Abstract
Melatonin (MT; N-acetyI-5-methoxytryptamine) is an amine hormone involved in abiotic stress resistance. Previous studies have confirmed that melatonin can promote seed germination, mediate physiological regulation mechanisms, and stimulate crop growth under stress. However, the osmotic regulation mechanism by which exogenous melatonin mediates salt tolerance in cotton is still largely unknown. To investigate the effect of salt stress on melatonin concentration in germinating cotton seeds, we analyzed melatonin content over time during seed germination under different treatments. Melatonin content reached its minimum at day 6, while cotton germination rates peaked at day 6, indicating melatonin content and seed germination are correlated. Then we investigated the effects of 10–100 μM melatonin treatments on membrane lipid peroxides and osmotic adjustment substances during cotton seed germination under salt stress. Salt stress led to electrolyte leakage (EL) as well as accumulations of hydrogen peroxide (H2O2), malondialdehyde (MDA), organic osmotic substances (i.e., proline, soluble sugars), and inorganic osmotic substances (i.e., Na+, Cl-). Meanwhile, the contents of melatonin, soluble proteins, and K+ as well as the K+/Na+ balance decreased, indicating that salt stress inhibited melatonin synthesis and damaged cellular membranes, seriously affecting seed germination. However, melatonin pretreatment at different concentrations alleviated the adverse effects of salt stress on cotton seeds and reduced EL as well as the contents of H2O2, MDA, Na+, and Cl-. The exogenous application of melatonin also promoted melatonin, soluble sugar, soluble proteins, proline, and K+/Na+ contents under salt stress. These results demonstrate that supplemental melatonin can effectively ameliorate the repression of cotton seed germination by enhancing osmotic regulating substances and adjusting ion homeostasis under salt stress. Thus, melatonin may potentially be used to protect cotton seeds from salt stress, with the 20 μM melatonin treatment most effectively promoting cotton seed germination and improving salt stress tolerance.
Introduction
Salt stress is a critical environmental factor that limits the agricultural productivity, survival, and geographical distributions of plants [1]. Approximately 950 million hm2 of land in the world is affected by salinization, of which about 90 million hm2 of impacted areas occur in China alone, placing great importance on the study of salt stress effects on plant growth and development [2]. Under salt stress, the oxidative reaction of free radicals in membrane lipids leads to the accumulation of more reactive oxygen radicals and hydrogen peroxide in plants, which causes cross-linking polymerization of proteins, nucleic acids, and other biomolecules as well as damage to the membrane system, which in turn increases malondialdehyde (MDA) content and electrolyte leakage (EL) as well as lipid peroxidation. The plant membrane system is one of the first sites initially injured by stress conditions, including high salinity [3–5]. Plants mainly resist salt stress damage through a series of physiological activities that includes osmotic regulation, ion transport, and hormone content change [6]. Osmotic regulators in plants mainly include organic osmotic regulators (proline, soluble sugar, soluble protein) and inorganic osmotic regulators (Na+, K+, Cl-), which can together increase cell fluid concentration and reduce osmotic potential as well as maintain intracellular homeostasis and enhance plant resistance to salt stress [7].
Melatonin is a well-known amine hormone, synthesized in chloroplasts and mitochondria, and it is found in most plants and animals [8–10]. Thus, endogenous melatonin is expected to occur in cotton seeds. Moreover, melatonin is an extremely efficient antioxidant that can effectively suppress H2O2 production through enhancing activities of CAT, POD, and APX under drought stress, improving antioxidant defense systems through MT-induced generation of NO and by lowering MDA and H2O2 levels, and regulating various physiological process in plants [11–13]. Accordingly, melatonin enhances plant growth and development under abiotic stresses (such as high salt, heavy metals, etc.) and acts as an osmotic regulatory substance in plants, enabling the maintenance of ion homeostasis and growth regulation [14]. In recent years, more plant science research has been focused on the role of melatonin, which is closely related to indole-3-acetic acid (IAA) in its chemical structure and metabolic pathways [15]. Previous studies have shown that exogenous melatonin can maintain high photosynthetic efficiency in tea plants through its effects on antioxidant systems against abiotic stress, which that enhance salt and cold tolerance [16]. The content of hydrogen peroxide and malondialdehyde in cucumber seedlings obviously increased under irrigation with salt solution, which indicated that salt treatment damaged cell membranes [17]. Furthermore, exogenous melatonin application can significantly reduce the accumulation of hydrogen peroxide and increase the activities of antioxidant enzymes [18]. Melatonin treatment has also been shown to significantly reduce electrolyte leakage in tomato plants under cadmium stress; however, it had no significant effect on tomatoes under normal conditions [19]. Salt stress severely inhibits the growth of soybean seedlings, and the application of exogenous melatonin can increase the soluble protein content of seedlings [20]. The appropriate application of melatonin has been shown to promote not only soluble protein content in Malus hupehensis but also the accumulation of soluble sugar in kiwifruit leaves, while increasing cell fluid concentrations and resistance to stress [21, 22]. Under salt stress, accumulation of Na+ under salt stress of plants leads to ion imbalances and toxicity, with K+ content decreasing as exogenous melatonin promotes the absorption of K+, suggesting that melatonin can regulate ion homeostasis or gene expression responses under salt stress to mitigate damage caused by stress [23–26].
Cotton (Gossypium hirsutum L.) is a major global crop, and it is widely cultivated in China. Under increasing soil salinization, the growth, yield, and quality of cotton harvests have been seriously affected. Seed germination is perhaps the most important and most complex process in the plant growth cycle, as it directly affects the development of cotton plants, which ultimately affects yields. Seed germination can be divided into three periods. First, seeds absorb water from the environment. In the second stage, seed germination is stimulated by various enzymes and hormones that prepare seeds for germination. Finally, the radicle breaks through the seed coat and lengthens. Accordingly, seed germination involves many processes [27]. While the response mechanism of cotton seed germination and seedlings to salt stress have been investigated, the mechanism by which exogenous melatonin influences osmotic control and thus the germination process of cotton seeds under salt stress remains unclear. Therefore, different melatonin concentration treatments were employed in this experiment to investigate the effects of exogenous melatonin on physiological activities such as membrane lipid peroxide, osmotic regulators, and ion homeostasis during the germination of cotton seeds under salt stress. This study provides some novel insights into salt tolerance mechanisms modulated by exogenous melatonin in seed germination.
Materials and methods
Reagents
All chemicals used in this study were of analytical grade. Melatonin (N-acetyl-5-methoxytryptamine) was purchased from Sigma-Aldrich (St. Louis, MO, USA). All other reagents were purchased from Sinopharm Chemical Reagent Beijing Co., Ltd, (Beijing, China).
Plant material
Cotton (Gossypium hirsutum L.) cultivar ‘GXM9’ seeds (provided by Guoxin Rural Technical Service Association of Hejian City, China) were used in the study. The experiment was conducted in the greenhouse facilities of Hebei Agricultural University in Baoding (38.85°N, 115.30°E) City, Hebei Province from September 2018 to May 2019.
Seed germination
Cotton seeds with full seed coats and of consistent size were disinfected with 75% ethanol for 17 min, rinsed in distilled water four times, and dried in a cool and ventilated area. All seeds were randomly divided into six groups, and the experimental treatments were as follows: (1) Control (Con), primed with water without salt treatment; (2) NaCl, primed with water and then treated with salt (150 mM NaCl, screened by the pretest); (3) MT10+NaCl, primed with 10 μM MT (melatonin) solution and then treated with salt (150 mM NaCl); (4) MT20+NaCl, primed with 20 μM MT solution and then treated with salt (150 mM NaCl); (5) MT50+NaCl, primed with 50 μM MT solution and then treated with salt (150 mM NaCl); and (6) MT100+NaCl, primed with 100 μM MT solution and then treated with salt (150 mM NaCl). Three replicates of 500 seeds were used for each treatment.
The cotton seeds were soaked in distilled water or one of the different concentrations of melatonin solutions for 24 h in 15-cm-diameter Petri dishes containing filter paper (Whatman International Ltd, Maidstone, UK), dried, and restored to their initial water content over the course of about 2 d. Seeds were then placed in a light culture box (25°C) and cultured in dark conditions for 6 d. Seeds were examined every two days and were considered germinated when the seed coat was broken and a radicle was visible. Germinated seeds were sampled from each treatment, rapidly frozen in liquid nitrogen, and stored at -80°C until analysis. All experiments were conducted in triplicate.
Determination of cotton seed germination rate
The number of cotton seeds germinated was recorded at 2, 4, and 6 d after seeds were placed in incubators. The following equation was used to calculated the germination rate:
Germination rate = total germinated seeds / total seeds × 100%.
Determination of melatonin content
Melatonin was extracted from cotton seeds using the Plant MT ELISA KIT (Shanghai MLBIO Biotechnology Co. Ltd., Shanghai, China) according to the manufacturer’s instructions. The samples to be tested were incubated with antibodies and measured at 450 nm with a microplate reader (Bio Tek Instrument, Inc., Winooski, VT, USA).
Determination of H2O2, MDA, and EL
Hydrogen peroxide content was determined according to the method used by Sun et al. [28] with some slight modifications. Two grams of cotton seeds were ground in a mortar, with 2 ml of acetone, followed by centrifugation at 10,000 rpm for 10 min. To the supernatant (i.e., hydrogen peroxide extract), acetone was added to reach a total volume of 3 ml. Then, 1 ml of extract, 3 ml of extraction agent, and 5 ml of distilled water were mixed and then centrifuged at 5000 rpm for 1 min, after which, 2 ml of working reagent was mixed. The light absorption value was then measured at 560 nm using a spectrophotometer (UV2450, Shimadzu Corp., Kyoto, Japan).
MDA content was measured according to the method described by Cui et al. [29] with some slight modifications. Cotton seeds were fully ground in pH 7.8 phosphate buffer, followed by centrifugation at 6000 rpm for 10 min. Two milliliters of supernatant was added to the scale test tube, to which 1 ml of 0.5% thiobarbituric acid and 3 ml of 5% trichloroacetic acid solution were added. The solution was heated in a boiling water bath for 10 min and then cooled rapidly. After centrifugation at 6000 rpm for 10 min, the light absorption value was measured at 532 nm and 600 nm using a spectrophotometer (UV2450, Shimadzu Corp.) with distilled water as the blank and 100% light transmittance.
EL was measured according to the assay described by Wu et al. [30] with some slight modifications. First, 0.1 g of fresh sample material was placed in a glass container, to which 30 ml of deionized water was added. The container was placed into a vacuum dryer, and air was extracted from the cells for 1 h. After standing for 5 min, sample conductivity was measured. Samples were sealed with foil, boiled in water for 30 min, and cooled, after which conductivity was measured. Finally, the relative conductivity was calculated.
Determination of osmotic regulators proline, soluble sugar, and soluble protein
The determination of proline content was measured according to the method by Bates et al. [31] with some slight modification. First, 0.3-g samples were cut into pieces and added to a mortar, to which an appropriate amount of 80% ethanol and a small amount of quartz were added prior to grinding the tissue into a homogenate. Then, the volume was filled with 80% ethanol to 25 ml and incubated in an 80°C watered bath for 20 min, after which 0.4 g of artificial zeolite and 0.2 g of activated carbon were added. Samples were subsequently oscillated and filtered, and 2 ml of the above extraction solution was transferred into a test tube, to which 2 ml of glacial acetic acid and 2 ml of indanone were added before being heated in boiling water for 15 min. After cooling, the light absorption values of the samples were measured at 520 nm using a spectrophotometer (UV2450, Shimadzu Corp.).
Soluble sugar content was determined using the anthraquinone colorimetric method [32]. Then, 0.3-g samples and 9 ml of distilled water were added to test tubes that were placed in a boiling water bath for 20 min and cooled. One milliliter of the supernatant and 5 ml of sulfuric acid-anthrone reagent were mixed, placed in a boiling water bath for 10 min, and cooled. The light absorption value of each sample was measured at 620 nm using a spectrophotometer (UV2450, Shimadzu Corp.).
Soluble protein content was determined using Coomassie brilliant blue. First, 0.3-g samples were ground into a homogenate with 5 ml of pH 7.8 phosphate buffer. The supernatant was centrifuged at 4000 rpm for 10 min, and to 0.1 ml of the supernatant, 9 ml of distilled water and 5 ml of Coomassie brilliant were added, followed by centrifugation again and then sample oscillation. After standing for 5 min, the light absorption value was measured at 620 nm using a spectrophotometer (UV2450, Shimadzu Corp.) and distilled water with Coomassie brilliant blue was used as the blank control.
Determination of ion content
To determine the Na+ and K+ contents of cotton seeds, well-ground samples were heated to 500°C for 6 h in a muffle furnace. To the resulting white ash, 5 ml of 2 M hot HCl was added, and the final volume was raised to 50 ml by adding distilled deionized H2O. The above solution was measured for atomic absorption (ZA3000, Hitachi, Ltd., Tokyo, Japan), and the obtained data were subjected to a final calculation. To determine Cl- content, we used a Cl- kit obtained from Nanjing Jiancheng Company (Nanjing, China).
Statistical analysis
The experiment was conducted according to a completely randomized design with three replicates. Analysis of variance (ANOVA) was conducted with SPSS software 21.0 (IBM Corp, Armonk, NY, USA). Differences among treatment means were assessed using Tukey’s honest significant different test considered significant at a p < 0.05 threshold.
Results
Exogenous melatonin promotes cotton seed germination under salt stress
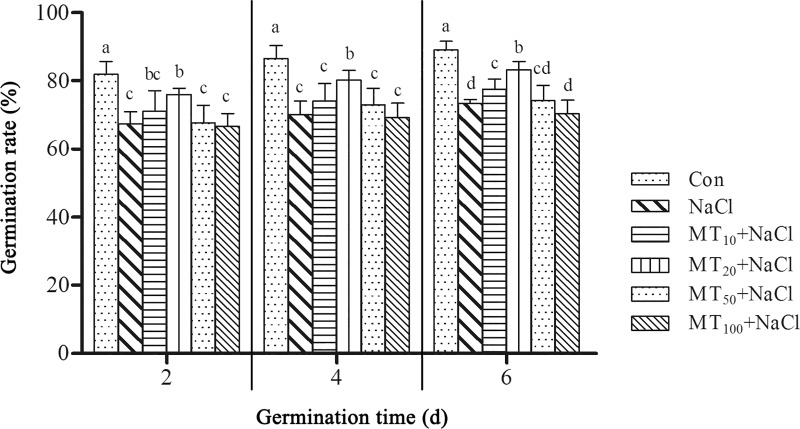
Seed germination is the most important stage in the life course of seeds, and it provides the nutritional basis for the growth and development of seeds into seedlings. We conducted an extensive set of germination assays using cotton seeds to examine the effects of different concentrations of melatonin on seed germination under treatment with 150 mM NaCl (screened by the pretest). As shown in Fig 1, as the germination assay continued, the cotton germination rate continued to increase, reaching its maximum at 6 d. The seed germination rate reached 89.00% under normal conditions (Con), while the germination rate was only about 73.30% under salt stress (NaCl) at 6 d, indicating that NaCl indeed inhibited cotton seed germination. When different concentrations of melatonin were applied, the cotton germination rate exhibited different trends. As melatonin concentrations increased, the cotton germination rate first rose and then decreased, indicating that melatonin affected seed germination in a dosage-dependent manner under salt stress. Low melatonin concentrations (i.e., MT10+NaCl and MT20+NaCl treatments) effectively promoted seed germination; however, the effect of high concentrations of melatonin (i.e., MT50+NaCl and MT100+NaCl treatments) on seed germination was not obvious. Among the treatments examined, the 20 μM melatonin pretreatment had the strongest effect in promoting cotton seed germination at different periods.
Fig 1. Effects of exogenous melatonin on GR in cotton seeds under salt stress.
The data are the means of six replicates (±SE), and treatments with different letters are significantly different at a p < 0.05 threshold.
Melatonin content of seed germinating under different treatments
The melatonin contents of cotton seeds under different treatments were determined. As show in Fig 2, as the germination assays continued, melatonin content in all treatments decreased and reached a minimum at day 6, which indicated that melatonin played a regulatory role in seed germination. At days 2, 4, and 6, the melatonin contents of NaCl stress seeds were significantly lower, by 9.28%, 15.48%, and 21.39% respectively, compared with those of Con seeds, which indicated that NaCl inhibited melatonin synthesis. After applying different exogenous concentrations of melatonin, the melatonin content was significantly higher than that of NaCl seeds. Notably, the melatonin content under the MT10+NaCl treatment was lowest; however, the melatonin content under the MT100+NaCl treatment was highest among all treatments, indicating that exogenous melatonin promoted the accumulation of endogenous melatonin.
Fig 2. Melatonin content of seeds germinating under different treatments.
The data are the means of four replicates (±SE), and treatments with different letters are significantly different at a p < 0.05 threshold.
Exogenous melatonin mitigates membrane permeability during cotton seed germination under salt stress
Exogenous melatonin reduces H2O2 content in cotton seeds under salt stress
Under stress, more H2O2 is produced, and these reactive oxygen species can produce toxic effects through cellular aerobic metabolism. As shown in Fig 3, as the germination assays continued, the hydrogen peroxide content of cotton seeds first sharply decreased and then increased. At 2, 4, and 6 d, the H2O2 contents of NaCl seeds were 6.28%, 60.54%, and 57.26% higher, respectively, compared with those of Con seeds, which indicated that H2O2 gradually accumulated in cotton seeds under salt stress. After applying different exogenous concentrations of melatonin, the H2O2 content was significantly lower than that of NaCl seeds. Notably, the H2O2 content decreased the most under the MT20+NaCl treatment, and it was 27.67%, 52.98%, and 78.69% lower than that of the NaCl treatment at 2, 4, and 6 d, respectively, indicating that the effect of 20 μM melatonin most obviously inhibited H2O2 accumulation.
Fig 3. Effects of exogenous melatonin on H2O2 content in cotton seeds under salt stress.
The data are the means of three replicates (±SE), and treatments with different letters are significantly different at a p < 0.05 threshold.
Exogenous melatonin decreases MDA accumulation in cotton seeds under salt stress
As shown in Table 1, during the seed germination assay, MDA contents under the NaCl treatment were significantly higher than those under the Con treatment, which were 13.36%, 23.70%, and 34.19% higher than those under the Con treatment at 2, 4, and 6 d, respectively, indicating that salt stress led to membrane lipid oxidation and increased MDA content. The MDA content of cotton seeds under exogenous melatonin application was significantly lower than that under the NaCl treatment. Among treatments, the 20 μM melatonin-treated seeds at 2, 4, and 6 d had MDA contents 39.46%, 27.62%, and 34.42% lower, respectively, than those of NaCl seeds. As melatonin concentration increased, MDA content increased slightly under MT50+NaCl and MT100+NaCl treatments, indicating that an appropriate melatonin concentration can effectively reduce membrane oxidation injury and protect cell structure stability.
Table 1. MDA content (μmol g-1) of melatonin-pretreated cotton seeds under salt stress.
| Treatment | 2 d | 4 d | 6 d |
|---|---|---|---|
| Control (Con) | 1.861±0.049b | 2.006±0.010b | 2.066±0.059b |
| NaCl | 2.110±0.152a | 2.481±0.088a | 2.772±0.104a |
| MT10+NaCl | 1.666±0.063c | 2.001±0.118b | 1.960±0.053bc |
| MT20+NaCl | 1.277±0.038d | 1.796±0.119c | 1.818±0.030d |
| MT50+NaCl | 1.812±0.014bc | 1.870±0.104bc | 1.867±0.061cd |
| MT100+NaCl | 1.868±0.064b | 1.936±0.057bc | 2.045±0.102b |
Cells followed by different letters within a column are significantly different at a p < 0.05 threshold.
Exogenous melatonin reduces cotton seed EL under salt stress
EL is an important index of cell membrane damage. During salt stress, membrane lipid peroxidation damages membranes and increases membrane permeability, resulting in solution extravasation and increased EL. As shown in Fig 4, EL was low under normal conditions (Con), which showed that the cell membranes of cotton seeds were relatively complete. Salt stress significantly increased EL in cotton seeds, which was 188.97% higher than that under the Con treatment. However, the EL of cotton seeds treated with exogenous melatonin was significantly lower. MT10+NaCl, MT20+NaCl, MT50+NaCl, and MT100+NaCl treatments had 40.69%, 60.46%, 58.44%, and 54.44% lower EL, respectively, compared with the NaCl treatment. The EL of cotton seeds under the MT20+NaCl, treatment was the lowest of all treatments across different periods, indicating that the appropriate melatonin concentration could effectively alleviate cell membrane damage to cotton seeds caused by salt stress.
Fig 4. Effects of exogenous melatonin on EL content in cotton seeds under salt stress.
The data are the means of three replicates (±SE), and treatments with different letters are significantly different at a p < 0.05 threshold.
Exogenous melatonin affects organic osmotic substance content during cotton seed germination under salt stress
Exogenous melatonin affects the proline content of cotton seeds under salt stress
Proline, an important osmotic regulator in plants, can improve the ability of plants to resist stress. As shown in Fig 5A, proline content decreased overall across the seed germination assay. Under salt stress (NaCl), proline content in cotton seeds decreased obviously, with concentrations of 473.39 μg g-1, 154.35 μg g-1, and 132.34 μg g-1, respectively, 2, 4, and 6 d into the germination assay. The proline contents of NaCl seeds were 21.30% and 3.71% higher and 1.75% lower respectively, compared to Con seeds at 2, 4, and 6 d, respectively, indicating that proline decreased gradually in cells. At 2 d, the proline content was significantly lower under the NaCl treatment than under the MT10+NaCl, MT50+NaCl, and MT100+NaCl treatments; however, proline contents were significantly higher under the MT20+NaCl treatments, indicating that exogenous melatonin had less effect on proline content initially (i.e., 2 d), while it eventually promoted the accumulation of proline in cotton seeds under salt stress (i.e., 4 and 6 d), which was associated with tolerance to salt stress. Notably, the proline content under the MT20+NaCl treatment was the highest across all treatments at different periods, being 3.76%, 63.24%, and 59.25% higher, respectively, at 2, 4, and 6 d compared with the NaCl seeds.
Fig 5.
Effects of exogenous melatonin on proline (A), soluble sugar (B), and soluble protein (C) contents of cotton seeds under salt stress. The data are the means of three replicates (±SE), and treatments with different letters are significantly different at a p < 0.05 threshold.
Exogenous melatonin affects the soluble sugar content of cotton seeds under salt stress
Soluble sugar is also an important osmotic regulator that provides energy for plant growth and development to ensure a well-functioning metabolism. As shown in Fig 5B, as the assay continues, the soluble sugar content overall decreased and then increased. Compared to Con seeds, the soluble sugar content in NaCl seeds was significantly higher, indicating that plant cells must accumulate some organic matter to cope with salt stress. However, the total accumulated sugar by itself was relatively limited, which may have been insufficient for germination. The soluble sugar content trends of cotton seeds differed among melatonin treatments. MT10+NaCl and MT100+NaCl seeds had lower sugar content than did NaCl seeds over the course of the assay, while that of MT20+NaCl seeds was significantly higher throughout the assay, by 11.43%, 9.32%, and 21.57%, respectively, at 2, 4, and 6 d. Additionally, the soluble sugar content of cotton seeds under the MT50+NaCl treatment was significantly higher than that of NaCl seeds at 4 and 6 d. This indicates that both low and high concentrations of melatonin (MT10+NaCl and MT100+NaCl, respectively) inhibited the accumulation of soluble sugar in cotton seeds, and intermediate concentrations of melatonin (MT20+NaCl and MT50+NaCl) were effective in promoting the accumulation of soluble sugar in cotton seeds.
Among treatments, the MT20+NaCl treatment had the highest soluble sugar content, indicating it is a suitable concentration of melatonin for improving salt stress resistance in cotton seeds.
Exogenous melatonin affects soluble protein content of cotton seeds under salt stress
Soluble proteins are among the main metabolites that accumulate in various species of higher plants in response to salt stress. Fig 5C shows soluble protein content reductions for the NaCl-treated seeds of 16.13%, 26.78%, and 29.49%, respectively, compared with Con seeds at 2, 4, and 6 d. Under all melatonin treatments, except for MT10+NaCl, the soluble protein contents were significantly higher than under the NaCl treatment. In particular, the soluble protein content under the MT20+NaCl treatment was the highest across all stages. This suggests that melatonin supported macromolecular structure proteins and played a role in maintaining cell stability.
Exogenous melatonin affects inorganic osmotic regulators in cotton seeds under salt stress
Exogenous melatonin affects Na+ and Cl- content of cotton seeds under salt stress
Na+ toxicity is one of the main components of salt stress in plants. As shown in Fig 6A, at 2, 4, and 6 d, the content of Na+ in NaCl seeds under salt stress was significantly higher, by 21.00%, 32.50%, and 62.11%, respectively, compared with Con seeds, resulting in an ion imbalance. After treatment with various concentrations of melatonin, Na+ content was significantly lower, demonstrating that melatonin slowed the rate of ions entering cells, thereby effectively protecting the cellular structure. Na+ contents under the MT20+NaCl treatment were 14.72%, 22.89%, and 28.40% lower at 2, 4, and 6 d, respectively, relative to the NaCl treatment, showing the most obvious Na+ accumulation inhibition among melatonin treatments. Fig 6B shows that not only Na+ content but also Cl- content increased significantly under salt stress. At 2, 4, and 6 d, the Cl- content under the NaCl treatment was 152.06%, 54.52%, and 109.90% higher, respectively, compared with Con seeds. The Cl- content decreased significantly under different concentrations of melatonin. In particular, the Cl- contents under the MT20+NaCl treatment were 46.86%, 31.04%, and 46.14% lower at 2, 4, and 6 d, respectively, relative to the NaCl treatment. The trend in Cl- content was similar to that in Na+ content, indicating that melatonin may reduce damage caused by Cl-, thus protecting cell membranes.
Fig 6.
Effects of exogenous melatonin on Na+ (A), Cl- (B), and K+ (C) content in cotton seeds under salt stress. The data are the means of six replicates (±SE), and treatments with different letters are significantly different at a p < 0.05 threshold.
Exogenous melatonin affects K+ content and the K+/Na+ balance of cotton seeds under salt stress
K is an essential element in plants, and high K+/Na+ balance can improve salt tolerance. As shown in Fig 6C, the K+ content of cotton seeds decreased significantly throughout the germination assay. Among the different melatonin treatments, K+ content trends over time differed. When treated with 20 μM melatonin (MT20+NaCl), K+ content increased by 21.20%, 33.72%, and 4.87% at 2, 4, and 6 d, respectively, compared with NaCl seeds, indicating that an appropriate concentration of melatonin can maintain ion homeostasis and relieve the toxicity of salt stress. As shown in Table 2, salt stress (i.e., NaCl treatment) resulted in significantly lower K+/Na+ balances, which were 49.19%, 43.30%, and 52.02% lower than those of Con seeds at 2, 4, and 6 d, respectively. However, the K+/Na+ balance increased significantly after the application of different melatonin concentrations. Notably, MT20+NaCl treatment increased the K+/Na+ balance by 42.21%, 73.44%, and 46.46% compared with NaCl at 2, 4, and 6 d, respectively. Accordingly, appropriate concentrations of melatonin can effectively control the rate of K+/Na+ intake and maintain a relatively high K+/Na+ balance, as well as protect intracellular ion homeostasis in cotton seed cells under salt stress.
Table 2. Effects of melatonin treatment on K+/Na+ balance in cotton seeds under salt stress.
| Treatment | 2 d | 4 d | 6 d |
|---|---|---|---|
| Control (Con) | 0.256±0.0036a | 0.145±0.0042a | 0.160±0.0082a |
| NaCl | 0.130±0.0253d | 0.082±0.0047d | 0.077±0.0017c |
| MT10+NaCl | 0.127±0.0118d | 0.104±0.0070c | 0.084±0.0138c |
| MT20+NaCl | 0.185±0.0126b | 0.143±0.0071a | 0.113±0.0049b |
| MT50+NaCl | 0.155±0.0139c | 0.089±0.0077d | 0.058±0.0038d |
| MT100+NaCl | 0.165±0.0102c | 0.119±0.0093b | 0.055±0.0051d |
Cells followed by different letters within a column are significantly different at a p < 0.05 threshold.
Discussion
In recent years, abiotic stress has markedly impaired crop yields [33]. Salt is one of the main factors affecting ecological environments and inhibiting crop growth. With the expansion of saline-alkali land areas in China, crop yields and quality have decreased gradually [34]. The salt stress damage to plants is mainly manifested through osmotic stress, ion stress, and antioxidant system and hormone signal transduction, among other phenomena [35, 7]. Various osmotic regulatory substances can be actively accumulated in plants, allowing them to cope with damaged cells and maintain homeostasis between the internal and external environments of cells, thus enabling plants to slowly adapt to salt stress [23]. A similar pattern is observed in salt-stress seeds, but with reduced growth rates and germination rates, indicating salt stress affects germination of cotton seeds. Meanwhile, the contents of H2O2, EL, and MDA increased and the content of organic osmotic substances in cotton seeds increased, such as proline and soluble sugar, which changed in the seeds upon exposure to saline stress. Moreover, the content of inorganic osmotic regulatory substances increased, including Na+ and Cl-, which changed in the seeds owing to a loss of control under saline stress; however, the content of soluble proteins and K+ as well as the K+/Na+ balance decreased. Together, these results indicate that salt stress led to membrane lipid peroxidation, which affected cotton seed germination, consistent with the results of Samea-Andabjadid et al. [5] and Castanares et al. [36].
Melatonin is a small molecular hormone found in plants and animals; it is a broad-spectrum growth regulator and antioxidant that resists peroxidation damage to plants caused by stresses such as salt and drought stresses [23]. Abiotic stress can induce changes in the melatonin content of plants [37]. The present study showed that melatonin levels decreased with time in the control (Fig 2), consistent with their consumption by antioxidant action. Salt stress can reduce melatonin levels in cotton seeds (Fig 2); as there are lower reactive oxygen species levels under higher melatonin treatments, it is more likely that it is consumed by antioxidant action of the melatonin, which is consistent with previous findings [17]. However melatonin content significantly increased after the application of exogenous melatonin, indicating that exogenous melatonin could induce endogenous melatonin accumulation [17]. Melatonin content reached its minimum on day 6, while the germination rate peaked at day 6 of the seed germination trial, indicating a relationship between melatonin content and seed germination. During germination, melatonin content decreased while alleviating the inhibitory effects of high salinity. However, melatonin content was not positively correlated with seed germination rate under salt stress. Low melatonin concentrations (i.e., MT10+NaCl and MT20+NaCl treatments) effectively promoted seed germination at all times, but not up to the control level, suggesting that melatonin alleviates some of the damage caused by salt stress, not completely eliminates it. Meanwhile, the effect of high concentrations of melatonin (i.e., MT50+NaCl and MT100+NaCl treatments) on seed germination was not obvious, demonstrating that 20 μM melatonin was the optimum concentration for promoting cotton seed germination under salt stress (Fig 1), which indicates that the effect of exogenous melatonin is closely related to its concentration. A higher concentration of melatonin is not conductive to the mitigation of salt stress, and it is necessary to select an appropriate concentration of melatonin based on the specific situation. Similar results have been reported in rice and rapeseed [38, 39].
It has been suggested that melatonin improves the redox state of cells, thereby decreasing levels of ROS and reactive nitrogen species and stabilizing biological membranes in plant cells. Previous studies have shown that cold stress induced a considerable accumulation of hydrogen peroxide in cucumber and watermelon seeds, and exogenous melatonin can effectively inhibit the accumulation of hydrogen peroxide [40, 41]. Exogenous melatonin reduced MDA content in Avena nuda under salt stress and increased tolerance to salt stress [42], and an appropriate concentration of melatonin was able reduce electrolyte exudation and effectively protect cell membranes in bermudagrass [43]. Additionally, melatonin-treated seedlings exhibited reduced oxidative damage through the inhibited overproduction of these ROS and MDA as well as EL. This may be owing to the role of melatonin in plants under abiotic stress, as it acts as an antioxidant that upregulates the expression of antioxidant enzymes, thereby reducing ROS levels [44]. In the present study, H2O2 was quite high in the control at 2 d, dropped drastically by 4 d, and rose again by 6 d. The amount of H2O2 in the salinity treatment at day 6 was less than that of the Control treatment at day 2, but the germination rate was significantly less than the control at all time points. The MT50+NaCl and MT100+NaCl treatments had substantially decreased H2O2 levels at 4 d and 6 d, but the germination rate was unimproved. Consequently, the amount of hydrogen peroxide may not be a particularly important variable, or the seed germination process may be quite tolerant to it (Fig 3). MDA and EL are important indicators of cell membrane stability. During the seed germination assay, salt stress led to membrane lipid oxidation and increased MDA and EL contents. However, the content of MDA and EL in cotton seeds decreased in response to melatonin treatment. The MDA and EL content of the 20 μM melatonin-treated seeds were significantly lower than those of NaCl-treated seeds, confirming that the effect of melatonin was dose dependent and that a suitable concentration of melatonin could reduce peroxidation damage to membrane lipids (Fig 4, Table 1). Based on these observations, an optimal melatonin concentration appears to mitigate the accumulation of H2O2, defending against oxidative stress, and too much melatonin disrupts ROS accumulation in germinating seeds, consistent with previous research [45].
The accumulation of osmotic regulators plays an important role in maintaining intracellular stability and protecting cells from salt stress and toxicity. Melatonin can promote the accumulation of proline in tomato seedlings under salt stress and also accelerates cucumber seed germination [44, 46]. In the present study, proline content dropped in the control over time, but increased under saline treatment by 2 d and then dropped to a similar level as the control by days 4 and 6, which indicates salt stress led to the accumulation of proline in the early stages of cotton seed germination. Proline content was increased by melatonin pretreatment, perhaps though melatonin regulating the related metabolism of osmotic substances and enhancing salt tolerance. Notably, MT20+NaCl increases proline levels at all times, contributing to more normal osmotic balance in seeds. Notably, increased melatonin concentration does not lead to more proline (Fig 5A), indicating that excessive supplemental melatonin inhibits the accumulation of proline in germination seeds.
In the present experiment, the soluble sugar content overall decreased first and then increased in saline treatments, indicating that salt stress led to the accumulation of soluble sugar in cotton seeds. The sugar content was increased by intermediate concentrations of melatonin pretreatment (MT20+NaCl and MT50+NaCl) or decreased by low and high concentrations of melatonin pretreatment (MT10+NaCl and MT100+NaCl, respectively), which indicates that melatonin regulated the observed change in osmotic substances to improve salt tolerance (Fig 5B). The soluble protein content of cotton seeds decreased as caused by the degradation of proteins under salt stress, and the soluble protein content increased obviously after melatonin treatment, likely as a consequence of melatonin inducing protein synthesis and inhibiting the degradation process, thus maintaining the physiological activity and stability of cells (Fig 5C). In this study, exogenous application of 20 μM melatonin is most efficient in increasing the concentration of proline, soluble sugar, and soluble protein and these substances need to be greater in the experimental treatments than in the control to improve osmotic balance in salt stress seeds (Fig 5). However, even in the control, these substances vary with time, indicating that the germination of cotton seeds is a complex process, which is similar to previous results [38].
The dynamic balance of ions in plant cells plays an important role in plant growth and development, which can protect enzyme activity, and maintains membrane potential and osmotic pressure, thus maintaining cell volumes. Previous studies have demonstrated the potential of exogenous melatonin application in mediating K+/Na+ homeostasis and relative uptake rates of K+ and Na+ under salt stress in sweet potato [47]. Under salt stress, Na+ and Cl- ions rush into the cells of seeds, causing the accumulation of Na+, affecting the absorption of K+ by plants, and causing ionic toxicity [48]. Maintaining a high K+/Na+ balance is essential to maintaining cell metabolism. Excessive salt ions disturb ion homeostasis and inhibit plant growth and development [49, 50]. Castanares et al. [36] concluded that salt stress leads to K+/Na+ imbalance, destroys cell membrane integrity, and reduces potassium retention, while exogenous melatonin effectively relieved this effect, which might be related to the activity of related enzymes in cells. Similarly, melatonin could significantly reduce Na+ accumulation and increase K+ content in maize seeds under salt stress [34]. Yu et al. [51] proposed a novel mechanism for melatonin-mediated salt tolerance, i.e., melatonin supplementation decreased the oxidative damage induced by salinity, perhaps by directly scavenging H2O2 or enhancing the activities of antioxidative enzymes. In addition, melatonin might control the expression of ion-channel genes (MdNHX1 and MdAKT1) under salinity and maintain ion homeostasis and thus improve salinity resistance in plants exposed to exogenous melatonin [52]. As the germination time continued in the present experiment, the Na+ content of cotton seeds increased continually, and the K+ content of cotton seeds decreased and then increased in the Con and NaCl treatments. The Na+ content of cotton seeds decreased obviously while K+ content of cotton seeds increased obviously under salt stress. Na+ content was significantly lower and K+ content and K+/Na+ balance were significantly higher after melatonin treatments compared to Con seeds across different stages (Fig 6A–6C, Table 2). This demonstrated that the application of melatonin alleviated the accumulation of Na+ by increasing K+ absorption to maintain K+/Na+ balance and enhance salt tolerance. The present study also confirms the beneficial effect of 20 μM melatonin treatment on maintaining ion balance under salt stress.
Conclusions
Exogenous melatonin was able to effectively alleviate salt stress damage and promote cotton seed germination by improving the physiological activity of cotton seeds by maintaining the K+/Na+ balance in vivo and promoting the content of melatonin and osmotic regulation substances. The 20 μM melatonin treatment was particularly effective in reducing the physiological damage caused by salt stress to cotton seeds and internally stabilizing cells, which was the most effective treatment in promoting seed germination under salt stress.
Supporting information
(PDF)
(XLSX)
Acknowledgments
The authors thank the anonymous reviewers for their valuable comments and suggestions. We also thank our laboratory members for their help and efforts. Li Chen and Liantao Liu contributed equally to this research.
Abbreviations
- MT
melatonin
- GR
germination rate
- H2O2
hydrogen peroxide
- MDA
malondialdehyde
- EL
electrolyte leakage
- ROS
reactive oxygen species
- IAA
indole-3-acetic acid
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the National Science Foundation of China (No. 31871569 and 31571610).
References
- 1.Liang W, Ma X, Wan P, Liu P. Plant salt-tolerance mechanism: A review. Biochem Biophys Res Commun. 2018, 495(1): 286–291. 10.1016/j.bbrc.2017.11.043 . [DOI] [PubMed] [Google Scholar]
- 2.Malcolm E, Sumner R N. Sodic soils-distribution, properties, management, and environment consequence [M]. New York: Oxford University, 1998. [Google Scholar]
- 3.Sapre S, Gontia-Mishra I, Tiwari S. Klebsiella sp. confers enhanced tolerance to salinity and plant growth promotion in oat seedlings (Avena sativa). Microbiological Research. 2018, 206: 25–32. 10.1016/j.micres.2017.09.009 . [DOI] [PubMed] [Google Scholar]
- 4.Cui F, Sui N, Duan G, Liu Y, Han Y, Liu S, et al. Identification of Metabolites and Transcripts Involved in Salt Stress and Recovery in Peanut. Frontiers in Plant Science. 2018; 9: 217 10.3389/fpls.2018.00217 . PMCID: PMC5827294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samea-Andabjadid Samira, Ghassemi-Golezani Kazem, Nasrollahzadeh Safar, Nosratollah Najafi. Exogenous salicylic acid and cytokinin alter sugar accumulation, antioxidants and membrane stability of faba bean. Acta biologica Hungarica. 2018, 69(1): 86–96. 10.1556/018.68.2018.1.7 . [DOI] [PubMed] [Google Scholar]
- 6.Hu T, Zhang GX, Zheng FC, Cao Y. Research Progress in Plant Salt Stress Response. Molecular Plant Breeding. 2018, 16 (09): 3006–3015. 10.13271/j.mpb.016.003006. [DOI] [Google Scholar]
- 7.Park Hee Jin, Kim Woe-Yeon, Yun Dae-Jin. A New Insight of Salt Stress Signaling in Plant. Molecules and Cells. 2016; 39(6): 447–459. 10.14348/molcells.2016.0083 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amao MB, Hernández-Ruiz J. Functions of melatonin in plants: a review. J Pineal Res. 2015, 59(2): 133–150. 10.1111/jpi.12253 . [DOI] [PubMed] [Google Scholar]
- 9.Pevet P, Klosen P, Felder-Schmittbuhl MP. The hormone melatonin: Animal studies. Best Pract Res Clin Endocrinol Metab. 2017, 31(6): 547–559. 10.1016/j.beem.2017.10.010 . [DOI] [PubMed] [Google Scholar]
- 10.Tan DX, Manchester LC, Liu X, Rosales-Corral SA, Acuna-Castroviejo D, Reiter RJ. Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J Pineal Res. 2013; 54(2):127–138. 10.1111/jpi.12026 . [DOI] [PubMed] [Google Scholar]
- 11.Li C, Tan DX, Liang D, Chang C, Jia D, Ma F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. Journal of Experimental Botany. 2015, 66(3): 669–680. 10.1093/jxb/eru476 . [DOI] [PubMed] [Google Scholar]
- 12.Kaya C, Okant M, Ugurlar F, Alyemeni MN, Ashraf M, Ahmad P. Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere. 2019, 225: 627–638. 10.1016/j.chemosphere.2019.03.026 . [DOI] [PubMed] [Google Scholar]
- 13.Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007; 42(1): 28–42. 10.1111/j.1600-079X.2006.00407.x . [DOI] [PubMed] [Google Scholar]
- 14.Timothy J. Flowers Timothy D. Colmer. Plant salt tolerance: adaptations in halophytes. Annals of Botany. 2015, 115: 327–331. 10.1210/jcem-33-6-992 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnao MB, Hernandez-Ruiz J. Melatonin and its relatoninship to plant hormones. Annals of Botany. 2018, 12; 121(2): 195–207. 10.1093/aob/mcx114 . PMCID: PMC5808790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Jiahao, Yang Yiqing, Sun Kang, Chen Yi, Chen Xuan, Li Xinghui. Exogenous Melatonin Enhances Cold, Salt and Drought Stress Tolerance by Improving Antioxidant Defense in Tea Plant (Camellia sinensis (L.) O. Kuntze). Molecules, 2019, 24(9), 1826 10.3390/molecules24091826 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang HJ, Zhang N, Yang RC, Wang L, Sun QQ, Li DB, et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J Pineal Res. 2014, 57(3): 269–79. 10.1111/jpi.12167 . [DOI] [PubMed] [Google Scholar]
- 18.Gong B, Yan Y, Wen D, Shi Q. Hydrogen peroxide produced by NADPH oxidase: a novel downstream signaling pathway in melatonin induced stress tolerance in Solanum Iycopersicum. Physiologia Plantarum. 2017, 160(4): 396–409. 10.1111/ppl.12581 . [DOI] [PubMed] [Google Scholar]
- 19.Li MQ, Hasan MK, Li CX, Ahammed GJ, Xia XJ, Shi K, et al. Melatonin mediates selenium-induced tolerance to cadmium stress in tomato plants. J Pineal Res.2016, 61(3): 291–302. 10.1111/jpi.12346 . [DOI] [PubMed] [Google Scholar]
- 20.Wei W, Li QT, Chu YN, Reiter RJ, Yu XM, Zhu DH, et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J Exp Bot. 2015, 66(3): 695–707. 10.1093/jxb/eru392 . PMCID: PMC4321538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang P, Sun X, Chang C, Feng F, Liang D, Cheng L. Delay in leaf senescence of Malus hupehensis by long-term melatonin application is associated with its regulation of metabolic status and protein degradation. J Pineal Res. 2013, 55(4): 424–434. 10.1111/jpi.12091 . [DOI] [PubMed] [Google Scholar]
- 22.Liang Dong, Shen Yanqiu, Ni Zhiyou, Wang Qin, Lei Zhi, Xu Nengqin, et al. Exogenous Melatonin Application Delays Senescence of Kiwifruit Leaves by Regulating the Antioxidant Capacity and Biosynthesis of Flavonoids. Frontiers in Plant Science. 2018; 9: 426 PMCID: PMC5896581. 10.3389/fpls.2018.00426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhan Haoshuang, Nie Xiaojun, Zhang Ting, Li Shuang, Wang Xiaoyu, Du Xianghong, et al. Melatonin: A Small Molecule but Important for Salt Stress Tolerance in Plants. International Journal of Molecular Sciences. 2019, 20(3), 709 10.3390/ijms20030709 . PMCID: PMC6387279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashraf M., Wu L. Breeding for salinity tolerance in plants. Critical Reviews in Plant Sciences. 1994, 13(1): 17–42. 10.1080/07352689409701906. [DOI] [Google Scholar]
- 25.Wu H H. Plant salt tolerance and Na+ sensing and transport. Crop Science Society of China. 2018, 215–225. 10.1016/j.cj.2018.01.003. [DOI] [Google Scholar]
- 26.Frans J. M. Maathuis. Sodium in plants: perception, signaling, and regulation of sodium fluxes. Journal of Experimental Botany. 2014, 65(3): 849–58. 10.1093/jxb/ert326 . [DOI] [PubMed] [Google Scholar]
- 27.Bewley J. Seed Germination and Dormancy. The Plant Cell. 1977, 9(7), 1055–1066. 10.1105/tpc.9.7.1055 . PMCID: PMC156979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun C, Lu L, Liu L, Liu W, Yu Y, Liu X. et al. Nitrate reductase mediated early nitric oxide burst alleviates oxidative damage induced by aluminum through enhancement of antioxidant defenses in roots of wheat (Triticum aestivum). New phytologist. 2014, 201(4): 1240–1250. 10.1111/nph.12597 . [DOI] [PubMed] [Google Scholar]
- 29.Cui G, Zhao X, Liu S, Sun F, Zhang C, Xi Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiology and biochemistry. 2017, 118: 138–149. 10.1016/j.plaphy.2017.06.014 . [DOI] [PubMed] [Google Scholar]
- 30.Wu Wenli, Zhang Qiang, Ervin Erik. H., Yang Zhiping, Zhang Xunzhong. Physiological Mechanism of Enhancing Salt Stress Tolerance of Perennial Ryegrass by 24-Epibrassinolide. Frontiers in Plant Sciences. 2017, 8: 1017 10.3389/fpls.2017.01017 . PMCID: PMC5474491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bates L. S., Waldren R. P., Teare I. D. Rapid determination of free proline for water-stress studies. Plant soil. 1973, 39: 205–217. 10.1007/BF00018060. [DOI] [Google Scholar]
- 32.Xu L, Islam F, Ali B, Pei Z, Li J, Ghani M A, et al. Silicon and water-deficit stress differentially modulate physiology and ultrastructure in wheat (Triticum aestivum L.). 3 Biotech. 2017, 7(4): 273 10.1007/s13205-017-0904-5 . PMCID: PMC5538996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You J, Chan Z. ROS Regulation During Abiotic Stress Responses in Crop Plants. Front Plant Sci. 2015, 6: 1092 10.3389/fpls.2015.01092 . PMCID: PMC4672674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen YE, Mao JJ, Sun LQ, Huang B, Ding CB, Gu Y, et al. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiologia plantarum. 2018, 164(3): 349–363. 10.1111/ppl.12737 . [DOI] [PubMed] [Google Scholar]
- 35.Deinlein U, Stephan AB, Horie T, Luo W, Xu G, Schroeder JI. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014. 19(6): 371–379. 10.1016/j.tplants.2014.02.001 . PMCID: PMC4041829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castanares Jose Luis, Bouzo Carlos Alberto. Effect of Exogenous Melatonin on Seed Germination and Seedling Growth in Melon (Cucumis melo L.) Under Salt Stress. Horticultural Plant Journal. 2019, 5 (2): 79–87. 10.1016/j.hpj.2019.01.002. [DOI] [Google Scholar]
- 37.Sharafi Yavar, Morteza Soleimani Aghdam Zisheng Luo, et al. Melatonin treatment promotes endogenous melatonin accumulation and triggers GABA shunt pathway activity in tomato fruits during cold storage. Food Chemistry. 2019, 254; 222–227. 10.1016/j.scienta.2019.04.056. [DOI] [Google Scholar]
- 38.Zeng L, Cai JS, Li JP, Lu G Y, Li CS, Fu GP, et al. Exogenous application of a low concentration of melatonin enhances salt tolerance in rapeseed (Brassica napus L.) seedlings. Journal of Integrative Agriculture. 2018, 17(2): 328–335. 10.1016/S2095-3119(17)61757-X. [DOI] [Google Scholar]
- 39.Liang C, Zheng G, Li W, Wang Y, Hu B, Wang H, et al. Melatonin delays leaf senescence and enhances salt stress tolerance in rice. Journal of Pineal Research. 2015, 59(1):91–101. 10.1111/jpi.12243 . [DOI] [PubMed] [Google Scholar]
- 40.Zhao HL, Ye L, Wang YP, Zhou XT, Yang JW, Wang JW, et al. Melatonin increase the Chilling tolerance of chloroplast in cucumber seedlings by regulating photosynthetic electron Flux and the Ascorbate-Glutathione Cycle. Frontiers in plant science. 2016, 7: 1814 10.3389/fpls.2016.01814 . PMCID: PMC5138187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, Chang J, Chen H, Wang Z, Gu X, Wei C, et al. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front Plant Sci. 2017, 1; 8: 295 10.3389/fpls.2017.00295 . PMCID: PMC5331065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao W, Feng Z, Bai Q, He JJ, Wang YJ. Melatonin-Mediated Regulation of Growth and Antioxidant Capacity in Salt-Tolerant Naked Oat under Salt Stress. International Journal of Molecular Sciences. 2019, 20(5), 1176 10.1007/bf00265803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi H, Ye T, Zhong B, Liu X, Chan Z. Comparative proteomic and metabolomics analyses reveal mechanisms of improved cold stress tolerance in bermudagrass (Cynodon dactylon (L.) Pers.) by exogenous melatonin calcium. Journal of integrative plant biology. 2014, 56(11): 1064–79. 10.1111/jipb.12167 . [DOI] [PubMed] [Google Scholar]
- 44.Siddiqui MH, Alamri S, Al-Khaishany MY, Khan MN, Al-Amri A, Ali HM, et al. Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int J Mol Sci. 2019, 16; 20(2). 10.3390/ijms20020353 . PMCID: PMC6358940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ke Q, Ye J, Wang B, Ren J, Yin L, Deng X, et al. Melatonin mitigate salt stress in wheat seedlings by modulating polyamine metabolism. Frontiers in plant science. 2018, 3; 9: 914 10.3389/fpls.2018.00914 . PMCID: PMC6037824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang N, Zhang HJ, Sun QQ, Cao YY, Li X, Zhao B, et al. Proteomic analysis reveals a role of melatonin in promoting cucumber seed germination under high salinity by regulating energy production. Scientific reports. 2017, 7(1): 503 10.1038/s41598-017-00566-1 . PMCID: PMC5428666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Y, Wang A, Li X, Kou M, Wang W, Chen X, et al. Melatonin-Stimulated Triacylglycerol Breakdown and Energy Turnover under Salinity Stress Contributes to the Maintenance of Plasma Membrane H+-ATPase Activity and K+/Na+ Homeostasis in Sweet Potato. Front Plant Sci. 2018, 9: 256 10.3389/fpls.2018.00256 . PMCID: PMC5835075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu JK. Regulation of ion homeostasis under salt stress. Current opinion in plant biology. 2003, 6(5): 441–445. 10.1016/s1369-5266(03)00085-2 . [DOI] [PubMed] [Google Scholar]
- 49.Li J, Liu J, Zhu T, Zhao C, Li L, Chen M. The Role of Melatonin in salt stress responses. International journal of molecular sciences. 2019, 20(7). 10.3390/ijms20071735 . PMCID: PMC6479358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan F, Lyu MJ, Leng BY, Zhu XG, Wang BS. The transcriptome of NaCl-treated Limonium bicolor leaves reveals the genes controlling salt secretion of salt gland. Plant molecular biology. 2016, 91(3): 241–256. 10.1007/s11103-016-0460-0 . [DOI] [PubMed] [Google Scholar]
- 51.Yu Y, Wang A, Li X, Kou M, Wang W, Chen X, et al. Melatonin-Stimulated Triacylglycerol Breakdown and Energy Turnover under Salinity Stress Contributes to the Maintenance of Plasma Membrane H+-ATPase Activity and K+/Na+ Homeostasis in Sweet Potato. Front Plant Sci. 2018, 27; 9:256 10.3389/fpls.2018.00256 . PMCID: PMC5835075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li C, Wang P, Wei Z, Liang D, Liu C, Yin L, et al. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J Pineal Res. 2012, 53(3): 298–306. 10.1111/j.1600-079X.2012.00999.x . [DOI] [PubMed] [Google Scholar]