Abstract
Growing interest in blood-borne microRNAs (miRNAs) as biomarkers has led to the introduction of a number of commercial kits for isolating small RNAs from plasma/serum. We sought to compare the efficacy of six such kits in isolating miRNAs from either whole plasma or a plasma-derived ultracentrifugation (UC) fraction from 2 healthy volunteers with some of the results being validated in 10 additional subjects. To assess the overall yield and concentration of isolated small RNAs, we measured the levels of one spiked-in and four endogenous miRNAs by quantitative reverse transcription and polymerase chain reaction (qRT-PCR). We also tested the performance of the Agilent Bioanalyzer small RNA assay with these RNA samples. Additionally, we tested the effects of hemolysis on measured miRNA levels in whole plasma and in the UC fraction. Both the efficiency of RNA isolation and the relative levels of specific miRNAs in different samples varied considerably between the tested extraction methods. Of all kits tested, the QIAGEN miRNeasy kits (Mini and Serum/Plasma kits) and the Macherey-Nagel NucleoSpin kit produced the highest RNA yields. The QIAGEN Exo kit produced lesser yields than what could be extracted from the UC fraction using the QIAGEN miRNeasy kits and the Macherey-Nagel NucleoSpin kit. Bioanalyzer results showed an average correlation of R2 = 0.8 with endogenous miRNA qRT-PCR results, for sample concentrations >40 pg/µl. The levels of the endogenous miRNAs measured in the two volunteer samples were compared with those in a larger group of subjects (n = 10) and found to be typical. Our comparison favors the use of the QIAGEN Serum/Plasma kit and the Macherey-Nagel NucleoSpin kit for plasma miRNA applications. Furthermore, extraction of miRNAs from the UC fraction results in higher yield than extraction from whole plasma.
Keywords: Biofluid, biomarkers, small RNAs, nucleic acids, purification, optimization, hemolysis
Introduction
Profiling microRNAs (miRNAs) and other small RNAs in biofluids, e.g., plasma/serum, has been attracting wide interest due to their potential as minimally invasive biomarkers of various diseases (e.g., [1–8]). These RNAs are shielded from degradation by several mechanisms, such as encapsulation in extracellular vesicles (EVs), among them exosomes [9–11], and binding to ribonucleoprotein or lipoprotein complexes [12,13]. Furthermore, circulating RNAs may serve as a novel mode of signaling between different tissues and cell types [10, 14–17]. However, circulating RNAs are present at very low levels compared to those in tissues or cells, and their accurate detection and quantification is challenging due to several compounding pre-analytical variables, such as sample hemolysis, platelet content, storage, and—notably—RNA isolation method [18–23]. Several commercial small RNA isolation kits, some of them biofluid specific, have been introduced in recent years, competing in efficacy, affordability, and ease of use. Here, we sought to compare the efficacy of six such kits (three from QIAGEN, one from Norgen Biotek, one from Zymo Research, and one from Macherey-Nagel) in isolating small RNAs from either whole plasma or a plasma-derived ultracentrifugation (UC) fraction from two healthy volunteers, with some of the results being substantiated in additional 10 subjects. Furthermore, we sought to quantify the impact of visible hemolysis on the miRNA content in whole plasma as well as in the UC fraction.
Materials and methods
Blood was collected from three groups of subjects. The workflow for the first two groups (kit comparison and miRNA level validation) is outlined in Fig. 1A. The workflow for the third group (the hemolysis test) is shown in Fig. 1B.
Figure 1.
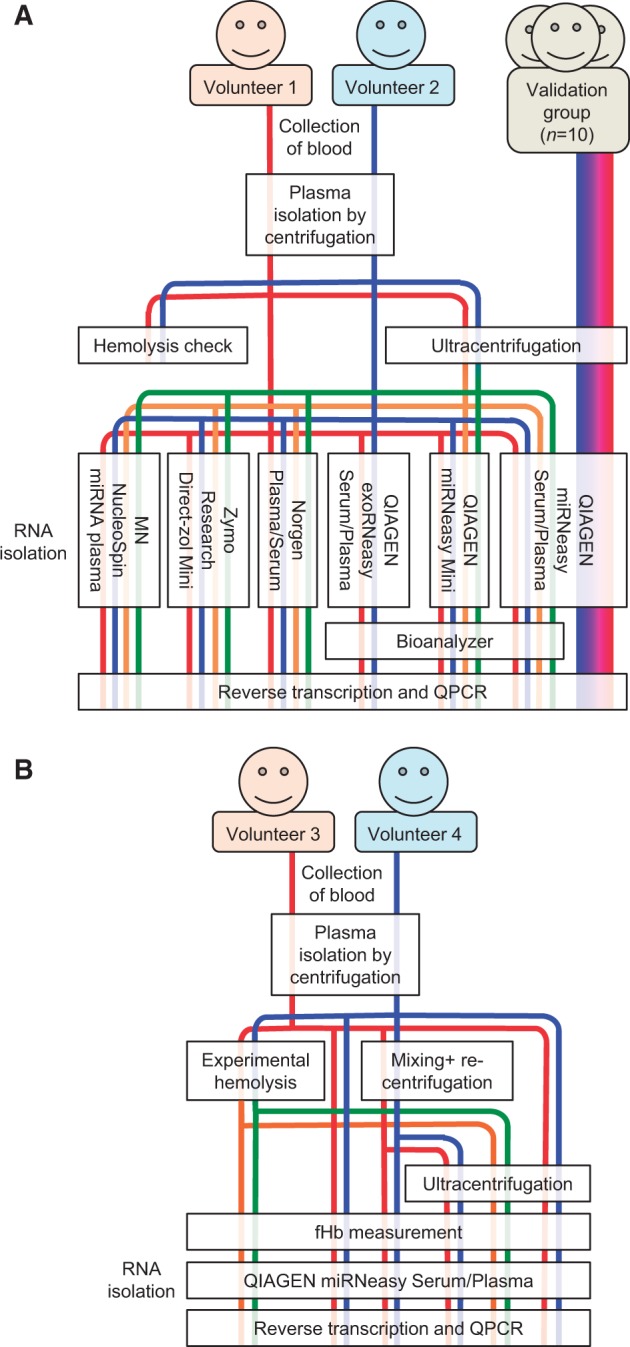
Graphic summary of workflows. (A) The workflow for the kit comparison group (n = 2) and miRNA level validation group (n = 10). (B) The workflow for the hemolysis test group (n = 2).
In the first (kit comparison) group, blood was collected from a cubital vein into K2EDTA BD Vacutainer tubes from two healthy Caucasian male volunteers (aged 37 and 60 years) at rest following an overnight fast. Plasma was immediately isolated by centrifugation at 2500 × g at 4°C for 10 min and stored in aliquots at −80°C. These samples were used to generate the data shown in Figs. 2–4B.
Figure 2.
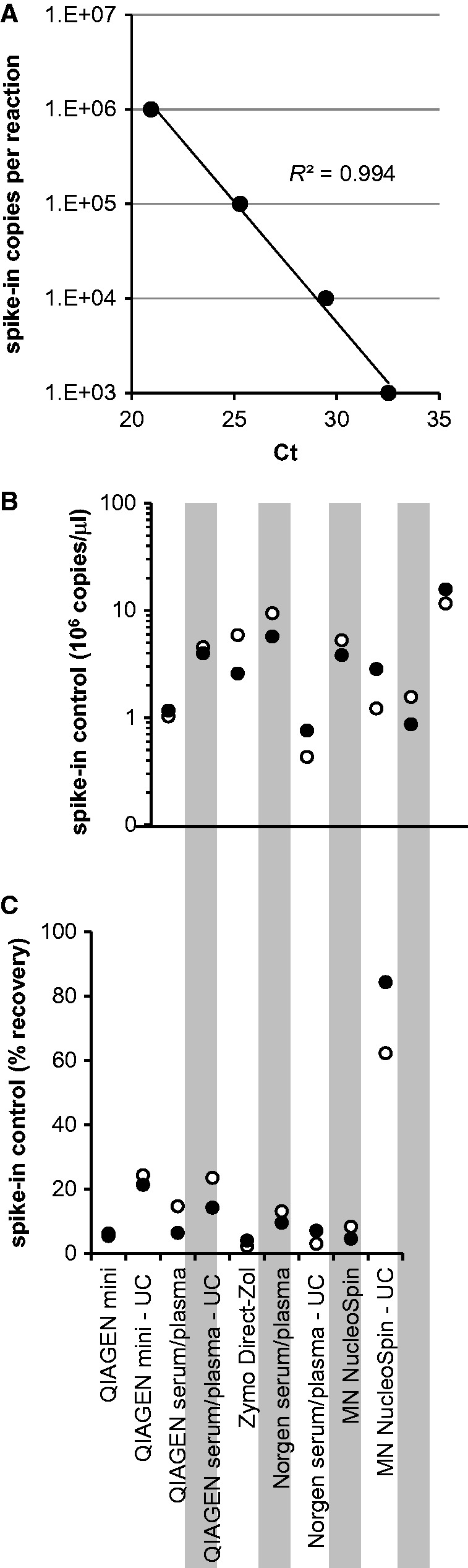
qRT-PCR for one spike-in control (cel-miR-39) in plasma samples from two donors. (A) Standard curve for spike-in control based on four dilutions spanning a 1000-fold difference in input copy number. (B) Concentration of spike-in control, in million copies per microliters. Note log scale. Both QIAGEN Mini and QIAGEN Serum/Plasma are miRNeasy kits. The QIAGEN Exo kit was not used in this assay. Black and white circles mark samples from two subjects, respectively. UC fractions processed with Zymo Direct-Zol yielded no quantitative results. (C) Overall recovery of spike-in control as percentage of input (equal to 5.6 × 108 copies/sample), in samples as in (B), accounting for elution volume. Black and white circles mark samples from two subjects, respectively.
Figure 3.
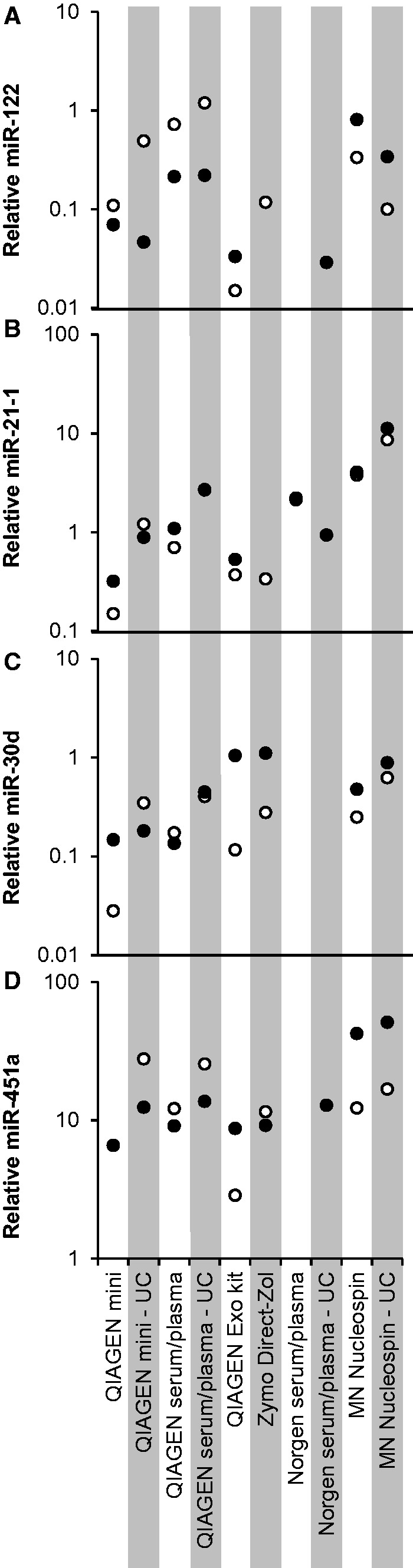
qRT-PCR for four endogenous miRNAs in plasma samples from two donors. (A–D) Relative concentration of four endogenous miRNAs: miR-122 (A), miR-21-1 (B), miR-30d (C), miR-451a (D). Levels are relative to the average of Ct values from all samples obtained with the QIAGEN miRNeasy Serum/Plasma kit plotted on a log scale. Both QIAGEN Mini and QIAGEN Serum/Plasma are miRNeasy kits. Black and white circles mark samples from two subjects, respectively. Where one or more circles are absent, one or both samples yielded no quantitative results. However, the miR-21-1 values from the two subjects in the QIAGEN miRNeasy Serum/Plasma UC and MN Nucleospin columns are close enough to overlap in the chart.
Figure 4.
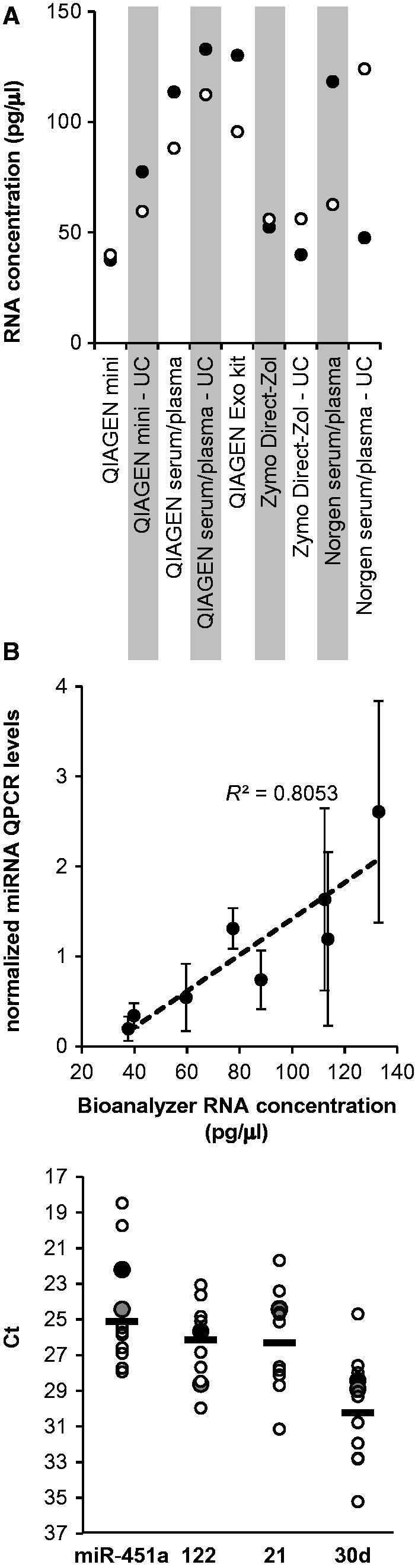
Agilent Bioanalyzer assessment of small RNA concentration and yield from plasma samples. (A) Small RNA concentrations obtained with the kits indicated, quantified using Bioanalyzer hardware and software. Both QIAGEN Mini and QIAGEN Serum/Plasma are miRNeasy kits. Black and white circles mark samples from two subjects, respectively. (B) Correlation between average RNA measurements as in A and average qRT-PCR results (as in Fig. 3) for three endogenous miRNAs (miR-122, miR-21-1, and miR-30d), in samples processed with the following QIAGEN miRNeasy kits: Mini and Serum/Plasma. miR-451a was excluded due to high variance between subjects. Mean ± SD from three miRNAs. Trend line (dashed)—least squares linear. (C) Comparison of the expression levels (indicated by Ct values) of four endogenous miRNAs (miR-122, miR-21-1, miR-30d, and miR-451a) between the UC fractions of the same two volunteer samples used elsewhere in this study, and an independent group of 10 additional subject samples (characterized in “Methods” section). All samples were processed using UC and the QIAGEN Serum/Plasma miRNeasy kit. Values from volunteers 1 and 2 are marked by larger black and gray circles, respectively. White circles mark values from the 10 additional subjects. Horizontal black bars indicate the 10-subject group means.
In the second (miRNA-level validation) group, stored (−80°C) ethylenediaminetetraacetic acid (EDTA) plasma from 10 subjects who participated in the FINE project was used in order to further validate some of the data obtained from the first group of subjects described above. The FINE project was a randomized control trial mainly addressing the interindividual variation in insulin sensitivity in sedentary male and the metabolic effects of 3 months of lifestyle intervention by increased physical activity. The study population consisted of healthy, sedentary Caucasian males, 20–40 years of age, body mass index (BMI) 25–30 kg/m2. Further details of the FINE project have been previously published [24, 25]. The plasma samples used in the current study were obtained after an overnight fast before start of the intervention program. Data from these samples are shown in Fig. 4C.
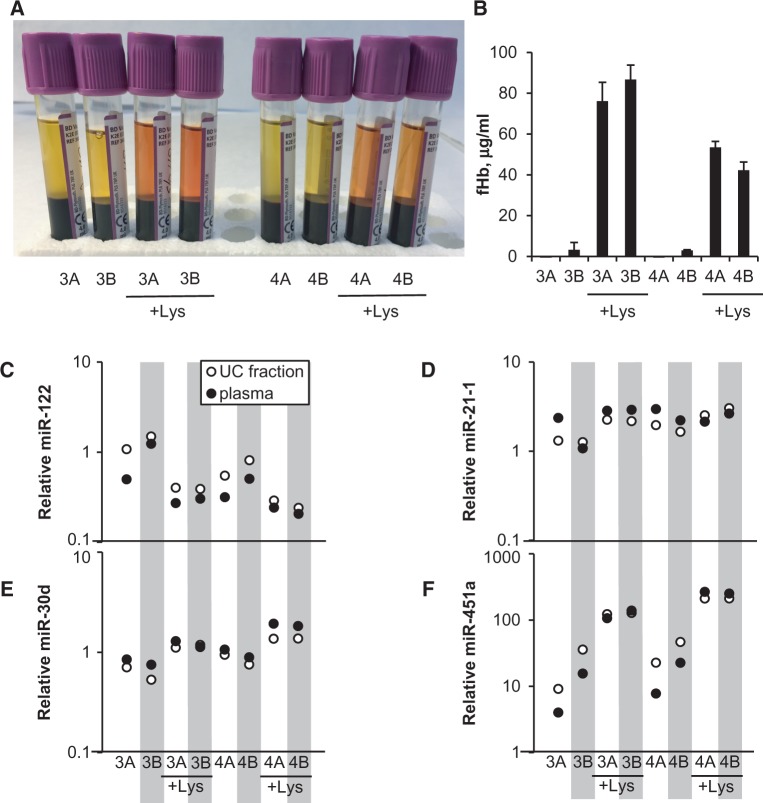
In the third (hemolysis test) group, blood was collected from a cubital vein into K2EDTA BD Vacutainer tubes in quadruple replicates from each of two healthy Caucasian volunteers (female and male, aged 55 and 61 years, respectively) at rest following an overnight fast. All four tubes from each donor were immediately centrifuged at 2500 × g at 4°C for 10 min. Plasma from the first tube (Control) in each subject set was stored in aliquots at −80°C. The second tube (Control recentrifuged) in both sets was inverted 10 times (to mix plasma and packed cells), recentrifuged at 2500 × g for 15 min and plasma stored in aliquots at −80°C. Tubes 3 and 4 in both sets were treated identically (duplicates). Following the first centrifugation, the packed erythrocytes at the bottom of the tube were partly hemolyzed by being drawn through a 23G 30-mm-long needle using a 2-ml syringe and then injected back into the Vacutainer tube, which was inverted 10 times to mix plasma and partly hemolyzed cells, recentrifuged at 2500 × g for 15 min and plasma stored in aliquots at −80°C. Data from these samples are shown in Fig. 5.
Figure 5.
Assessment of the effects of hemolysis on miRNA levels in plasma and UC fraction. (A) Photograph of the sample tubes after initial centrifugation to separate cells from plasma. The numbers 3 and 4 refer to two volunteers who contributed the samples. For non-hemolyzed samples, the suffixes A and B refer to the two methods of plasma preparation mentioned in “Methods” section (without and with mixing and recentrifugation, respectively). For hemolyzed samples (marked + Lys), the suffixes A and B indicate duplicates. (B) fHb estimate in plasma samples as shown in (A), using Harboe’s spectroscopic method (see “Methods” section). Mean + SD from duplicate measurements. (C–F) Relative concentration of four endogenous miRNAs: miR-122 (C), miR-21-1 (D), miR-30d (E), miR-451a (F) in RNA isolated from plasma samples as in (A) and their respective UC fractions. Levels are relative to the average of Ct values for all four miRNAs for the given sample, plotted on a log scale. Black and white circles mark whole plasma or UC fraction, respectively.
To obtain the UC fraction, 220 µl of plasma were diluted 1:20 with ice-cold PBS containing 1 mM EDTA in 5-ml Eppendorf tubes and centrifuged at 15 557× g for 1 min at 4°C to remove any larger particles. Four milliliters (corresponding to 200 µl diluted plasma) were then transferred to an UC tube and centrifuged at 200 000× g for 16–17 h at 2°C in a Beckman 70.1 Ti rotor. The supernatant was discarded, the tube immediately rinsed with 4 ml of ice-cold phosphate buffered saline (PBS) containing 1 mM EDTA and the tube wall dried with a couple of cotton swaps. The pellet (UC fraction) was resuspended in 200 µl of RNase-free water and stored at −80°C.
To assess the degree of hemolysis in plasma or UC samples, free hemoglobin (fHb) concentration was estimated using the Harboe method [24, 25]. Briefly, 5 µl plasma were diluted 1:11 in PBS, and absorption at the wavelengths of 380, 415, and 450 nm was measured in 96-well plate format in a Tecan Infinite M200 Pro spectrophotometer. Free hemoglobin concentration (fCHb, in milligrams per milliliters) was calculated using the formula CHb = 0.836[2A415 − (A380 + A450)] [25] The values were presented in micrograms per milliliters.
Total RNA isolation used the following kits, with the following elution volumes of nuclease-free water (according to manufacturers’ recommendations):
QIAGEN miRNeasy Mini Kit (Cat. 217004), 30 µl
QIAGEN miRNeasy Serum/Plasma kit (Cat. 217184), 14 µl
QIAGEN exoRNeasy Serum/Plasma Starter Kit (Cat. 77023), 14 µl
Norgen Biotek Plasma/Serum RNA Purification Mini Kit (Cat. 55000), 30 µl
Zymo Research Direct-zol™ RNA MiniPrep (Cat. R2050), 14 µl
Macherey-Nagel NucleoSpin™ miRNA Plasma kit (Cat. 740981.10), 30 µl
Each kit was used according to the manufacturer’s instructions, on both whole plasma and plasma-derived UC fractions from both volunteers, except the QIAGEN exoRNeasy kit which was used only on whole plasma samples (as it integrates EV isolation). Samples were lyzed in QIAzol lysis reagent (QIAGEN Cat. 79306) (for kits 1–3 and 5) or the respective lysis buffer included in the kits (for kits 4 and 6) and spiked with 5.6 × 108 copies of miRNeasy Serum/Plasma Spike-In Control (C. elegans miR-39 mimic, QIAGEN Cat. 219610) before proceeding with the extraction. After elution (using the volumes indicated above), samples were stored at −80°C.
Electrophoretic analysis was performed at the Genomics Center of the Silberman Institute of Life Sciences, Hebrew University, Jerusalem, Israel, using the Agilent Small RNA kit on an Agilent 2100 Bioanalyzer.
Reverse transcription (RT) and quantitative PCR (qPCR) for the Serum/Plasma Spike-In Control were performed using a QIAGEN miScript PCR Starter Kit (Cat. 218193) and the PCR primers provided with the spike-in control. RT, primer design, and qPCR for endogenous miRNAs were performed using SYBR Green chemistry and DNA primers as previously described [26, 27]; primers used are listed in Table 1. qPCR was performed in quadruplicates on an Applied Biosystems ABI-7900HT Sequence Detection System equipped with a 384-well block, using iTaq SYBR Green (BioRad). This qRT-PCR method is highly accurate and reproducible [26, 27], and was chosen over TaqMan chemistry due to lower cost and higher convenience. Results were analyzed with SDS 2.3 (Applied Biosystems) and Microsoft Excel. All qRT-PCR and fHb measurements are included in the Supplementary Data.
Table 1.
QPCR primers used
| Gene | Forward primer | Reverse Primer |
|---|---|---|
| miR-30d | AGTGTAAACATCCCCGACT | TCCAGTTTTTTTTTTTTTTTCTTCCA |
| miR-21-1 | GCAGTAGCTTATCAGACTGATG | GGTCCAGTTTTTTTTTTTTTTTCAAC |
| miR-122 | AGTGGAGTGTGACAATGGT | CCAGTTTTTTTTTTTTTTTCAAACACC |
| miR-451a | CAGAAACCGTTACCATTACTGA | GGTCCAGTTTTTTTTTTTTTTTAACTC |
Results
QIAGEN and Macherey-Nagel kits provide the best combination of concentration and quality of small RNAs
To assess the overall yield and concentration of isolated small RNAs, the concentration of a spike-in control (cel-miR-39) as well as four endogenous miRNAs present at different levels in plasma (miR-451a, miR-21-1, miR-30d, and miR-122) was quantified by qRT-PCR. The spike-in control measurements enabled absolute quantification of the oligo concentration in the isolates, and the percentage of recovery, based on a standard curve obtained from serial dilutions of the spike-in control oligo (Fig. 2A). Based on spike-in control amplification, the best RNA concentration and overall recovery from whole plasma was obtained by the QIAGEN miRNeasy Serum/Plasma kit and the Norgen kit (Fig. 2B–C). The UC fraction produced the most RNA (based on spike-in quantification) when processed with the Macherey-Nagel kit, followed by the QIAGEN Serum/Plasma kit.
The endogenous miRNA measurements showed the highest levels in the samples processed with the Macherey-Nagel kit and the QIAGEN miRNeasy Serum/Plasma kit (Fig. 3). The UC fraction sample, when processed with the Zymo Direct-Zol kit, failed to produce reliable quantification of the spike-in control and of any of the endogenous miRNAs; some of the endogenous miRNAs also failed to produce quantitative results in the whole plasma samples when processed with this kit. The samples processed by the Norgen kit, although showing adequate concentration and recovery of the spike-in control (Fig. 2B–C), failed to produce robust quantitative results for the endogenous miRNAs (Fig. 3), pointing toward a possible problem with RNA purity.
Higher recovery of miRNA from the UC fraction than from whole plasma
Although blood-borne small RNAs are known not to be exclusively carried by EVs, higher levels of all four endogenous miRNAs were measured in the UC fraction than in whole plasma, when using both QIAGEN miRNeasy kits and the Macherey-Nagel NucleoSpin kit (Fig. 3), suggesting a more efficient RNA isolation from these samples. The similarly higher concentration and recovery of the spike-in control in the UC samples (Fig. 2B– C) confirmed that more RNA is recovered from the UC fraction than from whole plasma, probably due to plasma components that interfere with the binding or elution of RNA in the column matrix.
QIAGEN Exo kit yields less RNA than can be recovered by UC
One of our aims was to evaluate the efficacy of the QIAGEN Exo kit in isolating small RNAs from EVs, as a substitute for the longer procedure of EV isolation by UC. Although all endogenous miRNAs tested could be quantified, their levels were generally lower when using the Exo kit, when compared with the levels obtained by extracting RNA with the QIAGEN Serum/Plasma kit from the UC fraction (Fig. 3). These results may be explained by non-EV carriers (such as high molecular weight lipoprotein complexes) being retained by UC but not the Exo kit, and are consistent with previous findings that only a fraction of plasma total miRNA is associated with EVs[11]. Another possible explanation is lower EV recovery by the Exo kit than by UC.
Electrophoretic separation analysis shows an average correlation of R2 = 0.8 with endogenous miRNA qRT-PCR results >40 pg/µl
The Agilent Bioanalyzer is a commonly used tool for RNA quantification and quality control and utilizes the principle of electrophoretic separation. The Bioanalyzer small RNA assay is designed to quantify RNA samples at concentrations exceeding 50 pg/µl, which limits the use of this method in quantifying biofluid-derived RNA, as previously reported [23]. To evaluate the performance of the Bioanalyzer with the rather dilute small RNAs obtained from the plasma samples using the different kits, several of the RNA samples were subjected to Bioanalyzer analysis, which returned concentrations in the order of 30–150 pg/µl (Fig. 4A). The Bioanalyzer concentration values obtained from samples processed with the three different QIAGEN miRNeasy kits were then compared with qRT-PCR results for three endogenous miRNAs (miR-122, miR-21-1, and miR-30d), in the same samples (a total of eight RNA samples). miR-451a was excluded due to high variance between subjects. qRT-PCR results, normalized to the different average expression levels for each miRNA, showed an average correlation of R2 = 0.8 to Bioanalyzer results at concentrations exceeding 40 pg/µl (Fig. 4B), suggesting that the Bioanalyzer is useful at least as a rough estimate of the amount of RNA extracted from rather small plasma volumes (200 µl).
The two volunteer samples show typical expression levels for the endogenous plasma miRNAs measured
As the different extraction methods were tested on identical samples, individual variability in miRNA levels could not cause any bias in method comparison. However, in order to ascertain that the individual miRNA levels in the samples from the two volunteers are indeed within the range commonly observed for these miRNAs, the levels of the same four endogenous plasma miRNAs (miR-451a, miR-122, miR-21-1, and miR-30d) were also measured by qRT-PCR in the UC fractions of plasma from a group of 10 additional volunteers (see “Methods” section). The resulting Ct values were compared to those obtained from the two volunteer samples used elsewhere in this study. As shown in Fig. 4C, the levels of all four endogenous miRNAs in the two volunteer samples were within the ranges of values of the larger group, and did not deviate from the group means by more than three cycles, which was typical of the overall variation within the larger group (SD values for miR-451a, miR-122, miR-21-1, and miR-30d were 3.27, 2.15, 2.85, and 3.09, respectively).
miR-451a is highly prevalent in non-hemolyzed plasma and its UC fraction
miR-451a is abundant in red blood cells (RBCs) [28, 29], which has led to its use as a marker of hemolysis in plasma-derived RNA samples. To rule out hemolysis as a source of miR-451a in our samples, fCHb in input plasma samples was estimated using the Harboe method [30, 31]. The resulting fHb estimates were 5 µg/ml for Volunteer 1 and <1 µg/ml for Volunteer 2. Thus, our data show that this miRNA is highly prevalent in the UC fraction of plasma as well as whole plasma prepared under well-controlled conditions with practically undetectable hemolysis. Although miR-451a levels were lowest in the samples processed with the Exo kit (Fig. 3D), they were still high compared to other miRNAs. These results suggest that miR-451a is not merely an indicator of hemolysis or RBC contamination, but rather an abundant miRNA in several compartments of human blood. RNA-seq analysis (data not shown) also indicates that miR-451a is consistently one of the three most abundant miRNAs in plasma, and specifically in the UC fraction.
Hemolysis dramatically affects the levels of miR-451a but not other tested miRNAs
To test the effect of hemolysis on miRNA levels, a new set of plasma samples was collected, with some of them undergoing controlled hemolysis (see “Methods” section and Fig. 1B). The hemolyzed samples had a clearly visible reddish tint (Fig. 5A). These samples were processed into RNA using the QIAGEN miRNeasy Serum/Plasma kit and the levels of the same four endogenous plasma miRNAs (miR-451a, miR-122, miR-21-1, and miR-30d) were measured by qRT-PCR. In parallel, aliquots of the whole plasma were used for fHb concentration estimates using the Harboe method. The hemolyzed samples produced fHb values in the range of 40–100 µg/ml, while the non-hemolyzed samples contained <5 µg/ml fHb (Fig. 5B). Hemolysis had a minor effect on the measured levels of three of the endogenous miRNAs, with miR-122 showing somewhat lower levels (Fig. 5C), miR-21 appearing practically unaffected (Fig. 5D), and miR-30d showing slightly higher levels in the hemolyzed samples (Fig. 5E) compared to non-hemolyzed controls. The exception was miR-451a, which was elevated approximately 10-fold in the hemolyzed samples compared to controls (Fig. 5F; note the logarithmic scale). This result is in line with the known high concentration of miR-451a in RBCs.
UC does not negate the effects of hemolysis on miRNA levels
We wished to test whether UC can separate the heavier miRNA carriers (such as vesicles) from the released contents of hemolyzed RBCs, and thus negate the effects of hemolysis on endogenous miRNA levels. To address this question, UC fractions from the same sample set as described above were processed into RNA using the QIAGEN miRNeasy Serum/Plasma kit and the levels of the same four endogenous plasma miRNAs (miR-451a, miR-122, miR-21-1, and miR-30d) were measured by qRT-PCR. For all four miRNAs, the measured levels were similar between the UC fractions and their respective whole plasma samples, including the hemolyzed ones (Fig. 5C–F). In particular, UC failed to decrease the high levels of miR-451a detected in the hemolyzed samples (Fig. 5F), indicating that most of the miR-451a molecules released from hemolyzed RBCs are bound to complexes, which are massive enough to be pelleted by UC, at least in our protocol.
Discussion
Growing interest in biofluid—contained small noncoding RNAs underscores the need for optimized isolation and quantification protocols. This need has led to the rapid development of commercial reagents, as well as to widespread “trial and error” testing for appropriate controls and workflows. Our present comparison suggests that the QIAGEN miRNeasy Serum/Plasma kit and the Macherey-Nagel NucleoSpin™ miRNA Plasma kit produce the best results in terms of miRNA yields and purity, among all kits tested, in agreement with others [32]. Additionally, our results indicate that UC can improve miRNA yields with these kits. This finding suggests that whole plasma may contain substances that somewhat decrease the efficacy of RNA isolation, and also that the bulk of plasma miRNAs is contained in vesicles or bound to complexes that are massive enough to be pelleted by UC. Supporting this notion, no robust miRNA expression was detected in plasma depleted from its UC fraction (data not shown). However, UC (at least in our protocol) did not remove miRNA-containing material that was released from lyzed RBCs in hemolyzed samples.
miR-451a, which is among the most prevalent miRNAs in plasma, was previously suggested as both a hemolysis marker and a normalizer for qRT-PCR measurements in non-hemolyzed samples [28]. Although our results confirm its high levels in non-hemolyzed samples, its even higher levels in RBCs make it extremely sensitive to even a very minor degree of hemolysis, perhaps even such as to be undetectable by spectroscopy. This is in agreement with previous findings [29] and underscores the need for attention to individual samples and thorough quality control in the choice of normalizers. Elevated miR-451a levels due to hemolysis, when this miRNA is used as a normalizer, will lead to a perceived decrease in the levels of most other miRNAs, and can thus be identified at the stage of data analysis. In general, our and others’ experience favors the use of global normalization, or at least using the average of multiple normalizers, for most qRT-PCR experiments.
Our current comparison also exemplifies the different sources of variation that gets introduced into the final result of small RNA quantification, whether by qRT-PCR or other methods (such as RNA-seq). Some of these variation sources, such as the difference in efficiency between isolation kits, are easily controlled by the experimental design; others have to be accounted for at the stage of data analysis. These include differences in plasma processing and preparation [33, 34], random differences in RNA purification efficiency between samples when using the same kit and protocol; differences between RT reactions and qPCR runs performed on the same sample at different times, etc. Furthermore, the plasma levels of many miRNAs differ between subjects (exemplified in Fig. 4C), and these differences are often dramatic when compared to the variation of expression in tissues or cells. Hence, study-specific optimization of normalization controls is often necessary.
Ethics statement
The study protocol was submitted to the Research Ethics Committees of the Capital Region of Denmark (H-1-2014-FSP-008) but did not require a formal approval due to the focus of the study being methodological development rather than a health science research project. Plasma samples used for generating the data presented in Fig. 4C were obtained from the FINE project, a study which was approved by the Research Ethics Committees of the Capital Region of Denmark (H-4-2009-089) and where the participants gave fully informed oral and written consent.
Supplementary Material
Acknowledgements
We thank Gerda Hau and Hila Yehuda for skilled technical assistance and we also thank the FINE team for collecting the blood samples used for generating the data presented in Fig. 4C.
Funding
A.M. was funded by the Gesher award from the Israel Cancer Research Fund (ICRF) and the Ministry of Science and Technology, Israel; by a Diabetes Research Grant from D-Cure and the Ministry of Health, Israel; by the European Union’s FP7-REGPOT-2012-2013-1, Agreement No 316157 (“CEREHA”); and by a Visiting Scientist Grant from the Danish Diabetes Academy (supported by the Novo Nordisk Foundation). T.P.’s work was carried out as part of the research program of the UNIK: Food, Fitness & Pharma for Health and Disease (see http://foodfitnesspharma.ku.dk). The UNIK project was supported by the Danish Ministry of Science, Technology and Innovation.
Author contributions statement
A.M. performed RNA isolation, qRT-PCR, and data analysis. T.P. collected and processed plasma samples. A.M. and T.P. both designed the study and wrote the manuscript.
Conflict of interest statement. None declared.
Supplementary data
Supplementary data is available at Biology Methods and Protocols online.
References
- 1. Gilad S, Meiri E, Yogev Y, et al. Serum microRNAs are promising novel biomarkers. PLoS One [Internet]. 2008;3:e3148 .http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2519789/ (15 October 2016, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pescador N, Pérez-Barba M, Ibarra JM, et al. Serum circulating microRNA profiling for identification of potential type 2 diabetes and obesity biomarkers. PloS One 2013;8:e77251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rawal S, Manning P, Katare R. Cardiovascular microRNAs: as modulators and diagnostic biomarkers of diabetic heart disease. Cardiovasc Diabetol 2014;13:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zampetaki A, Willeit P, Drozdov I, et al. Profiling of circulating microRNAs: from single biomarkers to re-wired networks. Cardiovasc Res 2012;93:555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao Z, Moley KH, Gronowski AM. Diagnostic potential for miRNAs as biomarkers for pregnancy-specific diseases. Clin Biochem 2013;46:953–60. [DOI] [PubMed] [Google Scholar]
- 6. Chevillet JR, Lee I, Briggs HA, et al. Issues and prospects of microRNA-based biomarkers in blood and other body fluids. Molecules 2014;19:6080–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shifeng H, Danni W, Pu C, et al. Circulating liver-specific miR-122 as a novel potential biomarker for diagnosis of cholestatic liver injury. PLoS One [Internet]. 2013;8:e73133 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3785475/ (15 October 2016, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grasedieck S, Sorrentino A, Langer C, et al. Circulating microRNAs in hematological diseases: principles, challenges, and perspectives. Blood 2013;121:4977–84. [DOI] [PubMed] [Google Scholar]
- 9. Cheng L, Sharples RA, Scicluna BJ, et al. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles 2014;3 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3968297/ (15 October 2016, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kosaka N, Iguchi H, Yoshioka Y, et al. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 2010;285:17442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chevillet JR, Kang Q, Ruf IK, et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci 2014;111:14888–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vickers KC, Palmisano BT, Shoucri BM, et al. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 2011;13:423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wagner J, Riwanto M, Besler C, et al. Characterization of levels and cellular transfer of circulating lipoprotein-bound microRNAs. Arterioscler Thromb Vasc Biol 2013;33:1392–400. [DOI] [PubMed] [Google Scholar]
- 14. Hulsmans M, Holvoet P. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovasc Res 2013;100:7–18. [DOI] [PubMed] [Google Scholar]
- 15. Camussi G, Deregibus MC, Bruno S, et al. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int 2010;78:838–48. [DOI] [PubMed] [Google Scholar]
- 16. Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654–59. [DOI] [PubMed] [Google Scholar]
- 17. Nazarenko I, Rupp A-K, Altevogt P. Exosomes as a potential tool for a specific delivery of functional molecules. Methods Mol Biol 2013;1049:495–511. [DOI] [PubMed] [Google Scholar]
- 18. Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat Rev Genet 2012;13:358–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McAlexander MA, Phillips MJ, Witwer KW. Comparison of methods for miRNA extraction from plasma and quantitative recovery of RNA from cerebrospinal fluid. Front Genet 2013;4:83 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3655275/ (15 October 2016, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sourvinou IS, Markou A, Lianidou ES. Quantification of circulating miRNAs in plasma: effect of preanalytical and analytical parameters on their isolation and stability. J Mol Diagn 2013;15:827–34. [DOI] [PubMed] [Google Scholar]
- 21. Spornraft M, Kirchner B, Haase B, et al. Optimization of extraction of circulating RNAs from plasma–enabling small RNA sequencing. PloS One 2014;9:e107259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moret I, Sánchez-Izquierdo D, Iborra M, et al. Assessing an improved protocol for plasma microRNA extraction. PloS One 2013;8:e82753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duy J, Koehler JW, Honko AN, et al. Optimized microRNA purification from TRIzol-treated plasma. BMC Genomics 2015;16:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenkilde M, Auerbach P, Reichkendler MH, et al. Body fat loss and compensatory mechanisms in response to different doses of aerobic exercise—a randomized controlled trial in overweight sedentary males. Am J Physiol Regul Integr Comp Physiol 2012;303:R571–79. [DOI] [PubMed] [Google Scholar]
- 25. Reichkendler MH, Rosenkilde M, Auerbach PL, et al. Only minor additional metabolic health benefits of high as opposed to moderate dose physical exercise in young, moderately overweight men. Obesity 2014;22:1220–32. [DOI] [PubMed] [Google Scholar]
- 26. Balcells I, Cirera S, Busk PK. Specific and sensitive quantitative RT-PCR of miRNAs with DNA primers. BMC Biotechnol 2011;11:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Busk PK. A tool for design of primers for microRNA-specific quantitative RT-qPCR. BMC Bioinformatics 2014;15:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kirschner MB, Kao SC, Edelman JJ, et al. Haemolysis during sample preparation alters microRNA content of plasma. PloS One. 2011;6:e24145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pritchard CC, Kroh E, Wood B, et al. Blood cell origin of circulating microRNAs: a cautionary note for cancer biomarker studies. Cancer Prev Res Phila Pa 2012;5:492–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harboe M. A method for determination of hemoglobin in plasma by near-ultraviolet spectrophotometry. Scand J Clin Lab Invest 1959;11:66–70. [DOI] [PubMed] [Google Scholar]
- 31. Fairbanks VF, Ziesmer SC, O’Brien PC. Methods for measuring plasma hemoglobin in micromolar concentration compared. Clin Chem 1992;38:132–40. [PubMed] [Google Scholar]
- 32. Monleau M, Bonnel S, Gostan T, et al. Comparison of different extraction techniques to profile microRNAs from human sera and peripheral blood mononuclear cells. BMC Genomics 2014;15:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Page K, Guttery DS, Zahra N, et al. Influence of plasma processing on recovery and analysis of circulating nucleic acids. PLoS One 2013;8:e77963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. El-Khoury V, Pierson S, Kaoma T, et al. Assessing cellular and circulating miRNA recovery: the impact of the RNA isolation method and the quantity of input material. Sci Rep 2016;6:19529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.