of testimonials and opinion pieces as positive (i.e., only discussed the benefits of 23andme), negative (i.e., primarily discussed drawbacks of 23andme), or neutral (i.e., discussed both benefits and drawback or provided neutral information). We also sought to identify inaccurate information (among tweets coded as “informational”) about 23andMe and the FDA decision, but did not identify inaccurate information in our subset. We compared how the “type of tweet” varied before-, during-, and after- the FDA announcement using chi-square tests. Similarly, we examined sentiment of testimonials and opinion pieces by time of tweet.
RESULTS
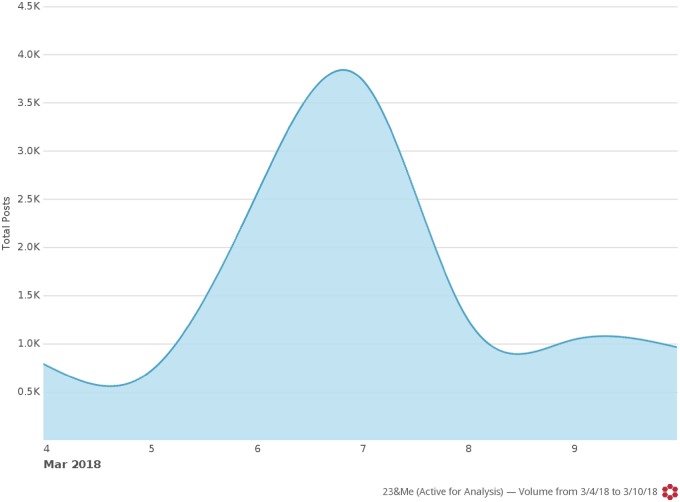
We identified 11 055 tweets about 23andMe across the week of FDA’s announcement. Twitter discourse about 23andMe and the FDA authorization peaked the day following their press release (7 March 2018; n = 3, 762) (Figure 1). The most influential Twitter accounts included large media outlets such as @CNN and @WSJ (Table 1).
Figure 1.
Twitter discourse about 23andMe during the week of the FDA’s authorization (Boston, MA, USA).
Table 1.
Top influencers among tweets identified during the study period
| Author | Name | Country | Posts | Followers | Postdate GMT | Post text |
|---|---|---|---|---|---|---|
| @CNN | CNN | USA | 172978 | 39629486 | 3/7 | Genetic testing company 23andMe has been give federal approval to sell at-home kits that test for three breast cancer gene mutations https://t.co/6yBLXbVtekhttps://t.co/l8dwUEOLNj |
| @Reuters | Reuters Top News | USA | 255347 | 19445229 | 3/6 | U.S. FDA allows 23andMe to sell test for 3 mutations of breast cancer gene https://t.co/WqRm9LjYIGhttps://t.co/enEbD3e9Ti |
| @WSJ | The Wall Street Journal | USA | 236287 | 15604458 | 3/9/ | The FDA's approval of 23andMe’s cancer-risk test is the latest example of the agency’s course reversal. But will consumer access empower people—or confuse them? https://t.co/bCPKxK7wehhttps://t.co/XOaxdSNNsW |
| @cnni | CNN International | UK | 148377 | 7610479 | 3/7 | Genetic testing company 23andMe has been give federal approval to sell at-home kits that test for three breast cancer gene mutations https://t.co/vtyVgfsXx6https://t.co/dow9Oqy54q |
| @people | People | USA | 201206 | 7883207 | 3/7 | 23andMe Kits Now FDA-Approved as a Genetic Test for Breast Cancer—But Is it Truly Accurate? https://t.co/Vrkrr38BhJ |
| @FortuneMagazine | FORTUNE | USA | 161218 | 2294324 | 3/7 | The new 23andMe FDA cancer screening approval is a game-changer for the company https://t.co/EicWJWx96j |
| @TechCrunch | TechCrunch | USA | 175442 | 10140863 | 3/7 | .@23andMe gets FDA green light for cancer risk test https://t.co/enbUle7k3b |
| @businessinsider | Business Insider | USA | 473990 | 2364108 | 3/7 | Genetic experts have a message for anyone thinking of taking 23andMe's new breast cancer test https://t.co/xEn5PqWBcUhttps://t.co/BVsQx3Utit |
| @newscientist | New Scientist | UK | 49518 | 3374271 | 3/9 | 23andMe’s breast cancer test may create false sense of security https://t.co/URadJ5YD3hhttps://t.co/pw5Ob1XqPG |
| @sfchronicle | San Francisco Chronicle | USA | 80776 | 145430 | 3/6 | #23andMe can now sell breast cancer genetic test with no prescription needed https://t.co/gO2uTdzngL |
The majority (67.8%, n = 5549) of discourse occurred in the United States, with the United Kingdom, and Canada comprising 6.1% and 3.4% of tweets, respectively (108 countries represented).
Most tweets (48.6%) were informational and 26.3% expressed opinions (about 23andMe and/or FDA authorization) or contained testimonials (personal experiences with genetic testing, 11.4%) (Table 2).
Table 2.
Qualitative findings and exemplar tweets
| Frequency (%) |
P-value | Example Tweet | ||||
|---|---|---|---|---|---|---|
| Overall | Before | During | After | |||
| Type of Tweet | <.001 | |||||
| Informational | 294 (48.6) | 19 (10.1) | 145 (74.7) | 130 (58.3) |
|
|
| Opinion | 90 (14.9) | 43 (22.9) | 13 (6.7) | 34 (15.2) | RT @DanaFarber 23AndMe at-home cancer test: Should you take it? Dana-Farber's Huma Q. Rana, MD, weighs in: https://t.co/wHMFcLNJDi | |
| Testimonial | 69 (11.4) | 35 (18.6) | 15 (7.7) | 19 (8.5) |
|
|
| Other (non-English, common language, NA) | 152 (25.1) | 91 (48.5) | 21 (10.9) | 40 (18.0) | Join our gene pool! 23andMe, Inc. is looking for Supply Chain Planner. Learn more or Jobvite a fr… https://t.co/AwKkXbaitE #job | |
| Sentimenta | .46 | |||||
| Positive | 32 (20.1) | 14 (17.9) | 9 (32.1) | 9 (17.0) |
|
|
| Neutral | 58 (36.5) | 28 (35.9) | 8 (28.6) | 22 (41.5) |
|
|
| Negative | 69 (43.4) | 36 (46.2) | 11 (39.3) | 22 (41.5) |
|
|
Among opinion and testimonial tweets only; chi-square.
The types of tweets varied over the period (P < 0.001); for example, 74.7% of tweets were informational on the day of the FDA announcement, versus 10.1% before and 58.3% following the announcement (Table 2). Among tweets expressing opinions or testimonials, 43.4% of tweets had a negative tone, 20.1% were positive, and the remaining were neutral. This sentiment (negative, positive, and neural) did not change across the time periods before, on the day of, or after the FDA announcement (P = .46). Tweets relaying opinions about 23andMe and the FDA announcement tended to be more negative than testimonials (78.3% vs 21.7%). Finally, we found 14.5% of tweets used 23andMe in everyday language, often as commentary about the ancestry, race, or ethnicity of an individual, group or oneself.
DISCUSSION
Online discussion about 23andMe increased after the FDA’s authorization for the company to return BRCA1/2 results for three specific pathogenic variants. Most of these tweets were informational in nature and were retweeted from news outlets. These tweets were neutral and informed the Twitter community about the FDA authorization (often referencing the FDA’s press release) and included discussion of the benefits and limitations of the authorized test. These findings align with other social media studies in which the majority of tweets about antimicrobial resistance were informational in nature,9 prominently from news sources.10 In contrast, a study about other health topics (elbow surgery, hereditary cancer) found that individual users from the general public11 and patient advocates and advocacy groups led discourse,12 demonstrating variation in how social media discourse rolls out by health topic. During our qualitative coding, we did not identify informational tweets that provided inaccurate information, suggesting that messaging by the FDA was largely adopted and shared via news outlets during the initial announcement of the authorization. Future studies should continue to monitor the accuracy of shared information over time and take a closer look at the accuracy of opinion pieces as well as user comprehension. In the case of the FDA authorization, tweets often provided additional information about potential harms of DTC genetic testing.
Tweets that shared opinions typically sought to inform the public regarding the potential harms of DTC testing. The most commonly referenced concern was that consumers may falsely assume that a negative 23andMe test result is definitive. Many of these tweets also discussed the need for consumers to consult with providers about their DTC genetic testing results, which aligns with recommendations by the American College of Medical Geneticists13 and public preferences.14 This suggests that with increasing demand for DTC testing, demand for genetic services follow-up may follow suit, and a continuing need to address the complex ethical, legal, and social implications of DTC testing. Testimonials were mostly positive in nature, often describing positive experiences with DTC testing or explaining why the new authorization may provide useful information for others. Interestingly, we did not find tweets during this period related to research; that is, no tweets provided data related to BRCA1/2 testing, FDA authorizations or 23andMe. This finding may be due to the larger reach of news organizations relative to that of individual researchers. Moving forward, researchers and practitioners should consider partnering with news organizations to engage in social media discourse related to research findings about DTC testing. Through this partnership researchers and practitioners can engage the public in discussion about how DTC testing may impact health beliefs, behaviors, and outcomes.8 This will be increasingly important in this era of precision medicine and DTC genetic testing.
While this study provides the first snapshot of social media discourse around the FDA authorization of BRCA1/2 testing through 23andMe, it is not without limitations. First, while we sought to use comprehensive and broad search terminology, relevant tweets may have been excluded or misclassified during qualitative coding. Future research should examine not only how information about the FDA authorization was disseminated on Twitter, but also how information sharing impacts knowledge, beliefs, and behaviors.
In conclusion, we see that information about the FDA’s authorization of DTC for three pathogenic BRCA1/2 variants greatly increased discussion of DTC genetic testing. As more genetic technologies are brought to the DTC market, social media sites, such as Twitter, and news outlets will play an important role in disseminating this information, providing a platform for information exchange, consumer testimonials, opinion pieces, and research.
ACKNOWLEDGMENTS
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Cancer Institute or the US Department of Health and Human Services. We acknowledge Destinee Tierra-Senay Williams for her editorial contribution in preparation for manuscript submission.
FUNDING
This work was funded by internal National Cancer Institute funds.
COMPETING INTERESTS
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.23andMe. 2018. https://www.23andme.com/ Accessed May 23, 2018.
- 2.Gutierrez A. Warning Letter CMS 415534 Document Number: GEN1300666 Re: Personal Genome Service (PGS). Silver Spring, MD: FDA, HHS; 2013.
- 3. FDA Authorizes, with Special Controls, Direct-to-Consumer Test that Reports Three Mutations in the BRCA Breast Cancer Genes 2018. Food and Drug Administration; 2018. https://www.fda.gov/news-events/press-announcements/fda-authorizes-special-controls-direct-consumer-test-reports-three-mutations-brca-breast-cancer. Accessed August 13, 2019.
- 4. Rutten LJF, Gullust SE, Naveed S, Moser RP.. Increasing public awareness of direct-to-consumer genetic tests: health care access, internet use, and population density correlates. J Cancer Epidemiol 2012; 2012: . 309109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Apathy NC, Menser T, Keeran LM, et al. Trends and gaps in awareness of direct-to-consumer genetic tests from 2007 to 2014. Am J Prev Med 2018; 54 (6): 806–13. [DOI] [PubMed] [Google Scholar]
- 6. Roberts MC, Taber JM, Klein W.. Engagement with genetic information and uptake of genetic testing: the role of trust and personal cancer history. J Cancer Educ 2018; 33 (4): 893–900. [DOI] [PubMed] [Google Scholar]
- 7. Noar SM, Althouse BM, Ayers JW, et al. Cancer information seeking in the digital age: effects of Angelina Jolie's prophylactic mastectomy announcement. Med Decis Making 2015; 35 (1): 16–21. [DOI] [PubMed] [Google Scholar]
- 8. Allen CG, Andersen B, Chambers DA, et al. Twitter use at the 2016 Conference on the Science of Dissemination and Implementation in Health: analyzing #DIScience16. Implement Sci 2018; 13 (1): 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andersen B, Hair L, Groshek J, et al. Understanding and diagnosing antimicrobial resistance on social media: a yearlong overview of data and analytics. Health Commun 2019; 34 (2): 248–58. [DOI] [PubMed] [Google Scholar]
- 10. Dyar OJ, Castro-Sanchez E, Holmes AH.. What makes people talk about antibiotics on social media? A retrospective analysis of Twitter use. J Antimicrob Chemother 2014; 69 (9): 2568–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramkumar PN, Navarro SM, Cornaghie MM, et al. Social media in shoulder & elbow surgery: an analysis of Twitter and Instagram. Int J Sports Med 2018; 39 (7): 564–70. [DOI] [PubMed] [Google Scholar]
- 12. Allen CG, Roberts MC, Andersen B, Khoury MJ.. Communication about hereditary cancers on social media: a content analysis of tweets about hereditary breast and ovarian cancer and lynch syndrome. J Cancer Educ 2018. doi: 10.1007/s13187-018-1451-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.ACMG Board of Directors. Direct-to-consumer genetic testing: a revised position statement of the American College of Medical Genetics and Genomics. Genet Med 2016; 18 (2): 207–8. [DOI] [PubMed] [Google Scholar]
- 14. Roberts JS, Gornick MC, Carere DA, et al. Direct-to-consumer genetic testing: user motivations, decision making, and perceived utility of results. Public Health Genomics 2017; 20 (1): 36–45. [DOI] [PubMed] [Google Scholar]