Abstract
Computerized clinical decision support (CDS) faces challenges to interoperability and scalability. Centralized, web-based solutions offer a mechanism to share the cost of CDS development, maintenance, and implementation across practices. Data standards have emerged to facilitate interoperability and rapid integration of such third-party CDS. This case report describes the challenges to implementation and scalability of an integrated, web-based CDS intervention for EMergency department-initiated BuprenorphinE for opioid use Disorder which will soon be evaluated in a trial across 20 sites in five healthcare systems. Due to limitations of current standards, security concerns, and the need for resource-intensive local customization, barriers persist related to centralized CDS at this scale. These challenges demonstrate the need and importance for future standards to support two-way messaging (read and write) between electronic health records and web applications, thus allowing for more robust sharing across health systems and decreasing redundant, resource-intensive CDS development at individual sites.
Keywords: clinical decision support systems, emergency department-initiated buprenorphine, buprenorphine, user-centered design, EHR interoperability
INTRODUCTION
Overview
Computerized clinical decision support (CDS) has proven potential to increase the adoption of evidence-based medicine.1,2 In particular, CDS has been successful in improving adoption of medication-related best practices.3–5 However, customizing local CDS solutions often involves significant costs due to the technical infrastructure and staff expertise required.6,7 In response, centralized web service-based CDS models have emerged as a mechanism to share the cost of development, maintenance, and implementation across practices.6 In addition, data standards such as Fast Healthcare Interoperability Resources (FHIR) and specifications such as Substitutable Medical Apps and Reusable Technology (SMART) and CDS-Hooks have been proposed to facilitate interoperability and rapid integration of these third-party CDS into electronic health record (EHR) systems.7
EMBED project context (EMergency department-initiated BuprenorphinE for opioid use Disorder)
Approximately 2.1 million Americans have opioid use disorder (OUD).8 The cost of the opioid crisis has been estimated to be greater than $500 billion per year.9 There were 47 600 opioid-related overdose deaths in the United States in 2017, representing 67.8% of all drug overdose deaths.10 People with OUD often seek care in the emergency department (ED) for complications and morbidity associated with opioid use, contributing to a 30% increase in ED visits for opioid overdose from 2016 to 2017.11 These visits offer an important opportunity to engage patients in treatment for OUD.12
Medications for OUD (MOUD), specifically opioid agonists such as methadone and buprenorphine/naloxone (BUP), can safely and effectively treat OUD by decreasing future opioid use, mortality, craving, and withdrawal symptoms while also increasing retention in treatment.13–15 A 2015 randomized clinical trial involving 329 patients with OUD showed that BUP could be safely initiated in the ED. Furthermore, patients in the BUP intervention group were twice as likely to be engaged in addiction treatment at 30 days (78% vs 37%).16 A retrospective cohort study of 17 568 adults who survived an opioid overdose showed that all-cause mortality at 12 months dropped from 4.7% (95% confidence interval 4.4–5.0) to 2.0% (95% confidence interval 1.3–2.7) in persons receiving BUP treatment.14
Despite this treatment effect, initiation of BUP has not been adopted as routine emergency care for people with OUD.17,18 This is partly due to emergency clinicians’ perception that the practice of initiation of BUP is unfamiliar, complicated, and time consuming, problems which are only exacerbated by the dynamic ED context.12,19 To overcome these barriers and leverage the ED as a critical opportunity to treat OUD, we developed a CDS solution to assist emergency clinicians with the processes and decisions that are necessary to initiate BUP for OUD patients such that this practice could be simplified to a few clicks in the EHR. The CDS was implemented with the goal of optimizing its usability, EHR integration, automation of EHR workflow, and scalability across a variety of healthcare systems. Here, we report the technical details, challenges, and lessons learned while implementing this CDS.
CASE DESCRIPTION
Setting
The EMergency department-initiated BuprenorphinE for opioid use Disorder (EMBED) CDS was developed to serve as the intervention in a pragmatic trial that compares the effectiveness of CDS with an automated and EHR-integrated workflow to usual care on the adoption of ED-initiation of BUP in emergency care.20–22 This 18-month parallel, cluster-randomized trial will evaluate the intervention in 20 EDs in 5 healthcare systems nationally.22 The EMBED intervention was intended to be vendor-agnostic and capable of integration within multiple healthcare systems; each health system in the trial utilizes a different EHR platform and/or different build of the same vendor’s product.
Integration needs for automated workflow
Full details of the user-centered design have been previously reported.20 Twenty-six ED physicians expressed needs in four key areas that would allow CDS to support ED-initiation of BUP: (1) identify patients appropriately, (2) avoid workflow disruptions, (3) streamline clerical burden, and (4) help users understand the treatment process. Specifically, the application would need to offer a patient-specific, rapid, integrated, automated care pathway as well as optional support in diagnosing OUD, assessing withdrawal severity, and motivating readiness for treatment.
Features
Due to the limited flexibility of CDS customization available from our EHR vendor at the time of initial development, an EHR-integrated web application was created with a graphical user interface similar to the final design prototype. This was intended to allow for future interoperability with other EHR platforms. As our primary health system used Epic (Epic Systems, Verona, WI), we first focused on integration with the Epic EHR.
First, we evaluated development of a SMART on FHIR application. At that time, Epic’s FHIR server did not support the requirements described above. After a comprehensive review of other application architectures, the decision was made to utilize the Epic Active Guidelines (AGL) framework. AGL provides multiple advantages compared to generic SMART on FHIR, primarily an ability to directly interact with Epic through a dedicated Application Programming Interface (API). While other major EHR vendors may support similar functionality in the future, the key limitation of AGL is that it is currently supported only by Epic.
AGL was used to seamlessly integrate the EMBED web application directly into the user’s EHR workflow. The application is accessible from the navigation bar directly in a patient’s chart. In technical terms, the web application is launched in a dedicated sandboxed environment using an iframe (ie, a page from one domain is framed inside a page from another domain). AGL was developed using an HTML5 web messaging protocol to allow secure communication between a trusted web application and the Epic AGL API. When launched, the application receives an authentication token which is used for direct communication between the web application and the EHR.
The EMBED web application automates a care pathway that includes patient-specific orders (ED medications, prescriptions, and referral) and documentation (a note in the chart reflecting the use of the app and discharge instructions). When the user launches one of seven potential care pathways in the web application, a message is sent back to the EHR with order preference and treatment pathway identification numbers (Figure 1). Epic’s Flowsheet functionality is used to select a documentation SmartText (ETX) corresponding to a numerical value (1–7). Another ETX pulls narrative details of the care pathway into a clinical note. To increase flexibility with documentation, an Epic SmartPhrase is also available for users to alternatively insert documentation of the selected care pathway.
Figure 1.
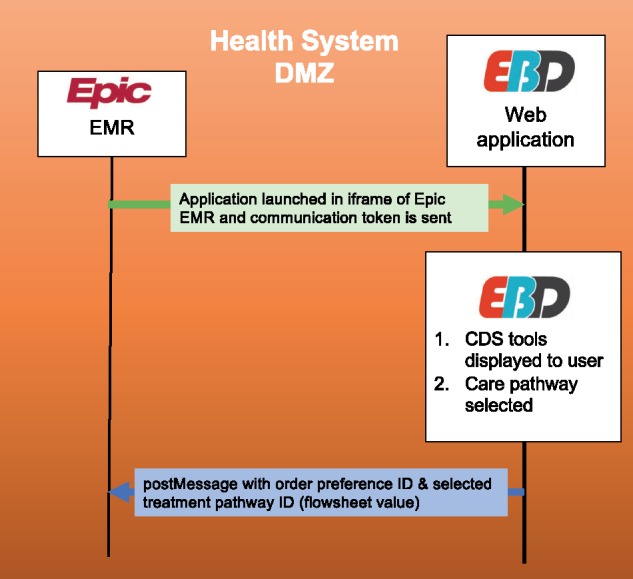
Data diagram of the integrated web application.
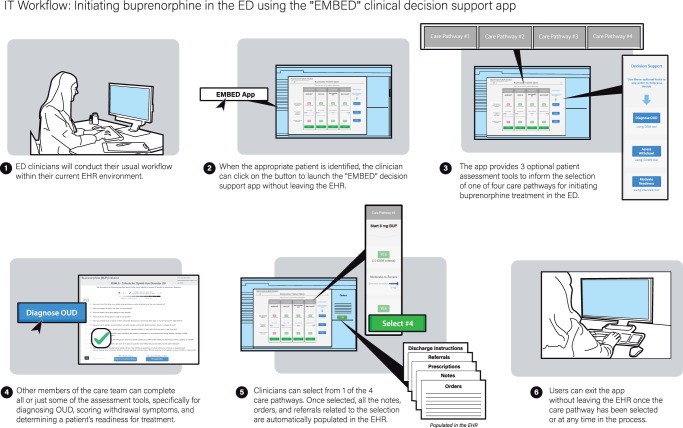
With these features, the EMBED intervention streamlines the process of BUP initiation in the ED effectively transforming a complicated algorithm into an automated workflow. From a clinician’s perspective, the “EMBED” button is clicked within a patient’s EHR chart. Next, they are guided through the process of choosing the best care pathway with the help of three optional decision support tools (Figure 2). These tools assist with diagnosing OUD, assessing withdrawal severity, and motivating patient readiness to begin treatment. Once a specific treatment pathway is launched, the application automatically sends the corresponding orders and prescriptions, completes documentation and discharge instructions, and provides choices for local referral sites. At any point, users may still alter these clinical activities to further customize them for a specific patient.
Figure 2.
Clinician workflow for EMBED intervention.
CHALLENGES & LESSONS LEARNED
Implementation of this user-centered CDS for ED-initiation of BUP for a large pragmatic trial faced multiple challenges. Despite the effectiveness of BUP–both long term and when initiated in the ED–there are multiple barriers to increasing adoption of ED-initiation of BUP into routine emergency care.23 A qualitative study of centralized CDS services implemented across four sites which analyzed clinical observations and 91 interviews previously identified challenges and lessons learned in eight domains including infrastructure, interface, people, workflow, environment, regulations, and monitoring.24 We add to this knowledge base by presenting the specific challenges and lessons learned in the EMBED implementation (Table 1) so that healthcare systems outside of the trial network can accelerate the adoption of the EMBED intervention–or similar interventions.
Table 1.
Issues, barriers, and solutions from the implementation and use of the EMBED intervention, an integrated web application for decision support and automation of EHR workflow to simplify the ED-initiation of BUP for OUD
| Issue | Barrier | Solution |
|---|---|---|
| Usability | Vendor-provided CDS tools have limited capabilities for interface customization to develop intuitive, efficient user interfaces and workflow | Created an integrated web application that embedded into EHR clinical workflow for the end-user |
| Needed to support health systems in the trial network using different EHR vendors (Epic and Cerner) | ||
| Two-way communication between EHR and web application | FHIR standard does not yet cover enough resources and two-way (“read /write”) communication for many FHIR resources is still not widely available. SMART on FHIR tools have limited capability to directly interact with an EHR API. Real-time interaction (order entry and documentation) cannot be pushed back to EHR from a web app. | Short-term: Epic AGL and flowsheet solution in main health system. Secondary health system has to rebuild web-app features in EMR vendor tool resulting in multiple separate instantiations of protocol. |
| Long-term: await maturation of FHIR, SMART on FHIR or similar standards for interoperability | ||
| Institutional IT support | Some secondary health systems reluctant to invest in hosting and supporting a custom web application on-premise | Designed a lightweight, single-page web application running on open source technologies, to maximize flexibility in hosting and minimize infrastructure requirements |
| Long-term: consider centralized, secure hosting of the web application in software as a service (SaaS) model | ||
| Security and privacy concerns from secondary health systems | Utilized secure methods that pass no protected health information outside the EHR to the web application. Achieved independent, third-party security audit and certification for the provided web application. | |
| Stakeholder buy-in and alignment | Informatics leadership in secondary systems reluctant to change established, local interfaces and workflows with which users are already familiar | Secondary health systems made local, pragmatic decisions on how the intervention was built in their system provided it allowed for decision support for diagnosing OUD, assessing withdrawal, and automating documentation, orders, prescriptions, referral, and discharge instructions |
| Informatics leadership reluctant to take responsibility for updating of local instance of EMBED CDS as protocol or other variables change over time (“technology debt”) | No solution until progress is made in standards-based interoperability | |
| Referral to community providers for continued medications for opioid use disorder (MOUD) | Limited availability of community providers of MOUD. Even if available, often not on the same vendor’s EHR product | Used platform-agnostic secure messaging |
| Engaged local care coordination teams | ||
| Piggy-backed onto other local MOUD initiatives | ||
| Governance | Prioritization of intervention required review by local committees in each health system. | Early engagement with informatics leadership to accelerate EHR prioritization requests and navigation of local governance norms to maximize the time available for local implementation of intervention |
| Sometimes multiple committees in the same system. | ||
| Human resources | Limited workforce available to make changes in local EHR build | Leveraged local physician builders, where available |
The automated and usable intervention that we have implemented has the potential to make ED-initiation of BUP simpler, provide the necessary information and tools for diagnosis of OUD, assessment of withdrawal and subsequent treatment algorithms thus helping to overcome the barrier of ED clinicians’ perceptions that this treatment protocol is unfamiliar, too complicated, and overly burdensome for use in crowded, chaotic EDs.12,20 A strength of the EMBED intervention is that it will be tested in a trial network that includes three healthcare systems using the Epic EHR and two using Cerner Millenium (Cerner Corporation, North Kansas City, MO).
Our user-centered design process revealed that EHR native tools were not expressive enough to address users’ needs for an efficient interface integrated into the workflow for ED-initiation of BUP. We evaluated the use of SMART on FHIR25 to allow two-way communication between a centralized application and EHRs within the trial network. Order entry had been identified as a critical component for integration in the design.20 At that time, Epic’s FHIR implementation did not support robust API’s for real-time order entry. In the primary EMBED health system, this challenge was overcome using Epic’s AGL framework and flowsheet functionality. This solution met local users’ needs and has been successfully launched.
Our initial CDS implementation took approximately 6 months in the primary health system and was then proposed for dissemination in four secondary health systems comprising the remainder of the trial network. However, informatics leaders in the secondary systems expressed reservation about this build due to: (1) the resources required for local customization and maintenance of a nonstandards-based intervention and (2) the security limitations and potential loss of control of a centralized, nonstandards-based solution hosted outside of their system. EHR vendors launched new tools after the primary EMBED implementation, such as Epic Smartsets or Cerner Care Pathways, to provide similar integrated, albeit less expressive CDS solutions. As a pragmatic trial, EMBED focuses on “identifying sustainable, generalizable, and evidence-based ways to improve healthcare” in real-world settings.26,27 Evaluating the durability of a nonstandards-based solution against a native solution, the secondary health systems made the pragmatic decision to move forward with native, integrated EHR solutions and added-on functionality as necessary to support all of the key components of the EMBED intervention. The interventions implemented in the secondary EMBED healthcare systems will have interfaces and workflows comparable to the primary site and familiar to local clinicians but, with continued maintenance, may diverge from each other over time.
Interventions utilizing the Enterprise Clinical Rules Service (ECRS), a centralized, web-based, shareable CDS service, addressed similar challenges to provide consistent, maintainable, and scalable CDS in a variety of settings.6 ECRS was successfully integrated in a 13-site trial to implement prediction rules in 19 029 children with minor blunt head trauma.28 At the time of that implementation, researchers hypothesized that maturation of standards for CDS services could decrease time-to-trial for multicenter evaluations.29 Although trials utilizing the ECRS demonstrated the feasibility of using remote decision support services for multicenter trials, these early efforts were burdened by the lack of integration standards for remote CDS as well as sharing packaged workflows. Since then, FHIR-based specifications, like CDS-Hooks, have matured and grown in adoption.6 While progress has been made, the EMBED implementation illustrates a continued need for standards to evolve to meet requirements for CDS at scale.
CONCLUSION
The EMBED implementation process elicited many challenges to interoperability and scalability. User-centered CDS can be created to support clinicians needs for initiating BUP therapy in ED patients with OUD. A scalable, centralized CDS solution that meets users’ needs for ED-initiation of BUP still remains elusive as it would, at a minimum, require maturation of tools and standards such as SMART on FHIR to include additional clinical activities, specifically order placement and referrals.
In response to these challenges, future work in the field should focus on updating interoperability standards themselves. The updated standards could then be used with existing services like ECRS to facilitate the sharing of new technology across health systems and decrease the redundant, resource-intensive development of programs at individual sites.
FUNDING
This work is supported within the National Institutes of Health (NIH) Health Care Systems Research Collaboratory by the NIH Common Fund through cooperative agreement U24AT009676 from the Office of Strategic Coordination within the Office of the NIH Director and cooperative agreement (UG3DA047003/UH3DA047003) as well as the National Heart, Lung and Blood Institute (T35HL007649) from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
AUTHOR CONTRIBUTIONS
ERM and YS significantly contributed to the conception and design of the study. WCH, OMA, AKM, and ERM drafted the initial manuscript. ERM, GD, TS, and YS developed the intervention. All authors revised the manuscript and approved the final version submitted for publication. ERM takes responsibility for all aspects of the work.
CONFLICT OF INTEREST
The authors have no financial conflicts or competing interests to disclose.
REFERENCES
- 1. Kawamoto K, Houlihan CA, Balas EA, et al. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ 2005; 330 (7494): 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garg AX, Adhikari NKJ, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA 2005; 293 (10): 1223–38. [DOI] [PubMed] [Google Scholar]
- 3. Bates DW, Teich JM, Lee J, et al. The impact of computerized physician order entry on medication error prevention. J Am Med Inform Assoc 1999; 6 (4): 313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuperman GJ, Bobb A, Payne TH, et al. Medication-related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc 2007; 14 (1): 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hemens BJ, Holbrook A, Tonkin M, et al. Computerized clinical decision support systems for drug prescribing and management: a decision-maker-researcher partnership systematic review. Implement Sci 2011; 6 (1): 1–17; doi: 10.1186/1748-5908-6-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldberg HS, Paterno MD, Rocha BH, et al. A highly scalable, interoperable clinical decision support service. J Am Med Inform Assoc 2014; 21 (e1): e55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mandl KD, Gottlieb D, Ellis A.. Beyond one-off integrations: a commercial, substitutable, reusable, standards-based, electronic health record–connected app. J Med Internet Res 2019; 21 (2): e12902.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahrnsbrak R, Bose J, Hedden SL, et al. Key Substance Use and Mental Health Indicators in the United States: Results from the 2016 National Survey on Drug Use and Health. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2017. [Google Scholar]
- 9.The Council of Economic Advisers (US). The Underestimated Cost of the Opioid Crisis Washington, DC: Executive Office of the President of the United States, Council of Economic Advisers; 2017.
- 10. Scholl L, Seth P, Kariisa M, et al. Drug and opioid-involved overdose deaths—United States, 2013–2017. MMWR Morb Mortal Wkly Rep 2019; 67 (5152): 1419–27; doi: 10.15585/mmwr.mm6751521e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vivolo-Kantor AM, Seth P, Gladden RM, et al. Vital signs: trends in emergency department visits for suspected opioid overdoses - United States, July 2016-September 2017. MMWR Morb Mortal Wkly Rep 2018; 67 (9): 279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. D’Onofrio G, McCormack RP, Hawk K.. Emergency Departments - A 24/7/365 option for combating the opioid crisis. N Engl J Med 2018; 379: 2487–90. [DOI] [PubMed] [Google Scholar]
- 13. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014; 6: CD002207. [DOI] [PubMed] [Google Scholar]
- 14. Larochelle MR, Bernson D, Land T, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med 2018; 169: 137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sullivan LE, Fiellin DA.. Narrative review: buprenorphine for opioid-dependent patients in office practice. Ann Intern Med 2008; 148 (9): 662–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA 2015; 313: 1636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duber HC, Barata IA, Cioè-Peña E, et al. Identification, management, and transition of care for patients with opioid use disorder in the emergency department. Ann Emerg Med 2018; 72: 420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Houry DE, Haegerich TM, Vivolo-Kantor A.. Opportunities for prevention and intervention of opioid overdose in the emergency department. Ann Emerg Med 2018; 71 (6): 688–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lowenstein M, Kilaru A, Perrone J, et al. Barriers and facilitators for emergency department initiation of buprenorphine: a physician survey. Am J Emerg Med 2019; 37: 1787–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ray JM, Ahmed OM, Solad Y, et al. Computerized clinical decision support system for emergency department–initiated buprenorphine for opioid use disorder: user-centered design. JMIR Hum Factors 2019; 6 (1): e13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ahmed OM, Mao JA, Holt SR, et al. A scalable, automated warm handoff from the emergency department to community sites offering continued medication for opioid use disorder: Lessons learned from the EMBED trial stakeholders. J Subst Abuse Treat 2019; 102: 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Melnick ER, Jeffery MM, Dziura JD, et al. User-centred clinical decision support to implement emergency department-initiated buprenorphine for opioid use disorder: protocol for the pragmatic group randomised EMBED trial. BMJ Open 2019; 9 (5): e028488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Volkow ND, Frieden TR, Hyde PS, et al. Medication-assisted therapies–tackling the opioid-overdose epidemic. N Engl J Med 2014; 370 (22): 2063–6. [DOI] [PubMed] [Google Scholar]
- 24. Wright A, Sittig DF, Ash JS, et al. Lessons learned from implementing service-oriented clinical decision support at four sites: a qualitative study. Int J Med Inform 2015; 84 (11): 901–11. [DOI] [PubMed] [Google Scholar]
- 25. Mandel JC, Kreda DA, Mandl KD, et al. SMART on FHIR: a standards-based, interoperable apps platform for electronic health records. J Am Med Inform Assoc 2016; 23 (5): 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roland M, Torgerson DJ.. Understanding controlled trials: what are pragmatic trials? BMJ 1998; 316 (7127): 285.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson KE, Tachibana C, Coronado GD, et al. A guide to research partnerships for pragmatic clinical trials. BMJ 2014; 349 (dec01 7): g6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dayan PS, Ballard DW, Tham E, et al. Use of traumatic brain injury prediction rules with clinical decision support. Pediatrics 2017; 139; doi: 10.1542/peds.2016-2709 [DOI] [PubMed] [Google Scholar]
- 29. Goldberg HS, Paterno MD, Grundmeier RW, et al. Use of a remote clinical decision support service for a multicenter trial to implement prediction rules for children with minor blunt head trauma. Int J Med Inform 2016; 87: 101–10. [DOI] [PubMed] [Google Scholar]