Abstract
Nucleic acid detection and quantification using a labeled DNA probe is a very common molecular biology procedure. Here, we describe a new method, based on commonly used laboratory solutions, for nucleic acid hybridization and detection with digoxigenin-labeled DNA probes. The protocol described is faster, more sensitive and much cheaper than a standard protocol using commercial solutions. Comparison with a classical radioactive detection method shows that the latter exhibits less background and shows a greater linear response. Hence, the proposed protocol may be routinely performed for qualitative detection of nucleic acid, but when precise signal quantitation needs to be obtained, radioactive probe hybridization associated to phosphorimaging technology is more reliable.
Keywords: nucleic acid hybridization, nucleic acid quantification, digoxigenin-labeled probe
Introduction
Detection of nucleic acids (DNA or RNA) bound to an hybridization membrane, using a specific probe, has been a widely used method in molecular biology laboratories for more than four decades [1]. Over the years, several radioactive or non-radioactive methods were designed to label probes. Random priming of a DNA molecule using random oligonucleotides as short primers is a popular technique aiming at obtaining single-stranded radioactive DNA probes exhibiting a high specific activity [2]. Such probes can be hybridized on denatured DNA or RNA molecules bound to a nylon membrane, in a sodium-phosphate buffer (also called “Church buffer”) [3]. The use of phosphor screen technology to quantify the amount of radioactivity at specific locations on the hybridization membrane has allowed for almost three decades precise and reliable quantification of DNA or RNA amounts, with high sensitivity on a linear range. However, manipulation of radioactive phosphorus is tedious, restricted to authorized areas and must be performed behind a protective screen by properly trained scientists. In addition, radioactive products handling and shipping are more and more regulated and their price has been dramatically increasing for the last 10 years, to such a point that regular ordering of such products weighs significantly on small to medium size laboratories budget. Non-radioactive hybridization methods have been developed for many years as alternatives to radioactive approaches. Enzymatic incorporation of one of the four dNTP covalently linked to biotin or digoxigenin into single-stranded DNA probes were commonly used to that end. Comparisons of both methods reached the conclusion that they were equally sensitive [4] but that digoxigenin probes were slightly more specific [5]. Several kits are commercially available to label DNA with digoxigenin-dUTP but this step may be easily implemented with homemade laboratory solutions [6]. However, subsequent steps of DNA hybridization, washes, antibody incubation, and washes are usually tedious, time consuming and expensive when using commercially available solutions. Here, we describe a simple and cheap protocol for non-radioactive probes in nucleic acid detection and quantification. All reagents can be simply prepared from stock solutions available in any molecular biology laboratory. Sensitivity was found to be generally better than with a commonly performed commercial protocol and comparable to radioactive probes coupled to phosphorimaging technology.
Materials and methods
Dot blots
Total genomic DNA was extracted from Saccharomyces cerevisiae (strain BY4741) and quantified on gel. Two-fold serial dilutions of this genomic DNA were made in 0.4 M NaOH, incubated at room temperature for 10 min, then spotted on Hybond-XL nylon membranes (Amersham RPN 203S), before proceeding to hybridization.
Radioactive probe synthesis
The probe was labeled by random priming as follows. A 750-bp DNA probe corresponding to the 5′ end of the ARG2 gene was amplified using oligonucleotides AR1 (AGGAGAATATTCGCGCATGAA) and AR2 (AAGATATCTCATCTTTTTTAACGT). After gel purification, 50–60 ng of this PCR product were mixed with 150 pmoles poly-deoxynucleotides hexamers (pd(N)6, Takara) in 15 μl of 1× random priming buffer (500 mM Tris–HCl (pH 7), 100 mM MgSO4, 1 mM DTT). This mix was denatured at 95°C for 5 min before being put on ice. To this denatured DNA, 3 μl of α 32 P dATP (6000 Ci/mmol), 1 μl of the three remaining dNTP (10 mM each) and 10 units of Klenow polymerase fragment were added. The probe was incubated at 37°C for 1 h, then denatured 5 min at 95°C before being added to the hybridization buffer. Alternatively, it could be purified on ProbeQuant G50 micro sepharose columns (GE Healthcare) to allow probe quantification. Specific activities ranged from 6, 3×107 to 1, 4×109 cpm/μg DNA, depending on the probe (mean ± 99% confidence interval = 5, 6×108 ± 1, 9×108). The whole labeled probe was used in each hybridization (3 000 000 cpm on the average).
Non-radioactive probe synthesis
Non-radioactive probes were synthesized from the same purified PCR fragment covering 750 bp of the ARG2 gene 5′ end. Labeling was performed in 100 μl volume reactions containing 50–60 ng ARG2 PCR template, 1 M of each AR1 and AR2 primers, 10 μl PCR DIG labeling mix (Roche 11 585 550 910), 10 μl × PCR buffer and 5 units of Taq polymerase (Thermo Fischer). The PCR program used started by an initial denaturation step at 98°C for 2 min, followed by 35 cycles of: 98°C denaturation for 30 s, 50°C annealing for 30 s, 72°C extension for 1 min. A final 10 min extension step at 72°C was added at the end. A 5 μl aliquot was loaded on gel for quantification and approximately 250 ng of the probe was used for hybridization, after a 5 min denaturation step at 95°C.
Probe hybridization
Hybridization of radioactive or non-radioactive probes was performed overnight, at 65°C in a rotating tube (Hybaid hybridization oven, Thermo Scientific) in 250 mM sodium phosphate buffer (pH 7.4), 7% SDS, 1 mM EDTA. Two washes were performed in 20 mM sodium phosphate buffer (pH 7.4), 1% SDS, 1 mM EDTA. When using the commercial protocol with DIG-labeled probes, the membrane was hybridized and washed as recommended by the manufacturer (Roche DIG Easy Hyb 11 796 895 001, see Table 1). Membranes hybridized with radioactive probes were exposed 4 or 20 h on a phosphor screen before quantification. Membranes hybridized with non-radioactive probes were subsequently treated with an anti-DIG antibody (see below).
Table 1:
Comparison of non-radioactive hybridization methods
| Commercial protocol | Time (°C) | Homemade protocol | Time (°C) | |
|---|---|---|---|---|
| Probe hybridization | Tampon DIG Easy Hyb | Overnight (42°C) | 250 mM sodium phosphate buffer, 7% SDS, 1 mM EDTA | Overnight (65°C) |
| Hybridization washes | SSC 2×, 0.1% SDS | 2×5′ (RT) | 20 mM sodium phosphate buffer, 1% SDS, 1 mM EDTA | 2×10′ (65°C) |
| SSC 1×, 0.1% SDS | 2×15′ (60°C) | |||
| B1 washing buffer | 1′ (RT) | |||
| Membrane blocking | 10× blocking solution diluted in B2 solution | 60′ (RT) | 75 mM maleic acid pH 7.5, 200 mM NaCl, 5% non-fat dry milk powder | 60′ (RT) |
| Antibody binding | B3 10× blocking solution diluted in B2 solution | 60′ (RT) | 75 mM maleic acid pH 7.5, 200 mM NaCl, 5% non-fat dry milk powder | 30′–60′ (RT) |
| Membrane washes | B1 washing buffer | 2×15′ (RT) | 75 mM maleic acid, 200 mM NaCl, 0.3% Tween 20a | 2×15′ (RT) |
| B4 detection buffer | 5′ (RT) | 100 mM Tris pH 9.5, 100 mM NaCl | 5′ (RT) |
RT, room temperature.
The tween is optional, it slightly reduces the background with some probes.
Revealing the probe with the anti-digoxigenin antibody
When using the commercial protocol, the membrane was treated as recommended by the manufacturer (Roche Wash and Block Buffer Set 11 585 762 001, see Table 1). When using the homemade protocol, the membrane was incubated in 100 ml of blocking buffer (75 mM maleic acid, 200 mM NaCl, pH adjusted to 7.5 with 32% NaOH solution), supplemented with 5% non fat dry milk powder, for 1 h at room temperature. The anti-digoxigenin antibody (Fab fragment, Roche 11 093 274 910) was diluted (1/10 000) in 50 ml blocking buffer supplemented with milk powder and incubated with the membrane for 30 min to 1 h at room temperature. Subsequently, two washes were performed in blocking buffer supplemented with 0.3% Tween 20 for 15 min each at room temperature. One quick final wash in 100 mM Tris–HCl (pH 9.5), 100 mM NaCl was performed for 5 min at room temperature before the chemiluminescent substrate (CSPD, Roche 11 655 884 001) was diluted (1:100 to 1:10 in 100 mM Tris–HCl pH 9.5, 100 mM NaCl for dot blots, or used undiluted for the Southern blot in Fig. 1) and poured on the membrane (1 ml/10 cm2). It was subsequently incubated during 5 min at room temperature, the CSPD was removed and a further 10 min incubation at 37°C was performed to enhance the chemoluminescent reaction, as recommended by the manufacturer.
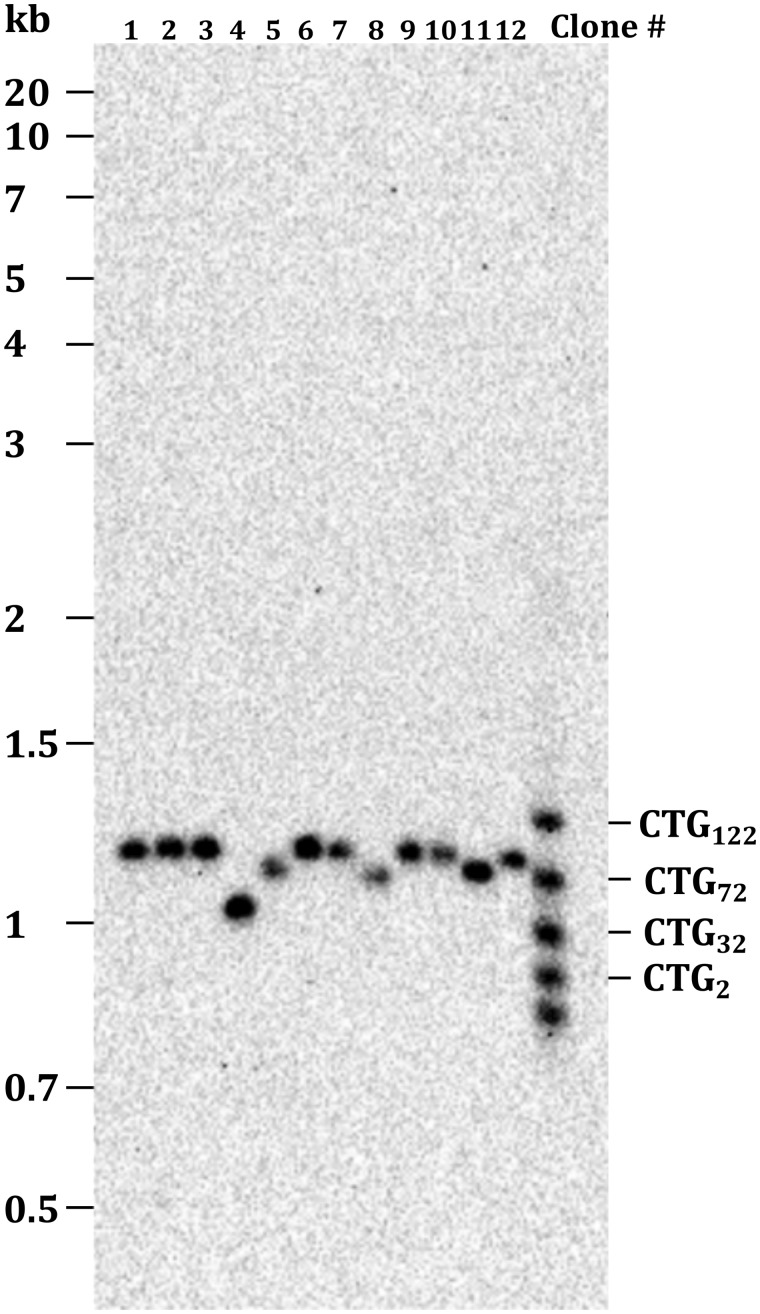
Figure 1:
Southern blot of S. cerevisiae BY4741 genomic DNA. Total genomic DNA was prepared from yeast independent colonies grown overnight in YPD medium as previously described [8] and 2–3 μg were digested with 20 units of SspI (NEBiolabs) during 4 h at 37°C. Digestions were loaded on a 1% agarose gel ran overnight at 1 V/cm. It was alkaline transfered on a Hybond-XL membrane (Amersham) and hybridized with the DIG-labeled SUP4 probe. The membrane was treated as described in the “Materials and methods” section, using our homemade protocol. The CSPD was used undiluted. The molecular weight marker on the left is the GeneRuler 1 kb Plus (Thermo Scientific #SM1331). The molecular weight ladder on the right is a homemade PCR product hybridizing with the probe and corresponding to different CTG trinucleotide repeat lengths, as described previously [9].
Signal quantification
Radioactive signals were read on a FujiFilm FLA-9000 phosphorimager with the Image Reader software and quantified with Multi Gauge (v 3.0). Light signals were detected on a ChemiDoc Imaging System (Bio-Rad) and quantified with the dedicated Image Lab software. Alternatively, an open source software ImageJ may also be used, for very similar results.
Results and discussion
The homemade non-radioactive protocol is faster, cheaper and more sentitive
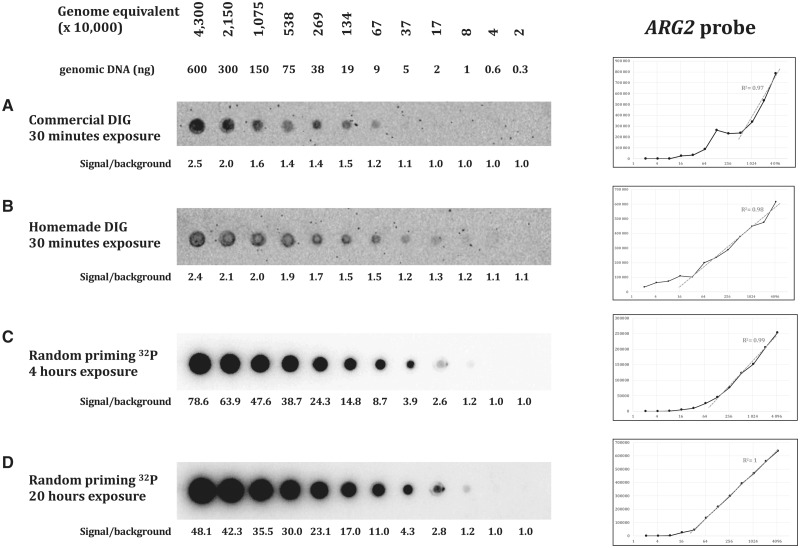
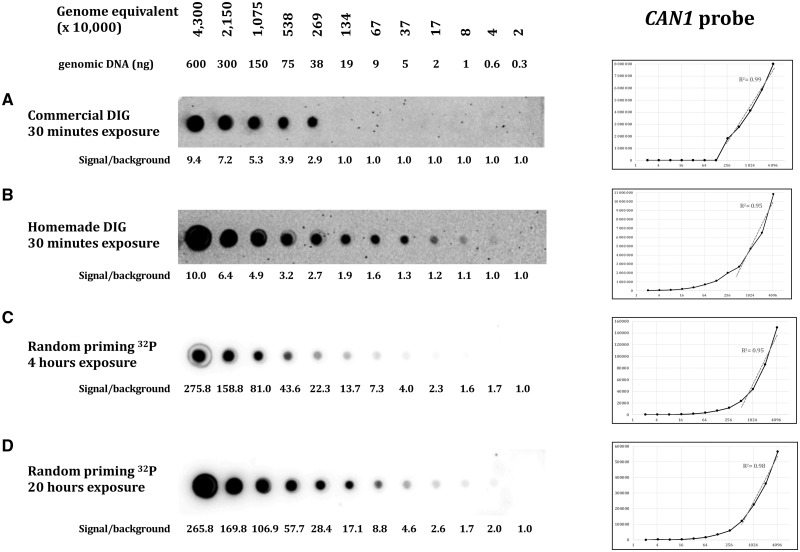
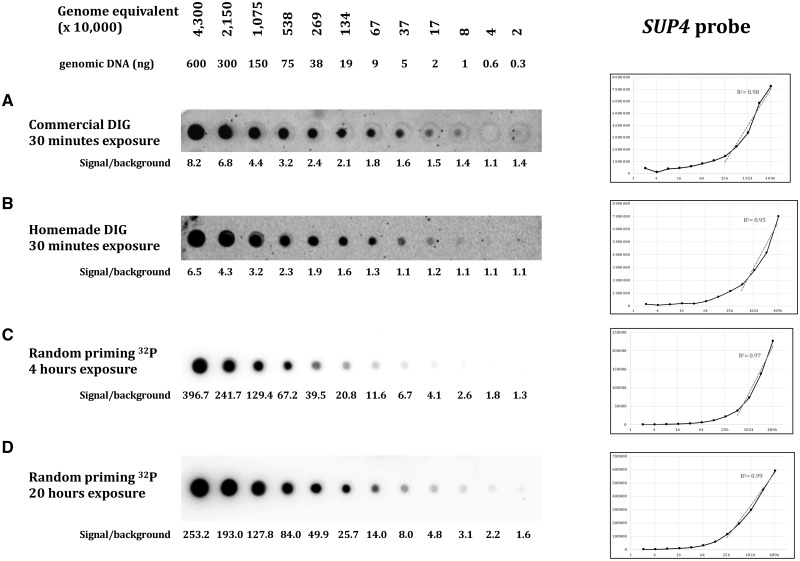
Three different probes were assayed, targeting three different loci: ARG2, CAN1, and SUP4. The yeast genome being AT-rich, the three probes show similar low GC content, respectively, 38, 33 and 34%. Probe lengths were 305 bp for CAN1, 488 bp for SUP4, and 751 bp for ARG2. Efficacy of both hybridization and wash protocols were compared on dot blots containing serial dilutions of S. cerevisiae total genomic DNA. Commercial and homemade protocols are extensively described in Table 1 and in the “Materials and methods” section. With the commercial protocol, the minimal amount of target molecules detected by the DIG-labeled probe was 370 000 genome equivalent (For S. cerevisiae (12.5-Mb genome), one genome equivalent corresponds to 0.0137 pg of genomic DNA, the amount needed to contain one genome copy, on average.) for ARG2 (5 ng DNA, Fig. 2A), 2 690 000 genome equivalent for the CAN1 probe (38 ng DNA, Fig. 3A) and 80 000 genome equivalent for the SUP4 probe (1 ng DNA, Fig. 4A). With our homemade protocol, sensitivity increased to 80 000 genome equivalent for ARG2 and CAN1 (1 ng DNA, Figs. 2B and 3B) and was unchanged for the SUP4 probe (Fig. 4B). Hence, our homemade protocol was found to be at least as sensitive as the commercial method (SUP4 probe) and at best 5–30 times more sensitive (ARG2 and CAN1 probes). Linearity of the signal response as compared to DNA amount was found to be in the same range. The ratio signal/background was very comparable with both methods and quite low in all three experiments, as shown by the high background visible. The total time spent on washes with our homemade protocol was 55 min altogether, for a 20-min time saving as compared to the commercial protocol. So, the use of our buffers and method was faster and generally more sensitive than the commercial solution used here.
Figure 2:
S. cerevisiae BY4741 total genomic DNA was 2-fold diluted and spotted on nylon membranes. From 600 to 0.3 ng genomic DNA, corresponding to 4, 3×107 to 20 000 genome equivalents were spoted. A Non-radioactive ARG2 DIG probe with commercial protocol, CSPD diluted 1:100, 30 min exposure on ChemiDoc. B Non-radioactive ARG2 DIG probe with homemade protocol, CSPD diluted 1:100, 30 min exposure on ChemiDoc. C Radioactive ARG2 probe, 4 h exposure on phosphor screen. D Radioactive ARG2 probe, 20 h exposure on phosphor screen. Signal quantification graphs are shown to the right, for each experiment. x-axis: genome equivalent; y-axis: signal quantification. Linearity is estimated by the correlation coefficient value of the linear part of the curve.
Figure 3:
Same as Fig. 2, with the CAN1 probe. CSPD diluted 1:10 in both DIG protocols.
Figure 4:
Same as Fig. 2, with the SUP4 probe. CSPD diluted 1:10 in both DIG protocols.
Radioactive hybridization is more specific, cleaner and more linear
In order to compare non-radioactive methods to a well-established protocol of hybridization with a radioactive probe, we hybridized similar dot blots with alpha-32P dATP labeled probes (“Materials and methods” section). Membranes were exposed 4 or 20 h on a phosphor imaging screen. The ARG2 and CAN1 radioactive probes were able to detect 80 000 genome equivalent (1 ng DNA), similar to what was achieved with our homemade DIG probe protocol. Sensitivity was slightly better with the SUP4 probe that was able to detect 20 000 genome equivalent (300 pg DNA) after 20 h of exposure (Fig. 4D). The ratio signal/background was extremely high, in all three experiments, as compared to what was obtained with non-radioactive protocols. The outcome was a cleaner and nicer image of each dot blot, with low background. Linearity of the signal response as compared to DNA amount was excellent, especially at the highest exposure time, for which it was almost perfectly linear, the CAN1 probe giving a slightly poorer linear response. This allowed for very precise quantification of the amount of DNA present on each blot. Therefore, we concluded that although the DIG probe was as sensitive as the 32 P probe, this last one was a much better choice when precise signal quantification was required.
The homemade non-radioactive protocol exhibits a good signal specificity
In order to check probe specificity, a Southern blot of total yeast genomic DNA was hybridized with a DIG-labeled SUP4 probe, in our homemade conditions. The yeast strain analyzed contained a CTG trinucleotide repeat integrated at the SUP4 locus, whose length varies among different subclones [7], as shown in Fig. 1. Length polymorphism corresponding to shorter CTG repeat tracts was clearly visible in four clones (4, 5, 8, and 11). The signal was unique and no other band was detected under or above the expected band, showing the good specificity of our homemade protocol.
Hybridization of nucleic acids to a specific labeled probe is very commonly used in molecular biology experiments such as dot blots, Southern or Northern blots. With the recent increase in price and regulation of radioactive compounds, non-radioactive probes such as those labeled with digoxigenin-dUTP are more and more attractive for molecular biology laboratories. Here, we show that using homemade buffers and solutions that are common and cheap, we achieved a more sensitive and faster detection than what was obtained with dedicated commercial solutions. The protocol was also greatly simplified, since the probe hybridization buffer was the same as the buffer used for radioactive probes (“Church buffer”), and only two different solutions were used for antibody blocking, binding, and washes, instead of four when using commercial solutions. In addition, our method was also cost-effective, since homemade buffers led to a 12-fold decrease in experimental cost as compared to commercial solutions. That last result will be a definitive asset for laboratories using hybridization methods on a regular basis.
Comparison of non-radioactive methods with 32 P probe hybridization showed a clear advantage to the latter for low background and high linearity of the response. It is particularly visible after 20 h of exposure, although sensitivity did not increase with longer exposure times. Despite the high signal level, the phosphor screen was remarkably not saturated, even for larger DNA amounts.
In conclusion, we recommend to use our non-radioactive detection protocol to obtain a fast, sensitive, and cost-effective response to nucleic acid hybridization, when qualitative rather than quantitative results are expected. However, as long as signal quantitation needs to be accurate, for example to compare relative levels of low intensity bands on a Southern or Northern blot, or to quantify pausing signal level with 2D gel electrophoresis [8], radioactive hybridization and phosphor screen technology must be chosen for reliable and reproducible results.
Author contributions
D.V., A.M., V.M., L.P., and W.V.-Z. ran hybridizations with radioactive or non-radioactive probes in different experimental conditions and quantified results. G.-F.R. wrote the manuscript.
Conflict of interest statement. None declared.
Funding
V.M. was supported by Fondation Guy Nicolas and Fondation Hardy. L.P. is the recipient of a PhD student CIFRE fellowship from SANOFI. W.V.-Z. is the recipient of a PhD student fellowship from the Ligue Nationale contre le Cancer.
References
- 1. Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 1975;98:503–17. [DOI] [PubMed] [Google Scholar]
- 2. Feinberg AP, Vogelstein B.. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 1983;132:6–13. [DOI] [PubMed] [Google Scholar]
- 3. Church GM, Gilbert W.. Genomic sequencing. Proc Natl Acad Sci USA 1984;81:1991–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McQuaid S, McMahon J, Allan GM.. A comparison of digoxigenin and biotin labelled DNA and RNA probes for in situ hybridization. Biotech Histochem 1995;70:147–54. [DOI] [PubMed] [Google Scholar]
- 5. Buti M, Jardi R, Rodriguez-Frias F. et al. Digoxygenin-labelled DNA-probe: a rapid non-radioactive method for hepatitis B virus DNA detection in serum. Eur J Clin Chem Clin Biochem J Forum Eur Clin Chem Soc 1991;29:731735.. [DOI] [PubMed] [Google Scholar]
- 6. Emanuel JR. Simple and efficient system for synthesis of non-radioactive nucleic acid hybridization probes using PCR. Nucleic Acids Res 1991;19:2790.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Richard GF, Viterbo D, Khanna V. et al. Highly specific contractions of a single CAG/CTG trinucleotide repeat by TALEN in yeast. PLoS One 2014;9:e95611.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Viterbo D, Michoud G, Mosbach V. et al. Replication stalling and heteroduplex formation within CAG/CTG trinucleotide repeats by mismatch repair. DNA Repair (Amst) 2016;42:94–106. [DOI] [PubMed] [Google Scholar]
- 9. Mosbach V, Poggi L, Viterbo D. et al. TALEN-induced double-strand break repair of CTG trinucleotide repeats. Cell Rep 2018;22:2146–59. [DOI] [PubMed] [Google Scholar]