Abstract
The objectives of the study were to determine the effect of steroidal implants on growth performance, carcass characteristics, and estradiol-17β (E2) concentrations in the blood and longissimus muscle of Holstein steers fed a grain-based diet. Seventy Holstein steers (average initial BW = 275 ± 6.4 kg, 10 to 12 mo of age) were assigned to treatments: (i) implanted with 80 mg of trenbolone acetate (TBA) and 16 mg of E2 (Component TE-IS with Tylan; Elanco Animal Health, Greenfield, IN) at the start of the trial (day 0), and reimplanted with 120 mg of TBA and 24 mg of E2 (Component TE-S with Tylan; Elanco Animal Health) on day 84 of the experiment; or (ii) no implant. Implanted Holstein steers were heavier (P ≤ 0.01) than nonimplanted Holstein steers in the middle (day 84) and at the end of the experiment (day 186). Implanting Holstein steers increased (P < 0.01) average daily gain (ADG) and dry matter intake (DMI) without affecting gain-to-feed ratio compared with nonimplanted animals. Carcasses from implanted Holstein steers had greater (P < 0.01) hot carcass weight (HCW) and longissimus muscle (LM) area than carcasses from nonimplanted steers. Implanting did not affect (P ≥ 0.21) other carcass characteristics. There was an increase (P = 0.03) of 1.3 pg of E2/g of muscle in implanted Holstein steers compared with that from nonimplanted Holstein steers. There was an implant × day interaction (P < 0.01) in serum E2 concentrations. Serum E2 concentrations were not altered in nonimplanted Holstein steers, whereas E2 concentration increased (P < 0.01) after steers were implanted, regardless of implant characteristics. Serum E2 peaked at 28 days after the first implant and then rapidly declined after day 56. In summary, steroidal implants administered on days 0 and 84 increased DMI, ADG, HCW, and LM area in Holstein steers compared with nonimplanted steers due to increased serum E2 concentrations. However, these changes did not improve feed efficiency or other carcass characteristics.
Keywords: carcass characteristics, feed efficiency, Holstein, hormones, implants, steers
INTRODUCTION
Steroidal implants have been used in beef cattle production to improve growth performance and feed efficiency for over 50 years (Preston, 1999). Moreover, 90% of all feedlot cattle in the U.S. receive implants (NAHMS, USDA, 2011). The effective period, also known as the payout period, of implants varies from 90 to 120 days (Mader, 1998). However, the payout of the implant is impacted by hormone concentrations in the implant, quality of the implanting technique (Mader, 1998), and cattle body weight (BW) at implantation (Smith et al., 2018).
Much of the available data on implants was conducted in beef breeds. However, Holstein animals represent 16% of the total slaughter cattle fed in the U.S. (Boykin et al., 2017). Therefore, the focus on technologies to enhance Holstein beef production has been growing. Torrentera et al. (2016) evaluated the effects of BW at time of implant on Holstein steers and concluded that implanting Holstein steers improved feedlot growth performance with no impact on carcass characteristics regardless of the BW at first implant. In addition, researchers affirmed that positive effects were additive when Holstein steers were implanted multiple times (Torrentera et al., 2017).
There is a dearth of information regarding circulating hormone concentrations in implanted Holstein steers and its correlation with growth performance, carcass characteristics, and payout period of the implant. Therefore, we hypothesized that implants would increase growth performance and carcass characteristics by increasing serum estradiol-17β (E2) in implanted Holstein steers compared with nonimplanted Holstein steers and that these effects would occur with minimal impact on longissimus muscle (LM) E2 concentrations. The objectives of the study were to determine the effect of hormone implants on growth performance, carcass characteristics, and E2 concentrations in the blood and LM of Holstein steers fed a grain-based diet.
MATERIALS AND METHODS
All procedures involving the use of animals were approved by the Pennsylvania State University Institutional Animal Care and Use Committee (#201800037) and followed the guidelines recommended in the Guide for the Care and Use of Agricultural Animals in Research and Teaching (Federation of Animal Science Societies, 2010).
Animal and Diet Management
Seventy Holstein steers (average initial BW = 275 ± 6 kg, 10 to 12 mo of age) were used for this experiment at The Pennsylvania Department of Agriculture Livestock Evaluation Center, Pennsylvania Furnace, PA. Steers were housed in a confinement barn. The facility was a gable roof barn with interior pen, constructed of metal gates and cables, on concrete floor (30.5 m × 7.5 m) that was open on the back side to an exterior gravel lot (30.5 m × 61 m). A feed alley on the interior of the building was equipped with GrowSafe automated feeding systems (Model 4000E, GrowSafe Systems Ltd., Airdrie, AB Canada), and there were six Growsafe feed bunks per pen. Individual animal feed intakes were monitored daily by trained personnel and considered acceptable if both 85% of the feed supplied and 90% of the feed that disappeared from the bunk within the pen could be attributed to animals assigned to those bunks via electronic identification. Data were discarded for the pens that did not meet these criteria on any given day. At the end of the experiment, 86% of the total fed days were included in the data to be reported for DMI values.
All Holstein steers were supplied by a single commercial farm in York, PA (~130 km. Seven days after arriving at the feedlot, steers were weighed on two consecutive days (days 0 and 1) to determine initial full BW. Steers were not denied feed prior to weighing. Steers were stratified by BW on day 0 and allotted to one of two pens (35 steers per pen) on day 1 such that each pen (one pen per treatment) had a similar initial average BW. Steers were assigned to one of two treatments: (1) Implanted with 80 mg of trenbolone acetate (TBA) and 16 mg of E2 (Component TE-IS with Tylan; Elanco Animal Health; Greenfield, IN) at the start of the trial (day 0), and reimplanted with 120 mg of TBA and 24 mg of E2 (Component TE-S with Tylan; Elanco Animal Health) on day 84 of the experiment; or (2) not implanted. Implant retention was checked at 14 and 28 days after each implantation to ensure that the implant was still placed in the ear of the animal. One steer lost its implant during the 28 days after first implantation, and that steer was reimplanted with the same implant on day 28. Steers were adapted to the diet over 21 days in a stepwise adaptation process. The initial diet contained 22.8% dry-rolled corn, 60% corn silage, 15% dried distillers grains with solubles, 2% supplement, and 0.2% urea (DM basis). Every 7 days, dry-rolled corn replaced 14.33% (DM basis) of the corn silage in the diet until the final diet was fed (Table 1). Steers full BW was recorded on days 14, 28, 56, 84, 98, 112, and 140 relative to trial initiation. Final BW were collected on two consecutives (days 185 and 186). Overall, ADG was calculated as the average of the two final BW minus the average of the two initial BW divided by the total days on feed (186 days). This calculated ADG for each steer was divided by the average daily DMI of each steer to calculate gain-to-feed ratio (G:F).
Table 1.
Composition of diet fed to Holstein steers
| Ingredients, % DM basis | |
|---|---|
| Cracked corn | 65.8 |
| Corn silage | 17.0 |
| Dried distillers grains with solubles | 15.0 |
| Mineral and vitamin supplement1 | 2.0 |
| Urea | 0.2 |
| Analyzed nutrient composition, % DM basis | |
| Dry matter | 71.9 |
| Crude protein | 11.8 |
| Neutral detergent fiber | 23.1 |
| Starch | 53.7 |
| Calculated composition 2 , Mcal/kg | |
| NEm | 2.07 |
| NEg | 1.41 |
1Mineral and vitamin supplement = 1,550 g/ton Rumensin 90 (198 g of monensin/kg of DM; Elanco Animal Health, Greenfield, IN), Ca 25%, NaCl 15%, Mg 1%, K 3.5%, Zn 1,000 mg/kg, Cu 180 mg/kg, Se 16 mg/kg, Vit A 130,000 IU/lb. (Agri-Basics, Inc.; Elizabethtown, PA)
2Calculated based on tabular values for individual ingredients (NRC, 2000).
Feed Sampling and Analysis
Samples of dietary ingredients were collected every 2 weeks throughout the feedlot phase. Sampled feed ingredients were dried for 72 h at 55 °C. Dried ingredient samples were composited over the course of the trial, and composited samples were ground through a Wiley mill (1-mm screen, Arthur H. Thomas, Philadelphia, PA). Ground ingredient samples were analyzed for NDF (using Ankom Technology method 6; Ankom200 Fiber Analyzer, Ankom Technology, Macedon, NY), starch and soluble sugars were determined by the method of Hall (2009), and total ash (500 °C for 12 h, HotPack Muffle Oven Model: 770750, HotPack Corp., Philadelphia, PA). All analyses were corrected for lab DM (24 h at 105 °C). Diets were adjusted weekly for DM changes (24 h at 105 °C).
Serum Estradiol-17β Concentration
Whole blood was collected on days 0, 14, 28, 56, 84, 98, 112, 140, and 185 relative to trial initiation. Whole blood was collected from the jugular vein into 15 mL red top vacutainer tubes (Becton, Dickinson and Co., Franklin Lakes, NJ) and allowed to clot for 24 h at 4 °C and subsequently centrifuged at 1,250 × g at 4 °C for 15 min. Serum was harvested and stored at −20 °C until analysis was performed by radioimmunoassay (RIA).
Serum estradiol-17β concentration was determined on all serum samples via RIA procedures using methods described by Perry and Perry (2008). Cross-reactivity of the antibody used was 100% for estradiol-17β, 6.5% for estriol, 5.2% for estradiol-17α, 0.6% for estrone, and <0.01% for aldosterone, androstenedione, cholesterol, progesterone, and testosterone. The intra- and interassay coefficients of variation for the estradiol-17β assay were 3.7% and 8.8%, respectively, and assay sensitivity was 0.4 pg/mL.
Carcass Data Collection
On day 186, steers were transported for 320 km to an abattoir (JBS Inc., Souderton, PA) and were humanely slaughtered under USDA inspection. On the same day, hot carcass weight (HCW) was collected. Carcasses were chilled for 48 h at −4 °C. Approximately 48 h postharvest, the carcasses were ribbed between the 12th and 13th ribs, and carcass data including 12th rib back fat thickness, percent of kidney, pelvic, heart fat (% KPH), marbling score, and LM area were collected by Penn State trained personnel at the plant. The USDA quality grade (QG) was assigned by the plant and the Yield Grade (YG) was calculated using the USDA equation (USDA, 1997).
Muscle Estradiol-17β and Intramuscular Fat Concentration
Approximately 48 h postharvest, an LM muscle sample (100 to 200 g from each carcass) was shaved from the 12th rib interface on one half of the carcass. Muscle samples were frozen at −20 °C. Prior to analyzes muscle samples were thawed at room temperature and ~2 g from the middle of each sample was removed, divided to retain duplicates, and refrozen at −20 °C for E2 analysis. Muscle samples were processed using a method similar to the one described by Blasco et al. (2007). The muscle was minced and then homogenized in 0.1 M Tris–HCl (pH = 9.6). Following homogenization, samples were digested with 1 mg of Subtilisin A for 2 h at 37 °C. Every 15 min during the digestion tubes were mixed with a vortex mixer for 1 min. After the Subtilisin A digestion, samples were cooled, and then that digest was extracted twice and 5 mL of MtBE were used to perform each extraction. The final extract was then assayed for estradiol-17β as described by Perry and Perry (2008). All samples were analyzed in duplicate in a single assay and the intra-assay CV was 3.5%. In addition, samples of known concentration of estradiol-17β (1 and 5 pg/mL) were added to the analysis and accurately recovered (120%; r = 0.79).
The external fat was removed from the remaining muscle tissue that was collected from the 12th rib interface and was ground and homogenized using a food processor, then refrozen at −20 °C for later analysis of intramuscular fat. Muscle intramuscular fat was extracted using petroleum ether (Ankom Method 2; Ankom Technology).
Statistical Analysis
The experimental design for this study was completely randomized. To evaluate the effects of implants on growth performance, carcass characteristics, and muscle E2, data were analyzed using the MIXED procedure of SAS (SAS Inst. Inc., Cary, NC). The model was:
where Yijk is the response variable; μ is the mean; Tj is the fixed effect of treatment; eijk is the experimental error. Steer was the experimental unit. Categorical carcass characteristics (QG) were analyzed using the GLIMMIX procedure of SAS (SAS Institute) using a binomial distribution and a Satterthwaite adjustment. Significance was declared at P ≤ 0.05. Trends are discussed at 0.05 < P < 0.10.
To evaluate the effects of hormone implant on serum E2 concentration over time, data were analyzed using the MIXED procedures of SAS (SAS Institute) with repeated measures. The statistical model for the effect of implant and day was:
where Yijk is the response variable; μ is the mean; Di is the fixed effect of day of collection; Tj is the fixed effect of implant treatment; (DT)ij is the fixed effect of the interaction of day of collection and implant treatment, eijk is the experimental error. Steer was the experimental unit. The covariance structure compound symmetry was selected based on the lowest Bayesian Information Criterion. Significance was declared at P ≤ 0.05. Trends are discussed at 0.05 < P < 0.10.
RESULTS AND DISCUSSION
Feedlot Performance
Since its approval by the United States Food and Drug Administration in 1991, the effect of TBA and E2 combination (TBA/E2) implants has been extensively studied on beef cattle breeds (Preston, 1999). However, there is a limited number of experiments evaluating the effects of steroidal implants in Holstein steers fed a grain-based diet.
By design, initial BW did not differ between implanted and nonimplanted Holstein steers (P = 0.83; Table 2). However, by day 84, implanted Holstein steers were 6.6% heavier (P = 0.01) than nonimplanted steers. Moreover, final BW of implanted Holstein steers was 10.2% heavier (P < 0.01) than that of nonimplanted cohorts. During the first 84 days of the trial, Holstein steers implanted with 80 mg of TBA + 16 mg of E2 had an 18.8% increase (P < 0.01) in ADG compared with nonimplanted Holstein steers. On day 84, steers were reimplanted with 120 mg of TBA + 24 mg of E2. Despite the decrease in the daily animal weight gain in both groups during the second phase of the experiment (85 to 186 days), ADG of implanted Holstein steers was 21.3% greater (P < 0.01) than nonimplanted steers. Overall, Holstein steers that received steroidal implants on days 0 and 84 had a 20% increase (P < 0.01) in ADG compared with nonimplanted Holstein steers (Table 2).
Table 2.
Effects of steroidal implants on feedlot performance of Holstein steers
| Nonimplanted | Implanted1 | SEM | P-value | |
|---|---|---|---|---|
| n, steers | 35 | 35 | ||
| Live weight2, kg | ||||
| Initial | 274 | 276 | 6.420 | 0.83 |
| 84 days | 413 | 440 | 7.364 | 0.01 |
| Final | 542 | 598 | 8.968 | <0.01 |
| ADG, kg/d | ||||
| Days 1 to 84 | 1.65 | 1.96 | 0.031 | <0.01 |
| Days 85 to 186 | 1.27 | 1.54 | 0.036 | <0.01 |
| Days 1 to 186 | 1.44 | 1.73 | 0.028 | <0.01 |
| DMI, kg/d | ||||
| Days 1 to 84 | 8.88 | 10.19 | 0.176 | <0.01 |
| Days 85 to 186 | 10.14 | 11.76 | 0.205 | <0.01 |
| Days 1 to 186 | 9.47 | 11.02 | 0.180 | <0.01 |
| G:F3 | ||||
| Days 1 to 84 | 0.188 | 0.193 | 0.003 | 0.35 |
| Days 85 to 186 | 0.125 | 0.131 | 0.003 | 0.15 |
| Days 1 to 186 | 0.154 | 0.158 | 0.002 | 0.31 |
1Holstein steers implanted with 16 mg of E2 and 80 mg of TBA (Component TE-IS with Tylan; Elanco Animal Health, Greenfield, IN) at the start of the trial (day 0), and 24 mg of E2 and 120 mg of TBA (Component TE-S with Tylan; Elanco Animal Health) on day 84 of the trial.2Initial and final BW were calculated at the average BW of days 0 and 1, and days 185 and 186, respectively. 3G:F was calculated as the steer average ADG divided by steer average DMI within each period (days 1 to 84; days 85 to 186; days 1 to 186).
In a review conducted by Duckett and Pratt (2014), the authors stated that when cattle received two consecutive doses of TBA/E2 implant ADG improved by 16% to 20% compared with nonimplanted cattle. Moreover, a 16% to 20% increase ADG in Holstein steers that received hormone implants (TBA/E2) vs. nonimplanted cohorts have been reported by Scheffler et al. (2003) and Torrentera et al. (2016). Evaluating the effects of hormone implants on calf-fed Holsteins fed a grain-based diet, Scheffler et al. (2003) concluded that three consecutive implants (120 mg of TBA + 24 mg of E2 on days 0, 112, and 224 of the trial) increased ADG by 16.4% when cattle were fed for 290 days. Recently, Torrentera et al. (2016) reported that ADG increased over nonimplanted cohorts by 16.7% in implanted (120 mg of TBA + 24 mg of E2), calf-fed Holsteins that were on trial for 224 days. Although the vast majority of the Duckett and Pratt (2014) review data were based on experiments using native, or beef breed animals, the 20% increase in ADG of implanted Holstein steers reported in the current experiment (Table 2) is consistent with previous research in both beef and Holstein steers.
The ADG response was driven in part by a consistent increase in DMI of implanted Holstein steers compared with their nonimplanted cohorts. Overall, DMI of implanted Holstein steers was 16.5% greater (P < 0.01) for the 186 days on feed compared with nonimplanted cohorts (Table 2). Because both DMI and ADG of implanted Holstein steers increased, the resulting feed efficiency, as measured by the ratio of gain divided by feed (G:F), did not differ (P ≥ 0.15) compared with nonimplanted Holstein steers.
Carcass Characteristics
Based on previous research, we had hypothesized an increase in HCW in implanted steers compared with nonimplanted cohorts. In fact, carcasses from cattle that were implanted had increased (P < 0.01) HCW and LM area by 11% and 6%, respectively, compared with carcasses from nonimplanted Holstein steers. Consistent with the current study, implanting both beef and dairy breeds has been shown to increase HCW and LM area (Sheffler et al., 2003; Duckett and Pratt, 2014; Torrentera et al., 2016). In the current study, steers that were implanted also had an 11% decrease (P = 0.05) in the percentage of KPH in the carcass compared with nonimplanted steers. Results on the effects of implants on KPH have been inconsistent in the literature, but often there are minimal or no effects of implants in both beef (Duckett and Pratt, 2014) and dairy breeds (Sheffler et al., 2003; Torrentera et al., 2016). The inconsistent KPH results can be attributed to the fact that KPH is a subjective measurement and is also expressed as a percentage of HCW.
Improvements in HCW and LM area observed with the use of implants are often associated with decreased marbling score or LM fat concentration (Duckett and Pratt, 2014). However, in the current study, there was no effect (P ≥ 0.21) of treatment on subjective marbling score or measured LM fat concentrations (Table 3). Similarly, treatment did not impact (P ≥ 0.34) dressing percentage, subcutaneous fat thickness, calculated YG or USDA QG. Perry et al. (1991) concluded that in order to achieve a similar small degree of marbling for choice USDA QG, implanted Holstein steers require an additional 25 to 45 kg of final live BW compared with nonimplanted cohorts. These values were reported in another study where similar marbling score and carcass characteristics occurred in implanted Holstein steers that were slaughtered 49 kg heavier than nonimplanted steers (Torrentera et al., 2016). Implanted Holstein steers from the current experiment were slaughtered 56 kg heavier than nonimplanted Holstein steers without negative impacts on marbling score, intramuscular fat deposition, YG, or USDA QG.
Table 3.
Effects steroidal hormone implant on Holstein steers carcass characteristics
| Nonimplanted | Implanted1 | SEM | P-value | |
|---|---|---|---|---|
| n, steers | 35 | 35 | ||
| HCW, kg | 305 | 338 | 5.495 | <0.01 |
| Dressing percent, % | 58.72 | 58.84 | 0.301 | 0.79 |
| Longissimus muscle area, cm2 | 68.25 | 72.36 | 1.114 | 0.01 |
| Marbling | 467 | 461 | 12.326 | 0.71 |
| Longissimus muscle fat concentration2, % | 5.6 | 5.0 | 0.357 | 0.21 |
| Fat thickness, cm | 0.61 | 0.55 | 0.040 | 0.34 |
| KPH, % | 2.86 | 2.56 | 0.109 | 0.05 |
| YG3 | 2.80 | 2.87 | 0.07 | 0.46 |
| Choice and above4, % | 87.88 | 80.00 | 5.681 | 0.39 |
1Holstein steers implanted with 16 mg of E2 and 80 mg of TBA (Component TE-IS with Tylan; Elanco Animal Health, Greenfield, IN) at the start of the trial (day 0), and 24 mg of E2 and 120 mg of TBA (Component TE-S with Tylan; Elanco Animal Health) on day 84 of the trial.
2Crude fat concentration as measured by petroleum ether extract (Ankom Method 2; Ankom Technology).
3Carcass yield grade was calculated (USDA, 1997).
4Percentage of carcasses grading USDA choice or above.
Muscle and Serum Estradiol-17β Concentration
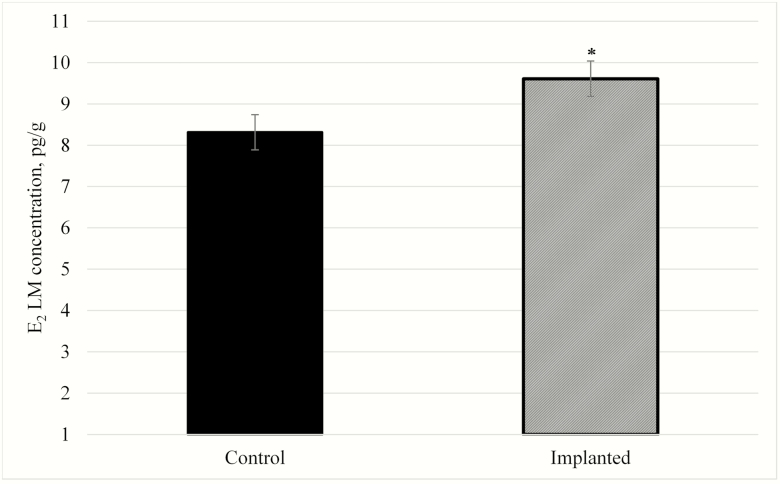
Hormone residues in the meat from implanted cattle are a concern among consumers. However, hormone residues in meat from implanted cattle rarely surpass the levels of a nonimplanted animal (Hartmann et al., 1998). In the current study, there was a 1.3 pg of E2/g increase (P = 0.03) in LM tissue from implanted Holstein steers compared with nonimplanted Holstein steers (Figure 1).
Figure 1.
Effects of steroidal implants on longissimus muscle (LM) E2 concentration (pg/g) of Holstein steers. Steers in this study were implanted (day 0 with 80 mg of TBA + 16 mg of E2; day 84 with 120 mg of TBA + 24 mg of E2; dashed bar) or nonimplanted (Control; solid bar). There was an effect of implant on muscle E2 concentration (P = 0.03). The error bars reflect the standard error of the mean (SEM = 0.426). The * above each bar reflects a significant (P < 0.05) difference between treatments.
Hartmann et al. (1998) stated that the major source of steroid hormone intake in human diets are milk products and plants, and that hormones from meat contribute to <25% of daily human intake. In addition, about 90% of the ingested hormones are inactivated in the liver (Hartmann et al., 1998). Therefore, no human health concerns are expected following consumption of meat produced from implanted animals.
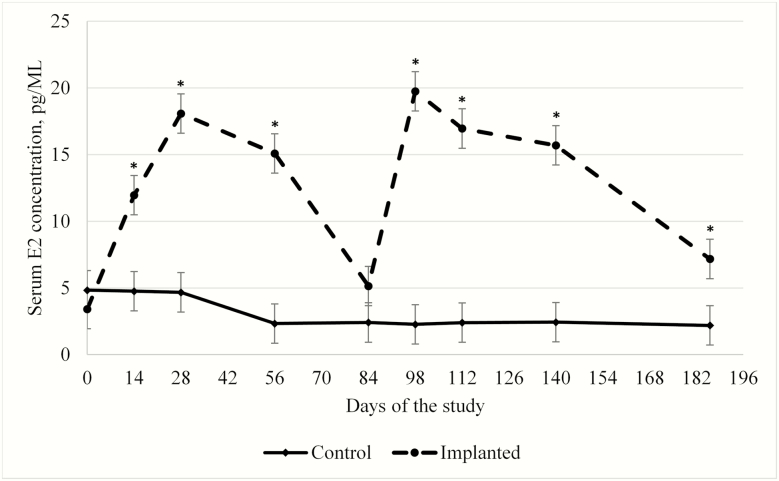
An implant × day interaction (P < 0.01; Figure 2) was observed on circulating serum E2 concentrations. Serum E2 concentrations between implanted and nonimplanted Holstein steers were similar at the beginning of the trial (3.4 vs. 4.8 pg/mL; P > 0.05); however, 14 days after receiving their first hormone implant, implanted Holstein steers had greater (P < 0.01) serum E2 concentrations than nonimplanted Holstein steers (12.0 vs. 4.8 pg/mL; P < 0.05). Serum E2 concentrations peaked in implanted Holstein steers on day 28 (18.1 vs. 4.7 pg/mL; P < 0.05), decreased slightly to day 56 (15.1 vs. 2.3 pg/mL; P < 0.05), and did not differ compared with serum E2 concentration in nonimplanted Holstein steers by day 84 (5.1 vs. 2.4 pg/mL; P > 0.05) after implantation.
Figure 2.
Effects of steroidal implants on serum E2 concentration (pg/mL) of Holstein steers over time. Steers in this study were implanted (day 0 with 80 mg of TBA + 16 mg of E2; day 84 with 120 mg of TBA + 24 mg of E2; dashed line) or nonimplanted (Control; solid line). There was an implant × day of the study interaction (P < 0.01). In addition, there were main effects of both implant (P < 0.01) and day of the study (P < 0.01). The error bars reflect the standard error of the mean associated with the interaction of implant × day of the study (SEM = 1.475). The * above each time point reflects a significant (P < 0.05) difference between treatments.
On day 84, implanted Holstein steers were reimplanted with Component TE-S with Tylan (120 mg of TBA + 24 mg of E2; Elanco Animal Health). Thus, at day 98 (14 days after second implantation) implanted Holstein steers had a second peak of serum E2 (19.8 vs. 2.3 pg/mL; Figure 2). From days 98 to 140, serum E2 concentration remained relatively stable. However, the serum E2 concentration of implanted Holstein steers decreased by half from days 140 to 186. Despite the decrease, serum E2 concentrations were still greater in implanted Holstein steers than nonimplanted Holstein steers at the end of the experiment (7.2 vs. 2.2 pg/mL; P < 0.05).
Although the mode of action of cattle implants (TBA/E2) is not well explained (Preston, 1999), the increase in serum E2 concentrations when implanted steers are compared with nonimplanted steers is well documented (Johnson et al., 1996; Parr et al., 2014; Smith et al., 2018). Johnson et al. (1996) stated that there is an increase in growth performance following the increase in serum E2 concentrations on implanted beef steers, particularly during the first 40 days after implantation. Therefore, measurements of serum E2 concentrations in implanted steers over time have been conducted to estimate the payout period, or the length of time before serum E2 concentrations return to normal, of each implant strategy.
Johnson et al. (1996) compared serum E2 concentrations at 0, 2, 21, 40, 115, and 143 days after implantation in implanted (120 mg of TBA + 24 mg of E2) and nonimplanted crossbred yearling, beef steers. The authors observed that 2 days after cattle were implanted, serum E2 concentrations were four times greater in implanted steers than nonimplanted steers. In addition, similar to what was observed in the current study (Figure 2), the peak of serum E2 concentration in implanted steers was reached within 2 to 4 weeks after implantation. The peak of serum E2 concentration is associated with maximum hormone release. Therefore, maximum animal growth response is expected when serum E2 peaks (Johnson et al., 1996). The slow decline in serum E2 after the peak generally maintains positive growth as long as hormone concentration remain elevated (Mader, 1998).
Once the payout period is over, serum E2 concentrations did not differ between treatments, and a decrease in animal performance from implanted steers was expected. The payout period of most TBA/E2 combination implants varies from 90 to 120 days and depends on implant design and the quality of implanting technique (Mader, 1998). Previous research reporting serum E2 concentration and implant payout period had been conducted only in beef breeds (Johnson et al., 1996; Preston, 1999; Smith et al., 2018).
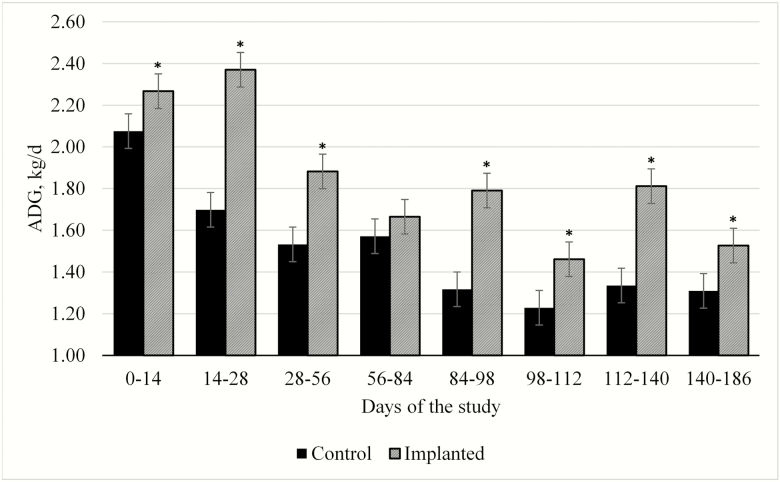
In the current trial, serum E2 concentrations of implanted and nonimplanted Holstein steers were similar by day 84 after implantation; and ADG was also similar from days 56 to 84 (Figure 3). Therefore, implants had a shorter payout period in the current trial than previously reported for beef breeds (Johnson et al., 1996; Mader, 1998; Preston, 1999). The second implant (120 mg of TBA + 24 mg of E2) increased serum E2 concentration and increased ADG similar to the first implant. But, the implant payout period for the second implant was greater than 100 days and more similar to periods previously reported in beef cattle breeds (Mader, 1998).
Figure 3.
Effects of steroidal implants on average daily gain (ADG) of Holstein steers (kg/d). Steers in this study were implanted (day 0 with 80 mg of TBA + 16 mg of E2; day 84 with 120 mg of TBA + 24 mg of E2; dashed bar) or nonimplanted (Control; solid bar). The error bars reflect the standard error of the mean (SEM = 0.083). The * above each bar reflects a significant (P < 0.05) difference between treatments.
The difference in payout period may be related to the difference in hormone concentrations between the first implant (80 mg of TBA + 16 mg of E2) and the second implant (120 mg of TBA + 24 mg of E2). However, another possible explanation could be that Holstein steers have a greater metabolic clearance of the implanted hormones than beef breeds due to the greater liver size of dairy breeds when compared with beef breeds (Taylor and Murray, 1991). However, liver size was not determined in the current study. Thus, the reduction in payout period for first implant compared with the literature pertaining to beef breeds remains unknown.
Implanting Holstein steers increased ADG and DMI with no impact on feed efficiency. Moreover, implants increased HCW and LM area with no detrimental effects to carcass quality. While implants increased muscle E2 concentrations, effects were minimal, despite the increase in circulating serum E2 concentration when cattle were implanted. Implanting Holstein steers with a more aggressive implant strategy had similar payout period to previously reported payouts in beef cattle breeds; however, the initial, more mild, implant had a shorter payout than that previously reported in beef breeds.
ACKNOWLEDGMENTS
The authors thank JBS USA for their support throughout the project. In addition, the authors acknowledge the contributions and support of the Pennsylvania Beef Producers Working Group. Finally, the authors thank the staff at the Pennsylvania Department of Agriculture Livestock Evaluation Center for the daily care and feeding of the animals used in this research project.
Conflict of interest statement. None declared.
LITERATURE CITED
- Blasco C. C. Poucke Van, and Van Peteghem C.. 2007. Analysis of meat samples for anabolic steroids residues by liquid chromatography/tandom mass spectrometry. J. Chromatogr. A, 1154:230–239. doi: 10.1016/j.chroma.2007.03.090 [DOI] [PubMed] [Google Scholar]
- Boykin C. A., Eastwood L. C., Harris M. K., Hale D. S., Kerth C. R., Griffin D. B., Arnold A. N., Hasty J. D., Belk K. E., Woerner D. R., . et al. 2017. National beef quality audit-2016: in-plant survey of carcass characteristics related to quality, quantity, and value of fed steers and heifers. J. Anim. Sci. 95:2993–3002. doi: 10.2527/jas.2017.1543 [DOI] [PubMed] [Google Scholar]
- Duckett S. K., and Pratt S. L.. 2014. Meat science and muscle biology symposium: anabolic implants and meat quality. J. Anim. Sci. 92:3–9. doi: 10.2527/jas.2013-7088 [DOI] [PubMed] [Google Scholar]
- FASS. 2010. Guide for the care and use of agricultural animals in agricultural research and teaching, 3rd ed.Champaign, IL: Consortium for Developing a Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. [Google Scholar]
- Hall M. B. 2009. Determination of starch, including maltooligosaccharides, in animal feeds: comparison of methods and a method recommended for AOAC collaborative study. J. AOAC Int. 92:42–49. [PubMed] [Google Scholar]
- Hartmann S., Lacorn M., and Steinhart H.. 1998. Natural occurrence of steroid hormones in food. Food Chem., 62:7–20. doi: 10.1016/S0308-8146(97)00150-7 [DOI] [Google Scholar]
- Johnson B. J., Anderson P. T., Meiske J. C., and Dayton W. R.. 1996. Effect of a combined trenbolone acetate and estradiol implant on feedlot performance, carcass characteristics, and carcass composition of feedlot steers. J. Anim. Sci. 74:363–371. doi: 10.2527/1996.742363x [DOI] [PubMed] [Google Scholar]
- Mader T. L. 1998. Implants. Vet. Clin. North Am. Food Anim. Pract. 14:279–290. doi: 10.1016/S0749-0720(15)30254-1 [DOI] [PubMed] [Google Scholar]
- National Animal Health Monitoring System (NAHMS), USDA. 2011. Feedlot 2011 part IV: health and health management on U.S. feedlots with a capacity of 1,000 or more head. Fort Collins, CO: National Animal Health Monitoring System. [Google Scholar]
- NRC. 2000. Nutrient requirements of beef cattle. 7th Rev. ed., 1996. Washington, DC: National Academic Press. [Google Scholar]
- Parr S. L., Brown T. R., Ribeiro F. R., Chung K. Y., Hutcheson J. P., Blackwell B. R., Smith P. N., and Johnson B. J.. 2014. Biological responses of beef steers to steroidal implants and zilpaterol hydrochloride. J. Anim. Sci. 92:3348–3363. doi: 10.2527/jas.2013-7221 [DOI] [PubMed] [Google Scholar]
- Perry T. C., Fox D. G., and Beermann D. H.. 1991. Effect of an implant of trenbolone acetate and estradiol on growth, feed efficiency, and carcass composition of Holstein and beef steers. J. Anim. Sci. 69:4696–4702. doi: 10.2527/1991.69124696x [DOI] [PubMed] [Google Scholar]
- Perry G. A., and Perry B. L.. 2008. Effect of preovulatory concentrations of estradiol and initiation of standing estrus on uterine pH in beef cows. Domest. Anim. Endocrinol. 34:333–338. doi: 10.1016/j.domaniend.2007.09.003 [DOI] [PubMed] [Google Scholar]
- Preston R. L. 1999. Hormone containing growth promoting implants in farmed livestock. Adv. Drug Deliv. Rev. 38:123–138. doi: 10.1016/s0169-409x(99)00012-5 [DOI] [PubMed] [Google Scholar]
- Scheffler J. M., Buskirk D. D., Rust S. R., Cowley J. D., and Doumit M. E.. 2003. Effect of repeated administration of combination trenbolone acetate and estradiol implants on growth, carcass traits, and beef quality of long-fed Holstein steers. J. Anim. Sci. 81:2395–2400. doi: 10.2527/2003.81102395x [DOI] [PubMed] [Google Scholar]
- Smith Z. K., Thompson A. J., Hutcheson J. P., Nichols W. T., and Johnson B. J.. 2018. Evaluation of coated steroidal implants containing trenbolone acetate and estradiol-17β on live performance, carcass traits, and sera metabolites in finishing steers. J. Anim. Sci. 96:1704–1723. doi: 10.1093/jas/sky095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, St. C. S., and Murray J. I.. 1991. Effect of feeding level, breed and milking potential on body tissues and organs of mature, nonlactating cows. Anim. Prod. 53:27–38. doi: 10.1017/S0003356100005948 [DOI] [Google Scholar]
- Torrentera N., Barreras A., Gonzales V., Plascencia A., Salinas J., and Zinn R. A.. 2016. Delay implant strategy in calf-fed Holstein steers: growth performance, growth rate and carcass characteristics. J. Appl. Anim. Res. 45:454–9. doi: 10.1080/09712119.2016.1210012 [DOI] [Google Scholar]
- Torrentera N., Plascencia A., Salinas-Chavira J., and Zinn R. A.. 2017. Influence of implant strategy on growth performance and carcass characteristics of calf-fed Holstein steers. Prof. Anim. Sci. 33:327–333. doi: 10.15232/pas.2016-01596 [DOI] [Google Scholar]
- U. S. Department of Agriculture. 1997. Standards for grades of carcass beef. Washington, DC: Agriculture Marketing Service, USDA. [Google Scholar]