Abstract
Although harmful consumption of alcohol and other drugs (both illicit and pharmaceutical) significantly contribute to global burden of disease, not all harms are captured within existing morbidity data sources. Indeed, harms occurring in the community may be missed or under-reported. This paper describes the National Ambulance Surveillance System, a unique Australian system for monitoring and mapping acute harms related to alcohol and other drug consumption. Data are sourced from paramedic electronic patient care records provided by ambulance services from across Australia. Coding occurs in a purpose-built system, by a team of specialised research assistants. Alcohol, and specific illicit and pharmaceutical drugs, rather than broad drug classes, are manually coded and the dataset is reviewed and cleaned prior to analysis. The National Ambulance Surveillance System is an ongoing, dynamic surveillance system of alcohol and other drug-related harms across Australia. The data includes more than 140 output variables per attendance, including individual substances, demographics, temporal, geospatial, and clinical data (e.g., Glasgow Coma Scale score, naloxone provision and response, outcome of attendance). The National Ambulance Surveillance System is an internationally unique population-level surveillance system of acute harms arising from alcohol and other drug consumption. Dissemination of National Ambulance Surveillance System data has been used to inform and evaluate policy approaches and potential points of intervention, as well as guide workforce development needs and clinical practice at the local and national level. This methodology could be replicated in other countries.
Introduction
Excessive consumption of alcohol and illicit drugs, and extra-medical use of pharmaceutical medications, are major avoidable risk factors for disease, illness, injury and death [1,2]. Globally, 38.3% of the population drink alcohol [3], though this is higher in Australia where, in 2016, 77.5% of adults reported that they consumed alcohol, and 25.5% reported risky drinking [4]. The World Health Organization estimated that approximately five per cent of the global population consumed drugs (substances under the control of international drug control conventions; including both illicit substances and extra-medical pharmaceutical use) at least once in 2015 [5]. Like alcohol, other drug consumption in Australia exceeds global averages, with 12.6% of Australians reporting past-year consumption of an illicit drug in 2016, and 4.8% reported extra-medical use of pharmaceuticals [4]. In the majority of both acute and chronic disease/illness categories, relationships between disease and the volume and/or pattern of alcohol and other drug (AOD) consumption exist, with increasing quantity of alcohol or riskier drug consumption patterns related to higher risk of subsequent disease or death [6].
AOD consumption has been identified as a causal or component risk factor in more than 200 disease and illness categories [6], and resulting harms can be acute or chronic [7]. For example, acute harms include injuries sustained while intoxicated, or unintentional overdose of both illicit and pharmaceutical drugs [8]. In terms of chronic harms, alcohol increases the risk of liver disease, mental disorders, heart disease, some cancers [9], and is the third leading risk factor for premature deaths and disabilities [9]. Chronic stimulant use is associated with increased risk of mental disorders, and opioid consumption is associated with an increased risk of mental disorders and blood borne viruses (associated with injecting drug use) [8]. To understand the impact of AOD consumption in the population, it is important that related harms are measured.
One means of understanding AOD-related harms is burden of disease frameworks, which provide a method for describing and quantifying the health burden of diseases and injuries, and the risk factors (such as AOD) that contribute to them [2,8]. However, not all harms associated with AOD consumption are routinely captured within burden of disease frameworks [10]; for example, many emergency department presentations that involve alcohol may be missed as these are typically classified by the specific presenting disease or injury. Only overdose or poisoning are likely to be captured by primary coding [11], leading to likely underestimation of AOD involvement in these presentations. Additionally, current burden of disease methodologies typically include hospital admission and coronial data, however the impact of AOD consumption on emergency health care responses (such as incidents attended by ambulance) are not currently captured, despite contributing to the overall burden [10].
A second means for quantifying the magnitude of harms is to use survey data. In Australia, instruments for measuring AOD-related harms at a population level include the National Drug Strategy Household Survey (NDSHS) [4] conducted every three years, and targeted surveys (e.g., the Australian Secondary School Drug Survey [12] aimed at students aged 12–18). However, population level surveys have limitations. Although recruitment aims for representative populations, survey frames exclude those outside of the school system or without stable housing. This excludes vulnerable and disadvantaged sub-groups, who may experience greater harms relative to consumption [13]. Additionally, surveys are reliant on self-reported consumption patterns, which are typically described over large intervals (e.g., the past 12 months), and may be prone to recall bias and reporting based on social desirability [14,15]. Furthermore, extremes of consumption within particular population sub-groups, or infrequent high consumption, may be masked by enquiring about average use over a period of time, without taking into account patterns of consumption [16]. Lastly, while instruments such as NDSHS enquire about extra-medical use of pharmaceuticals, medications are broadly categorised into drug classes, and specific medications are not captured [4].
A third means for measuring harms is to analyse administrative data sources, such as emergency department presentation or hospital admission data [17,18]. However, these data, classified using ICD-10, may not be sufficiently detailed to identify trends relative to specific substances [19]. Coronial or mortality data provide an additional source of information on harms [20], but these data only reflect mortality outcomes, and there is a significant reporting lag of two to three years [21]. Though these types of administrative data overcome issues of self-report, not all AOD-related harms will be treated in a hospital setting. For example, opioid poisoning treated with naloxone administration may occur in the community and those affected may not present to hospital, and therefore will not be captured in emergency department or hospital data [22].
Ambulance services are often the first (and frequently primary) contact with health services in the event of an acute AOD-related harm. However, despite ambulance services being available in many countries, their data is not routinely used to capture the breadth of acute AOD-related harms across the community. Ambulance patient care notes offer an additional source of information on acute AOD-related harms that may be missed in other datasets, especially as many of those attended by an ambulance are not transported to hospital. These clinical notes provide an important and rich data source which detail the nature and background to the attendance (including information about what was observed ‘on scene’ such as bystander accounts and evidence of drug paraphernalia), as well as the clinical outcome. In this way, coding paramedic clinical notes addresses an information gap and provides a robust source of information on AOD-related harms. This paper describes the development of the National Ambulance Surveillance System (NASS), an internationally unique system that captures acute harms related to AOD consumption by coding ambulance clinical records, allowing for examination of temporal and spatial trends related to individual substances.
Materials and methods
Data coverage and governance
“The Ambo Project”, established in 1998 with funding from the Victorian Department of Health and Human Services, began identifying and classifying AOD (both illicit and pharmaceutical)-related ambulance attendances in metropolitan Melbourne. This initial phase of this work, which primarily focussed on non-fatal heroin overdoses attended by ambulance in Metropolitan Melbourne only [23], served as the basis for NASS. The system underwent a two-phase expansion; in 2011 to incorporate regional Victoria and in 2012 to achieve national coverage, with inclusion of four Australian states (New South Wales, Queensland, Tasmania and Victoria) and two territories (Australian Capital Territory, Northern Territory). NASS captures 82.5% of Australia’s population; with plans to include the two remaining jurisdictions (Western Australia joined the system in 2018 and comparable data provision is anticipated in 2020, and negotiations with South Australia will recommence when an electronical clinical information system becomes available). Coded and categorised data are available for each jurisdiction from the date of jurisdictional project commencement, with complete annual data available for Victoria and data snapshots of one month per quarter (March, June, September and December) available for all other jurisdictions (Table 1). NASS is centrally administered and managed by Turning Point (a national addiction treatment, research and education centre), with data provision governed by agreements with each ambulance service. Rather than focusing on a few sub-types of AOD attendances (e.g., alcohol intoxication or heroin-related), NASS includes both illicit and pharmaceutical drug-related attendances and the inclusion of more than 140 variables, including patient demographics.
Table 1. Summary of coded data availability by jurisdiction.
| Jurisdiction | Population as at 30 June 2017 | Data collection start date | Number of attendances 2016–17 financial year * | Number of AOD-related attendances 2016 calendar year (4 snapshot months) ** |
|---|---|---|---|---|
| Australian Capital Territory | 411,667 | March 2013 | 42,098 | 1,130 |
| New South Wales | 7,861,674 | January 2013 | 774,137 | 13,806*** |
| Northern Territory | 247,491 | January 2015 | 33,760 | 2,883 |
| Queensland | 4,929,152 | January 2013 | 767,296 | 20,780 |
| Tasmania | 522,152 | March 2013 | 68,792 | 1,400 |
| Victoria | 6,321,648 | November 1998**** | 497,814 | 16,541 |
NB. All dates and numbers are correct as at 30 June 2018.
* Numbers include emergency and urgent incidents, in the Australian financial year (1 July to 30 June)
** Snapshot months: March, June, September, December in 2016 calendar year. Calendar year was used instead of financial year as 2017 data for Queensland is unavailable.
*** This includes cases completed on VACIS®, which is over 90% of all attendances
**** Regional Victoria data available from May 2011. Three months of missing data from October to December 2014 inclusive, due to paramedic industrial action
The overall project is approved through the Eastern Health Human Research Ethics Committee (HREC), with additional HREC approval for jurisdictional data provision, and requirements for informed consent were waived by these HRECs. Strict protocols are in place for data de-identification, confidentiality, storage, access and reporting. Patient identifiers are provided by some ambulance jurisdictions for the purposes of data linkage. On data receipt, these identifiers are stripped from the dataset and a unique statistical linkage key (SLK) created. Identifiers are held in a password protected, secure, separate database that is accessible only to database managers. All data is de-identified prior to coding. Of note, NASS spans additional coding modules including ambulance attendances related to mental health, self-harm, and violence.
Process overview
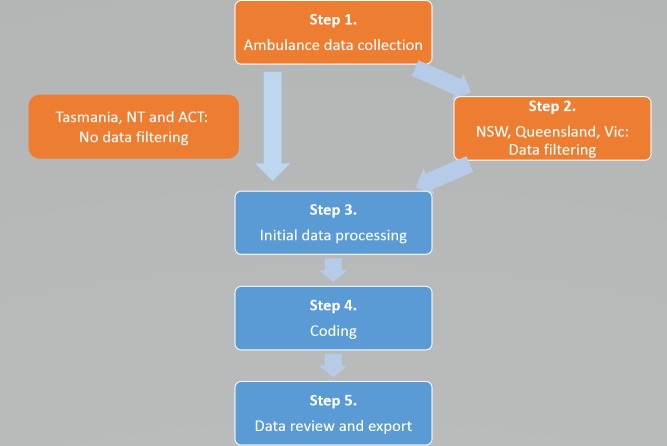
Fig 1 presents the five steps in NASS data collection and coding.
Fig 1. National Ambulance Surveillance System data collection and coding process.
Processes in orange occur at the jurisdictional ambulance services, with processes in blue occurring at Turning Point.
Step 1 –Ambulance data collection
VACIS® is the clinical record management system used by NASS contributing ambulance services with the exception of the Northern Territory’s use of Siren®, and a new system implemented in Queensland in 2017 (2016 Queensland data is reported in this manuscript, which is prior to their system migration). Although system specifics vary slightly the fundamentals remain the same. Paramedics create an electronic patient care record (ePCR) for each attendance, which includes clinically relevant information on patient demographics, attendance location and characteristics, clinical signs, treatment details, and outcomes. Validation rules, including mandatory fields that block progression until complete, and time-stamps to ensure details cannot be completed before attendance arrival, are built into the ePCR system, resulting in highly detailed and complete ePCRs. NASS is based entirely on coding these clinical records without placing any additional workload on paramedics. Prior to 2006, the Victorian ambulance service used paper-based records.
Step 2 –Filtering
Three of the six ambulance services (New South Wales, Queensland, and Victoria) undertake primary filtering prior to providing the ePCR to NASS. The filter (S1 File) is based on an automated keyword search to extract cases that potentially involve AOD consumption, using over-inclusive parameters to maximise probable case capture. VACIS® case capture, data matching and filtering have been previously described [24], and the filtering used in this project follows a similar process. Filtering is dynamic with manual reviewing in response to discrepancies in the project extract and comparison with other data systems, however cases may be missed, resulting in an underestimation of AOD-related cases. The remaining three ambulance services (Australian Capital Territory, Northern Territory and Tasmania) provide unfiltered priority 1 and 2 attendances.
Step 3 –Initial processing at Turning Point
Jurisdictional ambulance services extract case records from their data management systems and securely transfer these ePCRs to Turning Point. On receipt, data are checked for completeness and compared to the Turning Point database. Graphs of attendance numbers are created as a visual check for anomalous trends. Discrepancies are flagged for systematic investigation by the database manager. Once ePCRs pass all checks, they are appended to the data warehouse and prepared for manual coding.
Step 4 –Coding: Case ascertainment and case classification
Coding occurs in a purpose-built system by a team of research assistants (RAs) who manually scrutinise each ePCR to determine whether the case meets core inclusion criteria (case ascertainment) as well as to identify the substances involved in the attendance (case classification). The time to code a single ambulance attendance ranges from 1.28 to 4 minutes, and each RA codes approximately 180 attendances per day. Workflow is managed with the use of a data warehouse, with allocation of records based on reporting priorities.
Inclusion criteria are met if recent, inappropriate AOD use contributed to the ambulance attendance, using the following criterion: ‘Is it reasonable to attribute the immediate or recent (the past 24-hours) over or inappropriate AOD use as a contributing reason for the ambulance attendance?’ This information is ascertained from paramedic clinical assessment, patient self-report, information from third parties and evidence at the scene, as recorded in the clinical notes. Importantly, while AOD consumption must be a contributor to the ambulance attendance, it may not be the primary reason for the ambulance attendance.
Case classification occurs at the level of individual substances, including alcohol, 13 illicit drugs (individual substances presented in Table 2) and 82 pharmaceutical medications (individual medications presented in S1 Table), with each listed substance coded and routinely reported. For pharmaceutical medications (including over-the-counter), inappropriate use is defined as consumption contradictory to prescriber or manufacturer instructions. Specifically, consuming these substances in excess of the prescribed dose, drugs prescribed for another person, and/or consumption in combination with contraindicated substances is deemed inappropriate use. Adverse events following appropriate medication use are excluded. ‘Other pharmaceutical medication’ classification includes all substances prepared in pharmaceutical settings not further specified by codes, including over-the-counter medications not elsewhere specified, vitamins, and herbal supplements. For illicit substances, any consumption is classified as AOD-related. Substances not intended for human consumption and not captured elsewhere are classified as ‘other substances’.
Table 2. National Ambulance Surveillance System output variables, including scene patient and clincal details, alcohol, individual illicit drugs and pharmaceutical medication drug classes*.
| Case details | Patient details | Scene details | Physical condition | Illicit drugs | Pharmaceutical medications | Other substances | Intent of AOD poisoning |
|---|---|---|---|---|---|---|---|
| Case number | Gender | Public / private | Patient outcome | Methamphetamine | Opioid analgesics | Alcohol involved | Unintentional |
| Case date | Age | Indoor / outdoor | Pulse rate | Crystal methamphetamine | Other analgesics | Alcohol intoxication | Intentional |
| Case time | Residential postcode | Event postcode | Blood pressure | Cannabis | Benzodiazepines | Inhalant | Undetermined intent |
| Transport to hospital | Homelessness | Event coordinates | Respiratory rate | Synthetic cannabinoids | Anti-depressants | Other substance | |
| Reason for not transporting | Unemployment | Police co-attendance | Skin temperature | Emerging psychoactive substances | Anti-psychotics | ||
| Previous incarceration | Others on scene | Skin moisture | Cocaine | Anti-convulsants | |||
| Culturally and linguistically diverse | Minors on scene | Skin colour | 3,4-methylenedioxy-methamphetamine (MDMA) | Opioid pharmacotherapy treatments | |||
| Refugee background | GCS eye response | Gamma hydroxybutyrate (GHB) | Pharmaceutical stimulants | ||||
| GCS verbal response | Heroin | Peer administered naloxone | |||||
| GCS motor response | Ketamine | Other medication | |||||
| Naloxone administration | Lysergic acid diethylamide (LSD) | ||||||
| Naloxone dose | Mushrooms | ||||||
| Naloxone response | Other illicit drugs |
*Individual pharmaceutical drugs presented in S1 Table
Most individual substances are simply identified as ‘related’ to the ambulance attendance. The exceptions are alcohol, heroin, and pharmaceutical opioids. Attendances with any alcohol consumption, ranging from small (i.e., <1 standard drink) to large quantities are classified as ‘alcohol involved’, which is particularly useful when examining effects of possible interactions with other drugs. As blood alcohol levels are not completed by paramedics, an ‘alcohol intoxication’ proxy measure was devised. This distinction is based upon paramedic clinical assessment of intoxication, supported by the reported alcohol quantity consumed. Like other health professionals, paramedics undergo significant training to recognise the signs and symptoms of overdose, particularly for more common substances, in order to respond and provide treatment to the patient. This clinical knowledge is supplemented by their ability to draw on additional evidence available on scene that is not available in hospital or other clinical settings. For example, alcohol bottles, injecting equipment, medication bottles/vials/packets, friends, family and associates who can provide additional information, as well as signs and symptoms ensure that the clinical documentation of the case contains reliable information on substances involved in the attendance. Cases involving naloxone administration by paramedics with a positive response are classified as naloxone-responsive opioid-related cases. These are further defined as ‘heroin-‘, ‘specific pharmaceutical preparation-‘, or ‘unknown opioid-‘related cases.
To indicate collective impact of AOD consumption on an ambulance attendance, a supplementary classification of AOD use was defined and coded: unintentional AOD poisoning (overdose threshold met). Case inclusion criteria varies depending on the type of drug consumed, using proxy measures to identify cases with potential medical harms: (a) alcohol and/or illicit substances: potentially life threatening, identified by a clinical case involving a Glasgow Coma Scale (GCS) score of less than nine [25], low respiratory rate and/or paramedic concern for securing an airway; (b) pharmaceutical medications: concordant clinical picture of alcohol or illicit drug AOD poisoning, or the consumption of 10 or more times the typically prescribed dose for the specific preparation concerned. Case inclusion criteria for pharmaceutical drugs varied from that of alcohol and illicit substances due to the complexity of considering total drug effect for individual pharmaceutical medications, during the manual coding process. Case inclusion criteria also apply to coding categories used in the self-harm component of NASS: intentional AOD poisoning (AOD consumption with suicidal intent) and undetermined intent AOD poisoning (determination of intentional or unintentional AOD poisoning cannot be made); however, further explanation of these coding categories is beyond the scope of the present paper.
Step 5 –Data review and export
After a set of ePCRs are manually coded, the dataset is reviewed by project staff and extracted for data cleaning. The cleaning process is systematic, and each step must be completed before progression. A selection of summary variables are checked for missing data, and multiple ePCRs for the same patient are aggregated and duplicates removed. Data are then converted to a format suitable for analysis, exported, merged with the master dataset, and backed up. During this process, summary information from the data warehouse and master dataset are scrutinised to verify accuracy of the export process. Any unusual or unexpected results identified during analysis are re-reviewed to ensure data accuracy.
Coder training and validation
RAs undertake extensive, iterative training to ensure appropriate (a) case ascertainment and (b) case classification. RAs use coding rules and guidelines that detail the inclusion criteria, classification process, define case classification, and provide examples of common and uncommon attendances. New RAs are trained by senior researchers, and then paired with multiple experienced RAs on a rotating basis, until they and their senior coding partner are confident of coding quality. Senior researchers review records coded by new RAs to ensure inter- and intra-coder reliability. Following database changes (e.g., introduction of new variables), RAs are re-trained via workshops and “dummy case exercises”. Workshops are also used to identify coding difficulties, develop and test operational solutions and disseminate coding clarifications on an ongoing basis. If RAs are not confident in assigning codes for a specific attendance, the attendance is escalated to a senior researcher for review (this occurs in <1% of attendances), rules and guidelines clarified accordingly and disseminated to the coding workforce.
Coding audits are conducted on a routine bases to ensure inter-coder reliability. These provide a learning tool for RAs, and parameters may change over time based on the relative experience of the current coding team and introduction of new coding modules. Results of a recent coding audit are described. A maximum of 90 previously coded attendances per RA were extracted, from the Victoria Quarter 1 2017 dataset. The 90 attendances met 26 criteria for case classification. Each RA then re-coded a random selection of records where they were not the original coder. At that time, there were 23 individual RAs, and the records they re-coded came from an average of 9±3 (mean±standard deviation) other RAs. A total of 1,718 attendances were re-coded, which meant re-coding of 221,622 AOD variables, of which only 470 differences were identified. Differences were systematically identified, and reviewed by a senior researcher for personalised feedback to each RA and then followed up with systematic team training.
Analysis
Project success remains contingent on active partnerships with jurisdictional ambulance services. Senior staff adopt a collaborative and inclusive approach, ensuring project buy-in and commitment by implementing: (a) transparent data use processes communicated by routine reporting; (b) formal agreements to ensure data provision, analysis and reporting meet ambulance service requirements; (c) consistent opportunities for contribution, feedback and review of outputs; and (d) formal acknowledgement of partnerships in dissemination.
Time lags for data transfer vary across jurisdictional ambulance services, with most data available for coding between one-to-three months from the end of the data collection period. Data processing and coding at Turning Point ensures data consistency, quality, and timeliness. Generally, manual coding can be completed within one to two months of data acquisition, depending on the number of attendances and project reporting requirements.
Output variables
NASS captures more than 140 variables, including patient and scene details, physical condition of patient and substances consumed. Ethnicity is collected within the raw ambulance service data for some jurisdictions, however it is not collected reliably on a national basis and we do not report it. Not all ambulance services collect details on ethnicity as the time they are with the patient is limited and the details of ethnicity are often not clinically relevant to stabilising and treating the patient. Extensive sociodemographic variables are collected and coded, such as homelessness, unemployment, previous incarceration, and culturally and linguistically diverse and refugee backgrounds (Table 2).
Ambulance attendances are coded to the level of the individual substance, which is not routinely captured by other population-level data (for example, hospital and emergency department data). Output variables are summarised in Table 2. The column of pharmaceutical medications can be expanded into 82 individual commonly used medications within the broader drug classes presented (S1 Table). The surveillance system is flexible in that specific drugs can be added as they become of interest. Of note, some variables are not available from all ambulance systems (e.g. event coordinates by GPS only available from New South Wales and Victoria) due to jurisdictional system capacity and project agreements.
Relevant cases identified at each process step
Relevant cases identified at each step of the NASS process, using 12 months of Victorian data (2016–17 financial year), are presented in Table 3. The total rate of case ascertainment across the five steps of the process in this example year was 10.0%.
Table 3. Identification of relevant cases through the NASS process, Victoria, 2016–2017 financial year*.
| Process step | Number of ambulance attendances | Inclusion rate from preceding step |
|---|---|---|
| 1 –Ambulance data collection | 497,814 | N/A |
| 2 –Provided to Turning Point after filtering | 128,641 | 25.8% |
| 3 –Proceeded to coding after initial data processing | 128,641 | 100.0% |
| 4 –Case ascertainment (deemed to be AOD-related) | 50,685 | 39.4% |
| 5 –Available for analysis after data export | 49,647 | 98.0% |
*Australian financial year, from 1 July 2016 to 30 June 2017
Cases that do not meet inclusion criteria for AOD consumption at Step 4 are not discarded, but may be classified for inclusion in other components of NASS, including those focussing on mental health, self-harm or violence.
Demonstration of data utility
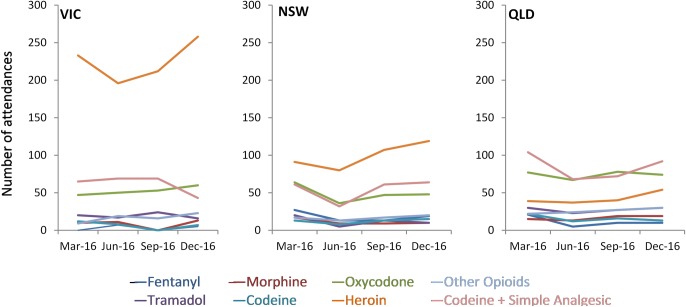
Fig 2 highlights capacity of NASS to analyse trends for individual substances and medications not captured in other population-level AOD data, and presents opioid-related ambulance attendances from the three largest jurisdictions (New South Wales, Queensland and Victoria). These features have important policy implications. For example, data have been used as supporting evidence in the selection of preparations to include in SafeScript, the real-time prescription monitoring program recently implemented in Victoria [26], as well as the evaluation of lock-out laws in Sydney’s entertainment districts [27].
Fig 2. Opioid-related ambulance attendances by jurisdiction, March, June, September and December 2016.
Australian Capital Territory, Northern Territory and Tasmania not presented due to small numbers.
Additionally, data have been used to elucidate gender and age affects in the context of attendances for AOD harms. Examples include examination of inhalant-related harms in young females, a group that is traditionally underrepresented in AOD research, with the majority of research to date relating only to young males [28]. Acute harms relating to AOD use and co-occurring self-harm and mental health symptomology in children under 12 years have also been described and compared with an older cohort, addressing a knowledge gap as people under 12 are excluded from routine survey research [29].
Results and discussion
We describe a novel method of monitoring, analysing and reporting acute AOD-related harms at a population level, using coded ambulance clinical records. Importantly, this project provides consistent, detailed, and timely data with spatial and temporal analysis capacity for AOD-related harms not captured by other systems. This project has been successfully established, with partnerships built across Australian ambulance services, to provide a national surveillance system of acute AOD-related harms with comprehensive coverage that includes diverse and hard-to-reach groups, such as drug-using populations, youth who are not captured by survey data or those experiencing acute issues that only present at a time of crises.
There are multiple benefits of undertaking monitoring in this way. NASS uses coded patient clinical notes from the ePCR, therefore there are no recruitment or self-selection biases, and there is no added workload for paramedics. A centralised coding system at Turning Point is efficient, timely, reliable and accurate and does not require duplication across jurisdictions. Reporting is highly specific with regards to substance type, and therefore policy, intervention and practice changes can be informed by examining emerging trends and employing a public health approach. NASS provides an internationally unique resource that sits alongside population measures of AOD use and burden of disease modelling, to provide an understanding of the impacts of AOD consumption. Data from NASS have informed public policy interventions and clinical practice related to AOD trends and harm [28–32]. Additionally, Victorian data are freely accessible and used by the public, policy makers, clinicians, and researchers via an online interactive website (www.AODstats.org.au). Spatial and temporal data can be used to inform service provision requirements, with highly granular data available (Fig 3). These data have clinical utility, and have been used to identify emerging trends related to the misuse of prescription medications (e.g., quetiapine [33] and pregabalin [34]), illicit drugs (e.g., GHB) [35] as well as mental health harms (e.g., methamphetamine in psychosis-related ambulance attendances [36]). Data can also be used to highlight population sub-groups at particular risk of harm, who may be hard-to-reach (e.g., substance use in young adolescents) [29]. Data has also been used to compare patterns of harms in substances and patterns of substances in specific harms [37].
Fig 3. The number of opioid-related ambulance attendances in one local government area in metropolitan Melbourne.
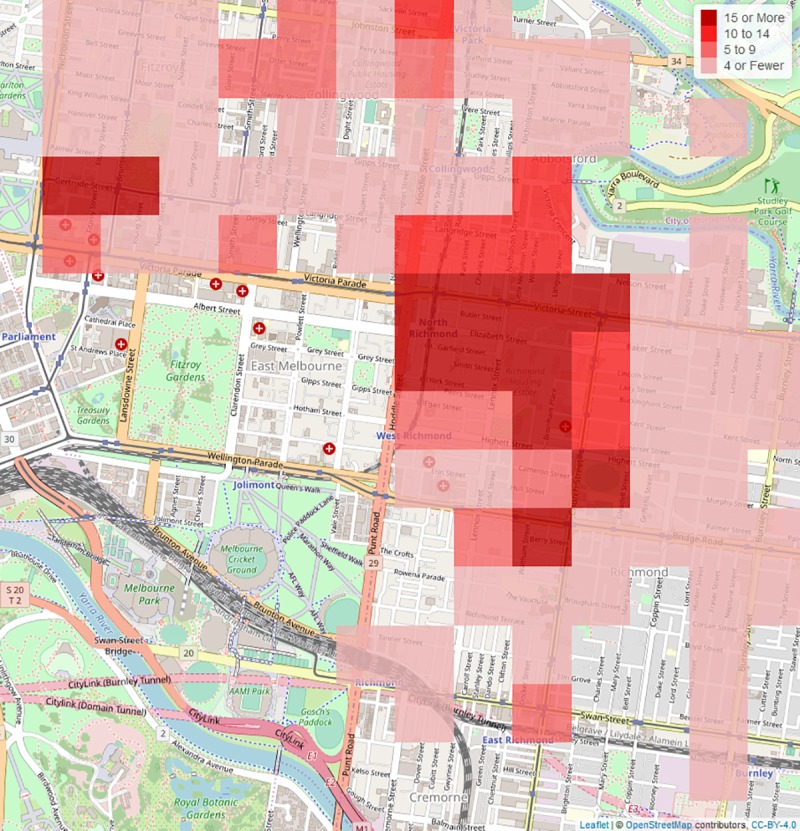
Ambulance attendances are shown within 250 metre squares, based on GPS data. The map source information is provided by OpenStreetMaps contributors.
In terms of limitations, the data do not capture attendances related to chronic harms; data only relate to acute AOD harms where an ambulance is called and attends. Like other coded health datasets, NASS is reliant on clinical information collected for operational rather than research purposes, with the potential for incomplete, inaccurate, or inconsistent recording of variables impacting data quality. Primary filtering of data may exclude cases of interest; however the filters are set to be intentionally broad to improve capture of relevant cases, and the potential for missing cases means that coded NASS data are conservative and under-estimate the number of AOD-related cases. Extensive ePCR scrutiny by trained staff coupled with collaborative communication between project staff and ambulance services is undertaken to minimise data inconsistencies or irregularities. Additionally, although ambulance services are considered universal in Australia, there are financial and geographic barriers to access in very remote areas of the country. NASS has been possible in Australia due to the nature of ambulance services providing a universal service at a state and territory level, covering a large proportion of the population using the same or similar ePCR systems. Other countries may face challenges introducing such a system due to large numbers of small ambulance services, lack of common ePCR systems, dependence on paper-based systems, or financial barriers to access.
Efforts to include the remaining two Australian jurisdictions are ongoing. A trial to code South Australia’s data found ongoing inclusion was unworkable due to additional resources associated with their paper-based patient clinical record system. Negotiations are expected to recommence when an ePCR system becomes operational in South Australia. Data provision from Western Australia commenced in late 2018, and comparable data provision is anticipated in 2020.
There are opportunities to improve NASS’s utility. Like the Australian hospital admission dataset, the NASS data presented are coded on the basis of episodes of care rather than individual patients. Although tracking individuals had not been possible, our data has accurately represented total service burden. A process is nearing completion for Victorian and NSW data to use a SLK to enable analysis by individuals rather than attendances. This SLK also enables linkage across health datasets, to track an individual’s trajectory through the health system to inform: (a) their treatment needs; (b) service provision; and (c) longer term outcomes, including death. This dataset will be improved by continuous coverage across jurisdictions other than Victoria, and inclusion of the remaining two Australian states. A target of future research is to develop rapid reporting methods, artificial intelligence and natural language processing to improve coding efficiencies so that emerging issues and patterns can be identified and reported faster. Coding for commonly co-occurring issues [2,38], such as mental health, violence, and self-harm, are conducted in complimentary modules of the project, and add significantly to the value of the data by capturing the nature and context of such presentations. In summary, we have demonstrated the utility of an internationally unique approach to quantify and monitor acute AOD-related harms in the community, providing a methodology that other countries and jurisdictions could adopt.
Conclusions
Excessive consumption of alcohol and illicit drugs, and extra-medical use of pharmaceutical medications, are major avoidable risk factors for disease, illness, injury and death. To understand the impact of AOD consumption and develop public health responses, it is important that related harms are measured. Coded ambulance attendance data offers a timely source of information on harms that may be missed in other datasets. This paper describes NASS, an internationally unique surveillance system that captures acute harms related to individual drugs using coded ambulance clinical records, reporting temporal and spatial trends within months of their occurrence. NASS has been used to inform and evaluate policy approaches and potential points of intervention at the local and national level, as well as guide workforce development needs and clinical practice.
Supporting information
(PDF)
(PDF)
Acknowledgments
We gratefully acknowledge our funders, project partners in the state and territory ambulance services, the paramedics who create the patient care records, the coding team at Turning Point, including the database manager Mr Mark Hoffmann, who together create this unique resource, and the patients upon whom this dataset is based. We also acknowledge the contribution of Professor Paul Dietze, who, during his time at Turning Point, established the first Ambo Project dataset for AOD in metropolitan Melbourne, upon which this project was built. The Fig 3 source map data copyrighted OpenStreetMap contributors and available from https://www.openstreetmap.org, and this complies with CC BY 4.0.
Data Availability
The datasets generated and analysed during the current study are not publicly available due to the need to protect privacy and confidentiality. Ambulance data are provided to Turning Point under strict conditions for the storage, retention and use of the data. The current approval permits storage of the data at one site, Turning Point, with any analysis to be undertaken onsite, no data to be removed, and no dissemination of unit level data. Researchers wishing to undertake additional analyses of the data are invited to contact Turning Point as the data custodians at aodstats@turningpoint.org.au.
Funding Statement
NAP is funded by the Victorian Department of Health and Human Services and the Commonwealth Department of Health (Australia). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Degenhardt L, Whiteford H, Hall WD. The Global Burden of Disease projects: What have we learned about illicit drug use and dependence and their contribution to the global burden of disease? Drug Alc Rev. 2014;33:4–12. [DOI] [PubMed] [Google Scholar]
- 2.Room R, Babor T, Rehm J. Alcohol and public health. Lancet. 2005; 365: 519–30. 10.1016/S0140-6736(05)17870-2 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Management of Substance Abuse—Facts and Figures Geneva 2018 [cited 2018 21 May 2018]. Available from: http://www.who.int/substance_abuse/facts/en/.
- 4.Australian Institute of Health and Welfare (AIHW). National Drug Strategy Household Survey (NDSHS) 2016—key findings Canberra: Australian Institute of Health and Welfare, 2017. [Google Scholar]
- 5.United Nations Office on Drugs and Crime. World Drug Report 2017. Vienna: United Nations, 2017. [Google Scholar]
- 6.Rehm J, Gmel GE, Gmel G, Hasan OSM, Imtiaz S, Popova S, et al. The relationship between different dimensions of alcohol use and the burden of disease—an update. Addiction. 2017;112: 968–1001. 10.1111/add.13757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogeil RP, Gao CX, Rehm J, Gmel G, Lloyd B. Temporal changes in alcohol‐related mortality and morbidity in Australia. Addiction. 2016;111: 626–34. 10.1111/add.13213 [DOI] [PubMed] [Google Scholar]
- 8.Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379: 55–70. 10.1016/S0140-6736(11)61138-0 [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Global status report on alcohol and health, 2014. Geneva: World Health Organization, 2014. [Google Scholar]
- 10.Ogeil RP, Matthews S, Lloyd B. Alcohol and burden of disease studies: Challenges in conceptualising and measuring harm. Aust Epidemiol. 2016;23: 49–52. [Google Scholar]
- 11.Saunders JB, Room R. Enhancing the ICD System in Recording Alcohol's Involvement in Disease and Injury. Alcohol Alcohol. 2012;47: 216–18. 10.1093/alcalc/ags024 [DOI] [PubMed] [Google Scholar]
- 12.White V, Williams T. Australian secondary school students’ use of tobacco, alcohol, and over-the-counter and illicit substances in 2014. Carlton, Victoria: Cancer Council Victoria; 2016. [Google Scholar]
- 13.Schmidt LA, Mäkelä P, Rehm J, Room R. Alcohol: equity and social determinants. In: Blas ESK A., editor. Equity, social determinants and public health programmes Geneva: World Health Organization; 2010. p. 11–29. [Google Scholar]
- 14.Stockwell T, Zhao J, Greenfield T, Livingston M, Meng Y. Estimating under‐and over‐reporting of drinking in national surveys of alcohol consumption: identification of consistent biases across four English‐speaking countries. Addiction. 2016;111: 1203–13. 10.1111/add.13373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magura S, Kang S-Y. Validity of self-reported drug use in high risk populations: a meta-analytical review. Subst Use Misuse. 1996;31: 1131–53. 10.3109/10826089609063969 [DOI] [PubMed] [Google Scholar]
- 16.Ogeil RP, Room R, Matthews S, Lloyd B. Alcohol and burden of disease in Australia: the challenge in assessing consumption. Aust NZ J Publ Health. 2015;39: 121–23. [DOI] [PubMed] [Google Scholar]
- 17.Camenga DR, Gaither J, Leventhal J, Ryan S. Increasing incidence of hospital admissions for opioid poisonings in adolescents and young adults: 2000–2009. Drug Alcohol Depend. 2015;146: e237. [Google Scholar]
- 18.Nambiar D, Stoové M, Hickman M, Dietze P. A prospective cohort study of hospital separations among people who inject drugs in Australia: 2008–2013. BMJ Open. 2017;7: e014854 10.1136/bmjopen-2016-014854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seth P, Rudd RA, Noonan RK, Haegerich TM. Quantifying the epidemic of prescription opioid overdose deaths. Am J Publ Health. 2018;108: 500–02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomes T, Mamdani MM, Dhalla IA, Cornish S, Paterson JM, Juurlink DN. The burden of premature opioid‐related mortality. Addiction. 2014;109: 1482–88. 10.1111/add.12598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Studdert DM, Walter SJ, Kemp C, Sutherland G. Duration of death investigations that proceed to inquest in Australia. Inj Prev. 2016;22: 314–20. 10.1136/injuryprev-2015-041933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chou R, Korthuis PT, McCarty D, Coffin PO, Griffin JC, Davis-O'Reilly C, et al. Management of Suspected Opioid Overdose With Naloxone in Out-of-Hospital Settings: A Systematic Review. Ann Int Med. 2017;167: 867–75. 10.7326/M17-2224 [DOI] [PubMed] [Google Scholar]
- 23.Dietze PM, Cvetkovski S, Rumbold G, Miller P. Ambulance attendance at heroin overdose in Melbourne: the establishment of a database of Ambulance Service records. Drug Alcohol Rev. 2000;19: 27–33. [Google Scholar]
- 24.Cox S, Martin R, Somaia P, Smith K. The development of a data-matching algorithm to define the ‘case patient’. Aust Health Rev 2013;37: 54–59. 10.1071/AH11161 [DOI] [PubMed] [Google Scholar]
- 25.Teasdale G, Murray G, Parker L, Jennett B. Adding up the Glasgow Coma Score. Acta Neurochir Suppl. 1979;28: 13–16. 10.1007/978-3-7091-4088-8_2 [DOI] [PubMed] [Google Scholar]
- 26.Liew D, Joules E, Booth J, Garrett H, Frauman A. Evidence to inform the inclusion of Schedule 4 prescription medications on a real-time prescription monitoring system 2017Department of Clinical Pharmacology and Therapeutics and Pharmacy Department. Austin Health; Melbourne, Victoria [Google Scholar]
- 27.Centre for Program Evaluation. Evaluation of the Sydney CBD Entertainment Precinct Plan of Management. 2016. Sydney, New South Wales: Department of Treasury NSW Government. [Google Scholar]
- 28.Crossin R, Scott D, Witt KG, Duncan JR, Smith K, Lubman DI. Acute harms associated with inhalant misuse: Co-morbidities and trends relative to age and gender among ambulance attendees. Drug Alcohol Depend. 2018;190: 46–53. 10.1016/j.drugalcdep.2018.05.026 [DOI] [PubMed] [Google Scholar]
- 29.Scott D, Crossin R, Ogeil R, Smith K, Lubman DI. Exploring Harms Experienced by Children Aged 7 to 11 Using Ambulance Attendance Data: A 6-Year Comparison with Adolescents Aged 12–17. Int J Environ Res Publ Health. 2018;15: 1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coomber K, Curtis A, Vandenberg B, Miller P, Heilbronn C, Matthews S, et al. Aggression and violence at ambulance attendances where alcohol, illicit and/or pharmaceutical drugs were recorded: a 5-year study of ambulance records in Victoria, Australia. Drug Alcohol Depend. 2019; 10.1016/j.drugalcdep.2019.107685 [DOI] [PubMed] [Google Scholar]
- 31.Curtis A, Droste N, Coomber K, Guadagno B, Mayshak R, Hyder S, et al. The Impact of Twenty-Four-Hour Public Transport in Melbourne, Australia: An Evaluation of Alcohol-Related Harms. J Stud Alcohol Drugs. 2019;80: 314–318. [PubMed] [Google Scholar]
- 32.Kaar SJ, Gao CX, Lloyd B, Smith K, Lubman DI. Trends in cannabis-related ambulance presentations from 2000 to 2013 in Melbourne, Australia. Drug Alcohol Depend. 2015;155: 24–30. 10.1016/j.drugalcdep.2015.08.021 [DOI] [PubMed] [Google Scholar]
- 33.Heilbronn C, Lloyd B, McElwee P, Eade A, Lubman DI. Trends in quetiapine use and non‐fatal quetiapine‐related ambulance attendances. Drug Alcohol Rev. 2013;32: 405–11. 10.1111/dar.12028 [DOI] [PubMed] [Google Scholar]
- 34.Crossin R, Scott D, Arunogiri S, Smith K, Dietze PM, Lubman DI. Pregabalin misuse‐related ambulance attendances in Victoria, 2012–2017: characteristics of patients and attendances. Med J Aust. 2018;210: 75–79. 10.5694/mja2.12036 [DOI] [PubMed] [Google Scholar]
- 35.Arunogiri S, Gao CX, Lloyd B, Smith K, Lubman DI. The role of methamphetamines in psychosis-related ambulance presentations. Aust NZ J Psychiat. 2015; 49:939–46. [DOI] [PubMed] [Google Scholar]
- 36.Arunogiri S, Moayeri F, Crossin R, Killian JJ, Smith K, Scott D, et al. Trends in gamma-hydroxybutyrate-related harms based on ambulance attendances from 2012 to 2018 in Victoria, Australia. Addiction. 2019; 10.1111/add.14848. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen S, Crossin R, Middleton M, Lam T, Wilson J, Scott D, et al. Comparing rates and characteristics of ambulance attendances related to extramedical use of pharmaceutical opioids in Australia from 2013–2018. Addiction. 2019; 10.1136/bmjopen-2019-029170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel V, Flisher AJ, Hetrick S, McGorry P. Mental health of young people: a global public-health challenge. Lancet. 2007;369: 1302–13. 10.1016/S0140-6736(07)60368-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available due to the need to protect privacy and confidentiality. Ambulance data are provided to Turning Point under strict conditions for the storage, retention and use of the data. The current approval permits storage of the data at one site, Turning Point, with any analysis to be undertaken onsite, no data to be removed, and no dissemination of unit level data. Researchers wishing to undertake additional analyses of the data are invited to contact Turning Point as the data custodians at aodstats@turningpoint.org.au.