Abstract
Purpose of Review
Smoking remains a leading preventable cause of premature death in the world; thus, developing effective and scalable smoking cessation interventions is crucial. This review uses the Obesity-Related Behavioral Intervention Trials (ORBIT) model for early phase development of behavioral interventions to conceptually organize the state of research of mobile applications (apps) for smoking cessation, briefly highlight their technical and theory-based components, and describe available data on efficacy and effectiveness.
Recent Findings
Our review suggests that there is a need for more programmatic efforts in the development of mobile applications for smoking cessation, though it is promising that more studies are reporting early phase research such as user-centered design. We identified and described the app features used to implement smoking cessation interventions, and found that the majority of the apps studied used a limited number of mechanisms of intervention delivery, though more effort is needed to link specific app features with clinical outcomes. Similar to earlier reviews, we found that few apps have yet been tested in large well-controlled clinical trials, although progress is being made in reporting transparency with protocol papers and clinical trial registration.
Summary
ORBIT is an effective model to summarize and guide research on smartphone apps for smoking cessation. Continued improvements in early phase research and app design should accelerate the progress of research in mobile apps for smoking cessation.
Keywords: Tobacco use, Smoking cessation, Smartphone apps, Mobile health, Mobile technology, ORBIT model
Introduction
Tobacco use and dependence is a leading cause of preventable death worldwide [1], and costs the global economy $422 billion in healthcare expenditures annually due to smoking-attributable diseases [2]. Smoking remains the leading preventable cause of premature death in the USA, with additional negative consequences to society such as absenteeism from work and increased health care cost and utilization [2]. Despite that interest in quitting is high across all sectors of society, evidence-based tobacco treatments remain underutilized and are implemented far below recommended funding levels [3]. Promoting more accessible evidence-based interventions is critical, including to subpopulations (e.g., psychiatric disorders) with the highest smoking prevalence [3, 4].
Current research suggests that mobile health technology (mHealth) for smoking cessation, such as smartphone apps, may be key in delivering wider-reaching treatment more efficiently [5]. Smoking cessation apps have a number of advantages for evidence-based treatment. Apps can deliver interventions in the individual’s natural context, potentially increasing real-world impact [6]. Further, by measuring behaviors in real time, apps increase ecological validity, reduce recall bias, and enable examination of behavioral patterns over time [7]. Apps also enable widespread distribution, improving dissemination [8–10] and overcoming barriers to treatment (e.g., transportation) [11, 12]. Apps can provide faster and more direct access to healthcare services and improve continuity of care. All these factors are key to cost-effectiveness [5, 9, 13]. Additionally, apps can be used to personalize or tailor treatments to the individual, such as tailored text messages and support based on user feedback [5, 9, 14], a major goal in precision science and medicine [15]. Finally, apps enable social networking, for example to share resources and experiences with other smokers who are trying to quit [16].
New smoking cessation apps are being released at a rapid rate: 400 apps were identified by review in 2013, and 546 apps were identified by review in 2017 [17, 18]. However, only a small portion of them has been empirically studied. Here, we present an empirical review of smoking cessation apps. We include reports on user-centered design research, pilot studies, and efficacy and effectiveness trials. mHealth is an interdisciplinary field that comprises not only behavior change technology but also the process of software design and engineering. User-centered design research ensures that app content can be received by the end user and have the intended effect. This is an important step prior to clinical trials to help ensure that the active ingredients of an intervention are implemented via the app. We also highlight available protocol papers, which report protocols for planned randomized controlled trials (RCTs) prior to reporting any results; an important step in scientific rigor and research transparency.
Overview of Review Methods
Articles were included if they (1) reported research on a smoking cessation app, (2) were peer-reviewed, and (3) were indexed in PubMed or Google Scholar. “Research” was defined as any empirical effort to develop, refine, or test an app. Key search terms were “smoking cessation applications” and “smoking cessation apps.” This search resulted in 349 articles. One-hundred twenty did not contain empirical studies, 56 were duplicates, 53 did not refer to smoking, and 78 did not refer to smoking cessation apps. A few additional apps were identified through literature review and targeted Google Scholar searches. A total of 33 apps were identified for review. The review focused on (1) quality of the programmatic effort to develop or study the app; (2) description of app features, including theoretical foundation; and (3) data supporting the effect of the app on clinical outcomes following recommended outcome criteria for smoking cessation trials [19•].
Programmatic effort was described based on the ORBIT model, a model for development of behavioral treatments for chronic conditions [20••], developed by the U.S. National Institutes of Health and Obesity-Related Behavioral Intervention Trials (ORBIT) consortium. ORBIT is focused on early and pre-efficacy phases of behavioral treatment development, while retaining terminology from the drug development model. The model provides a framework for research milestones prior to conducting large and costly efficacy or effectiveness trials. ORBIT defines four phases to optimize behavioral interventions: phase I involves intervention design (phase Ia) and refinement (phase Ib) using cost-efficient methods (e.g., reviews, qualitative research, single-case trials, design research); phase II involves initial testing such as feasibility, proof-of-concept, or pilot clinical studies; phase III involves testing efficacy (explanatory); and phase IV involves testing effectiveness (pragmatic). ORBIT is a useful model to guide the optimization of smoking cessation apps since there is a substantial amount of design and software research needed prior to testing these interventions in full-scale clinical trials.
As part of the iterative review process, we identified all the app features described in the literature and organized these into the following categories: psychoeducation about tobacco dependence, consequences, treatment; self-tracking of units of behavior (e.g., cigarettes smoked); personalized feedback including content customized to user (e.g., gender) or user responses (e.g., app surveys); social support via social media or networking; system of rewards (e.g., badges) for behaviors of importance; digital distractions to distract from craving or smoking; funnel-based apps provided new content contingent on completion of earlier content; geolocation to track user location; gamification used gaming to incentivize behavioral skills or knowledge gain [21] (note that this could include rewards, but rewards alone did not satisfy criteria for gamification); sensor-based just-in-time, defined as any feature that relied on an app sensor to provide just-in-time feedback to the user; and machine learning to tailor or deliver app content.
Results
Thirty-three apps were identified from the 55 articles that met review criteria (Table 1). This discrepancy resulted from several apps being studied in multiple research phases (e.g., design and efficacy). Almost half of the apps (46%) were first reported between 2017 and 2018. Half of the apps targeted smoking in the general population (n = 16 apps; see Section 3.1), and half targeted specific populations (n = 17 apps; see Section 3.2). Finally, the theoretical basis for behavior change was identified in all of the apps. Here, we provide a brief description of the apps identified for this review, followed by a discussion of the state of research.
Table 1.
Mobile applications for smoking cessation
| App | Population | Theory-based components | ORBIT model |
|---|---|---|---|
| Crush the Crave [22] | Young adults | Principles of persuasive technology and US Clinical Practice Guidelines (USCPG) | Phase I, II, IV |
| Crave-Out [23] | General population | Principles of classical conditioning, counter-purpose and relapse prevention model | Phase I, II |
| RxCoach [24] | General population | USCPG | Phase I, II |
| See Me Smoke Free [25] | Women | Cognitive behavioral therapy-framed guided imagery | Phase I, II |
| SmokeFree Baby [26] | Pregnant women | COMBa model and PRIMEa theory | Phase I, II |
| Distract Me [27] | General population | Relapse Prevention | Phase I |
| Inspired [28] | General population | Contingency management | Phase I |
| It’s about Two [29] | Pregnant women | Psychoeducation | Phase I |
| Kick.it [30] | Serious mental illness | Intervention Mapping and Principles of Persuasive Technology | Phase I |
| Learn to Quit [31] | Serious mental illness | Acceptance and commitment therapy, USCPG and applied behavior analysis | Phase I |
| MapMySmoke [32] | General population | Geospatial smoking behavior topography and individualized healthcare planning | Phase I |
| MyQuit USC [33] | Korean American young adults | Just-in-Time Adaptive Intervention (JITAI) approach | Phase I |
| Q Sense [34] | General population | Just-in-Time Adaptive Intervention (JITAI) approach | Phase I |
| Quit Genius [35] | General population | Cognitive-behavioral therapy | Phase Ib |
| Quittr [36] | General population | Motivational Affordances and Principles of Persuasive Technology | Phase I |
| SiS App [37] | Non-daily smokers | Positive Psychology and Intervention Mapping | Phase I |
| StopApp [38] | General population | Behavior Change Wheel (BCW) framework | Phase I |
| SmokeFree [39] | General population | PRIME theory | Phase II, IV |
| Smokerface [40] | Adolescent population | Theory of Planned Behavior | Phase II, IV (ongoing) |
| Clickotine [41] | General population | USCPG | Phase II |
| CO-ED based app [42] | Medical population | Principles of adult learning and instructional technology | Phase II |
| Pfizer meds [43] | General population | Varenicline adherence and educational information | Phase II |
| PSF-M [44] | HIV patients | Social Cognitive Theory | Phase II |
| REQ-Mobile [45] | Young adults | Cognitive-behavioral therapy, social cognitive theory, and modified version of transtheoretical model | Phase II |
| SmartQuit [46] | General population | Acceptance and commitment therapy | Phase II |
| Smart-T [47] | Low/middle income | Just-in-Time Adaptive Intervention (JITAI) approach | Phase II |
| SmokeBeat [48] | General population | Self-awareness via automated and real-time monitoring of smoking episodes | Phase II |
| SmokerFace-HIV [49] | HIV patients | Theory of Planned Behavior | Phase II |
| Stay Quit Coach [50] | PTSD patients | Relapse Prevention | Phase II |
| TControl [51] | General population | Healthcare coordination, social support, and positive reinforcement | Phase II |
| Craving to Quit [52] | General population | Mindfulness | Phase III |
| e-TIS [53] | General population | Social Cognitive Theory and Motivational Interviewing | Phase III (ongoing). |
| SSC App [54] | General population | Ottawa decision support framework | Phase IV |
Apps are sorted by ORBIT model phase and alphabetically
COMB model: ‘capability’, ‘opportunity’, ‘motivation’ and ‘behavior’ model; PRIME: ‘plans’, ‘responses’, ‘impulses’, ‘motives’, ‘evaluations’ theory of motivation
This study had elements of phase II of the ORBIT model; however, given the emphasis on user experience and usability, it was categorized as phase I
Apps Designed for the General Population
Clickotine
Clickotine delivers smoking cessation intervention components recommended by U.S. Clinical Practice Guidelines (USCPG) (e.g., the “5 A’s”) [55•] including breathing exercises; logging cravings, cigarettes, and feelings; receiving/responding to personalized messages; social support; and using quit smoking aids [41]. In an initial study (N = 416), self-reported 30-day smoking abstinence rates were 26% at 8 weeks [41].
Smartphone Smoking Cessation App (SSC App)
SSC App is described as a “decision aid with additional support” including information on pros/cons of quitting options, motivational messages, quitting diary, and quitting benefits tracker. SSC App was compared to an information-only app in an online, multi-country double-blind randomized controlled trial (RCT; N = 684). SSC App users reported greater continuous smoking abstinence rates (i.e., since quit date) at 6 months (10.2% vs 4.8%), and reported being more likely to have made an informed choice and feel confident about their quitting strategy [54].
Geo-Location Apps
Q Sense uses self-report data and geolocation to deliver tailored quit smoking messages. In a feasibility study in a convenience sample (N = 15), Q Sense was used for 3–6 weeks, and was found to be feasible and provide accurate and reliable identification of high-risk areas for smokers [34]. Smoking behavior, however, was underreported, and the authors suggested the use of app prompts to increase self-reporting compliance.
MapMySmoke uses 2 weeks of self-reported smoking and craving events with geolocation to inform a quit plan developed with the healthcare provider and then deliver support messages in a post-quit phase. Initial testing in the primary care setting (N = 8) demonstrated feasibility, in that users were able to log smoking events, and reported increased awareness of triggers and decreased craving and smoking [32].
StopApp was developed to increase motivation for uptake and attendance to smoking cessation services using evidence-based, personally tailored behavior change techniques with an online instant booking system. A user-centered design survey (N = 40) identified barriers to smoking cessation services such as lack of knowledge about services, beliefs that booking would be difficult, beliefs that services were not needed or would not be helpful, social stigma, and fear of cessation failure [38]. The user-centered design of StopApp was described in a subsequent report [56].
SmokeBeat uses data from smartbands to identify the hand-to-mouth gestures that characterize smoking and notify users of smoking incidences in real time. A pilot trial (N = 40) compared SmokeBeat smoking monitoring and notification to waitlist control across 30 days and found that SmokeBeat correctly detected smoking incidences (> 80%) with few false alarms. Furthermore, cigarettes per day were significantly reduced for SmokeBeat compared with wait-list control [48•]. Other smartwatch-based smoking monitoring devices are being developed and tested to work with cessation apps (e.g., [57]).
TControl is a multifeatured app developed to track smokers’ self-reported compulsion to smoke and provide tailored support via reinforcement and achievement messages, enable instant messaging with clinicians and other smokers, and provide information about nearest hospitals for tobacco treatment. A user-centered design study in a hospital setting (N = 31) suggested good usability standards, although 50% of patients reported needing some help to use the app [51].
E-Intervention Tabac Info Service (e-TIS) was developed by the French national smoking cessation service. The app uses personalized push notifications for questionnaires, advice, activities, and text messages, including content regarding tracking smoking and costs, decisional balance, quit date, nicotine replacement therapy, social support, craving, and other topics. Content was provided in four modules tailored to smokers’ stage of readiness to quit. The study protocol has been published for a two-arm pragmatic RCT (N = 3000) to compare the effects of e-TIS with treatment as usual on self-reported 7-day point prevalence abstinence at 6 months [53].
SmokeFree delivers a toolbox of behavior change techniques for smokers to achieve 28-day abstinence and monitor progress toward that goal. SmokeFree28 was tested in a pilot study [39] and in a RCT (N = 28,112) which compared a full and reduced version of the app. Self-reported continuous abstinence rates at 3 months were 19% versus 14% for the full and reduced app versions in those who completed follow-up (n = 2114) [58].
Cognitive-Behavioral Therapy (CBT)
Quit Genius (QG) is a gamified four-stage app using audio, exercises, and diary to deliver personalized CBT for self-reflection, changing thinking patterns, coping, problem solving, and mindfulness, for 8 weeks. A qualitative study (final N = explored users’ perceptions of QG versus the SmokeFree app (non-CBT-based), and found that QG was associated with more positive user responses and increased motivation to quit and willingness to use the app [35]. Another study surveyed current QG users (N = 190 survey completers) and found that 36% reported quitting smoking after using the app [59].
Mindfulness
SmartQuit delivers acceptance and commitment therapy, including using mindfulness skills to cope with cravings, emotions, and thoughts, and making value-guided committed behavior changes. In a pilot randomized controlled trial (N= 196), 13% of SmartQuit users reported 30-day point prevalence abstinence at two months versus 8% for the comparator, NCI QuitGuide [46]. A single-arm trial (N = 99) tested receptivity and smoking cessation with a second version of the app (SmartQuit 2.0), and found high satisfaction and usefulness ratings, as well as 21% of smokers reporting 7-day point prevalence abstinence at 2 months and 75% reporting smoking reductions [17].
CravingtoQuit teaches mindfulness training for smoking cessation including three standard mindfulness practices and an informal practice to recognize and work mindfully with cravings, and includes ecological momentary assessment (EMA) of smoking, craving, mood, and mindfulness [60•]. A full-scale two-arm RCT (N = 325) tested the efficacy of CravingtoQuit compared with an app delivering only EMA. Although 7-day point prevalence abstinence at 6 months did not differ between groups (18% self-reported, 11% biochemically verified overall), there was a significant reduction in the association between craving and smoking after treatment for CravingtoQuit versus control [52•].
Distraction-Based Apps
Crave-Out is multi-level pattern memory game designed to distract smokers during craving, with positive reinforcement and a link to a smoking cessation website. In a feasibility study (N = 30) with one 10-min laboratory session of game play, Crave-Out received positive user feedback (e.g., fun, challenging, distracted from cravings) and reduced cravings pre/post-game play [23].
Quittr is a mobile game app providing either distraction games or games that incentivize interaction with other app features including tracking quitting progress, support content, and educational material, to be used for 28 days. The app is currently undergoing late-stage development and beta-testing [36].
DistractMe provides smokers with access to distractions, tips to cope with cravings, and links to other smokers via comments. A qualitative 6-week user-centered design study (N = 14) tested how the app supported quitting. Results indicated that users engaged more with tips than distractions, although distractions facilitated content sharing. Although the initial idea of distraction through an app was appealing, smokers more commonly coped with cravings by paying attention to smoking and cravings rather than diverting their attention [27].
Contingency Management
Inspired is a contingency management app using game-based rewards to incentivize quitting and reduce the cost of contingency management for smoking cessation. A single-session feasibility study (N = 28) indicated that smokers found the app “fun” and reported being more likely to use Inspired than other smoking cessation aids, medications, or interventions [28].
Apps Designed for Targeted Populations
Women Smokers
See Me Smoke Free (SMSF) delivers guided imagery and behavior strategies to help women quit smoking, improve diet, and increase physical activity [61]. The app was refined iteratively based on prototype testing (N = 6) [25]. In a feasibility study (N = 73), use of the app was associated with improvements in all three targeted behaviors (smoking, diet, exercise), and self-reported 7-day abstinence at 3-months was 47% [62].
SmokeFree Baby delivers behavior change techniques for pregnant smokers targeting identity, health information, stress management, in-person support, and behavioral substitution [26]. The app underwent usability testing [26] and was then tested in a randomized full-factorial study (N = 565) designed to evaluate these five behavioral targets (modules). In that study, overall engagement with the app was low and no module had a significant impact on smoking abstinence during pregnancy [63].
It’s about Two—Baby & You describes tobacco risks and cessation strategies through a story of a young pregnant smoker. A cross-sectional study in a clinical setting was conducted (N = 210) in which women used the app on an iPad and completed a survey, and a subset participated in focus groups (n = 27). Most users provided positive feedback including increased interest in quitting and ideas on how to quit smoking [29].
Nondaily Smokers
Smiling Instead of Smoking (SiS) was developed using the intervention mapping framework and is a behavioral coach for quitting for nondaily smokers based on USCPG and positive psychology principles [37]. App development was based on a literature review, content analysis of available smoking cessation apps, and interviews with nondaily smokers undergoing a quit attempt (N = 38). The resulting app delivered proactive, tailored behavioral coaching; interactive tools; daily positive psychology exercises; and smoking self-monitoring [37]. No direct testing of the app has yet been reported.
Adolescents
Most smokers smoke their first cigarette in early adolescence; therefore, smoking interventions for youth are needed. SmokerFace targets adolescents’ interest in appearance by using “photoaging” to alter their “selfies” to depict future appearance if they smoke one pack daily. The app also portrays their appearance if they abstain from smoking. SmokerFace was initially tested in three schools (N = 125 students) by projecting the images in front of the whole class. The app was positively received (e.g., fun, motivating), but expensive to implement [64]. A study is underway testing a poster campaign for the app in 126 schools with long-term follow-up and biochemically verified abstinence [40•].
Young Adults
Young adults have high rates of smoking and mobile phone use and are consequently a promising target for smoking cessation apps [65, 66]. Crush the Crave (CTC) was developed based on USCPG and principles of persuasive technology for behavior change [22], and includes a quit plan, benefits of quitting, identifying triggers, tracking smoking and craving, tailored quit smoking messages, social networking, quit smoking information, and access to cessation services [67]. A two-arm RCT (N = 1599 young adults) compared the effects of CTC with self-help material and found that self-reported continuous abstinence at 6 months was not significantly different at 7.8% for CTC versus 9.2% for control [68]. A qualitative study of young adults participating in the RCT (N = 31) identified components of the app reported to be productive, such as documenting cigarettes smoked and cravings, or unproductive, such as social support components of the app, and found that some preferences for app components differed by gender [69].
Real e Quit (REQ-Mobile) delivers text messages based on social cognitive theory and the transtheoretical model to increase self-efficacy across stages of quitting, with additional content related to quitting benefits and strategies, coping, and nicotine replacement therapy. A RCT (N = 102 young adults) was conducted comparing the app to text messaging only and found that text messaging led to greater self-reported 30-day point-prevalence abstinence at 3 months [45].
MyQuit USC (MQU) is a tailored just-in-time adaptive intervention prototype for Korean American emerging adult smokers. Qualitative data from a user-centered design study highlighted that smoking episodes among this population are highly context-driven and there is a need for personalized cessation strategies for different contexts [33].
Serious Mental Illness
Learn to Quit was developed for persons with serious mental illness. The app was based on acceptance and commitment therapy in combination with USCPG. The app uses behavior analytic principles to increase app engagement and retention and comprehension of app content. A series of user-centered design studies and case studies [31•, 70, 71•] informed the app design, including app layer structure, the use of storytelling, successive approximations to increase mastery of smoking cessation skills, and symbolic rewards [31•]. A feasibility trial is currently underway.
Kick.it was developed using intervention mapping framework and persuasive system design for smoking cessation among young adults with serious mental illness. EMA is used to record smoking, craving, mood, and triggers, and the app then provides tailored feedback based on stored information, in addition to digital diversions and random content to promote engagement and sustain interest [30]. Pilot testing is underway [72].
Post-Traumatic Stress Disorder (PTSD)
Stay Quit Coach (SQC) was designed by the U.S. National Center for PTSD and provides evidence-based techniques addressing PTSD symptoms and smoking urges. A preliminary trial (N = 11) evaluated the usability and feasibility of SQC combined with mobile contingency management, quit smoking counseling and medications (“QUIT4EVER”), versus the same intervention without SQC, and found positive user experience feedback for the SQC app including helpfulness [50]. Another pilot study (N = 20) incorporated SQC into an 8-week in-person integrated care protocol, resulting in 35.3% biochemically verified 30-day point-prevalence abstinence at 3 months [73].
Medical Populations
The opportunity for smoking cessation intervention arises during hospitalization [74], and several apps have been developed to address this need. Computer-assisted Education System (CO-ED) delivers psychoeducation on the dangers of smoking and was tested in a hospital setting (N = 55) where smokers used CO-ED for up to 45 min and then completed a survey and semi-structured interview. CO-ED increased smoking knowledge, self-efficacy, and readiness to quit [42].
SmokerFace-HIV
Individuals who are HIV positive are twice as likely to smoke; therefore, smoking cessation aids for this population are needed [75]. A kiosk version (i.e., tablet projecting to a wall-mounted monitor) of the face-aging SmokerFace was developed for the waiting room of an HIV outpatient clinic and tested during a 19-day period during which patients tried the app and then completed an anonymous questionnaire (N = 187). Most smokers reported that the app was fun and motivated them to quit; nonsmokers reported the app motivated them to never take up smoking [49].
Positively Smoke Free-Mobile (PSF-M) is a mobile website that aims to assist persons living with HIV in quitting smoking over 42 days by delivering motivational/educational quit smoking sessions based on social cognitive theory, interactive quit smoking messaging around the quit date, access to a quitline, and other functions. A pilot RCT (N = 100) compared PSF-M to standard care (all were offered 3 months of nicotine patch, used by ~ 70%) and found some support for feasibility (moderate acceptance, adherence, engagement, and satisfaction) despite no difference in biochemically verified 7-day point prevalence abstinence rates at 3 months [44].
Adherence to Smoking Cessation Medication
RxCoach was designed to improve adherence to quit smoking medications (i.e., varenicline) by collecting medication information via self-report and barcode reader; and providing tailored adherence feedback, medication tracking, motivational messages, and tips to deal with cravings, side effects, and lapses; reminders for refills and appointments; and a direct link to a physician/pharmacist. RxCoach was refined through a focus group (N = 4) and two usability tests (N = 10 per study), followed by feasibility testing, which had low participation (N = 7, n = 5 retained) but good medication adherence (n = 4/5 participants reported current use of varenicline at 1 month) [24].
Pfizer meds was also developed for varenicline users to provide educational information and quit smoking support, including motivational support and medication information. Pfizer meds was tested in a prospective observational study (n = 131 survey completers at 3 months) and was found to have moderate levels of usability and user satisfaction [43].
Socioeconomic Status (SES)
Lower SES has been associated with lower cessation rates [76]; therefore, individuals with lower SES may benefit from technology-based interventions, which have high reach and are cost-effective. Smart-Treatment (Smart-T) uses EMA to track triggers, craving, smoking, and other factors, and deliver tailored, just-in-time quit smoking messages. An feasibility study in lower SES smokers (N = 59) tested use of Smart-T for 3 weeks with group counseling and pharmacotherapy and found high rates of app usage and EMA completion, and 20% biochemically verified 7-day point prevalence abstinence at 3 months [47]. A more recent study supported the approach of tailoring messages to specific triggers such as stress [77].
Discussion and Recommendations
Limited Range of App Features
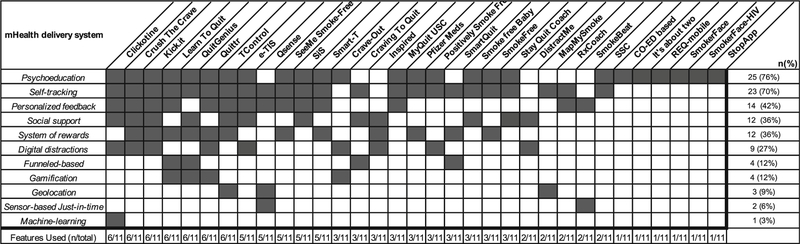
This review extracted app key features from the literature (Fig. 1). Across apps, we identified categories of app features for delivering intervention content and found that most apps used a limited range of features. For example, the majority of apps used psychoeducation (76%) and self-tracking (70%). Personalized feedback (42%) was used in conjunction with self-tracking by customizing the intervention to the user. Social support (36%) and implementing a system of rewards (36%) were used by a smaller portion of apps. Less commonly used features included geolocation (9%), just-in-time sensors (6%), and machine learning (3%). We note that additional features were not categorized in this review due to being underutilized and/or delivered separately from the app itself, such as live counseling, a component of some app-based interventions (e.g., Smart-T, QUIT4EVER/Stay Quit Coach).
Fig. 1.
Interventional features
This breakdown indicates that the majority of apps deliver interventions using features that require little software engineering, while more complex features (e.g., machine learning) are not yet widely implemented. Overall, there was considerable overlap on use of a limited number of app features. The majority of apps (63%) implemented no more than three features, while some apps implemented up to six features (Fig. 1). It is possible that over time apps will implement a wider range of features to deliver smoking cessation interventions, and/or that more efficacious app features will be identified and become the focus of treatment delivery.
Decisions regarding which features to implement for treatment delivery should be empirically driven. Early-phase studies (phase Ia and Ib of the ORBIT model) offer an ideal set of methodologies to inform these decisions in a cost-effective manner. Qualitative studies, user-centered design methods, and small-n studies can provide a rich set of observations to guide and validate decisions for a given app and population. Likewise, large-n studies, including newer methods such as multiphase optimization strategy (MOST); sequential, multiple assignment randomized trials (SMART); and micro-randomized trials (MRTs) [78], albeit more costly, should also provide valuable data to inform mHealth interventions. With one exception (i.e., SmokeFree Baby), these approaches are currently absent from the literature.
App Features Could Be Described More Consistently
Our review indicated that app features were often not well specified or described. Understanding the active ingredients of an app-based intervention requires a detailed account of app features and content. Reports of phase II or III trials are typically limited to a minimal description of app features, which is problematic when phase I research was not conducted or reported. Our review also identified inconsistencies in the vocabulary used to describe app features. For example, “gamification” was misused to describe games or digital diversions rather than the use of a game to enhance the acquisition of knowledge or skills [21]. Such inconsistencies can lead to confusion in the field about the design of app-based smoking cessation interventions. In particular, the field is lacking a complete understanding of which app features have been tested in which phase and how much evidence is available to support a given app feature, information that is critical to developing clinical practice guidelines in mHealth.
Further Programmatic Efforts Are Needed
This review used the ORBIT model to categorize available apps by their phase of development and testing (Table 1). Half of the papers identified in this review reported phase I studies, and among those, 23% progressed to a phase II study. The other half of papers first reported phase II studies such as proof-of-concept or two-arm pilot RCT without any previously reported design research. Finally, 31% of papers reported efficacy or effectiveness RCTs without reporting previous phase I or II research. This could be because earlier research phases were not conducted, or perhaps because they were conducted but not reported. While skipping research phases can be acceptable according to the ORBIT model, this can be problematic and cost-ineffective if the intervention needs to be re-designed to optimize its components. Not reporting user-centered design research can also be problematic because it limits the body of knowledge available to identify generalizable design features that could inform other interventions.
One approach gaining traction in clinical research more broadly, and also in mHealth, is the publication of protocol papers outlining the rationale, hypotheses, and methodology of a clinical trial prior to conducting the trial in order to reduce publication bias and improve reproducibility. Clinical trial pre-registration and evidence-based minimum reporting standards (e.g., Consolidated Standards of Reporting Trials [79]) also guide complete and transparent reporting and aid in interpreting clinical trials outcomes. Similarly, standards for evaluation of treatment feasibility (e.g., fidelity, adherence, acceptability) would improve the ability to interpret initial phase research of smoking cessation apps [80–82]. More generally, wider adoption of scientific transparency and data sharing should further improve the replicability and efficiency of mHealth research [83].
Overall, our review shows that there is a healthy amount of methodological diversity in smoking cessation app research [84], ensuring that the body of knowledge produced examines treatment development from multiple scientifically relevant perspectives (e.g., qualitative, efficacy, translational). However, we argue that greater programmatic efforts to organize the research endeavor so that design research is systematically conducted and precedes clinical research would improve the quality of these interventions. Lack of definition or optimization of an app design can lead to implementation failures. For example, an app might include theory-based components, but have a design with very low levels of usability. Conversely, some apps might have high usability, but incorporate content not supported by the scientific literature (e.g., astrology).
Few Studies Demonstrate Clinical Efficacy
Our review, similar to previous reviews [9, 11, 85], found only a few studies testing preliminary efficacy or efficacy of smoking cessation apps, despite apps being widely available and highly marketed. We found only four apps tested in Phase III or IV efficacy or effectiveness trials (Table 2). More specifically, only four well-powered studies have tested efficacy and effectiveness of mobile apps, two of them with positive findings and two reporting null results or a more efficacious control condition. Smoking abstinence rates ranged from 0.9% to 12% at trial endpoint [52•, 54, 58, 68]. Most of these trials used the app as stand-alone treatment, suggesting quit rates might be comparable to other non-app-based behavioral interventions for smoking cessation. Protocol papers and clinical trials registries indicate that there are additional clinical trials of smoking cessation apps underway.
Table 2.
Summary of phase III and phase IV studies of mobile apps in tobacco treatment
| Studies | Population | N | Comparison group | Main outcomes | End-point (months) | Final N | Abstinence verification | Quit rates (%) (experimental vs. control) | Relative risk (95% CI) Odds ratio (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| SSC App (BinDhim et al. 2018) [54] | General population | 684 | Static information app | Self-reported continuous abstinence | 6 | 583 | No | 7.3% vs. 3.2% | RR: 2.27 (1.09 to 4.86) |
| SmokeFree (Crane et al.2018) [58] | General population | 28,112 | Reduced app version | Self-reported continuous abstinence | 3 | 2114 | No | 1.6% vs. 0.9% | OR: 1.86 (1.49 to 2.31) |
| Craving to Quit (Garrison et al. 2018) [52] | General Population | 505 | Experience sampling app | Biochemically verified 7-day point prevalence abstinence | 6 | 325 | Yes | 9.8% vs. 12.1% | OR: 1.27 (0.62 to 2.57) |
| Crush the Crave (Baskerville et al. 2018) [22, 68] | Young adults | 1599 | Evidence-informed self-help booklet | Self-reported continuous abstinence | 6 | 725 | No | 7.8% vs. 9.2% | OR: 0.83 (0.59 to 1.18) |
An interesting question is whether a greater programmatic effort as suggested above would contribute to more positive clinical outcomes from smoking cessation apps. For example, the SSC App used a limited number of interventional features (Fig. 1), and was directly tested in a large multi-site phase IV effectiveness study, but showed positive results compared with control. SmokeFree, using three interventional features (Fig. 1), was tested in a phase II pilot study, and later in a large phase IV effectiveness trial, showing positive results compared with the control, but by a small margin. SmokeFree Baby underwent phase I research through a thematic analysis of think-aloud procedures with the final app and then was further evaluated in a large MOST design (N = 565; phase II) that systematically tested specific app features. This phase II study did not support the utility of specific app features, suggesting a need for more in-depth phase I work and/or refinements. Finally, Craving to Quit used a well-defined theoretical framework based on positive outcomes in an earlier in-person smoking cessation RCT, and was tested in a large phase IV effectiveness trial, but did not find significant differences in smoking cessation compared with control, although interesting mechanistic findings were reported.
To this question of whether a greater programmatic effort is needed, drug development research provides a useful parallel. In drug development, early phase research is conducted to evaluate the harm potential of novel drug compounds, as well as to understand key mechanisms of action, dosage, and unintended effects of the drug on other biological systems. This early phase research does not ensure effective translation from animal models to human trials. We argue that early phase research of smoking cessation apps is similarly key to understanding a range of factors including but not limited to cultural norms of the target population, impact of tone and style on the user, and whether the app promotes change consistent with theory-driven processes and clinically relevant outcomes (e.g., does the smoker take proximal steps in smoking cessation). These data are key to supporting whether an app is ready for a larger clinical study. However, phase I research may not directly indicate clinical impact of an app (e.g., SmokeFree Baby). In other words, phase I research may be a necessary but not sufficient step in smoking cessation apps research that establishes an empirical foundation for further app development and optimization if negative outcomes result from clinical trials.
Conclusions
Smoking cessation apps incorporate a diversity of mechanisms of delivery (i.e., features) to promote behavior change. Our review found that all app studies report some information on the theoretical basis of the intervention, an important sign of progress in the field [86]. However, it has been argued that existing behavior change theories may not be well suited to inform mHealth interventions as these become more interactive and adaptive [87]. Our review also found an increase in early-phase app development studies; however, there is still a lack of reporting of this stage of research. Finally, only a few studies have tested the efficacy and effectiveness of smoking cessation apps, and among those, only one app (Crush the Crave) conducted thorough early phase research. In an effort to increase access to smoking cessation treatment and curb the rates of disease caused by tobacco use and dependence, future studies should continue to standardize and optimize app development, testing, and reporting, to improve treatments and increase the transparency of this scientific process.
Acknowledgments
J.F.M. reports and ownership of a company that provided smoking cessation–related scientific consulting and market research to GSK—Consumer Healthcare.
R.V. reports grants from National Institutes on Drug Abuse, during the conduct of the study.
Footnotes
Compliance with Ethical Standards
Conflict of Interest
K.A.G. and E.C.-P. have nothing to disclose.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher's Disclaimer: Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.WHO Global Report: mortality attributable to tobacco Geneva, Switzerland: World Health Organization; 2012. Available from: https://www.who.int/tobacco/publications/surveillance/en/ [Google Scholar]
- 2.Goodchild M, Nargis N, d’Espaignet ET. Global economic cost of smoking-attributable diseases. Tob Control 2018;27(1):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Center for Chronic Disease Prevention and Health Promotion (U.S.) Office on Smoking and Health. The health consequences of smoking—50 years of progress: a report of the Surgeon General Atlanta GA: Centers for Disease Control and Prevention; 2014. Available from: http://www.ncbi.nlm.nih.gov/books/NBK179276/ [Google Scholar]
- 4.Centers for Disease Control and Prevention. Current cigarette smoking among adults—United States, 2016. Morbidity and Mortality Weekly Report 2018;67(2):53–9 [Accessed 2018 Nov 15]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattacharya A, Vilardaga R, Kientz JA, Munson SA. Lessons from practice: designing tools to facilitate individualized support for quitting smoking. ACM Trans Comput Hum Interact 2017;2017:3057–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vilardaga R, Bricker J, McDonell M. The promise of mobile technologies and single case designs for the study of individuals in their natural environment. J Contextual Behav Sci 2014;3(2):148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiffman S, Stone AA, Hufford M. Ecological momentary assessment. Annu Rev Clin Psychol 2008;4:1–32. [DOI] [PubMed] [Google Scholar]
- 8.Zeng EY, Vilardaga R, Heffner JL, Mull KE, Bricker JB. Predictors of utilization of a novel smoking cessation smartphone app. Telemed J E Health 2015;21(12):998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev 2016;4:CD006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.BinDhim NF, McGeechan K, Trevena L. Who uses smoking cessation apps? A feasibility study across three countries via smartphones. J Med Internet Res mHealth uHealth 2014;2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haskins BL, Lesperance D, Gibbons P, Boudreaux ED. A systematic review of smartphone applications for smoking cessation. Transl Behav Med 2017;7(2):292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heffner JL, Vilardaga R, Mercer LD, Kientz JA, Bricker JB. Feature-level analysis of a novel smartphone application for smoking cessation. Am J Drug Alcohol Abuse 2015;41(1):68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bert F, Giacometti M, Gualano MR, Siliquini R. Smartphones and health promotion: a review of the evidence. J Med Syst 2013;38(1): 9995. [DOI] [PubMed] [Google Scholar]
- 14.Ghorai K, Akter S, Khatun F, Ray P, Ghorai K, Akter S, et al. mHealth for smoking cessation programs: a systematic review. J Pers Med 2014;4(3):412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karkar R, Zia J, Vilardaga R, Mishra SR, Fogarty J, Munson SA, et al. A framework for self-experimentation in personalized health. J Am Med Inform Assoc 2015;23(3):440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McClure JB, Hartzler AL, Catz SL. Design considerations for smoking cessation apps: feedback from nicotine dependence treatment providers and smokers. J Med Internet Res mHealth uHealth 2016;4(1):e17 Published 2016 Feb 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bricker JB, Copeland W, Mull KE, Zeng EY, Watson NL, Akioka KJ, et al. Single-arm trial of the second version of an acceptance & commitment therapy smartphone application for smoking cessation. Drug Alcohol Depend 2017;170:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abroms LC, Lee Westmaas J, Bontemps-Jones J, Ramani R, Mellerson J. A content analysis of popular smartphone apps for smoking cessation. Am J Prev Med 2013;45(6):732–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. •.West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addict 2005;100(3):299–303Useful recommendations for reporting outcomes for smoking cessation.
- 20. ••.Czajkowski SM, Powell LH, Adler N, et al. From ideas to efficacy: the ORBIT model for developing behavioral treatments for chronic diseases. Health Psychol 2015;34(10):971–82Model used to define and guide phases of research on smoking cessation apps.
- 21.Landers RN. Developing a theory of gamified learning: linking serious games and gamification of learning. Simul Gaming 2014;45(6):752–68. [Google Scholar]
- 22.Baskerville NB, Struik LL, Dash D. Crush the crave: development and formative evaluation of a smartphone app for smoking cessation. J Med Internet Res mHealth uHealth 2018;6(3):e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeLaughter KL, Sadasivam RS, Kamberi A, English TM, Seward GL, Chan SW, et al. Crave-Out: a distraction/motivation mobile game to assist in smoking cessation. J Med Internet Res Serious Games 2016;4(1):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon JS, Armin JS, Cunningham JK, Muramoto ML, Christiansen SM, Jacobs TA. Lessons learned in the development and evaluation of RxCoach™, an mHealth app to increase tobacco cessation medication adherence. Patient Educ Couns 2017;100(4): 720–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armin J, Johnson T, Hingle M, Jr PG, Gordon JS. Development of a multi-behavioral mhealth app for women smokers. J Health Commun 2017;22(2):153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tombor I, Shahab L, Brown J, Crane D, Michie S, West R. Development of SmokeFree Baby: a smoking cessation smartphone app for pregnant smokers. Transl Behav Med 2016;6(4):533–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ploderer B, Smith W, Pearce J, Borland R. A mobile app offering distractions and tips to cope with cigarette craving: a qualitative study. J Med Internet Res mHealth uHealth 2014;2(2):e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raiff BR, Fortugno N, Scherlis DR, Rapoza D. A mobile game to support smoking cessation: prototype assessment. J Med Internet Res Serious Games 2018;6(2):e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dotson JAW, Pineda R, Cylkowski H, Amiri S. Development and evaluation of an iPad application to promote knowledge of tobacco use and cessation by pregnant women. Nurs Womens Health 2017;21(3):174–85. [DOI] [PubMed] [Google Scholar]
- 30.van Agteren JEM, Lawn S, Bonevski B, Smith BJ. Kick.it: the development of an evidence-based smoking cessation smartphone app. Transl Behav Med 2018;8(2):243–67. [DOI] [PubMed] [Google Scholar]
- 31. •.Vilardaga R, Rizo J, Zeng E, Kientz JA, Ries R, Otis C, et al. User-centered design of Learn to Quit, a smoking cessation smartphone app for people with serious mental illness. J Med Internet Res Serious Games 2018;6(1):e2.Example of phase Ia study (ORBIT model) to define the design characteristics of a smoking cessation app using user-centered design methodology.
- 32.Schick RS, Kelsey TW, Marston J, Samson K, Humphris GW. MapMySmoke: feasibility of a new quit cigarette smoking mobile phone application using integrated geo-positioning technology, and motivational messaging within a primary care setting. Pilot Feasibility Stud 2017;4(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerrada CJ, Dzubur E, Blackman KCA, Mays V, Shoptaw S, Huh J. Development of a just-in-time adaptive intervention for smoking cessation among Korean American emerging adults. Int J Behav Med 2017;24(5):665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naughton F, Hopewell S, Lathia N, Schalbroeck R, Brown C, Mascolo C, et al. A context-sensing mobile phone app (Q sense) for smoking cessation: a mixed-methods study. J Med Internet Res mHealth uHealth 2016;4(3):e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tudor-Sfetea C, Rabee R, Najim M, Amin N, Chadha M, Jain M, et al. Evaluation of two mobile health apps in the context of smoking cessation: qualitative study of cognitive behavioral therapy (CBT) versus non-CBT-based digital solutions. J Med Internet Res mHealth uHealth 2018;6(4):e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bindoff I, de Salas K, Peterson G, Ling T, Lewis I, Wells L, et al. Quittr: the design of a video game to support smoking cessation. J Med Internet Res Serious Games 2016;4(2):e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoeppner BB, Hoeppner SS, Kelly L, Schick M, Kelly JF. Smiling instead of smoking: development of a positive psychology smoking cessation smartphone app for non-daily smokers. Int J Behav Med 2017;24(5):683–93. [DOI] [PubMed] [Google Scholar]
- 38.Fulton E, Brown K, Kwah K, Wild S, Fulton EA, Brown KE, et al. StopApp: using the behaviour change wheel to develop an app to increase uptake and attendance at NHS stop smoking services. Healthcare 2016;4(2):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ubhi HK, Michie S, Kotz D, Wong WC, West R. A mobile app to aid smoking cessation: preliminary evaluation of SmokeFree28. J Med Internet Res 2015;17(1):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. •.Brinker TJ, Holzapfel J, Baudson TG, Sies K, Jakob L, Baumert HM, et al. Photoaging smartphone app promoting poster campaign to reduce smoking prevalence in secondary schools: the Smokerface randomized trial: design and baseline characteristics. BMJ Open 2016;6(11):e014288.Interesting approach to cost-effective dissemination of an anti-smoking app for adolescents via a poster campaign.
- 41.Iacoviello BM, Steinerman JR, Klein DB, Silver TL, Berger AG, Luo SX, et al. Clickotine, a personalized smartphone app for smoking cessation: initial evaluation. J Med Internet Res mHealth uHealth 2017;5(4):e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finkelstein J, Cha EM. Using a mobile app to promote smoking cessation in hospitalized patients. J Med Internet Res mHealth uHealth 2016;4(2):e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruno M, Wright M, Baker CL, Emir B, Carda E, Clausen M, et al. Mobile app usage patterns of patients prescribed a smoking cessation medicine: prospective observational study. J Med Internet Res mHealth uHealth 2018;6(4):e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shuter J, Kim RS, An LC, Abroms LC. Feasibility of a smartphone-based tobacco treatment for HIV-infected smokers. Nicotine Tob Res 2018. 10.1093/ntr/nty208. [DOI] [PMC free article] [PubMed]
- 45.Buller DB, Borland R, Bettinghaus EP, Shane JH, Zimmerman DE. Randomized trial of a smartphone mobile application compared to text messaging to support smoking cessation. Telemed J E Health 2014;20(3):206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bricker JB, Mull KE, Kientz JA, Vilardaga R, Mercer LD, Akioka KJ, et al. Randomized, controlled pilot trial of a smartphone app for smoking cessation using acceptance and commitment therapy. Drug Alcohol Depend 2014;143:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Businelle MS, Ma P, Kendzor DE, Frank SG, Vidrine DJ, Wetter DW. An ecological momentary intervention for smoking cessation: evaluation of feasibility and effectiveness. J Med Internet Res 2016;18(12):e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. •.Dar R. Effect of real-time monitoring and notification of smoking episodes on smoking reduction: a pilot study of a novel smoking cessation app. Nicotine Tob Res 2017;20(12):1515–8Promising new approach utilizing mobile wearable technology (smartbands) to monitor and intervene with smoking.
- 49.Brinker TJ, Brieske CM, Esser S, Klode J, Mons U, Batra A, et al. A face-aging app for smoking cessation in a waiting room setting: pilot study in an HIV outpatient clinic. J Med Internet Res 2018;20(8):e10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hicks TABS, Thomas SP, Wilson SM, Calhoun PS, Kuhn ER, Beckham JC. A preliminary investigation of a relapse prevention mobile application to maintain smoking abstinence among individuals with posttraumatic stress disorder. J Dual Diagn 2017;13(1): 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pifarré M, Carrera A, Vilaplana J, Cuadrado J, Solsona S, Abella F, et al. TControl: a mobile app to follow up tobacco-quitting patients. Comput Methods Prog Biomed 2017;142:81–9. [DOI] [PubMed] [Google Scholar]
- 52. •.Garrison KA, Pal P, O’Malley SS, Pittman BP, Gueorguieva R, Rojiani R, et al. Craving to quit: a randomized controlled trial of smartphone app-based mindfulness training for smoking cessation. Nicotine Tob Res 2018. 10.1093/ntr/nty126One author’s full-scale randomized controlled trial of smartphone app-based mindfulness training for smoking cessation.
- 53.Cambon L, Bergman P, Faou AL, Vincent I, Maitre BL, Pasquereau A, et al. Study protocol for a pragmatic randomized controlled trial evaluating efficacy of a smoking cessation e-’Tabac info service’: ee-TIS trial. BMJ Open 2017;7(2):e013604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.BinDhim NF, McGeechan K, Trevena L. Smartphone smoking cessation application (SSC App) trial: a multicountry double-blind automated randomized controlled trial of a smoking cessation decision-aid ‘app’. BMJ Open 2018;8(1):e017105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. •.Tobacco Use and Dependence Guideline Panel. Treating tobacco use and dependence: 2008 update Rockville MD: U.S. Department of Health and Human Services; 2008. May Available from: https://www.ncbi.nlm.nih.gov/books/NBK63952/.Evidence-based guidelines for treating tobacco use and dependence.
- 56.Fulton E, Kwah K, Wild S, Brown K, Fulton EA, Kwah KL, et al. Lost in translation: transforming behaviour change techniques into engaging digital content and design for the StopApp. Healthcare 2018;6(3):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skinner AL, Stone CJ, Doughty H, Munafò MR. StopWatch: the preliminary evaluation of a smartwatch-based system for passive detection of cigarette smoking. Nicotine Tob Res 2018;21:257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crane D, Ubhi HK, Brown J, West R. Relative effectiveness of a full versus reduced version of the ‘Smoke Free’ mobile application for smoking cessation: a randomized controlled trial. F1000Research 2018;7:1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin Y, Tudor-Sfetea C, Siddiqui S, Sherwani Y, Ahmed M, Eisingerich AB. Effective behavioral changes through a digital mHealth app: exploring the impact of hedonic well-being, psychological empowerment and inspiration. J Med Internet Res mHealth uHealth 2018;6(6):e10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. •.Garrison KA, Pal P, Rojiani R, Dallery J, O’Malley SS, Brewer JA. A randomized controlled trial of smartphone-based mindfulness training for smoking cessation: a study protocol. BMC Psychiatry 2015;15(1):83.Example “protocol paper” recommended to reduce reporting bias and increase transparency in reporting clinical trials of smartphone apps for smoking cessation.
- 61.Giacobbi P Jr, Hingle M, Johnson T, Cunningham JK, Armin J, Gordon JS. See me smoke-free: protocol for a research study to develop and test the feasibility of an mHealth app for women to address smoking, diet, and physical activity. JMIR Res Protoc 2016;5(1):e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gordon JS, Armin J, Hingle MD, Giacobbi P, Cunningham JK, Johnson T, et al. Development and evaluation of the see me smoke-free multi-behavioral mHealth app for women smokers. Transl Behav Med 2017;7(2):172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tombor I, Beard E, Brown J, Shahab L, Michie S, West R. Randomized factorial experiment of components of the SmokeFree Baby smartphone application to aid smoking cessation in pregnancy. Transl Behav Med 2018. 10.1093/tbm/iby073. [DOI] [PMC free article] [PubMed]
- 64.Brinker TJ, Seeger W, Buslaff F. Photoaging mobile apps in school-based tobacco prevention: the mirroring approach. J Med Internet Res 2016;18(6):e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pew Research Center, February, 2016, Smartphone ownership and internet usage continues to climb in emerging economies http://www.pewglobal.org/2016/02/22/smartphone-ownership-and-internet-usage-continues-to-climb-in-emerging-economies/ [Accessed 15 Nov 2018].
- 66.Center for Behavioral Health Statistics and Quality. (2017). 2016 National Survey on drug use and health: detailed tables Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- 67.Baskerville NB, Struik LL, Hammond D, Guindon GE, Norman CD, Whittaker R, et al. Effect of a mobile phone intervention on quitting smoking in a young adult population of smokers: randomized controlled trial study protocol. JMIR Res Protoc 2015;4(1): e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baskerville NB, Struik LL, Guindon GE, Norman CD, Whittaker R, Burns C, et al. Effect of a mobile phone intervention on quitting smoking in a young adult population of smokers: randomized controlled trial. J Med Internet Res mHealth uHealth 2018;6(10): e10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Struik LL, Bottorff JL, Baskerville NB, Oliffe JL. The Crush the Crave quit smoking app and young adult smokers: qualitative case study of affordances. J Med Internet Res mHealth uHealth 2018;6(6):e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vilardaga R, Rizo J, Kientz JA, McDonell MG, Ries RK, Sobel K. User experience evaluation of a smoking cessation app in people with serious mental illness. Nicotine Tob Res 2016;18(5):1032–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. •.Vilardaga R, Rizo J, Zeng E, et al. User-centered design of Learn to Quit, a smoking cessation smartphone app for people with serious mental illness. J Med Internet Res Serious Games 2018;6(1):e2.Example of phase Ib study (ORBIT model) to refine the design characteristics of a smoking cessation app using cost-effective single case design methodology.
- 72.Lawn S, van Agteren J, Zabeen S, Bertossa S, Barton C, Stewart J, et al. Adapting, pilot testing and evaluating the Kick.it app to support smoking cessation for smokers with severe mental illness: a study protocol. Int J Environ Res Public Health 2018;15(2):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herbst E, Pennington D, Kuhn E, McCaslin SE, Delucchi K, Batki SL, et al. Mobile technology for treatment augmentation in veteran smokers with posttraumatic stress disorder. Am J Prev Med 2018;54(1):124–8. [DOI] [PubMed] [Google Scholar]
- 74.Rigotti NA, Munafo MR, Stead LF. Smoking cessation interventions for hospitalized smokers: a systematic review. Arch Intern Med 2008;168(18):1950–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med 2015;162(5):335–44. [DOI] [PubMed] [Google Scholar]
- 76.Hiscock R, Bauld L, Amos A, Fidler JA, Munafò M. Socioeconomic status and smoking: a review. Ann N Y Acad Sci 2012;1248(1):107–23. [DOI] [PubMed] [Google Scholar]
- 77.Hébert ET, Stevens EM, Frank SG, Kendzor DE, Wetter DW, Zvolensky MJ, et al. An ecological momentary intervention for smoking cessation: the associations of just-in-time, tailored messages with lapse risk factors. Addict Behav 2018;78:30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med 2007;32(5 Suppl):S112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomized trials. BMJ 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Borrelli B The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent 2011;71(s1):S52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bowen DJ, Kreuter M, Spring B, Cofta-Woerpel L, Linnan L, Weiner D, et al. How we design feasibility studies. Am J Prev Med 2009;36(5):452–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch Gen Psychiatry 2006;63(5):484–9. [DOI] [PubMed] [Google Scholar]
- 83.Munafò MR, Nosek BA, Bishop DVM, Button KS, Chambers CD, Percie du Sert N, et al. A manifesto for reproducible science. Nat Hum Behav 2017;1(1):0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vilardaga R, Hayes SC, Levin ME, Muto T. Creating a strategy for progress: a contextual behavioral science approach. Behav Anal 2009;32(1):105–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thornton L, Quinn C, Birrell L, Guillaumier A, Shaw B, Forbes E, et al. Free smoking cessation mobile apps available in Australia: a quality review and content analysis. Aust N Z J Public Health 2017;41(6):625–30. [DOI] [PubMed] [Google Scholar]
- 86.Kumar S, Nilsen WJ, Abernethy A, Atienza A, Patrick K, Pavel M, et al. Mobile health technology evaluation: the mHealth evidence workshop. Am J Prev Med 2013;45(2):228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Riley WT, Rivera DE, Atienza AA, Nilsen W, Allison SM, Mermelstein R. Health behavior models in the age of mobile interventions: are our theories up to the task? Transl Behav Med 2011;1(1):53–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brewer JA, Mallik S, Babuscio TA, Nich C, Johnson HE, Deleone CM, et al. Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug Alcohol Depend 2011;119(1–2):72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]