Abstract
Background
Methotrexate (MTX) is an immunomodulator used for the treatment of pediatric inflammatory bowel disease (IBD). There are currently no RCTs that assess the treatment efficacy of methotrexate within the pediatric IBD patient population. This systematic review and meta-analysis assesses the efficacy of MTX therapy among the existing pediatric literature.
Methods
A systematic literature search was performed using MEDLINE and the Cochrane library from inception until March 2016. Synonyms for ‘pediatric’, ‘methotrexate’ and ‘IBD’ were utilized as both free text and MESH search terms. The studies included contained clinical remission (CR) rates for MTX treatment of pediatric IBD patients 18 yrs old, as mono- or combination therapy. Case studies with <10 patients were excluded. Quality assessment was performed with the Newcastle-Ottawa Scale. Meta-analysis calculated pooled CR rates. A random-effects meta-analysis with forest plots was performed using R.
Results
Fourteen (11 monotherapy, 1 combination therapy, 2 both; n = 886 patients) observational studies were eligible out of 202 studies. No interventional studies were identified. The pooled achieved CR rate for pediatric CD patients on monotherapy within 3-6 months was 57.7% (95% CI 48.2-66.6%), (P =0.22; I2 = 29.8%). The CR was 37.1% (95% CI 29.5-45.5%), (P = 0.20; I2 = 37.4%) for maintenance therapy at 12 months. Sub-analysis could not identify CR differences between MTX administration types, thiopurine exposure.
Conclusions
This meta-analysis demonstrated that, over 50% of pediatric Crohn’s disease patients induced with methotrexate achieved clinical remission, while 12-month remission rate was only 37%. Prospective controlled interventional trials should assess treatment efficacy among patient subgroups.
Keywords: Pediatric Crohn’s disease, methotrexate, immunomodulator, meta-analysis, systematic review
INTRODUCTION
Methotrexate (MTX) is a folate antagonist that is believed to have immunomodulator and anti-inflammatory properties when used for the treatment of Crohn’s disease (CD). Methotrexate is known to interfere with DNA and cellular metabolism, although its exact therapeutic mechanism in CD is unknown.
The efficacy of methotrexate has been demonstrated in randomized, double-blind, placebo-controlled trials for both induction and maintenance treatment among adult Crohn’s disease patients.1, 2 MTX is used as an immunomodulator among pediatric patients with CD, particularly as a thiopurine alternative for adolescent males. However, there have been no pediatric interventional trials of MTX for this indication.
There is a paucity of published research exploring the utilization of MTX as monotherapy for pediatric Crohn’s disease. Therefore, we performed a systematic review and meta-analysis to determine the efficacy of MTX in the treatment regimen of pediatric patients with CD.
METHODS
Study Selection
We conducted a systematic literature search and utilized the Preferred Reporting for Systematic Reviews and Meta-analyses (PRISMA) statement, the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) checklist, and the Cochrane handbook.3–5 All studies in any language published up to March 2016 were searched. Studies eligible for inclusion contained clinical remission (CR) rates for MTX treatment of CD patients ≤18 years old as either mono- or combination therapy. Studies with fewer than 10 patients were excluded.
Search Strategy
A systematic literature search was performed using MEDLINE, EMBASE, and the Cochrane library from inception until March 2016. Relevant articles and conference proceedings from the major gastrointestinal conferences [North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN), The European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), Digestive Diseases Week (DDW), American College of Gastroenterology (ACG), European Crohn’s and Colitis Organisation, Advances in IBD (AIBD)] were additionally searched. Synonyms for “pediatric,” “methotrexate,” and “IBD” were searched as free text and MESH terms.
Study Screening and Quality Assessment
Titles and abstracts were screened, and duplicates were excluded. Eligibility was assessed after retrieval of full texts. Two authors (R.C. and R.L.) independently reviewed the quality of all included articles. The Newcastle-Ottawa Scale (NOS) scale for cohort studies was utilized to assess the methodological quality and biases of studies.6 The NOS scale is a tool to assess and compare the methods of nonrandomized studies for meta-analysis on 3 distinct domains: selection, comparability, and outcome. The selection domain was further defined as study representativeness of the IBD cohort including age, sex disease behavior, and location. This domain also assessed ascertainment of MTX exposure (clinician-administered or patient-reported) confirmed that clinical remission was not present at study enrollment. The second domain, comparability, was scored on presence of concurrent therapy and type of administration mode (parenteral vs oral). Quality of outcome assessment included utilization of well-defined outcome measures, duration of follow-up, and adequacy of follow-up.
Data Extraction and Statistical Analysis
Two authors (R.C. and R.L.) independently extracted data using a preset data sheet into an Microsoft Excel (Microsoft, Redmont, WA, USA) spreadsheet. Demographics, study characteristics, study methods, and outcomes were extracted. Descriptive statistics were conducted. Clinical remission rates were calculated from the absolute number of enrolled patients and absolute number of patients who achieved and maintained clinical remission. Studies included in the analysis of induction of remission were considered if they reported CR rates within 6 months of MTX induction. Studies included for maintenance of remission were considered if they reported an absolute number of patients in remission at 12 months from MTX induction. Overall, studies were only included if they explicitly reported steroid-free clinical remission. Rates were expressed as percentages and odds ratio (ORs) with 95% confidence intervals (CIs). Proportions of clinical remission rates were pooled in a random-effects meta-analysis using the DerSimonian-Laird method with the R Project for Statistical Computing Package “Meta.”7 Meta-analyses for induction and maintenance of clinical remission were conducted separately. Subanalyses explored differences between anthropometric data, disease subtype, oral vs parenteral, mono- vs combination therapy with an anti–tumor necrosis factor (anti-TNF) agent, and thiopurine exposure. Statistical heterogeneity was assessed with the Cochran’s Q test (X2) and I2 statistic with a P < 0.1 level of significance. The I2 statistic assessed for degree of heterogeneity. Rates to assess heterogeneity were divided into 0%–40%, 30%–60%, 50%–90%, and 75%–100%, reflecting levels of low, moderate, substantial, and considerable heterogeneity, respectively, as per the Cochrane handbook.5
RESULTS
Study Selection
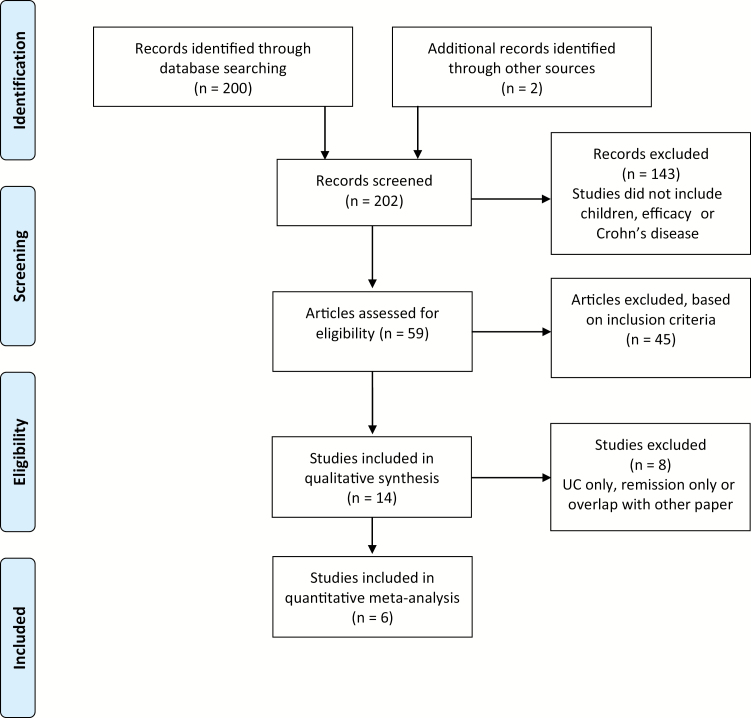
Two hundred two studies were identified. After review of titles and abstracts, 143 articles were excluded that did not meet our selection criteria. Another 45 articles were excluded after review of the full manuscript for various reasons, including (1) not meeting age criteria, (2) no analysis of MTX efficacy, (3) lack of included clinical information, or (4) inclusion of too few (<10) patients (Fig. 1). This left a total of 14 manuscripts that were further evaluated for qualitative synthesis. During qualitative synthesis, another 8 articles were excluded for the quantitative meta-analysis. Of these 8, 1 included clinical data for UC only,8 2 included data for combination therapy with anti-TNF therapy,9, 10 2 potentially included patients in remission who continued steroids,11, 12 and 3 only looked at outcomes for maintenance of remission.13–15 Overall, 6 manuscripts were eligible and included in the qualitative analysis and subsequent meta-analysis.16–21
FIGURE 1.
Flow chart of study selection.
Risk of Bias or Appraisal of the 6 Studies With NOS Score
Quality assessment scores between studies as measured by the Newcastle-Ottawa Scale ranged from 4 to 5 out of a maximum of 8 points (Table 1). All of the 6 studies included cohorts from academic tertiary care centers or university hospitals. All studies used well-defined outcome measures of clinical remission, ranging from physician global assessment (PGA) to Harvey-Bradshaw Index (HBI) and (Pediatric) Crohn’s Disease Activity Index ((P)CDAI) with or without report of steroid-free remission (Supplementary Table 1). However, only 1 study described that all other therapies (including corticosteroids) were discontinued at the start of MTX induction.17 Two of the studies determined complete MTX exposure with clinician-assisted administration rather than self-reported administration.20, 21
TABLE 1:
Newcastle-Ottawa Scale for Quality Assessment of Studies
| Study | Selection | Comparability | Outcome | Total Score | ||||
|---|---|---|---|---|---|---|---|---|
| Representativeness | Ascertainment of Exposure | Outcome Not Present at Start | Adjusted for Concomitant Treatment or Administration Mode | Assessment of Outcome | Duration of Follow-up | Adequacy of Follow-up | ||
| Uhlen et al., 200620 | * | * | - | - | * | * | * | 5 |
| Ravikumara et al., 200717 | * | - | - | * | * | * | * | 5 |
| Turner et al., 200719 | * | - | - | - | * | * | * | 4 |
| Weiss et al., 200921 | * | * | - | - | * | * | * | 5 |
| Boyle et al., 201016 | * | - | - | - | * | * | * | 4 |
| Turner et al., 201518 | * | - | - | - | * | * | * | 4 |
Max score was 8 (comparability can give 2 points max, the rest 1 point max).
Study Characteristics
The 6 manuscripts included in our meta-analysis were all retrospective cohort studies (half of which were multicenter cohort studies) (Tables 2 and 3)16–21. Of note, the most recent study (a retrospective multicenter propensity score study) reported outcomes after MTX use of at least 12 months and included data from previously published cohorts (Turner et al., 2007, and Weiss et al., 2009); for this reason, the latter studies were excluded from the maintenance meta-analysis.19, 21
TABLE 2:
Study Characteristics
| Name | Year | Retrospective/ Prospective | Study Type | Control Group | No. | Age, ya | Male | Induction Outcomeb | Maintenance Outcomeb |
|---|---|---|---|---|---|---|---|---|---|
| Uhlen20 | 2006 | Retrospective | Multicenter cohort | No | 61 | 11.1 ± 2.3 | 35 (57%) | 6 mo | 12 mo |
| Ravikumara17 | 2007 | Retrospective | Cohort | No | 10 | 15.8 (12–16.9) | 3 (30%) | Mean 12 wk (R: 8–14) | - |
| Turner19 | 2007 | Retrospective | Multicenter cohort | No | 60 | 13.8 ± 2.7 | 37 (62%) | 6 mo | - |
| Weiss21 | 2009 | Retrospective | Multicenter cohort | No | 25 | 14.5 ± 3.1 | 14 (56%) | Median 8 wk (R: 8–10) | - |
| Boyle16 | 2010 | Retrospective | Cohort | No | 27 | 13.8 ± 0.7 | 17 (63%) | 6 mo | 12 mo |
| Turner18 | 2015 | Retrospective | Cohort | Matched PS-score | 226 | 13.8 ± 2.8 | 141 (62%) | - | 12 mo |
aAge in years at MTX induction: median (range) or mean ± SD; matched propensity score (compared different administrations).
bTime in months, unless reported otherwise.
TABLE 3:
Study Characteristics–Continued
| Name | Year | Classification System | Severity/Activity | SC/IM/PO | Dose |
|---|---|---|---|---|---|
| Uhlen20 | 2006 | CD location and behavior | HBI (exact scores not mentioned) | SC (n = 10); IM (n = 51) | Weight-based categories |
| Ravikumara17 | 2007 | CD location | Active disease (>4 mos) | 9 IM/SC for 16 wk then PO; 1 PO only | Weight-based categories |
| Turner19 | 2007 | Location and behavior | Active (92%); CR at baseline (8%) | PO/SC | 15 mg/m2 or weight-based categories |
| Weiss21 | 2009 | Location and behavior | Thio refract/intolerant | SC (n = 19); PO (n = 6) | Median dose 12.5 mg/m2 (5 mg FA PO daily) |
| Boyle16 | 2010 | CD location | PGA: steroid-free remission (4%); mild (48%); moderate (30%); severe (4%) | SC (n = 26); PO (n = 1) | 10.0 ± 0.4 mg/m2 |
| Turner18 | 2015 | Disease location | PCDAI: mild 48% (109/226); mod– sev 45% (101/226) | PO; SC; SC then PO; (however, PO to SC was TX failure) | -<12.5 mg/m2 (42%) -12.5–17.5 mg/m2 (58%) |
Induction of Remission
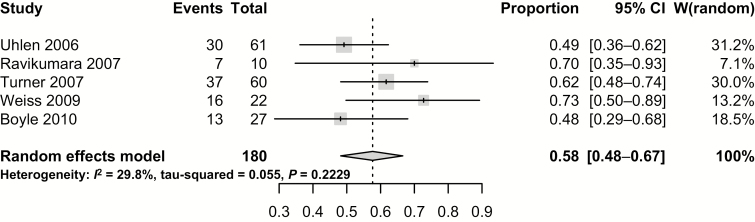
Studies reporting remission induction rates demonstrated an overall pooled clinical remission rate of 57.7% (95% CI, 48.2%–66.6%) (Fig. 2). This was a statistically homogenous analysis with low risk for statistical heterogeneity (Cochran’s Q, P = 0.22; I2 = 29.8%).
FIGURE 2.
Overall pooled clinical remission of induction rates.
In further subanalysis, 1 study reported outcomes for both oral (PO) and parenteral (SC) administration and could not identify CR differences between administration modes.19 When the authors looked at the indication for methotrexate use in relation to thiopurines, no studies reported outcomes for thiopurine exposed vs thiopurine-naïve patients. Among most studies, methotrexate was the next therapeutic step after exposure to thiopurines. Three of these studies made a further distinction between thiopurine failure and thiopurine intolerance. However, when these 3 studies were examined, there was no difference among CR rates between both indications (OR, 1.65; 95% CI, 0.71–3.83; P = 0.25; n = 3 studies).16, 17, 19
Maintenance of Remission
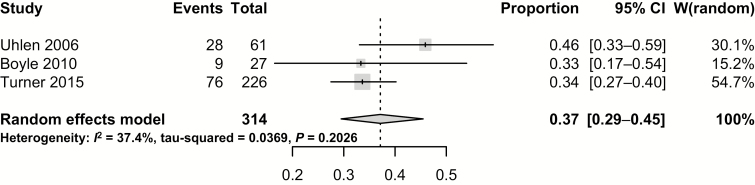
The second meta-analysis evaluated the pooled maintenance CR of studies at 12 months since induction of MTX. Of the 3 studies that reported outcomes at 12 months postinduction, the pooled maintenance CR was 37.1% (95% CI, 29.5%–45.5%) (Fig. 3).16, 18, 20 In this analysis, the risk for statistical heterogeneity was low to moderate, as per the Cochrane handbook (Cochran’s Q, P = 0.20; I2 = 37.4%). Again, only 1 study compared administration modality in the maintenance meta-analysis.18 This study by Turner et al., in collaboration with some of the same institutions included in Turner’s prior study, demonstrated no difference between PO and SC administrations.18
FIGURE 3.
Pooled clinical remission of 12-month maintenance rates.
Growth Velocity
Only 2 studies assessed growth velocity. Both of Turner’s papers included an assessment of height velocity.18, 19 Turner’s most recent paper demonstrated a comparatively lower height velocity among patients prescribed only PO MTX. In contrast, patients who were prescribed SC administration (either at induction or throughout treatment) demonstrated a comparatively higher height velocity.
DISCUSSION
This meta-analysis of pediatric patients who were administered MTX for the induction and maintenance of Crohn’s disease demonstrated a pooled clinical remission rate of 58% during induction within the first year and a pooled clinical remission rate of 37% of patients who maintained remission at 1 year after MTX induction. No randomized controlled or other interventional studies for the use of MTX among pediatric patients were identified.
Among the literature for adult patients with IBD, there is only 1 randomized controlled trial (RCT) that assessed the efficacy of methotrexate monotherapy for Crohn’s disease patients. This study of SC dosing by Feagan et al. found that 39% of patients achieved remission after the 16-week induction phase.2 An extension of this trial evaluated patients on MTX in clinical remission between weeks 16 and 24, after which they were randomized between methotrexate and placebo. In this follow-up study, 65% of the patients in the MTX group remained in clinical remission.1
There are several potential explanations for why the clinical remission rate in our study was higher than that demonstrated in the adult RCT by Feagan et al. There are methodological factors in the nature of primary studies, ranging from reporting bias to other unidentified confounding factors, differences in adult vs pediatric dosing, or administration methodology. In addition, variation in outcome measures (CDAI vs other clinical activity indices such as the PGA) could have led to a reporting bias. While the PGA has been shown to correlate with specific, more objective, disease outcomes, the measure itself is more subjective than other clinical disease indices (such as the CDAI), as it reflects the judgment of the physician based on a visual analog scale. As reported in the Newcastle-Ottawa Scale for quality assessment of studies (Supplementary Table 1), another factor that limits the comparability of these studies is the risk for concomitant therapies among their subjects (eg, steroids or other classes of therapies). This largely reflects the nature of retrospective studies, wherein patients were not stratified in different treatment arms. However, although these factors may have introduced additional heterogeneity in our analysis, this study reflects a real-world sampling of methotrexate treatment, and the risk of statistical heterogeneity in our meta-analysis was minimal. Furthermore, only studies that explicitly stated that patients in remission were “steroid-free” were included in our meta-analysis.
Each of these factors may have contributed to the differences described in the appraisal of studies. However, another explanation for disparate study outcomes could be that the population in Feagan’s study had a different phenotypic or genotypic “type” of Crohn’s disease, one that was less responsive to MTX. While at this time no studies have found genetic polymorphisms that have clinical associations with response to MTX among IBD patients, there is some evidence that genetic polymorphisms may play a role in general disease response.22, 23 Furthermore, certain metabolism pathways involved may have different rates among children and adults.23
The lower long-term clinical remission rate in this meta-analysis may be explained by the fact that the rates described here are from all patients reported who were included in the cohort studies. The maintenance phase in this analysis was defined as administration of MTX for the duration of 1 year, as some cohort studies did not report short-term outcomes. In Feagan’s maintenance trial, on the other hand, only patients who initially achieved remission were included.1 The intention-to-treat remission rate at 1 year among all patients who were administered MTX was not reported in the RCTs.1, 2 There is only 1 pediatric study in which patients achieving remission were then followed prospectively. This study demonstrated similar rates of clinical response to Feagan et al., with a 69% remission rate for patients at 1 year after MTX induction.14
This meta-analysis included studies with varied methodological and treatment approaches, including variation in MTX dose, administration mode, and time period of the induction phase. In addition, although we attempted to address these differences in clinical heterogeneity, no conclusions can be drawn from an analysis of these factors, including administration mode, as data from individual studies were too limited. However, as seen in the forest plot and funnel plot, the outcomes are nevertheless homogenous and the statistical heterogeneity is minimized, which strengthens the implications of our findings overall.
While all of the studies in the meta-analysis assessed clinical remission, an additional manuscript (not included in our quantitative analysis) was identified that assessed mucosal healing among patients in remission. Results from this research demonstrated that when a portion (approximately 50%) of these patients underwent endoscopy, 88% (7/8 patients) were found to have macroscopic (SES-CD score of 0) and microscopic healing.14 However, among the patients with a clinical relapse who underwent endoscopy, all had mucosal lesions and disease recurrence.14 In addition to remission, other secondary outcomes (such as differences in height velocity) were explored. However, as only Turner’s studies included height velocity, the data presented in the Results section related to these findings are limited and should be interpreted with caution.
While this meta-analysis focused on MTX monotherapy for Crohn’s disease patients, 1 of the included studies in the induction analysis included patients with a previous history of infliximab (IFX) failure.21 However, these patients completed a drug washout at the time of induction of the MTX monotherapy phase. Three additional patients (not included in our meta-analysis) in this study were started on MTX while maintained with IFX; only 1 of those had a response to the addition of MTX.21 An additional literature search identified 2 small studies that included methotrexate as part of combination therapy with an anti-TNF alpha inhibitor.9, 10 Given this amount of interest in using MTX as an alternative immunomodulator to thiopurines in combination with anti-TNF and potentially other biological therapies, there is clearly a need for more studies in pediatrics in this area. In addition, studies with ulcerative colitis (UC) patients were excluded from our meta-analysis as this is a different IBD entity with a likely different mechanism of action. Inclusion in this study would have likely made our meta-analysis too heterogeneous. Though safety outcomes were not included in this meta-analysis, a recently published meta-analysis specifically examined hepatotoxicity among pediatric IBD patients exposed to methotrexate.24
CONCLUSIONS
In conclusion, this systematic review and meta-analysis of MTX therapy for the treatment of Crohn’s disease demonstrates a high rate of initial remission among pediatric patients with Crohn’s disease. It provides an overview of published cohort studies in this patient population and suggests that methotrexate may have an important role in the treatment regimen for pediatric Crohn’s disease. Furthermore, well-executed prospective pediatric RCTs are needed to better elucidate the efficacy of methotrexate in pediatric CD, such as the currently active COMBINE trial (NCT02772965). In addition, it will be important to design future trials that incorporate therapeutic drug monitoring and clarification of the role of MTX as concomitant therapy.
Supplementary Material
Conflicts of interest: R.J.C. and R.C.L.: none. M.C.D.: consultant for Abbvie, Janssen, UCB, Takeda, Celgene, Pfizer, Gilead, Lilly. D.T.R.: consultations and grants from Abbvie, Takeda, Pfizer, Janssen.
Supported by: No funding declared.
REFERENCES
- 1. Feagan BG, Fedorak RN, Irvine EJ, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s Study Group investigators. N Engl J Med. 2000;342:1627–32. [DOI] [PubMed] [Google Scholar]
- 2. Feagan BG, Rochon J, Fedorak RN, et al. Methotrexate for the treatment of Crohn’s disease. The North American Crohn’s Study Group investigators. N Engl J Med. 1995;332:292–7. [DOI] [PubMed] [Google Scholar]
- 3. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 5. Deeks JJ, Higgins J, Altman DG. Analysing data and undertaking meta‐analyses. In: Jonathan J Deeks, Julian PT Higgins and Douglas G Altman; on behalf of the Cochrane Statistical Methods Group (eds). Cochrane Handbook for SystematicReviews of Interventions: Cochrane Book Series. Chichester, UK: John Wiley & Sons, 2008;243–26. [Google Scholar]
- 6. Wells G, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. The Ottawa Hospital Research Institute, 2000. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 7. Schwarzer G. Meta: an R package for meta-analysis. R News. 2007;7:40–5. [Google Scholar]
- 8. Aloi M, Di Nardo G, Conte F, et al. Methotrexate in paediatric ulcerative colitis: a retrospective survey at a single tertiary referral centre. Aliment Pharmacol Ther. 2010;32:1017–22. [DOI] [PubMed] [Google Scholar]
- 9. Absah I, Faubion WA Jr. Concomitant therapy with methotrexate and anti-TNF-α in pediatric patients with refractory Crohn’s colitis: a case series. Inflamm Bowel Dis. 2012;18:1488–92. [DOI] [PubMed] [Google Scholar]
- 10. Willot S, Noble A, Deslandres C. Methotrexate in the treatment of inflammatory bowel disease: an 8-year retrospective study in a Canadian pediatric IBD center. Inflamm Bowel Dis. 2011;17:2521–6. [DOI] [PubMed] [Google Scholar]
- 11. Garrick V, Atwal P, Barclay AR, et al. Successful implementation of a nurse-led teaching programme to independently administer subcutaneous methotrexate in the community setting to children with Crohn’s disease. Aliment Pharmacol Ther. 2009;29:90–6. [DOI] [PubMed] [Google Scholar]
- 12. Mack DR, Young R, Kaufman SS, et al. Methotrexate in patients with Crohn’s disease after 6-mercaptopurine. J Pediatr. 1998;132:830–5. [DOI] [PubMed] [Google Scholar]
- 13. Haisma SM, Lijftogt T, Kindermann A, et al. Methotrexate for maintaining remission in paediatric Crohn’s patients with prior failure or intolerance to thiopurines: a multicenter cohort study. J Crohns Colitis. 2015;9:305–11. [DOI] [PubMed] [Google Scholar]
- 14. Hojsak I, Mišak Z, Jadrešin O, et al. Methotrexate is an efficient therapeutic alternative in children with thiopurine-resistant Crohn’s disease. Scand J Gastroenterol. 2015;50:1208–13. [DOI] [PubMed] [Google Scholar]
- 15. Sunseri W, Hyams JS, Lerer T, et al. ; Pediatric Inflammatory Bowel Disease Collaborative Research Group Retrospective cohort study of methotrexate use in the treatment of pediatric Crohn’s disease. Inflamm Bowel Dis. 2014;20:1341–5. [DOI] [PubMed] [Google Scholar]
- 16. Boyle B, Mackner L, Ross C, et al. A single-center experience with methotrexate after thiopurine therapy in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2010;51:714–7. [DOI] [PubMed] [Google Scholar]
- 17. Ravikumara M, Hinsberger A, Spray CH. Role of methotrexate in the management of Crohn disease. J Pediatr Gastroenterol Nutr. 2007;44:427–30. [DOI] [PubMed] [Google Scholar]
- 18. Turner D, Doveh E, Cohen A, et al. Efficacy of oral methotrexate in paediatric Crohn’s disease: a multicentre propensity score study. Gut. 2015;64:1898–1904. [DOI] [PubMed] [Google Scholar]
- 19. Turner D, Grossman AB, Rosh J, et al. Methotrexate following unsuccessful thiopurine therapy in pediatric Crohn’s disease. Am J Gastroenterol. 2007;102:2804–12; quiz 2803, 2813. [DOI] [PubMed] [Google Scholar]
- 20. Uhlen S, Belbouab R, Narebski K, et al. Efficacy of methotrexate in pediatric Crohn’s disease: a French multicenter study. Inflamm Bowel Dis. 2006;12:1053–7. [DOI] [PubMed] [Google Scholar]
- 21. Weiss B, Lerner A, Shapiro R, et al. Methotrexate treatment in pediatric Crohn disease patients intolerant or resistant to purine analogues. J Pediatr Gastroenterol Nutr. 2009;48:526–30. [DOI] [PubMed] [Google Scholar]
- 22. Becker ML, Leeder JS. Developmental pharmacogenetics in pediatric rheumatology: utilizing a new paradigm to effectively treat patients with juvenile idiopathic arthritis with methotrexate. Hum Genomics Proteomics. 2010;2010:257120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Korbel L, George M, Kitzmiller J. Clinically relevant pharmacogenomic testing in pediatric practice. Clin Pediatr (Phila). 2014;53:831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Valentino PL, Church PC, Shah PS, et al. Hepatotoxicity caused by methotrexate therapy in children with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2014;20:47–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.