Abstract
BACKGROUND:
Patients with cancer-related pain are underrepresented in the opioid literature despite high opioid exposure and numerous risk factors for adverse opioid outcomes, including unnecessary persistent opioid use. The objective of this study was to determine the extent, historical trends, and predictors of new-onset persistent opioid use among older adult women after active breast cancer treatment.
METHODS:
Using Surveillance, Epidemiology, and End Results–Medicare data for opioid-naive women diagnosed with stage 0 to III breast cancer at the age of 66 to 90 years between 2008 and 2013, this study estimated overall and quarterly adjusted probabilities of new-onset persistent opioid use, which was defined as receiving ≥90 days’ supply of opioids in the year after active breast cancer treatment. Sensitivity analyses were conducted with an alternative definition of persistent opioid use: any opioid fill 90 to 180 days after active cancer treatment.
RESULTS:
Nearly two-thirds of the subjects received prescription opioid therapy during cancer treatment. Quarterly probabilities of new-onset persistent opioid use after active treatment ranged from 2% to 4%; in sensitivity analyses, the alternative outcome definition resulted in predicted probabilities ranging from 11.4% to 14.7%. Subjects with more advanced disease, a higher comorbidity burden, a low-income status, and greater opioid exposure during active cancer treatment were more likely to develop persistent opioid use.
CONCLUSIONS:
Persistent opioid use was an infrequent occurrence among older adult patients with breast cancer completing cancer treatment between 2008 and 2013. This finding was encouraging because of the concerning opioid trends seen in noncancer populations. However, opportunities to further mitigate unsafe opioid use as a complication of cancer care, including standardization of persistent opioid use definitions, should be explored.
Keywords: breast, Medicare, opioid analgesics, pain management, survivorship
INTRODUCTION
Improving the quality and safety of opioid prescribing remains a necessary strategy for curbing the opioid crisis in America. Prescription opioid overdoses claimed more than 17,000 lives in 2016 and have quadrupled since 1999.1,2 The misuse of prescription opioids, which encompasses taking opioids not as prescribed, using someone else’s opioid medication, and taking opioids for their euphoric effects, is also associated with an increased risk of engaging in heroin and illicit fentanyl use, which caused an additional 35,000 deaths in 2016.2–4
However, recent opioid prescribing recommendations, including the Guideline for Prescribing Opioids for Chronic Pain from the Centers for Disease Control and Prevention,5 are drawn from a body of evidence that is widely considered to be limited and of low quality.6,7 Notably, individuals with a history of cancer are largely absent from the opioid outcomes literature despite representing a sizeable, high-risk population.8 In 2016, there were an estimated 16 million cancer survivors living in the United States.9 This number is expected to grow 30% over the next 10 years. Patients with cancer experience a high prevalence of chronic pain after completing active treatment10–12 and comorbid mental health conditions associated with an increased risk for opioid addiction and overdose.13–19
With nearly two-thirds of people with cancer surviving at least 10 years beyond their diagnosis,20 it is crucial that we understand the burden of high-risk opioid use among cancer survivors resulting from their disease and its treatment. New-onset persistent opioid use specifically is being increasingly recognized as a serious and common health care complication demanding concerted prevention strategies, including strategies for patients undergoing curative cancer treatment.21,22
The purpose of this study was to determine the extent, historical trends, and predictors of new-onset persistent opioid use during the first year after the completion of active breast cancer treatment among older adult women. Older adult breast cancer survivors represent one of the largest subsets of the cancer survivor population. Older adults also have unique opioid-related risks and considerations. Despite having the lowest opioid overdose mortality rate of any age group,23 adults who are 65 years old or older have experienced sharp increases in opioid-related hospitalizations and emergency department visits24 and opioid use disorder treatment admissions in recent years.25 Older adults are physiologically more vulnerable to adverse effects of opioids than other age groups because of slower opioid metabolism and clearance,26 and they more often obtain opioids for misuse from physician prescriptions than younger adults.27 Findings from this research are necessary to inform strategies to prevent high-risk opioid use and outcomes as a complication of cancer care, particularly among older adults. This study will also help to address major gaps in knowledge about the broader impacts of the opioid crisis in the United States on populations with cancer-related pain.
MATERIALS AND METHODS
Data
Our study was performed with Surveillance, Epidemiology, and End Results (SEER)-Medicare linked data for the years 2007–2014.28 The SEER-Medicare database links cancer registry data from 19 large cancer registries across the United States with Medicare administrative claims data. This study was approved by the institutional review boards of the University of Kansas Medical Center and Medical College of Wisconsin.
Study Data and Cohort
We conducted a retrospective cohort study examining the probability and predictors of new-onset persistent opioid use in the year following the end of active breast cancer treatment. We defined the end of active cancer treatment as the last day following the breast cancer diagnosis date on which a subject recorded receipt of chemotherapy, radiation, or curative cancer surgery. We included women who had been diagnosed with stage 0 to III breast cancer between January 1, 2008, and December 31, 2013, at the age of 66 to 90 years and who had completed active cancer treatment by December 31, 2013. This ensured a full 12 months of prediagnosis observation of baseline covariates and a full 12 months of follow-up observation after active cancer treatment. Subjects had no prior cancer diagnoses. We excluded women with a prescription opioid claim in the 3 months leading up to their cancer diagnosis date to better ensure that any opioid use observed after their diagnosis was associated with breast cancer treatment. For a reliable assessment of study measures across the baseline, active treatment, and follow-up periods, subjects were required to have continuous Medicare Part A and B coverage from 12 months before their breast cancer diagnosis through the 12 months following the end of their active breast cancer treatment; Medicare Part D coverage was required from 3 months before the diagnosis through 12 months after the end of treatment. We excluded individuals who died or recorded a second primary cancer diagnosis during the 12-month follow-up period after the end of active cancer treatment or who did not have a full year of follow-up before December 31, 2014.
Measures
The primary outcome was new-onset persistent opioid use, which was defined as a ≥90-day opioid supply from prescription opioid claims recorded during the 12-month follow-up period after the end of active breast cancer treatment. For pain management regimens with concomitant use of long-acting and immediate-release opioids, we used the cabinet supply approach29 to determine total days’ supply for overlapping opioid prescriptions sharing the same active ingredient, route, and formulation. For example, a subject with a 5-day period with overlapping prescription claims for extended-release oxycodone tablets and immediate-release hydromorphone tablets would contribute 5 total days to her persistent opioid use outcome for that overlapping period because we assumed that these were intended to be used simultaneously. Alternatively, a subject with 5 days of overlap between 2 immediate-release oxycodone tablet prescription claims would contribute 10 total days to her persistent opioid use outcome for that overlapping period because we assumed consecutive use. In addition, we assessed the extent of patients’ opioid therapy received during their active cancer treatment phase by determining the percentage of active cancer treatment days for which patients had a prescription opioid supply available. We also measured select patient-level demographic, clinical, and treatment characteristics, as shown in Table 1.
TABLE 1.
Patient Demographic and Clinical Characteristics by Chemotherapy Status
| Characteristic | Total (n = 24,631) | No Chemotherapy (n = 20,712) | Chemotherapy (n = 3919) | P |
|---|---|---|---|---|
| Age, No. (%)a | ||||
| 66–75 y | 13,970 (56.7) | 10,846 (52.4) | 3124 (79.7) | |
| 76–85 y | 8894 (36.1) | 8142 (39.3) | 752 (19.2) | <.001 |
| 86–90 y | 1767 (7.2) | 1724 (8.3) | 43 (1.1) | |
| Race/ethnicity, No. (%) | ||||
| White, non-Hispanic | 20,212 (82.1) | 17,093 (82.5) | 3119 (79.6) | <.001 |
| Black, non-Hispanic | 1557 (6.3) | 1264 (6.1) | 293 (7.5) | |
| Hispanic | 1374 (5.6) | 1085 (5.2) | 289 (7.4) | |
| Other | 1488 (6.0) | 1270 (6.1) | 218 (5.6) | |
| Rural residence, No. (%)b | 4314 (17.5) | 3604 (17.4) | 710 (18.1) | .29 |
| % with high school education in census tract of residence, No. (%)c | ||||
| Quartile 1 | 4135 (16.8) | 3441 (16.6) | 694 (17.7) | <.001 |
| Quartile 2 | 5813 (23.6) | 4801 (23.2) | 1012 (25.8) | |
| Quartile 3 | 6782 (27.5) | 5733 (27.7) | 1049 (26.8) | |
| Quartile 4 | 7901 (32.1) | 6737 (32.5) | 1164 (29.7) | |
| Median household income in census tract of residence, No. (%)c | ||||
| Quartile 1 | 4363 (17.7) | 3663 (17.7) | 700 (17.9) | .337 |
| Quartile 2 | 5954 (24.2) | 4992 (24.1) | 962 (24.5) | |
| Quartile 3 | 6585 (26.7) | 5510 (26.6) | 1075 (27.4) | |
| Quartile 4 | 7729 (31.4) | 6547 (31.6) | 1182 (30.2) | |
| Low-income subsidy recipient, No. (%) | 5595 (22.7) | 4710 (22.7) | 885 (22.6) | .845 |
| Charlson Comorbidity Index, No. (%) | ||||
| 0 | 12,862 (52.2) | 10,645 (51.4) | 2217 (56.6) | |
| 1 | 6808 (27.6) | 5733 (27.7) | 1075 (27.4) | <.001 |
| ≥2 | 4961 (20.1) | 4334 (20.9) | 627 (16.0) | |
| Breast cancer stage, No. (%) | ||||
| 0 | 3594 (14.6) | 3573 (17.3) | 21 (0.5) | <.001 |
| I | 12,597 (51.1) | 11,581 (55.9) | 1016 (25.9) | |
| II | 6838 (27.8) | 4855 (23.4) | 1983 (50.6) | |
| III | 1602 (6.5) | 703 (3.4) | 899 (22.9) | |
| Tumor size, No. (%) | ||||
| ≤2 cm | 17,243 (70.0) | 15,489 (74.8) | 1754 (44.8) | |
| >2 to <5 cm | 6110 (24.8) | 4384 (21.2) | 1726 (44.0) | <.001 |
| ≥5 cm | 1278 (5.19) | 839 (4.05) | 439 (11.2) | |
| Curative surgery, No. (%) | ||||
| No or minimal surgery | 1789 (7.3) | 1602 (7.7) | 187 (4.8) | |
| Partial mastectomy | 14,043 (57.0) | 12,276 (59.3) | 1767 (45.1) | <.001 |
| Mastectomy | 8799 (35.7) | 6834 (33.0) | 1965 (50.1) | |
| Reconstructive surgery, No. (%) | 867 (3.5) | 593 (2.9) | 274 (7.0) | <.001 |
| Radiation, No. (%) | 13,280 (53.9) | 10,670 (51.5) | 2610 (66.6) | <.001 |
| Hormone therapy, No. (%) | 4406 (17.9) | 3539 (17.1) | 867 (22.1) | <.001 |
| Active cancer treatment duration, mean (SD), d | 71.7 (74.1) | 48.5 (46.0) | 194 (74.1) | <.001 |
| Proportion of active cancer treatment with opioid supply, No. (%) | ||||
| 0% | 9933 (40.3) | 9237 (44.6) | 696 (17.8) | |
| >0% to 10% | 8854 (35.9) | 6349 (30.7) | 2505 (63.9) | <.001 |
| >10% | 5844 (23.7) | 5126 (24.7) | 718 (18.3) | |
Age refers to the age at breast cancer diagnosis.
Rural residence was defined as residence in a county with a Rural-Urban Continuum code of 4 to 9.
Quartiles for census tract–level demographic measures were constructed within the state of residence.
Statistical Analysis
We described outcomes and demographic, clinical, and treatment characteristics for the cohort overall and by chemotherapy status. We subset the cohort by receipt of chemotherapy because, in prior literature, chemotherapy treatment has been a strong predictor of opioid use among patients with cancer.21,30 For our primary analysis, we estimated longitudinal trends, measured at the calendar quarter level, of the probability of developing new-onset persistent opioid use after active breast cancer treatment overall and by chemotherapy status with a modified Poisson model.31 Using the modified Poisson model, we predicted the probability of opioid prescribing for each quarter between 2008 and 2013, while controlling for all other covariates.31 We obtained adjusted predicted values via marginal standardization.32 In addition, on the basis of the modified Poisson model, we estimated the relative risk (RR) of the outcome associated with the demographic, clinical, and treatment characteristics across the full study period for the entire cohort and by chemotherapy status.33 Statistical significance was assumed at P < .05. Analyses were performed with Stata (StataCorp, College Station, Texas) and SAS (version 9.4; SAS Institute, Inc, Cary, North Carolina). We adhered to Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines for observational cohort studies.34
Sensitivity Analysis
There is a lack of consensus about how to define persistent opioid use in claims data. The primary study analyses were replicated with an alternative outcome definition of new persistent opioid use: the receipt of any prescription opioid claim in the 90- to 180-day period following the index date at the start of follow-up. These findings are reported in the supporting information and are discussed in the main body of this article. The purpose of these sensitivity analyses is to aid in the interpretation of our primary findings in the context of a growing body of literature that often uses this alternative definition of persistent opioid use. We anticipated the alternative outcome definition to be a more inclusive measure of persistent opioid use than our chosen measure of a 90-day opioid supply received in a 12-month period.21,35–38
RESULTS
We analyzed a cohort of 24,631 older adult women who were opioid-naive before receiving a new diagnosis of stage 0 to III breast cancer between 2008 and 2013 (see Supporting Fig. 1 for the cohort selection algorithm). Fifty-seven percent of the overall cohort were diagnosed between the ages of 66 and 75 years, and more than 80% were white (Table 1). Two-thirds were diagnosed with stage 0 or I breast cancer, and most underwent either partial mastectomy (57%) or full mastectomy (36%). Only 16% received chemotherapy, whereas half received radiation, 18% initiated hormone therapy, and 3.5% received reconstructive surgery. The mean length of active cancer treatment was 72 days for the full cohort. Forty percent did not receive any opioid therapy during active cancer treatment; nearly one-quarter received opioid therapy for more than 10% of their active cancer treatment period.
Across the entire study period, nearly half the cohort filled at least 1 opioid prescription after active cancer treatment, whereas 2.8% developed new-onset persistent opioid use (Table 2). Persistent opioid use was more common among those who received chemotherapy than those who did not (4.9% vs 2.4%; P < .01) even though there were fewer chemotherapy patients filling any opioid prescription after active treatment (41% of the chemotherapy group vs 49% of the nonchemotherapy group; P < .01). A higher proportion of the subjects who received chemotherapy filled an opioid prescription during their active cancer treatment in comparison with the larger nonchemotherapy subset (82% vs 55%; P < .01).
TABLE 2.
Opioid Prescription Receipt Across Active Cancer Treatment Phases by Chemotherapy Status
| Total (n = 24,631) | No Chemotherapy (n = 20,712) | Chemotherapy (n = 3919) | P | |
|---|---|---|---|---|
| Any opioid prescription filled, No. (%) | ||||
| After diagnosis, before active treatment | 3038 (12.3) | 2446 (11.8) | 592 (15.1) | <.001 |
| During active treatment | 14,698 (59.7) | 11,475 (55.4) | 3223 (82.2) | <.001 |
| After active treatment | 11,677 (47.4) | 10,060 (48.6) | 1617 (41.3) | <.001 |
| New persistent opioid use, No. (%) | 699 (2.8) | 507 (2.4) | 192 (4.9) | <.001 |
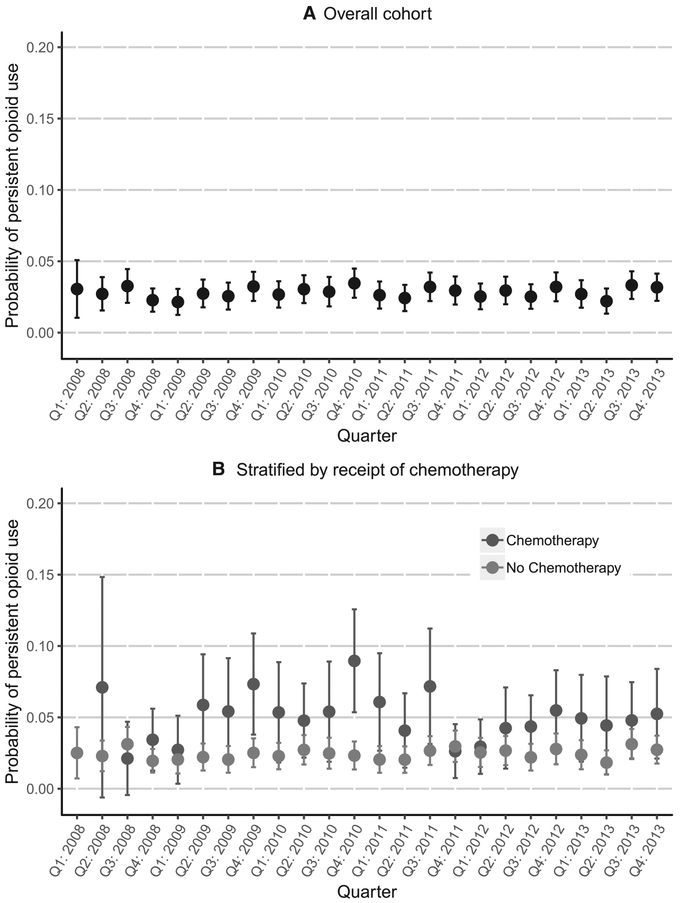
Figure 1 depicts the adjusted longitudinal trends for the predicted probability of experiencing new-onset persistent opioid use after the conclusion of active cancer treatment for the overall cohort and by chemotherapy status. The estimated quarterly predicted probability of new-onset persistent opioid use after active breast cancer treatment in the overall cohort was steady from 2008 to 2013 and ranged from 2.2% to 3.6%. There were no statistically significant differences in new-onset persistent opioid use across the study period. Quarterly probabilities of new-onset persistent opioid use were also low and consistent for the larger nonchemotherapy subset of the cohort and ranged from 2% to 3%, whereas those who received chemotherapy generally had higher and more varied probabilities of new-onset persistent opioid use over time.
Figure 1.
Adjusted quarterly probability of new-onset persistent opioid use from 2008 to 2013: (A) overall and (B) by chemotherapy status. The predicted probability of persistent opioid use was generated on the basis of marginal standardization (also called standardized predictive margins) with adjustments for demographic, clinical, and treatment characteristics. Quarter 1 in 2008 is not shown because of the small sample size.
In adjusted pooled analyses across the full study period, age, chemotherapy status, type of surgical intervention, radiation, hormone therapy, and tumor size were not significantly associated with the risk of new-onset persistent opioid use (Table 3). The risk of new-onset persistent opioid use significantly increased as the breast cancer stage, baseline comorbidity burden, and length of active cancer treatment increased. Black women were less likely to develop new-onset persistent opioid use than white women (RR, 0.62; 95% CI, 0.46–0.84; P < .01), as were women who did not receive the low-income subsidy or lived in areas with a higher median household income. Compared with those who did not receive any opioid prescriptions during active cancer treatment, those who had an opioid supply for >0% to 10% of their active cancer treatment period had a 40% lower risk of developing new-onset persistent opioid use (95% CI, 0.46–0.78; P < .01), whereas there was a significant positive association with new-onset persistent opioid use at higher opioid supply levels during active cancer treatment. Receiving an opioid supply for more than 10% of active cancer treatment days was associated with a 4-fold increase in the risk of developing new-onset persistent opioid use (RR, 4.18; 95% CI, 3.49–5.02; P < .01).
TABLE 3.
Generalized Linear Model Estimates of the Risk of New-Onset Persistent Opioid Use From 2008 to 2013 by Chemotherapy Status
| Total (n = 24,631) | No Chemotherapy (n = 20,712) | Chemotherapy (n = 3919) | ||||
|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| Agea | ||||||
| 66–75 y | Reference | Reference | Reference | |||
| 76–85 y | 1.05 | 0.88–1.25 | 1.17 | 0.96–1.43 | 0.68 | 0.42–1.09 |
| 86–90 y | 0.98 | 0.71–1.36 | 1.09 | 0.78–1.53 | 0.40 | 0.06–2.84 |
| Race/ethnicity | ||||||
| White, non-Hispanic | Reference | Reference | Reference | |||
| Black, non-Hispanic | 0.62 | 0.46–0.84 | 0.51 | 0.35–0.76 | 0.89 | 0.50–1.58 |
| Hispanic | 0.84 | 0.61–1.17 | 0.94 | 0.66–1.35 | 0.61 | 0.29–1.26 |
| Other | 0.62 | 0.41–0.93 | 0.57 | 0.36–0.92 | 0.82 | 0.33–2.05 |
| Rural residenceb | 0.98 | 0.77–1.25 | 0.97 | 0.73–1.28 | 1.06 | 0.62–1.80 |
| % with high school education in census tract of residencec | ||||||
| Quartile 1 | Reference | Reference | Reference | |||
| Quartile 2 | 1.10 | 0.87–1.38 | 1.13 | 0.86–1.48 | 0.99 | 0.59–1.64 |
| Quartile 3 | 1.33 | 1.02–1.73 | 1.35 | 1.00–1.83 | 1.25 | 0.71–2.18 |
| Quartile 4 | 1.06 | 0.77–1.45 | 1.04 | 0.72–1.51 | 1.08 | 0.53–2.19 |
| Median household income in census tract of residencec | ||||||
| Quartile 1 | Reference | Reference | Reference | |||
| Quartile 2 | 0.85 | 0.68–1.07 | 0.81 | 0.63–1.04 | 1.07 | 0.64–1.80 |
| Quartile 3 | 0.64 | 0.49–0.83 | 0.61 | 0.45–0.82 | 0.85 | 0.47–1.54 |
| Quartile 4 | 0.68 | 0.50–0.92 | 0.63 | 0.44–0.88 | 0.98 | 0.49–1.95 |
| Low-income subsidy recipient | 1.97 | 1.65–2.37 | 2.08 | 1.69–2.56 | 1.52 | 1.01–2.28 |
| Charlson Comorbidity Index | ||||||
| 0 | Reference | Reference | Reference | |||
| 1 | 1.63 | 1.35–1.98 | 1.74 | 1.38–2.18 | 1.30 | 0.89–1.90 |
| ≥2 | 2.17 | 1.78–2.65 | 2.35 | 1.86–2.97 | 1.56 | 1.01–2.40 |
| Breast cancer stage | ||||||
| 0 | Reference | Reference | Reference | |||
| I | 1.39 | 1.03–1.89 | 1.31 | 0.96–1.78 | Stages 0 and I combined | |
| II | 1.95 | 1.39–2.75 | 1.78 | 1.23–2.57 | 1.14 | 0.68–1.91 |
| III | 3.55 | 2.34–5.37 | 3.77 | 2.32–6.13 | 2.27 | 1.21–4.24 |
| Tumor size | ||||||
| ≤2 cm | Reference | Reference | Reference | |||
| >2 to <5 cm | 1.09 | 0.84–1.41 | 1.09 | 0.78–1.52 | 1.09 | 0.71–1.69 |
| ≥5 cm | 1.00 | 0.69–1.45 | 1.03 | 0.63–1.69 | 0.89 | 0.47–1.70 |
| Curative surgery | ||||||
| No or minimal surgery | Reference | Reference | Reference | |||
| Partial mastectomy | 1.10 | 0.79–1.54 | 1.13 | 0.75–1.70 | 0.59 | 0.27–1.29 |
| Mastectomy | 1.06 | 0.75–1.50 | 1.15 | 0.79–1.68 | 0.80 | 0.35–1.86 |
| Reconstructive surgery | 1.26 | 0.83–1.90 | 1.42 | 0.80–2.52 | 0.95 | 0.51–1.77 |
| Chemotherapy | 1.21 | 0.88–1.65 | N/A | N/A | ||
| Radiation | 1.02 | 0.81–1.28 | 0.97 | 0.70–1.34 | 0.69 | 0.42–1.14 |
| Hormone therapy | 0.92 | 0.73–1.16 | 0.97 | 0.74–1.28 | 0.65 | 0.44–0.97 |
| Days of active cancer treatment | 1.00 | 1.00–1.00 | 1.00 | 1.00–1.01 | 1.00 | 1.00–1.01 |
| Proportion of active cancer treatment with opioid supply | ||||||
| 0% | Reference | Reference | Reference | |||
| >0% to 10% | 0.60 | 0.46–0.78 | 0.68 | 0.49–0.92 | 1.97 | 0.78–5.01 |
| >10% | 4.18 | 3.49–5.02 | 3.27 | 2.67–4.01 | 33.06 | 13.61–80.31 |
Abbreviations: N/A. not applicable; RR, relative risk.
Models were also adjusted for the calendar quarter of the end of active cancer treatment and the Surveillance, Epidemiology, and End Results tumor registry region.
Age refers to the age at breast cancer diagnosis.
Rural residence was defined as residence in a county with a Rural-Urban Continuum code of 4 to 9.
Quartiles for census tract–level demographic measures were constructed within the state of residence.
Among the 3919 women who received chemotherapy, the risk of new-onset persistent opioid use was 35% lower for those with hormone therapy treatment (RR, 0.65; 95% CI, 0.44–0.97; P = .03). A diagnosis of stage III disease was associated with a greater risk of new-onset persistent opioid use in comparison with those with stage 0 or I disease (RR, 2.27; 95% CI, 1.21–4.24; P = .01). New-onset persistent opioid use was also more likely as the duration of the active cancer treatment period increased. Obtaining a prescription opioid supply for more than 10% of the chemotherapy subpopulation’s active treatment days increased the risk of new-onset persistent opioid use 33-fold (RR, 33.06; 95% CI, 13.61, 80.31; P < .01). Receipt of the low-income subsidy was associated with a 50% greater risk of the outcome, as well (95% CI, 1.01–2.28; P = .04).
Sensitivity Analysis
In sensitivity analyses evaluating an alternative persistent opioid use outcome measure, which was defined as an opioid claim recorded 90 to 180 days after the end of active cancer treatment, longitudinal trends in the predicted probability of new persistent opioid use were consistent from 2008 to 2013 at a larger magnitude in comparison with the primary analyses (Supporting Fig. 2). The quarterly probability of developing new persistent opioid use after active breast cancer treatment in the full cohort ranged from 11.4% to 14.7%. In the chemotherapy subset, the predicted probability ranged from 12.3% to 23.6%. In pooled analyses using the alternative outcome definition, we estimated similar findings for the associations of census tract–level median household income, comorbidity status, tumor stage, low-income subsidy, length of active cancer treatment, and proportion of active treatment days with opioid supply with persistent opioid use in comparison with the analyses using the primary outcome definition (Supporting Table 1). However, in sensitivity analyses, partial mastectomy and full mastectomy were protective of the alternative new-onset persistent opioid use definition in comparison with no to minimal surgery, as were radiation treatment and belonging to the older age categories. Black race was no longer significantly associated with new persistent opioid use. Receipt of reconstructive surgery was newly associated with an increased risk of persistent opioid use when the alternative outcome definition was used (RR, 4.56; 95% CI, 3.83–5.42; P < .01).
DISCUSSION
We examined longitudinal trends of new-onset persistent opioid use in the year following the completion of active breast cancer treatment among older adult women. To our knowledge, this is the first study to describe historical patterns of persistent opioid use in any clinical population. Overall, 1 in 35 older adult women who were not using prescription opioids before their breast cancer diagnosis went on to exhibit persistent opioid use after finishing their breast cancer treatment. From 2008 to 2013, the predicted probability of new-onset persistent opioid use after breast cancer treatment varied little and hovered close to 3%.
In 2018, nearly half of the estimated 266,120 women diagnosed with invasive breast cancer were older adults,39 and 90% of these women are expected to survive for at least 5 years after their diagnosis.40 With nearly two-thirds of our previously opioid-naive cohort experiencing new exposure to prescription opioids as part of their cancer diagnosis and treatment, it is imperative that providers routinely assess the risk of inadvertent downstream persistent opioid use at the outset of cancer care. In April 2019, the Centers for Disease Control and Prevention issued a clarification regarding their opioid prescribing guidelines, which stated that the guidelines do not apply to patients undergoing active cancer treatment.41 However, the Centers for Disease Control and Prevention stated that cancer pain management after active cancer treatment should be informed by guidelines from the American Society for Clinical Oncology8 and the National Comprehensive Cancer Network,42 which urge cautious use of opioids to minimize the risk of abuse and addiction after active cancer treatment.
Regardless, it was encouraging to find a low and historically stable probability of new-onset persistent opioid use after breast cancer treatment among older adult women. It is also possible that the new-onset persistent opioid use we observed in the year after active breast cancer treatment may have a legitimate indication for some of these 2% to 3% of women in some circumstances. However, our administrative data and the lack of consensus about the appropriate role of opioids in treating chronic pain precluded us from determining the clinical appropriateness of chronic opioid therapy at the patient level.
That said, there is likely room for improvement in preventing unnecessary persistent opioid use and its potentially adverse clinical consequences. We identified predictors in this population that could be useful in targeting efforts to prevent unnecessary persistent opioid use after breast cancer treatment. Patients who had multiple comorbid conditions at the time of their breast cancer diagnosis and those diagnosed with more advanced disease were at significantly greater risk of new-onset persistent opioid use. Low-income individuals were also much more likely to develop chronic opioid use. Providers should also be cognizant that greater opioid exposure during active cancer treatment, measured as the proportion of the active treatment period with an opioid supply available, increases the likelihood of conferring persistent opioid use after active cancer treatment. However, we recommend more detailed analyses in the future of the intensity and temporality of opioid use during active cancer treatment and its association with long-term opioid use in survivorship to assess the robustness of our measure of opioid exposure during active cancer treatment.
Our findings also underscore the lack of consensus around persistent opioid use measurement. We observed a large spectrum in the observed prevalence of new-onset persistent opioid use in our cohort between the outcome definition used in our primary analyses (90-day prescription opioid supply received in a 12-month period) and the alternative definition often used in recent literature (any opioid fill 90 to 180 days after the initial opioid exposure). The alternative outcome definition resulted in an observed probability of new-onset persistent opioid use (12%–15%) that was more than 5 times higher than the probability with our primary outcome definition (2%–3%).
We cannot deem any measure of persistent opioid use preferable to another on the basis of this study alone. However, it is notable that this wide variability in persistent opioid use prevalence across definitions of the measure could have implications for the perceived severity of the problem and effectiveness of interventions designed to prevent persistent opioid use. The alternative definition used in our sensitivity analyses may be beneficial because it would have greater sensitivity at capturing individuals who could be screened for a potential intervention. However, it identifies a larger subset of the population that initiates opioid therapy as persistent opioid users and may inflate the perceived severity of the problem at a population level and dissuade providers from prescribing opioid therapy when it is clinically appropriate. Conversely, our primary definition of persistent opioid use—or more restrictive definitions that have been used previously43–45—may risk understating the problem and cause some high-risk patients to be overlooked for interventions to prevent unnecessary persistent opioid use. Further work is needed to determine optimal strategies for identifying problematic persistent opioid use and how these measures should be tailored on the basis of pain indications and population characteristics.
Our study is subject to multiple limitations. As discussed previously, our primary outcome definition of new-onset persistent opioid use has high variability based on the measure’s definition, so comparisons with previous studies measuring long-term opioid use should be made with caution. Our findings may not be generalizable to patient populations with other cancer types or in other age groups. The nature of administrative claims data allowed us to observe only opioid prescription dispensations covered by a Medicare Part D prescription drug plan, so we could not confirm that patients consumed all prescription opioids that they filled or whether they consumed opioids obtained from other sources. We were also unable to assess the clinical appropriateness of persistent opioid use or examine the effects of the timing, intensity, and duration of pain experienced by subjects on their opioid use patterns.
In conclusion, new-onset persistent opioid use in the first year after active breast cancer treatment occurred in approximately 3% of older adult women who were opioid-naive before a new diagnosis of stage 0 to III breast cancer. The probability of developing persistent opioid use in this population has remained steady since 2008. Although persistent opioid use was an infrequent consequence of breast cancer diagnosis and treatment, the high rates of breast cancer diagnosis and long-term survival as well as current uncertainty about what constitutes problematic long-term opioid use patterns may necessitate increased scrutiny of the appropriate role of prescription opioids in managing acute and chronic cancer-related pain.
Supplementary Material
FUNDING SUPPORT
This work was supported by the American Cancer Society (RSG-11-098-01-CPHPS) and the National Institute on Minority Health and Health Disparities (grant 1R01MD010728-01 to Joan M. Neuner). Andrew W. Roberts was supported by a Clinical and Translational Science Awards grant from the National Center for Advancing Translational Sciences awarded to the University of Kansas for Frontiers: University of Kansas Clinical and Translational Science Institute (KL2TR002367). Aaron N. Winn was supported by the National Center for Research Resources, the National Center for Advancing Translational Sciences, and the Office of the Director of the National Institutes of Health (grant KL2TR001438).
Footnotes
Additional supporting information may be found in the online version of this article.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants—United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018;67:349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hedegaard H, Warner M, Minino AM. Drug overdose deaths in the United States, 1999–2016. NCHS Data Brief. 2017;294:1–8. [PubMed] [Google Scholar]
- 3.Cicero TJ, Ellis MS, Harney J. Shifting patterns of prescription opioid and heroin abuse in the United States. N Engl J Med. 2015; 373:1789–1790. [DOI] [PubMed] [Google Scholar]
- 4.Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. JAMA. 2016; 315:1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frieden TR, Houry D. Reducing the risks of relief—the CDC opioid-prescribing guideline. N Engl J Med. 2016;374:1501–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsen Y The CDC guideline on opioid prescribing: rising to the challenge. JAMA. 2016;315:1577–1579. [DOI] [PubMed] [Google Scholar]
- 8.Paice JA, Portenoy R, Lacchetti C, et al. Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34:3325–3345. [DOI] [PubMed] [Google Scholar]
- 9.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. [DOI] [PubMed] [Google Scholar]
- 10.Bennett M, Paice JA, Wallace M. Pain and opioids in cancer care: benefits, risks, and alternatives. Am Soc Clin Oncol Educ Book. 2017; 37:705–713. [DOI] [PubMed] [Google Scholar]
- 11.Hamood R, Hamood H, Merhasin I, Keinan-Boker L. Chronic pain and other symptoms among breast cancer survivors: prevalence, predictors, and effects on quality of life. Breast Cancer Res Treat. 2018; 167:157–169. [DOI] [PubMed] [Google Scholar]
- 12.van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 2016;51:1070–1090.e9. [DOI] [PubMed] [Google Scholar]
- 13.Jones SM, LaCroix AZ, Li W, et al. Depression and quality of life before and after breast cancer diagnosis in older women from the Women’s Health Initiative. J Cancer Surviv. 2015;9:620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell AJ, Ferguson DW, Gill J, Paul J, Symonds P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. Lancet Oncol. 2013;14:721–732. [DOI] [PubMed] [Google Scholar]
- 15.Zhao G, Okoro CA, Li J, White A, Dhingra S, Li C. Current depression among adult cancer survivors: findings from the 2010 Behavioral Risk Factor Surveillance System. Cancer Epidemiol 2014;38:757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edlund MJ, Martin BC, Fan MY, Devries A, Braden JB, Sullivan MD. Risks for opioid abuse and dependence among recipients of chronic opioid therapy: results from the TROUP study. Drug Alcohol Depend. 2010;112:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman KE, McCarthy EP, Recklitis CJ, Ng AK. Psychological distress in long-term survivors of adult-onset cancer: results from a national survey. Arch Intern Med. 2009;169:1274–1281. [DOI] [PubMed] [Google Scholar]
- 18.Manchikanti L, Giordano J, Boswell MV, Fellows B, Manchukonda R, Pampati V. Psychological factors as predictors of opioid abuse and illicit drug use in chronic pain patients. J Opioid Manag. 2007;3:89–100. [DOI] [PubMed] [Google Scholar]
- 19.Rice JB, White AG, Birnbaum HG, Schiller M, Brown DA, Roland CL. A model to identify patients at risk for prescription opioid abuse, dependence, and misuse. Pain Med. 2012;13:1162–1173. [DOI] [PubMed] [Google Scholar]
- 20.Noone A, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2015. Bethesda, MD: National Cancer Institute; 2018. [Google Scholar]
- 21.Lee JS, Hu HM, Edelman AL, et al. New persistent opioid use among patients with cancer after curative-intent surgery. J Clin Oncol. 2017;35:4042–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saraswathula A, Chen MM, Mudumbai SC, Whittemore AS, Divi V. Persistent postoperative opioid use in older head and neck cancer patients. Otolaryngol Head Neck Surg. 2019;160:380–387. [DOI] [PubMed] [Google Scholar]
- 23.Gomes T, Tadrous M, Mamdani M, Paterson M, Juurlink DN. The burden of opioid-related mortality in the United States. JAMA Netw Open. 2018;1:e180217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss AJ, Heslin KC, Barrett ML, Izar R, Bierman AS. Opioid-Related Inpatient Stays and Emergency Department Visits Among Patients Aged 65 Years and Older, 2010 and 2015: Statistical Brief #244. Rockville, MD: Agency for Healthcare Research and Quality; 2006. [PubMed] [Google Scholar]
- 25.Huhn AS, Strain EC, Tompkins DA, Dunn KE. A hidden aspect of the U.S. opioid crisis: rise in first-time treatment admissions for older adults with opioid use disorder. Drug Alcohol Depend. 2018;193:142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith HS. Opioid metabolism. Mayo Clinic Proc. 2009;84:613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schepis TS, McCabe SE, Teter CJ. Sources of opioid medication for misuse in older adults: results from a nationally representative survey. Pain. 2018;159:1543–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Cancer Institute. SEER-Medicare Linked Database. Accessed May 1, 2019 https://healthcaredelivery.cancer.gov/seermedicare/
- 29.Mosher HJ, Richardson KK, Lund BC. The 1-year treatment course of new opioid recipients in Veterans Health Administration. Pain Med. 2016;17:1282–1291. [DOI] [PubMed] [Google Scholar]
- 30.Cass AS, Alese JT, Kim C, et al. Analysis of opioid use following curative cancer treatment at a large urban safety-net hospital. Clin J Pain. 2018;34:885–889. [DOI] [PubMed] [Google Scholar]
- 31.Zou G A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 32.Williams R Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J. 2012;12:308–331. [Google Scholar]
- 33.Efron B An Introduction to the Bootstrap. New York, NY: Chapman and Hall; 1993. [Google Scholar]
- 34.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 35.Bennett KG, Harbaugh CM, Hu HM, et al. Persistent opioid use among children, adolescents, and young adults after common cleft operations. J Craniofac Surgery. 2018;29:1697–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brescia AA, Harrington CA, Mazurek A, et al. Factors associated with new persistent opioid usage after lung resection. Ann Thorac Surg. 2019;107:363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152:e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harbaugh CM, Nalliah RP, Hu HM, Englesbe MJ, Waljee JF, Brummett CM. Persistent opioid use after wisdom tooth extraction. JAMA. 2018;320:504–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Cancer Institute. Cancer Stat Facts: Female Breast Cancer. Accessed May 1, 2019 https://seer.cancer.gov/statfacts/html/breast.html
- 40.National Cancer Institute. SEER Cancer Statistics Review, 1975–2015. Accessed May 1, 2019 https://seer.cancer.gov/csr/1975_2015/results_merged/sect_04_breast.pdf
- 41.American Society for Clinical Oncology. CDC issues clarification on guideline for prescribing opioids for chronic pain in patients with cancer and sickle cell disease. Published April 9, 2019. Accessed May 8, 2019 https://www.ascopost.com/News/59920
- 42.National Comprehensive Cancer Network. Adult Cancer Pain (Version 2.2019). Published March 2019. Accessed May 8, 2019 https://www.nccn.org/professionals/physician_gls/pdf/pain.pdf
- 43.Connolly J III, Javed Z, Raji MA, Chan W, Kuo YF, Baillargeon J. Predictors of long-term opioid use following lumbar fusion surgery. Spine. 2017;42:1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim SC, Choudhry N, Franklin JM, et al. Patterns and predictors of persistent opioid use following hip or knee arthroplasty. Osteoarthritis Cartilage. 2017;25:1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raebel MA, Newcomer SR, Bayliss EA, et al. Chronic opioid use emerging after bariatric surgery. Pharmacoepidemiol Drug Saf. 2014; 23:1247–1257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.