Abstract
Volatile organic compounds (VOCs), produced and emitted through the metabolism of cancer cells or the body’s immune system, are considered novel cancer biomarkers for diagnostic purposes. Of late, a large number of work has been done to find a relationship between VOCs’ signature of body and cancer. Cancer-related VOCs can be used to detect several types of cancers at the earlier stages which in turn provide a significantly higher chance of survival. Here we aim to provide an updated picture of cancer-related VOCs based on recent findings in this field focusing on cancer odor database.
Keywords: cancer odor, cancer diagnosis, volatile organic compounds, lung cancer, aldehydes
Introduction
Cancer is one of the leading causes of death and a major public health problem worldwide. The development of a reliable method for diagnosis and treatment of cancer has been the subject of numerous recent studies [1–3].
A solid body of research literature shows that the chance of survival in people with different types of cancer is increased with earlier detection. Today, most diagnostic tools are unable to detect cancers at the very early stages of disease progression. Moreover, some of those tools are invasive and may present clinical risks for the patient. Therefore, the demand for alternative methods for cancer diagnosis has increased in recent years [4–7].
The discovery of cancer biomarkers opened a new realm of possibilities for cancer detection. Various types of biomarkers including proteins, peptides, metabolites, DNA, RNA, and whole cells are thought to assist in the diagnosis of cancer [8, 9]. With the development of technology and with a greater understanding of cancer itself, the discovery of biomarkers has accelerated tremendously. As a result, a number of potential biomarkers are being reported every year and some of them have been introduced into clinical practice. Finding potential biomarkers that are able to be used for the detection of cancers at the onset of disease holds promise for the future treatment of cancers [9].
To achieve this goal, different methods have been applied up to now. The detection of different types of cancer, including lung [10, 11], colon [12, 13], breast [14, 15], pancreatic [16, 17], prostate [12, 18], and head and neck [19], using volatile organic compounds (VOCs), has attracted the attention of scientists in recent years [15, 17, 20–22]. These studies usually involve profiling VOCs present in biological samples from cancer patients and healthy people and comparing the pattern of VOCs in healthy and cancer groups.
The entire set of VOCs generated by an organism is called “volatilome” or “volatome” and the study of volatilome is known as “volatilomics” [23]. VOCs generated through the metabolism of cells release into the blood and are excreted through the exhaled breath or body fluids. The volatilomic profile of different biological matrices can be efficiently identified by analytical methods.
It has been well documented that VOCs can give useful information about the metabolic state of an organism [24, 25]. The VOCs of our body reflect biochemical reactions caused by biological activities such as cell death, oxidative stress, or inflammation. Disease-related VOCs may be part of the cascade of the reactions that occur during the response of the body to the damage [24]. Therefore, the VOCs can be used as novel biomarkers for diagnostic purposes.
In the last decade, a considerable amount of effort has been directed toward finding the relationship between the VOCs’ signature of the body and the presence of cancer. There are many reports investigating VOCs associated with different types of cancers, known as cancer odor; however, these works only refer to their results or compare their results with other limited works. There are no comprehensive analyses of all of the available data about cancer-related VOCs. To address this problem, we have developed cancer odor database (COD), a comprehensive database of cancer-related VOCs [26].
The COD is a web-based database that contains comprehensive information of the VOCs associated with cancer manually extracted from literature. The database contains >1300 records with 19 critical features for each record and provides an excellent overview of volatile organic metabolites of cancer. The COD is freely available for noncommercial purposes online at http://bioinf.modares.ac.ir/software/cod. In this article, we will focus on VOCs associated with different types of cancer obtained from the COD database and review their origins, biological matrices, and methods of detection.
Biological matrices
VOCs produced by the body are first released into the circulatory system. They can then enter the air in the lungs or biofluids. Research on cancer-related VOCs has been performed using various human matrices including blood, breath, urine, bile, feces, saliva, and vocal fold lesions.
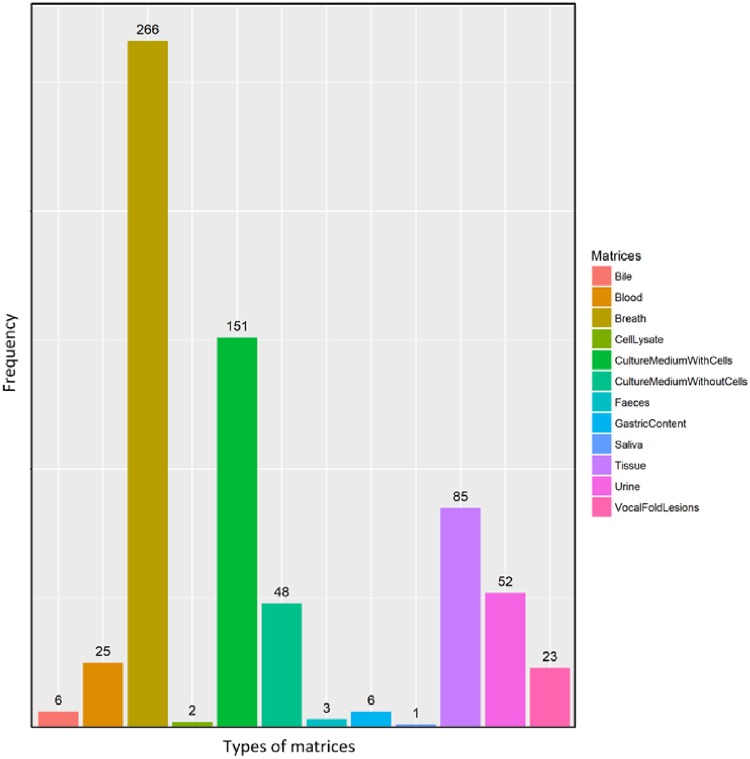
Evaluation of the COD database indicates that the most extensive research in cancer-related VOCs has been on exhaled breath, and especially for the detection of lung cancer (Fig. 1). Noninvasive methods, like breath analysis, are preferred for diagnosis due to the ease of sampling procedures, and low cost, the breath can be sampled and analyzed in real time [27]. Finally, the breath is less complex than other matrices like blood, which eliminates the need for preprocessing of samples.
Figure 1:
Frequency of VOCs detected in various types of biological matrices.
Blood has been used as metrics for VOC collection in a number of cancer studies [28–30]. Obtaining blood samples can be costly and time-consuming and may not be well tolerated by patients in comparison to obtaining breath or urine samples. It is worth noting that changes in the temperature and pH of blood samples can alter the VOC profile.
Several studies have also investigated VOCs in urine or feces samples of patients with various types of cancers [31, 32]. These two matrices are available in large volumes and their sample collection is noninvasive.
The kidneys concentrate analytes in the urine before it is excreted from the body which is an advantage over other biofluids. However, the drugs administered to a patient might influence VOCs in the urine. There are also some problems that may limit using feces as matrices, e.g. VOCs in the feces may be affected or generated by intestinal flora or infectious diseases.
There are several studies investigating cancer-related VOCs in vitro. In vitro experiments allow for better control of experimental variables. In addition, the results of in vitro studies can be easily interpreted, due to the absence of various parameters such as gender and age. Investigation of tumor cells in vitro makes it easier to directly recognize VOCs related to cancer among a large number of metabolites produced by the cells [33, 34]. On the contrary, several VOCs are produced through reactions in other organs. Therefore, studying the cancer cells alone will not capture information arising from the secondary interaction of VOCs with other organs [35–37].
The concentration of VOCs in human breath, blood, and urine is in the range of ppm to ppt [38]. Therefore, a preconcentration step is usually necessary prior to the analysis. In recent years, considerable efforts have been directed toward improvements in the sampling of volatile compounds and preconcentration technologies. Solid-phase microextraction (SPME) is a simple, fast, economic, and solvent-free preconcentration technique, which is widely used for the analysis of VOCs in biological samples. SPME is typically coupled with a separation technique for analysis of biological samples.
Analytical methods
Techniques for detection of cancer-related VOCs in biological samples can be broadly divided into two groups: those using analytical instruments and those using sensor and electronic nose systems [39].
Various analytical instruments for determining VOCs have been used including gas chromatography–mass spectrometry, ion mobility spectrometry, field asymmetric ion mobility spectrometry, selected ion flow tube mass spectrometry (SIFT-MS), proton transfer reaction–mass spectrometry (PTR-MS), gas chromatography–flame ionization detection, and comprehensive 2D gas chromatography [7].
Based on information from the COD database, gas chromatography coupled to mass spectrometry has been the main analytical method for detection of VOCs, however, other methods like PTR-MS and SIFT-MS have also been widely employed [26].
The abovementioned methods are commonly combined with separation and preconcentration methods like solid-phase extraction [40, 41]. Two points should be taken into account with regard to preconcentration methods. First, the presence of exogenous compounds must be minimized through experimental procedures. And second, the pH of biological fluids is another parameter that must be considered because it may influence the microextraction process [42–44].
Recently, sensors and electronic noses have shown promise as an alternative to traditional diagnostic tools. They have attracted a great deal of interest due to the advantages of high sensitivity, portability, low cost, and ease-of-use [45, 46]. Sensor-based techniques have great potential in clinical point-of-care use [47]. Various types of gas sensors including metal oxide chemiresistive sensors, nanomaterial-based chemiresistive sensors, piezoelectric sensors, colorimetric sensors, metal–organic frameworks, silicon nanowire field-effect transistor, and olfactory receptor-based sensors have been developed in order to detect cancer-related VOCs [7].
Important cancer-related VOCs and their origin
The primary goal of this study was to find potentially significant cancer-related VOCs based on existing reports. Cancer biomarkers can be classified into two main categories: general cancer biomarkers and biomarkers for a specific cancer type. An analysis of the COD data shows that some VOCs only contribute to a particular type of cancer and can be considered a specific biomarker for that type of cancer, while other VOCs are associated with several types of cancers and can be considered as generic cancer biomarkers.
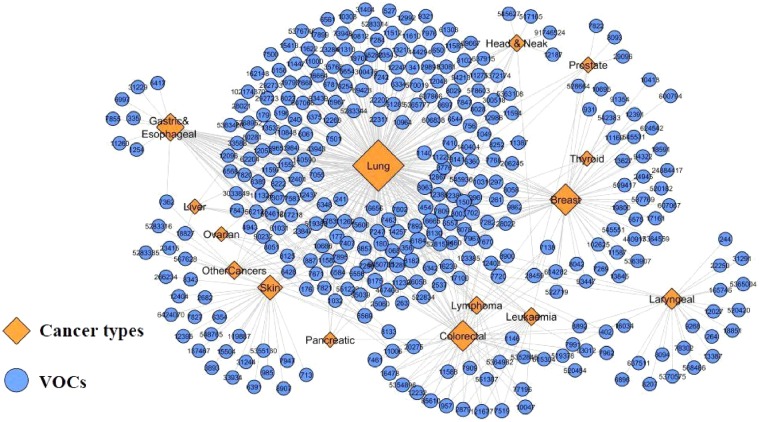
In order to find these cancer-related VOCs, the network of VOCs and their corresponding cancer type was constructed. Then, VOCs that contribute to more than three types of cancer were analyzed. Figure 2 shows the bipartite VOCs-cancer network corresponding to the VOCs observed in more than two different types of cancer. The blue circles and orange diamond represent VOCs and cancer types, respectively, and edges correspond to the association of VOCs with cancers.
Figure 2:
The network representations of cancer-related VOCs (shown by their PubChem ID) and cancer types obtained from analyzing the COD database.
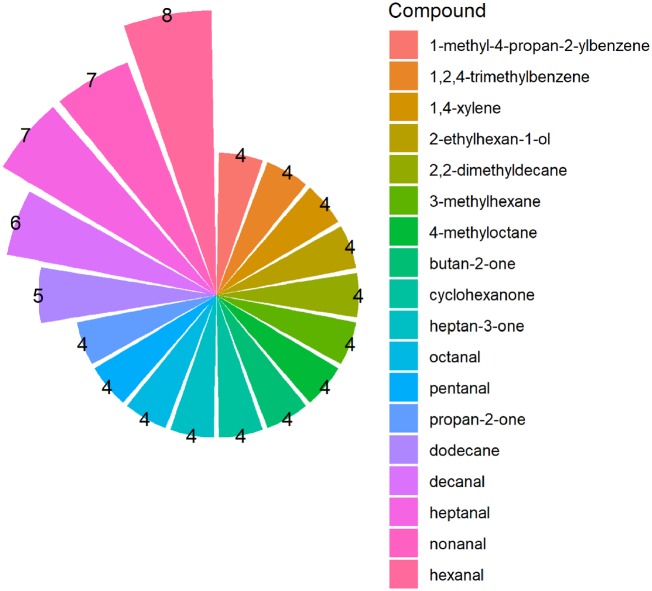
Figure 3 shows the VOCs ranked based on their association with three to eight types of cancer. It was observed that the VOCs in Fig. 3 can be classified by their chemical functional into five main groups: aldehydes (heptanal, hexanal, decanal, nonanal, pentanal, and octanal), ketones (acetone, 3-heptanone, 2-butanone, and cyclohexanone), alcohols (2-ethylhexanol), hydrocarbons (dodecane, 3-methylehexan, 4-methyloctane, and 2,2-dimethyldecane), and aromatic compounds (1,2,4-trimethylbenzene, 1-methyl-4-propan-2-ylbenzene, and p-xylene).
Figure 3:
VOCs related to four or more than four types of cancer. The number of different types of cancer in which the VOCs are contributed is indicated.
Indeed, these VOCs may be involved in some pathways involved in various types of cancer and can be considered as excellent general cancer biomarkers for early detection or risk prediction. A combination of general cancer biomarkers and specific biomarkers would be very promising for cancer detection.
The origin of many of the VOCs observed in different cancers has not been clearly defined. Understanding the metabolic pathways that lead to the production or elimination of these VOCs will result in a better understanding of the biochemical changes that occur in cancers. In the next section, we aim to shed light on the abovementioned significant VOCs and elucidate the biochemical pathways that they are involved in.
Aldehydes
As shown in Fig. 3, all the VOCs associated with more than five types of cancers (heptanal, hexanal, decanal, and nonanal) belong to the chemical class of aldehydes. Among these cancer-related aldehydes, hexanal has been reported for eight different cancer types thus showing its importance as a potential cancer biomarker. Several aldehydes including acetaldehyde, benzaldehyde, hexanal, heptanal, and octanal can be found in all human body matrices including breath, blood, saliva, skin secretions, urine, and feces [38].
A number of studies suggest a significant enhancement of aldehydes in cancer patients compared to healthy controls [48, 49]. Aldehydes are slightly soluble in blood and can be found in breath in just minutes after their formation in tissues [50]. There are some reports implying that there is no relationship between the level of aldehydes (in breath) and the age or gender of the patient [51–54].
Aldehydes are produced by different mechanisms. They can originate from dietary sources [55], metabolized alcohols, and smoking. One of the main sources of aldehydes is their generation as secondary oxidation products. Production of monofunctional C3–C10 aldehydes such as n-hexanal, n-heptanal, n-nonanal, and n-decanal is related to the reduction of hydroperoxides by cytochrome P450 (CYP450) through the lipid-oxidation of omega-3 and -6 polyunsaturated fatty acids (PUFAs) like linoleic or arachidonic acid [56–58].
CYP450 enzymes, a multigene family of constitutive and inducible enzymes found in many tissues with the highest concentration and activity in the liver, play a central role in human physiology. CYP is responsible for the oxidative metabolism of various endogenous and exogenous compounds. CYP450 enzymes play an important role in the Phase I metabolism of a broad range of different compounds. They oxidize these compounds to more hydrophilic products and hence facilitate their excretion [59, 60].
The P450s play a key role in cancer formation and are involved in tumor initiation or promotion, because of their ability to activate or deactivate most carcinogens. Reactive oxygen species (ROS) can be generated from the reactions mediated by CYP450s which are known to be overexpressed in cancer cells. It has been shown that the CYP450 family 1B1 is overexpressed in several types of tumor cells and associated with angiogenesis. The CYP2E1, another CYP450 family member, is one of the most active members of the family in terms of ROS production. They induce the generation of ROS which causes effects such as DNA damage, autophagy, unfolded protein response, enhanced angiogenic responses, and endoplasmic reticulum (ER) stress. CYP2E1 gene overexpression has been observed in malignant tissues in comparison with normal ones resulting in an increased level of inflammatory cytokines in the tumor microenvironment [61]. CYP2E1-mediated ROS generation can contribute to tumor development through different pathways [60]. Targeting of CYP450s in cancer therapy is very attractive.
It has also been found that the amount of saturated lipids in cancer cell membranes is greater than that in normal cells [62, 63]. Therefore, increased aldehyde production in cancer patients may be due to the changes in membrane lipid composition as well as increased oxidative stress in tumor cells. Furthermore, increased levels of certain unsaturated fatty acids in the membranes of tumor cells may increase the production of certain aldehydes through lipid peroxidation.
Aldehyde dehydrogenases (ALDHs) and alcohol dehydrogenases (ADHs) are two abundant enzymes in the human liver [64, 65]. Aldehydes can be irreversibly oxidized to carboxylic acids by ALDHs or reduced to their corresponding alcohols by ALHs [64].
ADH, which catalyzes the conversion of alcohol to aldehyde, is thought to play a role in the formation of both local and distant metastasis [66]. It has been found that ADH in the ovary of rats can metabolize alcohols to toxic aldehydes which could lead to cell damage [67–69]. Comparison of aldehydes levels in the reported cancer cell lines and cell lines treated with ADH inhibitors like 4-methylpyrazole might aid in a detailed understanding of the role of ADH. It has been demonstrated that downregulation of 10-formyltetrahydrofolate dehydrogenase in ovarian cancer cells leads to increased aldehydes levels [70]. Overexpression of ALDHs has also been shown in lung cancer cells [71]. Therefore, the concentration of cancer-related aldehydes can be reduced or increased in different reported cases.
Due to a decreased level of some types of aldehydes in the headspace of cancer cells compared to medium control, it has been suggested that these aldehydes are consumed or taken up by the cancer cells. Uptake of several volatile compounds has been reported in various cancer cell lines such as A-549, RPE, BEAS2B, CALU-1, and NCI-H1666 [72–75].
Saturated aldehydes are known to be taken up more easily than unsaturated ones. Hexanal can be metabolized in HepG2 cell cultures [76]. The uptake of volatiles by HepG2 cells from the culture medium may provide insight into the metabolism of these cells. Aldehydes can be metabolized by human hepatocellular carcinoma cells [76, 77]. However, the consumption of aldehydes in the headspace of the cells in vitro is not specific for cancer cells and can be seen in noncancerous cells [73, 74, 78].
Decreased concentrations of aldehydes may be attributed to the higher activity of ALDH in cancer cells [72, 79, 80]. For instance, overexpression of this enzyme has been reported in several tumor cells including non-small cell lung cancer (NSCLC) cell line [80] and esophageal cancer cells [81].
It has been speculated that the reason for a significantly decreased level of decanal in the headspace of lung cancer cells is related to mitochondrial defects in lung cancer cells which leads to a decreased level of ROS in the microenvironment of the cells and as a consequence, decreased lipid peroxidation [82]. On the contrary, an increase in the level of several aldehydes has been reported for several cancer types. The study of two main categories of lung cancers, namely small-cell lung cancer (SCLC) and NSCLC, has shown that the level of hexanal, as a general marker of oxidative stress, in the breath of SCLC patients is more than that of NSCLC patients. This can be partly explained by the higher activity of SCLC cells in terms of proliferation and metabolism compared to NSCLC cells [83, 84].
An increase in the level of hexanal and acetaldehyde has been identified in the headspace of the human promyelocytic leukemia cell line, HL60 [85]. It is known that human blood cells are able to metabolize ethanol to acetaldehyde [86, 87]. Neutrophils can oxidize amino acids and produce aldehydes [88]. Also, it has been suggested that generated ozone in neutrophils can react with cellular fatty acid resulting in the oxidation of omega-6 unsaturated fatty acids and the production of hexanal [85].
Levels of acetaldehyde may be modulated by a balance between its production of alcohol by ADHs and its elimination by ALDHs which change it to acetate. Therefore, the levels of acetaldehyde in biological matrices are closely related to the balance between ADHs and ALDHs activities and the metabolism of ethanol.
Due to the ability to form various types of DNA adducts, acetaldehyde has been classified as Class I carcinogen for humans. Acetaldehyde inhibits the enzymes involved in DNA repair leading to impaired DNA damage response. The link between acetaldehyde and several cancers like gastric cancer is evident [89].
Since tobacco smoke contains acetaldehyde, the high concentration of it in cancer patients may show a relationship between smoking and cancer. However, further investigation is needed to identify the exact role of aldehydes in cancer cell metabolism and their function in different types of cancer. It should be mentioned that gut flora can generate acetaldehyde from ethanol [90]. Formaldehyde and acetaldehyde are also present in the environment [91, 92].
It has been reported that the production of acetaldehyde is more efficient in the 3D culture models in comparison with 2D ones [93]. This could be due to the fact that 3D in vitro culture models mimic some properties of biological systems.
Benzaldehyde is another important aldehyde which is involved in several metabolic pathways, such as glycolysis/gluconeogenesis, tryptophan metabolism, and fatty acid metabolism [94].
Moreover, some aldehydes like nonanal have been considered biomarkers of apoptosis. It has been demonstrated that the levels of nonanal, 1,3-bis(1,1-dimethylethyl)-benzene, and 2,6-bis(1,1-dimethylethyl)-2,5-cyclohexadiene-1,4-dione significantly increase during apoptosis [95].
The general problems of analysis of aldehydes are their low concentrations in biological matrices and their high tendency to react with other compounds or breakdown during sample preparation or storage. However, high molecular weight aldehydes, like hexanal, heptanal, octanal, and nonanal are more stable than low molecular weight ones [96]. Fuchs et al. addressed this problem through the transformation of reactive aldehydes into stable oximes by means of on-fiber-derivatization [51]. Oximes are produced by the reaction of hydroxylamine with aldehydes or ketones. VOCs that have been reported as biomarkers for at least five types of cancers, namely hexanal, heptanal, octanal, and nonanal, are all high molecular weight aldehydes with weak polarity.
Ketones
Production of ketones is also closely related to the higher oxidation rate of fatty acids that are observed in several cancers [24, 97]. The acetyl-CoA, which is a substrate for ketogenesis, is mainly formed as the major product of β-oxidation of long-chain fatty acids in mitochondria. β-oxidation of branched fatty acids (e.g. valproic acid) results in the formation of heavier ketones (3-heptanone) [98]. Ketones in biological matrices may also originate from exogenous sources like food or the ambient air pollution [55].
The ADHs are known to catalyze the oxidation of aliphatic alcohols to ketones, with a wide range of chain lengths. Although the primary alcohols are the most preferred substrates for ADHs, metabolization of secondary alcohols (e.g. 2-propanol and 2-octanol) to ketones (e.g. acetone and 2-octanone) by ADHs have also been reported. In several types of cancers, like liver cancer, it has been demonstrated that the ADHs activity in the affected tissues is significantly higher than healthy ones. Production of ketones in human hepatocellular carcinoma cells can be attributed to metabolization of long-chain secondary alcohols in culture medium by highly active ADHs in the cells [76].
Acetone, the simplest ketone, is derived from decarboxylation of acetoacetate and the dehydrogenation of isopropanol. Acetone is a product of the spontaneous breakdown of acetoacetate and gives a distinctive odor to the breath when exhaled by the lungs. In the human body, acetone is mainly produced by decarboxylation of acetoacetate which is formed by both lipolysis and the breakdown of ketogenic amino acids. Decarboxylation of acetoacetate to acetone may occur either in an enzyme-catalyzed way, by acetoacetate decarboxylase [99–101], or by nonenzymatic reactions [102].
The oxidation of isopropanol (2-propanol) is another source of acetone. Isopropanol is metabolized by ADH to form acetone [103]. ADH dominantly catalyzes oxido-reduction reactions between acetone and isopropanol.
Mitochondrial oxidation of fatty acids generates acetyl COA that can enter the Krebs cycle. Cancer cells exhibit altered glucose metabolism known as the Warburg effect in which their energy production shifts from the Krebs cycle to glycolysis. Therefore, acetone and other ketone bodies (acetoacetate and β-hydroxybutyrate) are produced by the hepatocytes from excess acetyl-COA which in turn results in an increased level of acetone in the body.
CYP2E1 plays an important role in the degradation of acetone through the conversion of acetone into acetol and acetone is also considered the physiological inducer for CYP2E1 [104, 105].
The evidence revealed that VOCs, like acetone, can be produced by a wide variety of anaerobic and aerobic bacteria [106]. It has been proposed that a considerable fraction of ketones in urine arise from bacterial action in the gut.
Since the concentration of acetone in bodily fluids or breath changes during some activities like fasting, exercising, and food consumption, acetone is not recommended by some researchers to be used as a biomarker [107, 108].
Cyclohexanone, another important cancer-related VOC, might be formed from the oxidation of cyclohexane. Different amounts of cyclohexanone have been found in exhaled breath of healthy and chronic obstructive pulmonary disease patients [76].
2-Nonanon, 3-heptanone, and 4-heptanone are three other ketones that are considered cancer biomarkers. 2-Nonanon can be produced from nonane metabolism by CYP450 [109–111].
The origin of 4-heptanone is still unknown. Previous studies have shown that 4-heptanone can be produced from the in vivo metabolism of plasticizers in the body [31, 112].
Hydrocarbons
A potential source of saturated hydrocarbons (e.g. C3–C11) may be the lipid peroxidation process. However, this mechanism is probably irrelevant to the presence of branched hydrocarbons. The unmetabolized hydrocarbons excrete to the blood, and consequently to urine and/or breath. The concentrations of volatile hydrocarbons in biological matrices depend on their solubility in the different biological media. Hydrocarbons, with low solubility in the blood, instantly pass into the breath.
Alkanes are mainly formed during lipid peroxidation of PUFA constituents of biological membranes leading to the degradation of phospholipids and eventually cellular deterioration [52, 113]. Alkanes can be also produced in association with hepatic ethanol metabolism by ADHs.
The increased level of several alkanes (such as dodecane and pentane) and methylated alkane (e.g. 3-methylhexane) has been reported in the patients with different types of cancers [114–116]. Altered activity of CYP450 might be the reason for significant changes in the levels of alkanes and methylalkanes in patients suffering from cancer [117]. There is some controversy about the origin of methylated alkanes. It is thought to be secondary products of oxidative stress [118] by some researchers; however, others disagree with this hypothesis [119]. Isoprene (2-methyl-1,3-butadiene) can be formed by enzymatic or nonenzymatic pathways. Isoprene is produced along the mevalonate pathway of cholesterol synthesis [120]. Dimethylallyl pyrophosphate can be converted to isoprene nonenzymatically.
In human liver microsomes, CYP450 oxidizes isoprene mainly to 3,4-epoxy-3-methyl-1-butane, and 3,4-epoxy-2-methyl-1-butene, which are further hydrolyzed to vicinal diols (2-methyl-3-buten-1,2-diol and 3-methyl-3-buten-1,2-diol) [76]. A very low level of isoprene in gastric cancer tissue compared to a higher amount of that in healthy gastric tissue has been reported. This might be due to the ROS damaging effects happening in the gastric cancer tissue [121]. Also, isoprene is present in cigarette smoke [122] and can be considered as an exogenous compound. Some VOCs, like isoprene, can be stored in different tissues. Depending on the types of VOCs and the storage capacity of the tissue, the release time of VOCs varies. The amount of isoprene in the human body easily changes during exertion of a physical effort [123–126]. Even a few legs or arm contractions can lead to elevation of isoprene levels in exhaled breath [38]. It has been proposed that isoprene is stored in muscles and released from the working muscles through exercise [127].
Aromatics
The origin of aromatic compounds like p-xylene is still unknown and they have been considered as possible environmental contaminants (e.g. cigarette smoke) in previous reports. For instance, benzene derivatives have been observed in the breath of patients with lung cancer. They are also found in the breath of smokers. Some researchers believe that as the amount of several aromatic compounds (such as benzene, toluene, and 2,5-dimethylfuran) increases in the breath of smokers versus nonsmokers and lung cancer patients; they can be considered as tobacco-related carcinogens [24, 128].
Alcohols
One of the main sources of alcohols in body fluids is diet. Alcohols from the ingestion of food and beverages are absorbed through the gastrointestinal tract and then released into the bloodstream. However, they can also be detected in feces, urine, breath, skin secretions, milk, and saliva [24].
In addition, alcohols can be produced from the metabolism of hydrocarbons. As mentioned earlier, the metabolization of alcohol is mainly catalyzed by ADHs [55, 129, 130]. In the cytosol of hepatocytes, ethanol is metabolized to acetaldehyde, a carcinogenic compound, by ADHs. Acetaldehyde is rapidly oxidized to acetate by ALDHs in the mitochondria. Acetate may be released to the blood or metabolized further to form CO2, H2O, or fatty acids as well as entering into intermediary metabolism as acetyl-CoA [130].
CYP450 isoenzymes are also involved in the oxidation of alcohols. CYP2E1, 3A4, and 1A2 are predominantly found in the ER, contribute to alcohol metabolism [130]. Generally, when the level of alcohol is elevated, CYP2E1 is induced, resulting in oxidation of the excessive amount of alcohol to acetaldehyde, and generation of ROS [130].
Obvious changes (decreases or increases) in levels of ethanol have been observed in several cancers including lung, liver, colorectal, and gastric cancers. Increasing evidence suggests that alcohol may induce carcinogenesis through aberrant DNA methylation [131]. PPAR-a, SREBP-1c, and PNPLA3 are some genes affected by chronic alcohol consumption [130]. The reduced levels of ethylhexanol in blood and breath of patients with papillary thyroid carcinoma and colorectal cancer, respectively, have been reported. This can be due to the consumption of ethylhexanol by the cells during tumor cell proliferation. Further investigations have identified the releasing of 2-ethyl-1-hexanol from NCI-H2087 lung cancer cell line [78].
The activity and expression of specific enzymes that regulate the metabolism of ethanol are influenced by genetic polymorphisms [129]. Moreover, alcohol metabolism might be affected by a different amount of water and fat in the bodies of different people and different genders [55].
Conclusion and future research
VOCs contain valuable information about biological processes inside the cells. The association between cancer and VOCs produced in the human body has attracted a lot of interest. Finding distinguishable VOC fingerprints or chemical groups related to cancers may lead to the early detection of cancers, the unraveling of the mechanisms of cancer development and progression, and eventually making the manipulation of the altered pathways possible. Cellular events or biochemical pathways associated with the cancer initiation and progression, such as the altered activity of the CYP450 system, can be translated into VOC profile. Therefore, analysis of VOCs in breath or body fluids has the potential to achieve cancer detection at very early stages. In addition, changes in signal transduction, gene regulation, and cellular proliferation can be followed by the VOC pattern of cells or organisms. VOCs enter into the blood system and are released later through breath, urine, feces, and skin. In contrast to the determination of most of the traditional biomarkers, measurement of exhaled VOC levels in breath is completely noninvasive and offers the potential for the development of screening tests and for disease monitoring.
Although significant efforts have been made by various research groups in the past decade to find new VOC cancer biomarkers, they have not been prospectively compared or analyzed in various cancers. Herein, we analyzed the COD database containing detailed information about cancer-related VOCs to identify significant VOCs associated with cancer and their origin.
Some of these compounds appear in more than one cancer, while some are unique compounds. Surveying these VOCs can be used to discriminate patients from healthy individuals. A comprehensive analysis of the COD database has revealed that a total of 18 VOCs from five major chemical compound categories are reported in various types of cancers and can be considered as important VOCs biomarkers. Highlighted VOCs includes six aldehydes (heptanal, hexanal, decanal, nonanal, pentanal, and octanal), four ketones (acetone, 3-heptanon, 2-butanone, and cyclohexanone), one alcohol (2-ethylhexanol), four hydrocarbons (dodecane, 3-methylehexan, 4-methyloctane, and 2,2-dimethyldecane), and three aromatic compounds (1,2,4-trimethylbenzene,1-methyl-4-propan-2-ylbenzene, and p-xylene). There are several mechanisms for the production of hydrocarbons, aldehydes, aromatics, alcohols, and ketones which have been comprehensively reviewed in this article.
As discussed in this report, along with the benefits of VOCs as potential cancer biomarkers, there are some challenges for cancer diagnosis based on VOCs. The major problem is the strong effect of other parameters on VOCs pattern. VOCs can originate from both endogenous and exogenous sources. Food consumption, medications, physical activities, smoking, other noncancer diseases, and normal gut bacterial flora can all change the pattern of VOCs.
Another limitation arises from the fact that the sites and the origins of many VOCs are not clear. The information provided here is particularly useful for scientists investigating cancer-related VOCs, and for researchers who work on the development of sensors and electronic nose systems for cancer detection. However, there appear to be gaps in the studies that have been investigating VOCs as cancer biomarkers. Filling these gaps might help to solve the puzzle of cancer-related volatile compounds. We hope this article will stimulate new quantitative experimental and theoretical studies of cancer-related VOCs and be valuable for improving current cancer researches.
Acknowledgement
We are grateful to Geoffrey Barrow for language editing.
Funding
This work was supported by the Research Council of Tarbiat Modares University.
Reference
- 1. Kantarjian HM, Prat F, Steensma DP. et al. Cancer research in the United States: a critical review of current status and proposal for alternative models. Cancer 2018;124:2881–9 [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Center MM, DeSantis C. et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010;19:1893–907 [DOI] [PubMed] [Google Scholar]
- 3. Sung H, Siegel RL, Rosenberg PS. et al. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health 2019;4:e137–47 [DOI] [PubMed] [Google Scholar]
- 4. Hiller K, Metallo CM.. Profiling metabolic networks to study cancer metabolism. Curr Opin Biotechnol 2013;24:60–8 [DOI] [PubMed] [Google Scholar]
- 5. Chaturvedi VK, Singh A, Singh VK. et al. Cancer nanotechnology: a new revolution for cancer diagnosis and therapy. Curr Drug Metab 2018;20:416–29. [DOI] [PubMed] [Google Scholar]
- 6. Kaboli PJ, Rahmat A, Ismail P. et al. MicroRNA-based therapy and breast cancer: a comprehensive review of novel therapeutic strategies from diagnosis to treatment. Pharmacol Res 2015;97:104–21 [DOI] [PubMed] [Google Scholar]
- 7. Sun X, Shao K, Wang T.. Detection of volatile organic compounds (VOCs) from exhaled breath as noninvasive methods for cancer diagnosis. Anal Bioanal Chem 2016;408:2759–80 [DOI] [PubMed] [Google Scholar]
- 8. Music M, Prassas I, Diamandis EP.. Optimizing cancer immunotherapy: is it time for personalized predictive biomarkers? Crit Rev Clin Lab Sci 2018;55:466–79 [DOI] [PubMed] [Google Scholar]
- 9. Roointan A, Ahmad Mir T, Ibrahim Wani S. et al. Early detection of lung cancer biomarkers through biosensor technology: a review. J Pharm Biomed Anal 2019;164:93–103 [DOI] [PubMed] [Google Scholar]
- 10. Rudnicka J, Kowalkowski T, Buszewski B.. Searching for selected VOCs in human breath samples as potential markers of lung cancer. Lung Cancer 2019;135:123. [DOI] [PubMed] [Google Scholar]
- 11. Jia Z, Patra A, Kutty VK. et al. Critical review of volatile organic compound analysis in breath and in vitro cell culture for detection of lung cancer. Metabolites 2019;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mozdiak E, Wicaksono A, Covington J. et al. Colorectal cancer and adenoma screening using urinary volatile organic compound (VOC) detection: early results from a single-centre bowel screening population (UK BCSP). Tech Coloproctol 2019;23:343–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McFarlane M, Millard A, Hall H. et al. Urinary volatile organic compounds and faecal microbiome profiles in colorectal cancer. Colorectal Dis 2019; In press. DOI: 10.1111/codi.14739. [DOI] [PubMed] [Google Scholar]
- 14. Woollam M, Teli M, Angarita-Rivera P. et al. Detection of volatile organic compounds (VOCs) in urine via gas chromatography-mass spectrometry QTOF to differentiate between localized and metastatic models of breast cancer. Sci Rep 2019;9:2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Phillips M, Cataneo RN, Cruz-Ramos JA. et al. Prediction of breast cancer risk with volatile biomarkers in breath. Breast Cancer Res Treat 2018;170:343–50 [DOI] [PubMed] [Google Scholar]
- 16. Princivalle A, Monasta L, Butturini G. et al. Pancreatic ductal adenocarcinoma can be detected by analysis of volatile organic compounds (VOCs) in alveolar air. BMC Cancer 2018;18:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Markar SR, Brodie B, Chin ST. et al. Profile of exhaled‐breath volatile organic compounds to diagnose pancreatic cancer. Br J Surg 2018;105:1493–500 [DOI] [PubMed] [Google Scholar]
- 18. Bond A, Greenwood R, Lewis S. et al. Volatile organic compounds emitted from faeces as a biomarker for colorectal cancer. Aliment Pharmacol Ther 2019;49:1005–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Opitz P, Herbarth O.. The volatilome–investigation of volatile organic metabolites (VOM) as potential tumor markers in patients with head and neck squamous cell carcinoma (HNSCC). J Otolaryngol Head Neck Surg 2018;47:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou J, Huang Z-A, Kumar U. et al. Review of recent developments in determining volatile organic compounds in exhaled breath as biomarkers for lung cancer diagnosis. Anal Chim Acta 2017;996:1–9 [DOI] [PubMed] [Google Scholar]
- 21. Chang JE, Lee DS, Ban SW. et al. Analysis of volatile organic compounds in exhaled breath for lung cancer diagnosis using a sensor system. Sens Actuat B Chem 2018;255:800–7 [Google Scholar]
- 22. Di Lena M, Porcelli F, Altomare D.. Volatile organic compounds as new biomarkers for colorectal cancer: a review. Colorectal Dis 2016;18:654–63 [DOI] [PubMed] [Google Scholar]
- 23. Cumeras R. Volatilome metabolomics and databases, recent advances and needs. Curr Metab 2017;5:79–89 [Google Scholar]
- 24. Hakim M, Broza YY, Barash O. et al. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem Rev 2012;112:5949–66 [DOI] [PubMed] [Google Scholar]
- 25. Janfaza S, Khorsand B, Zahiri J. et al. Identification of important volatile organic compounds as cancer biomarkers. In 7th Iranian Bioinformatics Conference Iran: Tarbiat Modares University, 2017
- 26. Janfaza S, Banan Nojavani M, Khorsand B. et al. Cancer Odor Database (COD): a critical databank for cancer diagnosis research. Database 2017;2017:137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang R, Huang W, Li G. et al. Noninvasive strategy based on real-time in vivo cataluminescence monitoring for clinical breath analysis. Anal Chem 2017;89:3353–61 [DOI] [PubMed] [Google Scholar]
- 28. Wang C, Li P, Lian A. et al. Blood volatile compounds as biomarkers for colorectal cancer. Cancer Biol Ther 2014;15:200–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xue R, Dong L, Zhang S. et al. Investigation of volatile biomarkers in liver cancer blood using solid‐phase microextraction and gas chromatography/mass spectrometry, rapid communications in mass spectrometry: an International Journal Devoted to the Rapid Dissemination of Up‐to‐the‐Minute Research in Mass. Rapid Commun Mass Spectrom 2008;22:1181–6 [DOI] [PubMed] [Google Scholar]
- 30. Deng C, Zhang X, Li N.. Investigation of volatile biomarkers in lung cancer blood using solid-phase microextraction and capillary gas chromatography–mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2004;808:269–77 [DOI] [PubMed] [Google Scholar]
- 31. Silva C, Passos M, Camara J.. Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. Br J Cancer 2011; 105:1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Batty CA, Cauchi M, Lourenço C. et al. Use of the analysis of the volatile faecal metabolome in screening for colorectal cancer. PLoS One 2015;10:e0130301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang G, Li Y, Liu M. et al. Determination of volatile organic compounds in SW620 colorectal cancer cells and tumor-bearing mice. J Pharm Biomed Anal 2019;167:30–7 [DOI] [PubMed] [Google Scholar]
- 34. Thriumani R, Zakaria A, Hashim YY. et al. A study on volatile organic compounds emitted by in-vitro lung cancer cultured cells using gas sensor array and SPME-GCMS. BMC Cancer 2018;18:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith EA, Macfarlane G.. Dissimilatory amino acid metabolism in human colonic bacteria. Anaerobe 1997;3:327–37. [DOI] [PubMed] [Google Scholar]
- 36. Bäckhed F, Ley RE, Sonnenburg JL. et al. Host-bacterial mutualism in the human intestine. Science 2005;307:1915–20 [DOI] [PubMed] [Google Scholar]
- 37. Garner CE, Smith S, de Lacy Costello B. et al. Volatile organic compounds from feces and their potential for diagnosis of gastrointestinal disease. FASEB J 2007;21:1675–88 [DOI] [PubMed] [Google Scholar]
- 38. de Lacy Costello B, Amann A, Al-Kateb H. et al. A review of the volatiles from the healthy human body. J Breath Res 2014;8:014001. [DOI] [PubMed] [Google Scholar]
- 39. Amal H, Leja M, Funka K. et al. Detection of precancerous gastric lesions and gastric cancer through exhaled breath. Gut 2016;65:400–7 [DOI] [PubMed] [Google Scholar]
- 40. Ferreira SL, Junior MMS, Felix CS. et al. Multivariate optimization techniques in food analysis–a review. Food Chem 2019;273:3–8 [DOI] [PubMed] [Google Scholar]
- 41. Azzouz A, Kailasa SK, Lee SS. et al. Review of nanomaterials as sorbents in solid-phase extraction for environmental samples. TrAC Trends Anal Chem 2018;108:347 [Google Scholar]
- 42. Zhang Z, Pawliszyn J.. Headspace solid-phase microextraction. Anal Chem 1993;65:1843–52 [Google Scholar]
- 43. Schmidt K, Podmore I.. Solid phase microextraction (SPME) method development in analysis of volatile organic compounds (VOCS) as potential biomarkers of cancer. J Mol Biomark Diag 2015;6:253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li J, Chen N, Tian Y. et al. Solid-phase microextraction of volatile organic compounds in headspace of PM-induced MRC-5 cell lines. Talanta 2018;185:23–9 [DOI] [PubMed] [Google Scholar]
- 45. Brooks SW, Moore DR, Marzouk EB. et al. Canine olfaction and electronic nose detection of volatile organic compounds in the detection of cancer: a review. Cancer Invest 2015;33:411–9 [DOI] [PubMed] [Google Scholar]
- 46. Janfaza S, Nojavani MB, Nikkhah M. et al. A selective chemiresistive sensor for the cancer-related volatile organic compound hexanal by using molecularly imprinted polymers and multiwalled carbon nanotubes. Microchim Acta 2019;186:137. [DOI] [PubMed] [Google Scholar]
- 47. Razavi H, Janfaza S.. Medical nanobiosensors: a tutorial review. Nanomed J 2015;2:74–87 [Google Scholar]
- 48. Guadagni R, Miraglia N, Simonelli A. et al. Solid-phase microextraction–gas chromatography–mass spectrometry method validation for the determination of endogenous substances: urinary hexanal and heptanal as lung tumor biomarkers. Anal Chim Acta 2011;701:29–36 [DOI] [PubMed] [Google Scholar]
- 49. Hopkinson RJ, Schofield CJ.. Deciphering Functions of Intracellular Formaldehyde: Linking Cancer and Aldehyde Metabolism. Washington, DC: ACS Publications, 2018 [DOI] [PubMed] [Google Scholar]
- 50. Mazzone PJ. Analysis of volatile organic compounds in the exhaled breath for the diagnosis of lung cancer. J Thorac Oncol 2008;3:774–80 [DOI] [PubMed] [Google Scholar]
- 51. Fuchs P, Loeseken C, Schubert JK. et al. Breath gas aldehydes as biomarkers of lung cancer. Int J Cancer 2010;126:2663–70 [DOI] [PubMed] [Google Scholar]
- 52. Phillips M, Cataneo RN, Greenberg J. et al. Effect of age on the breath methylated alkane contour, a display of apparent new markers of oxidative stress. J Lab Clin Med 2000;136:243–9 [DOI] [PubMed] [Google Scholar]
- 53. Phillips M, Cataneo RN, Greenberg J. et al. Increased oxidative stress in younger as well as in older humans. Clin Chim Acta 2003;328:83–6 [DOI] [PubMed] [Google Scholar]
- 54. Phillips M, Greenberg J, Cataneo RN.. Effect of age on the profile of alkanes in normal human breath. Free Radic Res 2000;33:57–63 [DOI] [PubMed] [Google Scholar]
- 55. Haick H, Broza YY, Mochalski P. et al. Assessment, origin, and implementation of breath volatile cancer markers. Chem Soc Rev 2014;43:1423–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kinter M. Analytical technologies for lipid oxidation products analysis. J Chromatogr B Biomed Sci Appl 1995;671:223–36 [DOI] [PubMed] [Google Scholar]
- 57. Esterbauer H, Schaur RJ, Zollner H.. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 1991;11:81–128 [DOI] [PubMed] [Google Scholar]
- 58. Pryor WA, Das B, Church DF.. The ozonation of unsaturated fatty acids: aldehydes and hydrogen peroxide as products and possible mediators of ozone toxicity. Chem Res Toxicol 1991;4:341–8 [DOI] [PubMed] [Google Scholar]
- 59. Coon MJ. Cytochrome P450: nature’s most versatile biological catalyst. Annu Rev Pharmacol Toxicol 2005;45:1–25 [DOI] [PubMed] [Google Scholar]
- 60. Singh S, Rajendran R, Kuroda K. et al. Oxidative stress and breast cancer biomarkers: the case of the cytochrome P450 2E1. J Cancer Metastasis Treat 2016;2:268–76 [Google Scholar]
- 61. Gonzalez FJ. Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutat Res Fundamen Mol Mech Mutagen 2005;569:101–10 [DOI] [PubMed] [Google Scholar]
- 62. Burns CP, Spector AA.. Membrane fatty acid modification in tumor cells: a potential therapeutic adjunct. Lipids 1987;22:178. [DOI] [PubMed] [Google Scholar]
- 63. Baronzio G, Freitas I, Griffini P. et al. Omega-3 fatty acids can improve radioresponse modifying tumor interstitial pressure, blood rheology and membrane peroxidability. Anticancer Res 1994;14:1145–54 [PubMed] [Google Scholar]
- 64. Crabb DW, Matsumoto M, Chang D. et al. Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology. Proc Nutr Soc 2004;63:49–63 [DOI] [PubMed] [Google Scholar]
- 65. Klyosov AA. Kinetics and specificity of human liver aldehyde dehydrogenases toward aliphatic, aromatic, and fused polycyclic aldehydes. Biochemistry 1996;35:4457–67 [DOI] [PubMed] [Google Scholar]
- 66. Ma S, Chan KW, Lee T-W. et al. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res 2008;6:1146–53 [DOI] [PubMed] [Google Scholar]
- 67. Holmes RS. Electrophoretic analyses of alcohol dehydrogenase, aldehyde dehydrogenase, aldehyde oxidase, sorbitol dehydrogenase and xanthine oxidase from mouse tissues. Comp Biochem Physiol B 1978;61:339–46 [DOI] [PubMed] [Google Scholar]
- 68. Messiha F. Subcellular alcohol and aldehyde-dehydrogenases in the genital system of the female rat. Neurobehav Toxicol Teratol 1983;5:247–50 [PubMed] [Google Scholar]
- 69. Mapoles JE, Iwahashi M, Lucas D. et al. Acetaldehyde exposure causes growth inhibition in a Chinese hamster ovary cell line that expresses alcohol dehydrogenase. Alcoholism Clin Exp Res 1994;18:632–9 [DOI] [PubMed] [Google Scholar]
- 70. Krupenko SA, Oleinik NV.. 10-formyltetrahydrofolate dehydrogenase, one of the major folate enzymes, is down-regulated in tumor tissues and possesses suppressor effects on cancer cells. Cell Growth Differ 2002;13:227–36 [PubMed] [Google Scholar]
- 71. Chang JW, Jeon HB, Lee JH. et al. Augmented expression of peroxiredoxin I in lung cancer. Biochem Biophys Res Commun 2001;289:507–12. [DOI] [PubMed] [Google Scholar]
- 72. Brunner C, Szymczak W, Höllriegl V. et al. Discrimination of cancerous and non-cancerous cell lines by headspace-analysis with PTR-MS. Anal Bioanal Chem 2010;397:2315–24 [DOI] [PubMed] [Google Scholar]
- 73. Filipiak W, Sponring A, Filipiak A. et al. TD-GC-MS analysis of volatile metabolites of human lung cancer and normal cells in vitro. Cancer Epidemiol Prevent Biomarkers 2010;19:182–95 [DOI] [PubMed] [Google Scholar]
- 74. Filipiak W, Sponring A, Mikoviny T. et al. Release of volatile organic compounds (VOCs) from the lung cancer cell line CALU-1 in vitro. Cancer Cell Int 2008;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sponring A, Filipiak W, Ager C. et al. Analysis of volatile organic compounds (VOCs) in the headspace of NCI-H1666 lung cancer cells. Cancer Biomark 2011;7:153–61 [DOI] [PubMed] [Google Scholar]
- 76. Mochalski P, Sponring A, King J. et al. Release and uptake of volatile organic compounds by human hepatocellular carcinoma cells (HepG2) in vitro. Cancer Cell Int 2013;13:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang G, Ma L, Xie Y-K. et al. Esophageal cancer tumorspheres involve cancer stem-like populations with elevated aldehyde dehydrogenase enzymatic activity. Mol Med Rep 2012;6:519–24 [DOI] [PubMed] [Google Scholar]
- 78. Sponring A, Filipiak W, Mikoviny T. et al. Release of volatile organic compounds from the lung cancer cell line NCI-H2087 in vitro. Anticancer Res 2009;29:419–26 [PubMed] [Google Scholar]
- 79. Barash O, Peled N, Tisch U. et al. Classification of lung cancer histology by gold nanoparticle sensors. Nanomed Nanotechnol Biol Med 2012;8:580–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Patel M, Lu L, Zander DS. et al. ALDH1A1 and ALDH3A1 expression in lung cancers: correlation with histologic type and potential precursors. Lung Cancer 2008;59:340–9 [DOI] [PubMed] [Google Scholar]
- 81. Moreb JS, Zucali JR, Ostmark B. et al. Heterogeneity of aldehyde dehydrogenase expression in lung cancer cell lines is revealed by Aldefluor flow cytometry-based assay. Cytometry B Clin Cytom 2007;72:281–9 [DOI] [PubMed] [Google Scholar]
- 82. Gatenby RA, Gillies RJ.. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004;4:891. [DOI] [PubMed] [Google Scholar]
- 83. Soomro IN, Whimster WF.. Growth fraction in lung tumours determined by Ki67 immunostaining and comparison with agnor scores. J Pathol 1990;162:217–22 [DOI] [PubMed] [Google Scholar]
- 84. Soomro IN, Holmes J, Whimster WF.. Predicting prognosis in lung cancer. Use of proliferation marker Ki67 monoclonal antibody. J Pak Med Assoc 1998;48:66–8 [PubMed] [Google Scholar]
- 85. Shin HW, Umber BJ, Meinardi S. et al. Acetaldehyde and hexanaldehyde from cultured white cells. J Transl Med 2009;7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wickramasinghe SN, Bond AN, Sloviter HA. et al. Metabolism of ethanol by human bone marrow cells. Acta Haematol 1981;66:238–43 [DOI] [PubMed] [Google Scholar]
- 87. Bond AN, Wickramasinghe SN.. Investigations into the production of acetate from ethanol by human blood and bone marrow cells in vitro. Acta Haematol 1983;69:303–13 [DOI] [PubMed] [Google Scholar]
- 88. Hazen SL, Hsu FF, d’Avignon A. et al. Human neutrophils employ myeloperoxidase to convert α-amino acids to a battery of reactive aldehydes: a pathway for aldehyde generation at sites of inflammation. Biochemistry 1998;37:6864–73 [DOI] [PubMed] [Google Scholar]
- 89. Linderborg K, Marvola T, Marvola M. et al. Reducing carcinogenic acetaldehyde exposure in the achlorhydric stomach with cysteine. Alcohol Clin Exp Res 2011;35:516–22 [DOI] [PubMed] [Google Scholar]
- 90. Feng S, Plunkett SE, Lam K. et al. A new method for estimating the retention of selected smoke constituents in the respiratory tract of smokers during cigarette smoking. Inhal Toxicol 2007;19:169–79 [DOI] [PubMed] [Google Scholar]
- 91. Gilbert NL, Guay M, David Miller J. et al. Levels and determinants of formaldehyde, acetaldehyde, and acrolein in residential indoor air in Prince Edward Island, Canada. Environ Res 2005;99:11–7 [DOI] [PubMed] [Google Scholar]
- 92. Lovreglio P, Carrus A, Iavicoli S. et al. Indoor formaldehyde and acetaldehyde levels in the province of Bari, South Italy, and estimated health risk. J Environ Monit 2009;11:955–61 [DOI] [PubMed] [Google Scholar]
- 93. Rutter AV, Chippendale TWE, Yang Y. et al. Quantification by SIFT-MS of acetaldehyde released by lung cells in a 3D model. Analyst 2013;138:91–5 [DOI] [PubMed] [Google Scholar]
- 94. Zimmermann D, Hartmann M, Moyer MP. et al. Determination of volatile products of human colon cell line metabolism by GC/MS analysis. Metabolomics 2007;3:13–7 [Google Scholar]
- 95. Pyo JS, Ju HK, Park JH. et al. Determination of volatile biomarkers for apoptosis and necrosis by solid-phase microextraction–gas chromatography/mass spectrometry: a pharmacometabolomic approach to cisplatin’s cytotoxicity to human lung cancer cell lines. J Chromatogr B 2008;876:170–4 [DOI] [PubMed] [Google Scholar]
- 96. Li J, Peng Y, Liu Y. et al. Investigation of potential breath biomarkers for the early diagnosis of breast cancer using gas chromatography–mass spectrometry. Clin Chim Acta 2014;436:59–67 [DOI] [PubMed] [Google Scholar]
- 97. Broza YY, Kremer R, Tisch U. et al. A nanomaterial-based breath test for short-term follow-up after lung tumor resection. Nanomed Nanotechnol Biol Med 2013;9:15–21 [DOI] [PubMed] [Google Scholar]
- 98. Erhart S, Amann A, Haberlandt E. et al. 3-Heptanone as a potential new marker for valproic acid therapy. J Breath Res 2009;3:016004. [DOI] [PubMed] [Google Scholar]
- 99. Koorevaar G, Van Stekelenburg GJ.. Mammalian acetoacetate decarboxylase activity: its distribution in subfractions of human albumin and occurrence in various tissues of the rat. Clin Chim Acta 1976;71:173–83 [DOI] [PubMed] [Google Scholar]
- 100. López-Soriano F, Alemany M, Argilés J.. Rat acetoacetic acid decarboxylase inhibition by acetone. Int J Biochem 1985;17:1271–3 [DOI] [PubMed] [Google Scholar]
- 101. Lopez-Soriano F, Argilés J.. Rat acetoacetate decarboxylase: its role in the disposal of 4C-ketone bodies by the fetus. Horm Metab Res 1986;18:446–9 [DOI] [PubMed] [Google Scholar]
- 102. Pedersen KJ. The ketonic decomposition of beta-keto carboxylic acids. J Am Chem Soc 1929;51:2098–107 [Google Scholar]
- 103. Nordmann R, Ribiere C, Rouach H. et al. Metabolic pathways involved in the oxidation of isopropanol into acetone by the intact rat. Life Sci 1973;13:919–32 [DOI] [PubMed] [Google Scholar]
- 104. Gonzalez F. The molecular biology of cytochrome P450s. Pharmacol Rev 1988;40:243–88 [PubMed] [Google Scholar]
- 105. Bondoc FY, Bao Z, Hu WY. et al. Acetone catabolism by cytochrome P450 2E1: studies with CYP2E1-null mice. Biochem Pharmacol 1999;58:461–3 [DOI] [PubMed] [Google Scholar]
- 106. Sohrabi M, Zhang L, Zhang K. et al. Volatile Organic Compounds as Novel Markers for the Detection of Bacterial Infections. Clin Microbial 2014;3:151 [Google Scholar]
- 107. Lagg A, Taucher J, Hansel A. et al. Applications of proton transfer reactions to gas analysis. Int J Mass Spectr Ion Proc 1994;134:55–66 [Google Scholar]
- 108. Smith D, Wang T, Spanel P.. On-line, simultaneous quantification of ethanol, some metabolites and water vapour in breath following the ingestion of alcohol. Physiol Meas 2002;23:477. [DOI] [PubMed] [Google Scholar]
- 109. Edwards JE, Rose RL, Hodgson E.. The metabolism of nonane, a JP-8 jet fuel component, by human liver microsomes, P450 isoforms and alcohol dehydrogenase and inhibition of human P450 isoforms by JP-8. Chem Biol Interact 2005;151:203–11 [DOI] [PubMed] [Google Scholar]
- 110. Leung T, Rajendran R, Singh S. et al. Cytochrome P450 2E1 (CYP2E1) regulates the response to oxidative stress and migration of breast cancer cells. Breast Cancer Res 2013;15:R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Thomas RD, Green MR, Wilson C. et al. Cytochrome P450 expression and metabolic activation of cooked food mutagen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in MCF10A breast epithelial cells. Chem Biol Interact 2006;160:204–16 [DOI] [PubMed] [Google Scholar]
- 112. Walker V, Mills GA.. Urine 4-heptanone: a β-oxidation product of 2-ethylhexanoic acid from plasticisers. Clin Chim Acta 2001;306:51–61 [DOI] [PubMed] [Google Scholar]
- 113. Kneepkens CM, Ferreira C, Lepage G. et al. The hydrocarbon breath test in the study of lipid peroxidation: principles and practice, clinical and investigative medicine. Med Clin Exp 1992;15:163–86 [PubMed] [Google Scholar]
- 114. Phillips M, Cataneo RN, Saunders C. et al. Volatile biomarkers in the breath of women with breast cancer. J Breath Res 2010;4:026003. [DOI] [PubMed] [Google Scholar]
- 115. Handa H, Usuba A, Maddula S. et al. Exhaled breath analysis for lung cancer detection using ion mobility spectrometry. PLoS One 2014;9:e114555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Schallschmidt K, Becker R, Jung C. et al. Comparison of volatile organic compounds from lung cancer patients and healthy controls—Challenges and limitations of an observational study. J Breath Res 2016;10:046007. [DOI] [PubMed] [Google Scholar]
- 117. Speiser D, Schneider A, Staeck O. et al. Volatile organic compounds (VOCs) in exhaled breath of patients with breast cancer in a clinical setting. Ginekologia polska 2012;83:730–36 [PubMed] [Google Scholar]
- 118. Phillips M, Cataneo RN, Greenberg J. et al. Effect of oxygen on breath markers of oxidative stress. Eur Respir J 2003;21:48. [DOI] [PubMed] [Google Scholar]
- 119. Frank Kneepkens CM, Lepage G, Roy CC.. The potential of the hydrocarbon breath test as a measure of lipid peroxidation. Free Radic Biol Med 1994;17:127–60 [DOI] [PubMed] [Google Scholar]
- 120. Cailleux A, Cogny M, Allain P.. Blood isoprene concentrations in humans and in some animal species. Biochem Med Metab Biol 1992;47:157–60 [DOI] [PubMed] [Google Scholar]
- 121. Chen Y, Zhang Y, Pan F. et al. Breath analysis based on surface-enhanced Raman scattering sensors distinguishes early and advanced gastric cancer patients from healthy persons. ACS Nano 2016;10:8169–79 [DOI] [PubMed] [Google Scholar]
- 122. Wehinger A, Schmid A, Mechtcheriakov S. et al. Lung cancer detection by proton transfer reaction mass-spectrometric analysis of human breath gas. Int J Mass Spectrom 2007;265:49–59 [Google Scholar]
- 123. King J, Kupferthaler A, Frauscher B. et al. Measurement of endogenous acetone and isoprene in exhaled breath during sleep. Physiol Meas 2012;33:413–28 [DOI] [PubMed] [Google Scholar]
- 124. King J, Kupferthaler A, Unterkofler K. et al. Isoprene and acetone concentration profiles during exercise on an ergometer. J Breath Res 2009;3:027006. [DOI] [PubMed] [Google Scholar]
- 125. King J, Mochalski P, Kupferthaler A. et al. Dynamic profiles of volatile organic compounds in exhaled breath as determined by a coupled PTR-MS/GC-MS study. Physiol Meas 2010;31:1169–84 [DOI] [PubMed] [Google Scholar]
- 126. King J, Koc H, Unterkofler K. et al. Physiological modeling of isoprene dynamics in exhaled breath. J Theoretic Biol 2010;267:626–37 [DOI] [PubMed] [Google Scholar]
- 127. Koc H, King J, Teschl G. et al. The role of mathematical modeling in VOC analysis using isoprene as a prototypic example. J Breath Res 2011;5:037102. [DOI] [PubMed] [Google Scholar]
- 128. Ulanowska A, Kowalkowski T, Trawińska E. et al. The application of statistical methods using VOCs to identify patients with lung cancer. J Breath Res 2011;5:046008. [DOI] [PubMed] [Google Scholar]
- 129. Matejcic M, Gunter MJ, Ferrari P.. Alcohol metabolism and oesophageal cancer: a systematic review of the evidence. Carcinogenesis 2017;38:859–72 [DOI] [PubMed] [Google Scholar]
- 130. Patel VB. Molecular Aspects of Alcohol and Nutrition: A Volume in the Molecular Nutrition Series . Cambridge, MA: Academic Press, 2015 [Google Scholar]
- 131. Varela-Rey M, Woodhoo A, Martinez-Chantar ML. et al. Alcohol, DNA methylation, and cancer. Alcohol Res Curr Rev 2013;35:25. [DOI] [PMC free article] [PubMed] [Google Scholar]