Abstract
OBJECTIVES:
Approximately 25% of patients with irritable bowel syndrome-diarrhea (IBS-D) have increased total fecal bile acids (BA) and serum C4 (surrogate for BA synthesis). BA synthesis-related genes (KLB and FGFR4) are associated with colonic transit (CT) in IBS-D. Our aims were: (i) to compare phenotype and pathophysiology in IBS-D patients with increased or normal fecal excretion or synthesis of BA; and (ii) to explore association of variations in two candidate bile-acid synthesis genes (KLB and FGFR4) in these two subgroups of IBS-D.
METHODS:
A total of 64 IBS-D patients underwent on one occasion: fasting serum C4 and FGF19, total fecal fat and BA excretion, CT, intestinal and colonic permeability, and candidate genotyping (rs17618244 (KLB), rs351855 (FGFR4)). Colonic sensation and tone were measured in 47 of the IBS-D patients. IBS-D subgroups were identified by fecal BA > 2,337 mM per 48 h or by serum C4 > 47.1 ng/ml.
RESULTS:
IBS-D patients with fecal BA > 2,337 mM per 48 h (19/54) had significantly greater body mass index, fecal fat, percent chenodeoxycholic acid (CDCA) in feces, and intestinal permeability, and borderline increased CT (P= 0.13). Those IBS-D patients with serum C4 > 47.1 ng/ml (13/54) had increased total fecal BA excretion and borderline increased colonic permeability. Variants in genes involved in feedback regulation of BA synthesis (KLB, P = 0.06 and FGFR4, P= 0.09) were potentially associated with the subgroup with elevated serum C4.
CONCLUSIONS:
IBS-D with increased BA excretion or synthesis is associated with significant pathophysiological changes relative to patients with normal BA profile. BA diarrhea is identified more effectively with total fecal BA than with serum C4.
INTRODUCTION
Approximately 25% of patients with irritable bowel syndrome-diarrhea (IBS-D) have increased total fecal bile acids (BA) or other evidence of bile acid malabsorption, most readily demonstrated using 75SeHCAT retention test (1). Although therapeutic trial with BA sequestrants is still the predominant method to make a tentative diagnosis of BA diarrhea (BAD), two serological tests may soon be widely available to facilitate the identification of BAD. Serum 7α-OH-4-cholesten-3-one (C4) is a surrogate for hepatic BA synthesis rate; serum C4 is elevated in patients with BA malabsorption (2 ) and in a subset of patients with IBS-D (3). A second blood test (4) is based on the seminal documentation by Walters et al. (5) of a molecular mechanism (deficiency of fibroblast growth factor 19 (FGF19) secretion by ileal enterocytes) in patients with BAD. This was confirmed by other groups (3,6). In a recent comparison between serum FGF19 and 75SeHCAT retention test results, receiver operating characteristic curve analysis showed that FGF19 ≤145 pg/ml could predict 75SeHCAT values <10% or <5% in 58% (95% confidence interval 42–72) and 67% (95% confidence interval 38–87) of patients; moreover, 15 of 16 patients with serum FGF19 ≤145 pg/ml responded to BA sequestrants (4). Two BA-related genes, klotho B (KLB) and fibroblast growth factor receptor 4 (FGFR4), are associated with accelerated small intestinal or colonic transit (CT) in IBS-D (7,8). It is unclear whether BAD alters the pathophysiology in patients presenting with the symptom phenotype of IBS-D. Such insights may enhance the therapeutic approach to IBS-D, with greater emphasis on the specific treatment of the BAD contributing to the phenotype of IBS-D (9).
Our hypothesis was that increased fecal BA or increased serum C4 are associated with significant differences in quantitative traits such as colonic sensorimotor and intestinal or colonic permeability compared with patients with normal BA excretion and synthesis. The aims of the study were: (i) to compare phenotype, colonic motor or sensory functions, and permeability in IBS-D patients having increased fecal BA or increased serum C4 with patients having normal BA excretion and synthesis; and (ii) to explore associations of genetic variations in KLB and FGFR4 in these two subgroups of IBS-D.
METHODS
Study design
We appraised bowel functions, total fecal BA, colonic motility, sensation and permeability, and BA synthesis and excretion in 64 patients with IBS-D (by Rome III criteria) in order to identify the patients with IBS-D who had evidence suggestive of BAD, based on increased fecal total BA excretion or increased BA synthesis.
Patient selection
Patients were recruited by public advertisement or by invitation to participate from a database of ~ 1,200 patients with IBS living in communities within ~120 miles of Mayo Clinic in Rochester, MN.
Characterization of bowel function
A validated diary-based questionnaire was used to characterize IBS symptoms and particularly bowel functions (10). Participants also completed the Hospital Anxiety and Depression Inventory (11).
Measurements of quantitative traits
All participants underwent measurement of quantitative traits by methods that have been previously used extensively and validated in our laboratory. These are described briefly here:
(i) Fasting serum C4 (sampled in the morning) measured by high-performance liquid chromatography/tandem mass spectrometry (12). Serum C4 is a validated method for BAM. In head-to-head comparisons with the 75SeHCAT retention test, increased serum C4 had a sensitivity of 90% and a specificity of 79% in diagnosing BAM (13) where shorter retention half-time of 75SeHCAT is associated with increased level of C4, and it had 98% negative predictive value and 74% positive predictive value for diagnosis of BAM (14).
Based on the method adapted from Galman et al. (15) and using high-performance liquid chromatography/tandem mass spectrometry, we used serum C4 to screen for high BA synthesis (12). The lowest limit of detection of serum C4 using this assay is 0.04ng/ml (12).
(ii) Fasting serum FGF19 measured by a commercial enzyme-linked immunosorbent assay (FGF19 Quantikine Enzyme-Linked Immunosorbent Assay Kit; R&D Systems, Minneapolis, MN) as in prior studies (3,6).
As there are diurnal variations in measurements of C4 (16) and FGF19 (17), these measurements were conducted on fasting blood samples.
(iii) Total and main fecal BA excretion (per 48 h on 100 g fat diet, measured by high-performance liquid chromatography/tandem mass spectrometry) (3,18,19). This assay was adapted from a method used with serum samples (20). We have previously documented the analysis and analytical performance (18); the lower limit of quantitation of each of the individual measured BAs (cholic acid, deoxycholic acid, chenodeoxycholic acid, and lithocholic acid) is 0.06 μmol (in methanol extract).
(iv) Fecal fat measured by nuclear magnetic resonance spectrometry at Mayo Clinic’s Department of Laboratory Medicine and Pathology.
(v) Overall CT by scintigraphy (geometric center (GC)) at 24 and 48 h (21). We have extensively validated the measurement of CT by scintigraphy including both accelerated and delayed CT, inter- and intra-individual coefficient of variation, effect sizes demonstrable and statistical power based on defined sample sizes, and responsiveness of the primary end points to pharmacological effects of medications including correct prediction of efficacy of those medications in subsequent phase IIB or III clinical trials (22–25).
(vi) Intestinal and colonic permeability: we administered 1g lactulose and 0.2 g mannitol in 240 ml water to study permeability (26). We had previously identified increased small bowel permeability in IBS-D compared with healthy controls (27). The 0–2h urine reflects most closely small intestinal permeability, and the 8–24 h urine reflects colonic permeability; the validated high-performance liquid chromatography/tandem mass spectrometric method was used (26). Participants were all nonsmokers and had not ingested during the 2-week period before these measurements nonsteroidal anti-inflammatory drugs or other drugs that could interfere with the interpretation of the results. We have previously (27,28) demonstrated that, in the context of non-inflammatory, nonulcerated bowel disorders like IBS, there is little lactulose absorbed and excreted (~2% of the mass administered orally) compared with mannitol (~25% of oral load). Therefore, the primary end point used to assess permeability was urine mannitol excretion.
In addition, 47 of the 64 patients with IBS-D also consented to undergo measurement of colonic sensation and postprandial tone by barostat; all colonic motility and sensation data garnered were included in the analysis (29,30).
Genotyping of venous blood DNA
DNA was extracted from venous blood, and candidate genotype analysis was conducted using established PCR-based methods: rs17618244 (KLB) and rs351855 (FGFR4). These candidate genes were selected based on prior studies from our group documenting relationship between BA-related genes and small intestinal or colonic transit (8,7). The methods have been published elsewhere (7). However, there is evidence from exome DNA sequence study (31) that other variants in KLB (rs1015450, downstream) and FGFR4 rs434434 (intronic), rs1966265, and rs351855 (nonsynonymous)) were associated with CT (FGFR4 rs1966265) and fecal bile acids (KLB rs1015450). There is also evidence that subgroups of patients, based on CT and fecal BA excretion, were significantly associated with all three FGFR4 single-nucleotide variants (31). In addition, an analysis of a 633-person cohort showed that FGFR4 rs434434 was associated with symptom phenotype (31), and FGFR4 rs1966265 and rs351855 modulate KLB rs1768244 association with 24-h CT in IBS-D (7). Therefore, we conducted further genotyping of these polymorphisms to assess the relationships with IBS-D and BAD. We did not assess the association with other genes involved in the enterohepatic circulation for two reasons: first, the other genes were not previously shown to be associated with CT (7); and second, the limited number of patients with these quantitative measurements adequately supported the study of three main genes of interest. For all gene variants tested, we have previously reported (7,31) that all were in Hardy–Wein-berg equilibrium in healthy participants from the same region (with similar ethnic and racial distribution to that of the cohort reported here).
Statistical power and sample size
The sample size was based on the previously observed results of the primary end points in volunteers in our lab (data in Table 1 show mean±s.d.). The estimated effect sizes are based on a two-sample t-test with the number per group shown in Table 1 (i.e., 30 vs. 30 or 20 vs. 40, which were the predicted proportions of numbers of patients with evidence of increased BA excretion or synthesis). The effect size is the difference in group means as a percentage of the overall mean.
Table 1.
Sample size estimates to detect effect sizes with 80% power
| Effect size detectable with 80% power: |
|||||
|---|---|---|---|---|---|
| Response | Meana | s.d.a | COV%a | 20 vs. 40 | 30 vs. 30 |
| Ascending colon T1/2, h | 14.9 | 9.2 | 62 | 49% | 46% |
| Colon GC24 h | 3.53 | 0.87 | 25 | 20% | 18% |
| Serum C4, ng/ml | 17 | 10 | 59 | 46% | 43% |
| Urine mannitol, 8–24 hb | 65.9 | 54.2 | 82 | 65% | 60% |
| Urine mannitol, 0–2 hb | 29.8 | 11.9 | 40 | 32% | 29% |
COV, coefficient of variation; GC, geometric center.
Based on pooled data from health and irritable bowel syndrome-diarrhea (IBS-D) (12,21,27); the table uses s.d. rather than s.e.m. so that the reader can calculate the coefficient of variation.
Mannitol excretion based on data acquired in our lab in health and IBS-D (27).
Statistical analysis
Bowel functions, individual fecal BA, CT, colonic compliance, postprandial tone and sensation, and intestinal permeability were compared in IBS-D subgroups identified by fecal BA excretion > 2,337mM per 48 h or by serum C4 >47.1 ng/ml based on 90th percentile of healthy volunteers studied in our laboratory (45 and 163 subjects, respectively) (3,12). The 90th percentile was used to define the upper limit of normal range, consistent with the observation that a normal distribution requires sampling of 500 normal people (32). We used Fisher’s exact test to assess associations of subgroups based on BA biology and the two BA-related genes (2 degree of freedom general genetic model). Biological samples were missing in 10 participants; therefore, the analysis was based on all 54 with complete data for serum C4, fecal BA excretion, and all the quantitative traits. The associations between fecal BA excretion or serum C4 and CT (at 48 h and ascending colon emptying T1/2) were assessed using Spearman’s correlation coefficients.
RESULTS
Characterization of phenotype and pathophysiology
Table 2 summarizes number of bowel movements, stool consistency, CT (GC48 h), small intestinal permeability, fecal fat, serum FGF19 and C4, and total fecal BA in the 64 patients with IBS-D. Data for the 54 who had all blood and stool measurements available are also presented in Table 2 and illustrate that there were no appreciable differences between the groups.
Table 2.
Characteristics of phenotype, bile acid measurements, colonic transit, and intestinal and colonic permeability in all 64 participants and in the 54 participants with complete blood and stool data
| Data mean±s.e.m. | IBS-D | IBS-D with complete blood and stool data |
|---|---|---|
| N | 64 | 54 |
| Gender (F/M) | 59/5 | 49/5 |
| Age, years | 41.9±1.5 | 40.6±1.6 |
| BMI, kg/m2 | 29.7±0.9 | 29.8±1.1 |
| Anxiety score (HAD) | 4.0±0.5 | 3.8±0.5 |
| Depression score (HAD) | 1.6±0.2 | 1.5±0.2 |
| No. of BM/day | 2.26±0.1 | 2.32±0.1 |
| BM form | 4.76±0.1 | 4.81±0.1 |
| Fecal fat (g/day) | 9.1±0.9 | 9.3±1.0 |
| Serum C4 (ng/ml) | 34.7±3.6 | 32.7±3.4 |
| Serum FGF19 (ng/ml) | 118.8±10.8 | 120.5±11.9 |
| Total fecal BA (mM) | 2,495±382 | 2,515±429 |
| Mean % fecal LCA/CDCA/DCA/CA | 30/6/53/8 | 30/6/53/8 |
| Colonic transit GC24 | 2.85±0.2 | 2.98±0.2 |
| Colonic transit GC48 | 4.18±0.12 | 4.26±0.13 |
| Urine mannitol, 0–2 h | 444.3±75.2 | 452.7±77.8 |
| Urine mannitol, 8–24 h | 45.5±5.0 | 45.7±5.2 |
BA, bile acid; BM, bowel movements; BMI, body mass index; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; F, female; FGF19, fibroblast growth factor 19; GC, geometric center; HAD, Hospital Anxiety and Depression; IBS-D, irritable bowel syndrome-diarrhea; LCA, lithocholic acid; M, male. Note that there are no appreciable differences in the measurements of the two groups.
Comparison of IBS-D subgroups based on fecal total BA excretion
In the group (19/54) with fecal BA > 2,337 mM per 48 h (90%ile of normal value), there were significantly greater body mass index, fecal fat, percent chenodeoxycholic acid (CDCA) and cholic acid in stool, and intestinal permeability (Table 3). There was a reciprocal lowering of stool lithocholic acid percentage. In the group with fecal BA, there were borderline accelerations of CT at 24 and 48 h (both P = 0.13) and postprandial colonic tone in the first 30 min after meal (P = 0.07), but no differences in postprandial colonic phasic motility index or colonic sensation. The numerically different serum C4 levels in the two groups based on total fecal BA excretion were not statistically significant (P = 0.228).
Table 3.
Comparison of phenotype and quantitative traits according to the BA profile based on either total fecal excretion of BA per 48 h or hepatic BA synthesis, based on fasting serum C4
| Data mean±s.e.m. | Fecal BA ≥90% | Fecal BA < 90% | Serum C4 ≥90% | Serum C4 < 90% |
|---|---|---|---|---|
| N | 19 | 35 | 13 | 41 |
| Age, years | 39.6±2.5 | 41.1±2.1 | 46.8±2.9 | 38.6±1.8 |
| BMI, kg/m2 | 32.7±1.9* | 28.2±1.3 | 33.5±2.8 | 28.6±1.1 |
| Anxiety score (HAD) | 2.8±0.5 | 4.3±0.7 | 3.6±0.8 | 3.8±0.6 |
| Depression score (HAD) | 1.3±0.4 | 1.6±0.3 | 1.8±0.5 | 1.4±0.3 |
| No. of BM/day | 2.5±0.3 | 2.2±0.2 | 2.3±0.2 | 2.3±0.2 |
| BM form | 4.8±0.2 | 4.8±0.1 | 4.7±0.3 | 4.8±0.1 |
| Fecal fat (g/day) | 12.4±2.2* | 7.6±1.0 | 8.7±1.8 | 9.5±1.3 |
| Serum C4 (ng/ml) | 34.5±4.7 | 25.3±3.1 | 70.0±6.0 | 20.9±1.5 |
| Serum FGF19 (pg/ml) | 126.7±22.3 | 117.2±13.9 | 103.7±28.2 | 125.9±12.9 |
| Total Fecal BA (mM) | 5,287±913 | 1,010± 114 | 3,152±819# | 2,313±503 |
| Mean % fecal LCA/CDCA/DCA/CA | 24*/8*/51/13** | 34/5/54/5 | 27/4/59*/6 | 31/6/51/8 |
| Colonic transit GC24 | 3.4±0.3& | 2.75±0.2 | 2.97±0.4 | 2.99±0.2 |
| Colonic transit GC48 | 4.5±0.2& | 4.12±0.2 | 4.38±0.3 | 4.22±0.2 |
| Postprandial/fast. colonic tone, % | 20.2±3.1 | 26.2±3.5 | 18.1±5.2 | 25.7±2.7 |
| 1 h postprandial colonic MI (phasic) | 15.0±0.1 | 14.8±0.1 | 14.6±0.2 | 15.0±0.1@ |
| Colon sensation threshold, first | 13.5±2.4 | 12.2±2.2 | 18.0±4.7 | 10.8±1.4 |
| Colon sensation threshold, gas | 23.2±3.5 | 21.2±3.0 | 25.3±5.8 | 20.8±2.3 |
| Colon sensation threshold, pain | 40.2±3.6 | 46.1±2.8 | 45.3±4.9 | 43.0±2.6 |
| Urine mannitol, 0–2h | 526±25** | 415±110 | 620±280 | 399±50 |
| Urine mannitol, 8–24h | 46.1±7.6 | 45.4±6.9 | 49.8±7.2@ | 44.4±6.4 |
BA, bile acid; BM, bowel movements; BMI, body mass index; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; Fast., fasting; FGF19, fibroblast growth factor 19; GC, geometric center; HAD, Hospital Anxiety and Depression; LCA, lithocholic acid; MI, motility index.[2]Note that normal laboratory values for colonic transit at 24 and 48 h in 220 healthy volunteers were 2.4±0.06 (s.e.m.) and 3.6±0.08, respectively.
P < 0.05;
P < 0.01;
P=0.06;
P=0.10;
P=0.13.
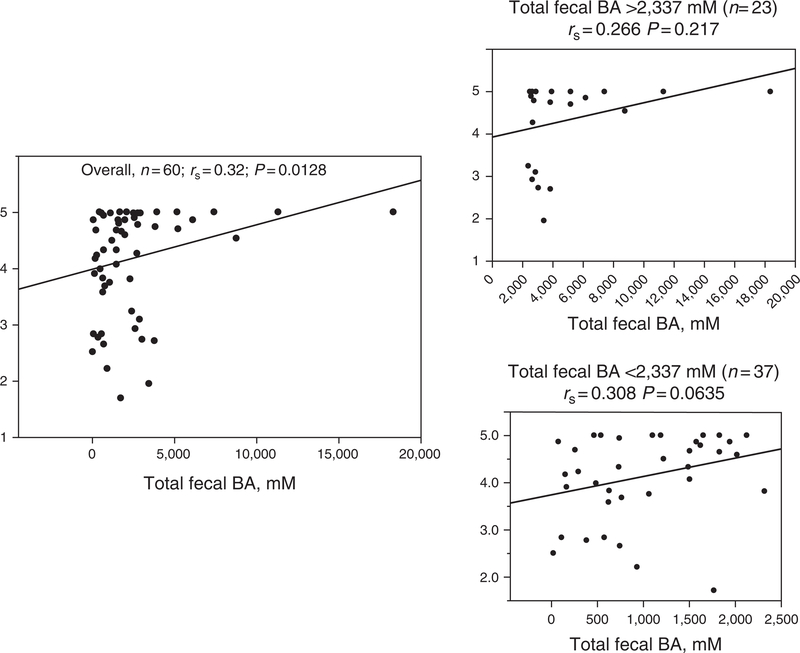
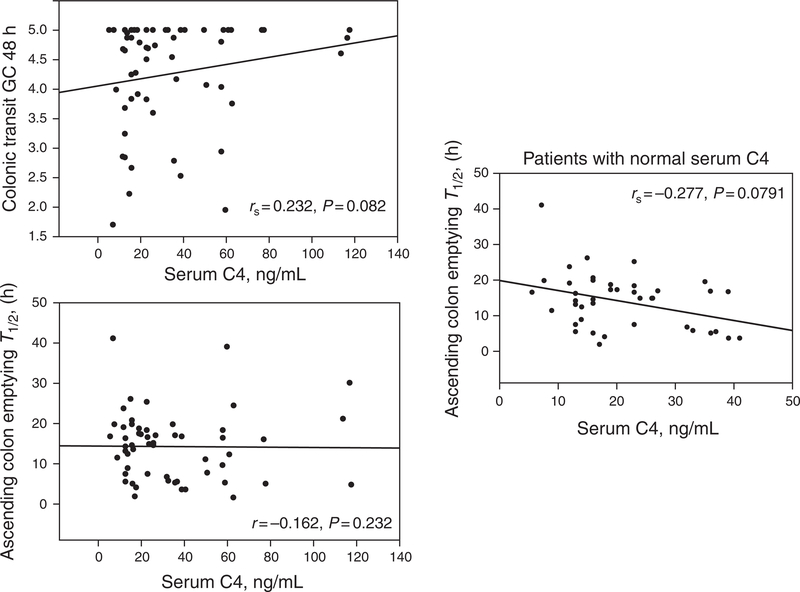
There was a significant overall correlation between total fecal BA excretion and CT at 48 h (rs = 0.32, P = 0.0132). This was predominantly observed in patients with total fecal BA excretion within the normal range (Figure 1). The overall correlation between fasting serum C4 and CT at 48 h (Figure 2) was borderline (rs = 0.232, P =0.082); the association of serum C4 and CT appeared to be associated predominantly with ascending colon emptying T1/2 (rs= −0.277, P = 0.0791) in patients with serum C4 within the normal range (<47.1 ng/ml).
Figure 1.
Relationship of fecal bile acid (BA) excretion and colonic transit at 48 h by Spearman’s correlation. Note the significant overall relationship between fecal BA excretion and overall colonic transit, and the apparently greater relationship in patients with normal total fecal BA excretion (< 2,337 mM).
Figure 2.
Relationship of fasting serum C4 and colonic transit at 48 h and ascending colon emptying T1/2 by Spearman’s correlation. GC, geometric center. Note the borderline relationship between serum C4 and overall colonic transit and the inverse relationship with ascending colon emptying T1/2 apparent only in patients with normal C4 (< 47.1 ng/ml).
Comparison of IBS-D subgroups based on serum C4
In the subgroup (13/54) with serum C4 >47.1 ng/ml (90%ile of normal value), there were increased total fecal BA excretion and percentage of deoxycholic acid (DCA) in stool (Table 4). There was borderline increase in colonic mucosal permeability but no significant differences in CT, colonic tone, phasic motility, or sensation.
Table 4.
Comparison of phenotype and quantitative traits according to the BA profile based on BOTH total fecal excretion of BA per 48 h or hepatic BA synthesis (fasting serum C4)
| Data mean±s.e.m. | Fecal BA and serum C4 > 90% | Fecal BA and serum C4 < 90% |
|---|---|---|
| N | 5 | 27 |
| Age, years | 47.8±2.3 | 39.6±2.3 |
| BMI, kg/m2 | 31.8±3.6 | 26.3±0.9 |
| Anxiety score (HAD) | 3.8±1.1 | 4.5±0.8 |
| Depression score (HAD) | 1.6±0.7 | 1.4±0.4 |
| No. of BM/day | 1.6±0.2 | 2.1±0.2 |
| BM form | 4.1±0.5 | 4.7±0.1 |
| Fecal fat (g/day) | 8.0±3.3 | 7.1±1.1 |
| Serum C4 (ng/ml) | 63.2±3.7 | 19.2±1.7 |
| Serum FGF19 (pg/ml) | 140.3±50.4 | 128.0±14.9 |
| Total fecal BA (mM) | 5,556±1,682 | 820±120 |
| Mean % fecal LCA/CDCA/DCA/CA | 31/5/52/9 | 36/5/51/5 |
| Colonic transit GC24 | 2.9±0.7 | 2.7±0.2 |
| Colonic transit GC48 | 4.0±0.6 | 4.0±0.2 |
| Postprandial/fast. colonic tone, % | 17.8±9.6 | 28.9±4.1 |
| Colon sensation threshold, first | 20.8±7.4 | 10.8±2.1 |
| Colon sensation threshold, gas | 27.2±8.8 | 20.2±3.1 |
| Colon sensation threshold, pain | 37.6±9.5 | 44.4±3.5 |
| Urine mannitol, 0–2 h | 373±59 | 309±44 |
| Urine mannitol, 8–24 h | 58.0±15.7 | 45.8±8.7 |
BA, bile acid; BM, bowel movements; BMI, body mass index; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; Fast., fasting; FGF19, fibroblast growth factor 19; GC, geometric center; HAD, Hospital Anxiety and Depression; LCA, lithocholic acid; MI, motility index.
Comparison of IBS-D subgroups based on elevated fecal BA excretion and serum C4
In the subgroup (5/54) with fecal BA >2,337mM per 48 h and serum C4 > 47.1 ng/ml, there were no significant differences in the quantitative measurements compared with the group of patients with neither parameter elevated (27/54), except for the expected higher serum C4 and total fecal BA excretion (Table 4).
Relationship of BA-related genes and subgroups of IBS-D based on total fecal BA or serum C4
Rs17618244 or rs1015450 (KLB), and rs351855, rs 1966265, or rs434434 (FGFR4) were not significantly associated with subgroups based on increased fecal total BA (Table 5); however, gene variants in KLB (rs1015450 (P=0.06)) and FGFR4 (rs1966265 (P = 0.09)) were borderline significantly associated with the subgroup defined by elevated serum C4 level.
Table 5.
Associations of IBS-D subgroups (increased fecal excretion or synthesis of bile acids and serum C4) with specific SNP genotypes
| Genotype | Elevated total fecal BA subgroup | Elevated C4 subgroup |
|---|---|---|
| KLB rs17618244 | 0.79 | 1.00 |
| KLB rs1015450 | 0.16 | 0.06 |
| FGFR4 rs351855 | 0.85 | 0.14 |
| FGFR4 rs1966265 | 0.43 | 0.09 |
| FGFR4 rs434434 | 0.55 | 0.46 |
BA, bile acid; FGFR4, fibroblast growth factor receptor 4; IBS-D, irritable bowel syndrome-diarrhea; KLB, klotho B; SNP, single-nucleotide polymorphism.
P values based on Fisher’s exact test.
DISCUSSION
Overall differences in the pathophysiological features associated with IBS-D subgroups
Our study shows that there are some quantitative differences in the pathophysiological features associated with IBS-D in patients with increased fecal BA excretion or increased BA synthesis. The proportion of IBS-D patients in whom there was evidence of increased BA excretion or synthesis in the current, prospectively studied patient cohort is consistent with the 25–35% reported in the literature or in prior studies (1), and with other recent papers in the literature (33,34). In a prior study (35), the increased colonic exposure to bile acids was also shown to influence bowel function and CT time (significant only in the left colon) in patients with IBS. The same authors observed response to open-label treatment with the BA sequestrant, colestipol, supporting the role of bile acids in the bowel dysfunction in these patients (35).
In assessing the pathophysiological associations of the two approaches to identifying BA component to the diarrhea in IBS-D patients, this study shows that those with fecal BA > 2,337 mM per 48 h had higher fecal fat, percent CDCA in feces, and intestinal permeability, and had borderline increased CT (P= 0.13), suggesting that steatorrhea, secretion (by the increased proportion of the secretory BA, CDCA, and increased permeability) and, possibly, accelerated CT may be inferred by the finding of increased fecal total BA.
We observed increased proportion of the secretory bile acid, chenodeoxycholic acid, and the nonsecretory lithocholic and cholic acids in the patients with increased fecal BA excretion. There was a nonsignificant mean difference of 0.7 GC units in CT at 24 h. The facts that BA malabsorption is so prevalent in patients with clinical symptoms of IBS-D and that other symptom and pathophysiological traits were not different in the two groups suggest that BAD should be excluded in patients presenting with symptoms of IBS-D, especially if there is an insufficient response to first-line therapy, such as with loperamide.
Colonic transit
Overall, these patients with IBS-D had acceleration in CT (36) with colonic geometric center at 24 and 48 h of 2.98±0.2 (s.e.m.) and 4.26±0.13 respectively; normal laboratory values for CT at 24 and 48 h in 220 healthy volunteers reported from our laboratory were 2.4±0.06 and 3.6±0.08, respectively (37). The current analysis shows only borderline significance (P = 0.13) for the comparison in CT between high compared with normal fecal BA excretion groups, despite 0.7 GC unit difference in mean colonic GC at 24 h; this may reflect insufficient power with the sample size studied. Table 1 predicted that a comparison of 20 vs. 40 patients (rather than 15 vs. 39 patients) would be sufficient to demonstrate an effect size of 20% in the colonic GC at 24 h. The observed difference in the means of colonic GC24 in the two groups was 24% (0.65/2.75), suggesting that the lack of difference in CT in the two groups was because of the larger observed s.d. with reduced statistical power. The statistically significant correlations between overall CT and fecal BA excretion or serum C4 suggest that colonic BA content is associated with CT, consistent with prior studies showing that ileocolonic delivery of chenodeoxycholate accelerated CT in healthy controls (6) and patients with IBS-constipation (38), and the retardation of CT with the BA sequestrant colesevelam in IBS-D (6). The effect of BA on CT appears to be saturable as there was a positive correlation of fecal bile excretion in the normal range with CT and an inverse correlation between serum C4 and ascending colon emptying time. In contrast, in patients with increased BA synthesis, there was no significant correlation with CT, suggesting another mechanism contributes to their rapid CT.
These data are also consistent with our recent finding on principal components analysis of patients with the symptom phenotype of IBS-D that showed at least two identifiable subgroups (31): first, a fast CT group of patients with modest increase in fecal BA and normal serum C4 (median 21.1 (interquartile range 12.9, 29.0)) and markedly accelerated transit at 24 h (4.57 (interquartile range 3.10, 4.79)) in whom the increased fecal BA may represent “spillage” of BA; second, increased BA synthesis diarrhea patients with increased serum C4 (median 68.0 (33.1, 75.2)) in whom CT is modestly accelerated (median 2.41 (interquartile range 1.62, 3.54)). The relationship between fecal BA and CT is consistent with documented effects of intraluminal administration of 1 mM CDCA and induction of high-amplitude propagated pressure waves in the colon (39), and the administration of 500 to 1,000 mg (1.2–2.4mM) sodium chenodeoxycholate in a delayed-release capsule that induced bowel function and accelerated CT in health and IBS-constipation (6,38).
We assessed whether having both increased serum C4 and total fecal BA excretion would result in differences in the quantitative traits measured. However, the sample size of only five patients with both increased serum C4 and total fecal BA excretion was certainly not large enough to appraise whether the combination of increased BA synthesis and excretion actually affected the phenotype of IBS-D. Studies in larger numbers of patients would be required to appraise the impact of both increased serum C4 and total fecal BA excretion.
Recent data suggest that these prokinetic effects may be mediated through the GPBAR1 receptor (40). The presence of excess BA in feces was associated with elevated fecal fat, percentage of secretory BA in stool, increased intestinal permeability, and borderline increased CT.
Steatorrhea
The cause of steatorrhea in patients with increased fecal BA excretion may reflect lower critical micellar concentration because of relative BA deficiency due to chronic loss of BA in stool. Though pancreatic exocrine insufficiency might be considered, the fecal fat excretion in the overall IBS-D group is still consistent with the range reported in experimental osmotic diarrhea, that is, up to ~23.5 g/day (41). Nevertheless, fecal fat was significantly higher in patients with high fecal BA excretion than those with normal fecal BA excretion, suggesting that these patients may, in fact, develop a relative BA deficiency causing low-grade steatorrhea. It is conceivable that these patients may benefit from dietary fat restriction or supplementation of a nonsecretory BA such as ursodeoxycholic acid (UDCA). This may benefit patients by enhancing the formation of micelles to facilitate fat absorption in the small bowel or to attenuate colonic epithelial secretion (42). It is relevant to note that the concentrations at which UDCA has its antisecretory actions (50 μM-1 mM) are not achievable normally in vivo. Thus, our study shows that only ~3% of the average 2,500 mM excreted BA is in the form of UDCA, and it is conceivable that the levels observed in feces represent, at least in part, the effects of bacterial β-epimerization of CDCA reaching the colon; hence, the concentration of UDCA reaching the colon is likely to be lower than 50mM. This is consistent with the prior report by De Kok et al. (43). Therapeutic supplementation of UDCA in healthy volunteers (e.g., 300 and 600 mg/day) significantly increased the concentrations in the colonic lumen and serum (44,45). In fact, as UDCA is bacterially metabolized to lithocholic acid in the colon (which does not have antisecretory action, but is the main metabolite detected in human studies of UDCA supplementation) (44), it is likely that the main effect of supplementation of UDCA will be to enhance fat absorption. The antisecretory effects of UDCA could be achieved by supplementation of 6α-MUDCA, a 6-methylated derivative of UDCA that is completely resistant to bacterial dehydroxylation (46), as demonstrated in mice in vivo ( 42 ).
Increased intestinal permeability
The reason for increased small intestinal permeability is not explored in the current studies. Our hypothesis is that increased colonic BA (and associated increased C4 and hepatocyte BA synthesis) was associated with increased concentrations of BA in the small bowel. It has been demonstrated that hydrophobic BA increased small intestinal permeability, in part through nicotinic cholinergic mechanisms or changes in small intestinal brush border membrane fluidity and fragility (47–51).
In patients with serum C4 > 47.1 ng/ml, there are increased total fecal BA excretion and borderline increased colonic permeability that may reflect higher fluid and electrolyte secretions and contribute to the loose stool consistency of patients with IBS-D.
Screening for BA diarrhea in patients with diarrhea-predominant IBS
BAD may be more effectively assessed with total fecal BA than with serum C4. Thus, our study identified increased fecal BA excretion in 19/54 patients with IBS-D and increased serum C4 in 13/54 of those patients. The group of patients with increased serum C4 had elevated fecal BA excretion. In contrast, patients with increased fecal BA excretion did not necessarily have increased serum C4; these patients had a nonstatistically increased serum FGF19 level, suggesting that the increased intraluminal BA (that ultimately caused increased total fecal BA excretion) stimulated ileal production of the hormone, FGF19 that reduced hepatocyte synthesis of C4, a surrogate of BA synthesis.
Genetic variation and control of BA homeostasis
The study explored genetic variation in KLB and FGFR4, and borderline univariate associations were identified between KLB (rs1015450 (P = 0.06)) and FGFR4 (rs1966265 (P = 0.09)) and the subgroup of IBS-D patients with elevated fasting serum C4, but not with increased fecal BA excretion. We had previously observed significant associations of KLB rs1015450 (31) and FGFR4 rs1966265 (3) with fecal BA excretion. The KLB rs1015450 is intronic and its functional significance has not yet been demonstrated. On the other hand, FGFR4 rs1966265 results in a valine to isoleucine change at position 10, and this single-nucleotide polymorphism appears to be functionally relevant, in view of the recent association with alveolar function ( 51 ).
The current data on associations of these single-nucleotide polymorphisms with BA homeostasis are hypothesis generating; if confirmed, this would be consistent with the known role of KLB and FGFR4 proteins in the feedback inhibition of hepatocyte BA synthesis. Thus, FGF19, secreted by enterocytes and reaching the liver through the portal venous return, normally binds to the FGFR4 receptor with interaction with KLB protein, and results in the inhibition of CYP7A1 in the BA synthesis pathway. Deficiencies of KLB or FGFR4 result in reduced inhibition of CYP7A1 and, hence, greater BA synthesis. This is indirectly reflected in the higher fasting serum C4 measurements. These genetic associations are of potential biological relevance, given the fact that the observations were based on 13 patients with elevated serum C4 and 41 with normal C4 measurements.
Strengths and limitations
The strengths of this study include the in-depth analysis of the phenotype of IBS-D and the use of two validated markers to identify the contribution of BAs (reflecting predominantly synthesis and excretion of the bile acids) to the overall phenotype. This is the first documentation of BA dysregulation and the associated quantitative manifestations in IBS-D.
A limitation of our study is that only 54 of the 64 patients with IBS-D were willing to participate in the 48-h fecal collections; regrettably, noninvasive 75SeHCAT retention test based on imaging is not available to be used as an alternative in the United States. In addition, we conducted multiple comparisons to characterize the quantitative traits between health and IBS and between patients with high compared with normal BA excretion. Importantly, for each physiological trait examined (e.g., CT, intraluminally measured colonic motility, or permeability), we had prespecified no more than two quantitative traits (e.g., CT at 24 and 48 h). The observed borderline associations with gene variants in KLB and FGFR4 provide the basis for further hypotheses-testing studies; if confirmed in a larger sample of patients, these findings may provide an alternative approach to screening patients for BAD and serve as complementary tests to measurements of serum C4 (12) and FGF19 (4), given the questions raised regarding the cost effectiveness of 75SeHCAT retention test and the logistic difficulties associated with 48-h stool collections (52).
In conclusion, IBS-D with increased BA excretion or synthesis (relative to normal BA profile) is associated with quantitative changes in fecal fat excretion, CT, and intestinal permeability, but there are no easily identifiable traits to distinguish the two groups. Given the high prevalence of BAD among patients with IBS-D (25–33% in the current cohort based on serum C4 and fecal BA excretion, respectively), there should be liberal screening for BAD in patients with IBS-D who do not respond to symptomatic treatment, such as with loperamide. The clinical relevance of finding BAD in IBS-D will be enhanced with future randomized, controlled studies that incorporate treatment, including sequestration of BA, or farnesoid X receptor agonists such as obeticholic acid (53). Such studies will build on evidence from open-label studies with colestipol (35) or smaller mechanistic studies with colesevelam (6), and may usher in a more specific approach of treatment targeting the BAD in patients with IBS-D who have either increased fasting serum C4, reduced serum FGF19 (4), increased 48-h total fecal BA, or reduced 75SeHCAT retention. Finally, our studies show that, in a setting where 75SeHCAT retention test is not available, BAD is identified more effectively with total fecal BA than with serum C4.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
-
✓
Approximately 25–33% of patients with irritable bowel syndrome-diarrhea (IBS-D) have bile acid malabsorption.
-
✓
Bile acid diarrhea is associated with increased fecal bile acid excretion.
-
✓
Serum C4 is a noninvasive screen for hepatic synthesis of bile acids.
WHAT IS NEW HERE
-
✓
IBS-D with increased bile acid excretion or synthesis is associated with more severe pathophysiology compared with IBS patients with normal bile acid profile.
-
✓
Bile acid diarrhea is identified more effectively by fecal bile acid excretion than serum C4.
-
✓
Genes in feedback regulation of bile acid synthesis may be associated with the IBS-D subgroup with elevated serum C4.
ACKNOWLEDGMENTS
We thank Mrs Cindy Stanislav for secretarial assistance and the nursing staff of the Mayo Clinic CCaTS for assistance with patient care.
Guarantor of the article: Michael Camilleri, MD.
Specific author contributions: M. Camilleri: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and study supervision; I. Busciglio: technical support (study coordinator, colonic motility) and revision of the manuscript; A. Acosta: conduct of the study (research fellow) and revision of the manuscript; A. Shin: conduct of the study (research fellow) and revision of the manuscript; P. Carlson: technical support (genotyping) and revision of the manuscript; D. Burton: technical support (scintigraphy, colonic motility) and acquisition of data; M. Ryks: technical support (scintigraphy) and acquisition of data; D. Rhoten: technical support (scintigraphy, colonic motility) and acquisition of data; J. Lamsam: technical support (serum C4 measurements); A. Lueke: technical support (fecal bile acid measurements); L.J. Donato: technical support (fecal bile acid measurements); A.R. Zinsmeister: analysis and interpretation of data and critical revision of the manuscript for important intellectual content.
Financial support: Camilleri is supported by grants R01-DK92179 from National Institutes of Health and CCaTS NIH grant UL1 TR000135.
Footnotes
CONFLICT OF INTEREST
Potential competing interests: None.
REFERENCES
- 1.Wedlake L, A’Hern R, Russell D et al. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2009;30:707–17. [DOI] [PubMed] [Google Scholar]
- 2.Eusufzai S, Axelson M, Angelin B et al. Serum 7 alpha-hydroxy-4-cholesten-3-one concentrations in the evaluation of bile acid malabsorption in patients with diarrhoea: correlation to SeHCAT test. Gut 1993;34:698–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong BS, Camilleri M, Carlson P et al. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol 2012;10:1009–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pattni SS, Brydon WG, Dew T et al. Fibroblast growth factor 19 in patients with bile acid diarrhoea: a prospective comparison of FGF19 serum assay and SeHCAT retention. Aliment Pharmacol Ther 2013;38:967–76. [DOI] [PubMed] [Google Scholar]
- 5.Walters JR, Tasleem AM, Omer OS et al. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol 2009;7:1189–94. [DOI] [PubMed] [Google Scholar]
- 6.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol 2010;8:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong BS, Camilleri M, Carlson PJ et al. A klothop variant mediates protein stability and associates with colon transit in irritable bowel syndrome with diarrhea. Gastroenterology 2011;140:1934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camilleri M, Vazquez-Roque MI, Carlson P et al. Association of bile acid receptor TGR5 variation and transit in health and lower functional gastrointestinal disorders. Neurogastroenterol Motil 2011;23:995–9, e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walters JR, Pattni SS. Managing bile acid diarrhoea. Therap Adv Gastroenterol 2010;3:349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talley NJ, Phillips SF, Melton J III et al. A patient questionnaire to identify bowel disease. Ann Intern Med 1989; 111:671–4. [DOI] [PubMed] [Google Scholar]
- 11.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- 12.Camilleri M, Nadeau A, Tremaine WJ et al. Measurement of serum 7α-hydroxy-4-cholesten-3-one (or 7αC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil 2009;21:734–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sauter GH, Munzing W, von Ritter C et al. Bile acid malabsorption as a cause of chronic diarrhea: diagnostic value of 7alpha-hydroxy-4-cholesten-3-one in serum. Dig Dis Sci 1999;44:14–9. [DOI] [PubMed] [Google Scholar]
- 14.Brydon WG, Nyhlin H, Eastwood MA et al. Serum 7 alpha-hydroxy-4-cholesten-3-one and seleno-homocholyltaurine (SeHCAT) whole body retention in the assessment of bile acid induced diarrhoea. Eur J Gastroenterol Hepatol 1996;8:117–23. [DOI] [PubMed] [Google Scholar]
- 15.Gälman C, Arvidsson I, Angelin B et al. Monitoring hepatic cholesterol 7alpha-hydroxylase activity by assay of the stable bile acid intermediate 7alpha-hydroxy-4-cholesten-3-one in peripheral blood. J Lipid Res 2003;44:859–66. [DOI] [PubMed] [Google Scholar]
- 16.Abrahamsson H1, Ostlund-Lindqvist AM, Nilsson R et al. Altered bile acid metabolism in patients with constipation-predominant irritable bowel syndrome and functional constipation. Scand J Gastroenterol 2008;43:1483–8. [DOI] [PubMed] [Google Scholar]
- 17.Gälman C, Angelin B, Rudling M. Pronounced variation in bile acid synthesis in humans is related to gender, hypertriglyceridaemia and circulating levels of fibroblast growth factor 19. J Intern Med 2011;270:580–8. [DOI] [PubMed] [Google Scholar]
- 18.Shin A, Camilleri M, Vijayvargiya P et al. Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2013;11:1270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vijayvargiya P, Camilleri M, Shin A et al. Methods for diagnosis of bile acid malabsorption in clinical practice. Clin Gastroenterol Hepatol 2013;11: 1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tagliacozzi D, Mozzi AF, Casetta B et al. Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: a simple and rapid one-step method. Clin Chem Lab Med 2003;41:1633–41. [DOI] [PubMed] [Google Scholar]
- 21.Deiteren A, Camilleri M, Bharucha AE et al. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterol Motil 2010;22:415–23, e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camilleri M, McKinzie S, Busciglio I et al. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2008;6:772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manabe N, Wong BS, Camilleri M et al. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil 2010;22:293–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zinsmeister AR, Burton D, Camilleri M. Pharmacodynamic and clinical endpoints for functional colonic disorders: statistical considerations. Dig Dis Sci 2013;58:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camilleri M. Scintigraphic biomarkers for colonic dysmotility. Clin Pharmacol Ther 2010;87:748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camilleri M, Nadeau A, Lamsam J et al. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterol Motil 2010;22:e15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao AS, Camilleri M, Eckert DJ et al. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am J Physiol 2011; 301:G919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vazquez-Roque MI, Camilleri M, Smyrk T et al. Association of HLA-DQ gene with bowel transit, barrier function, and inflammation in irritable bowel syndrome with diarrhea. Am J Physiol 2012;303:G1262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odunsi ST, Camilleri M, Bharucha AE et al. Reproducibility and performance characteristics of colonic compliance, tone and sensory tests in healthy humans. Dig Dis Sci 2010;55:709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravi K, Bharucha AE, Camilleri M et al. Phenotypic variation of colonic motor functions in chronic constipation. Gastroenterology 2010;138:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camilleri M, Klee EW, Shin A et al. Irritable bowel syndrome-diarrhea: characterization of genotype by exome sequencing, and phenotypes of bile acid synthesis and colonic transit. Am J Physiol 2014;306:G13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altman DG, Bland JM. The normal distribution. BMJ 1995;310:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurien M, Evans KE, Leeds JS et al. Bile acid malabsorption: an under-investigated differential diagnosis in patients presenting with diarrhea predominant irritable bowel syndrome type symptoms. Scand J Gastroenterol 2011;46:818–22. [DOI] [PubMed] [Google Scholar]
- 34.Gracie DJ, Kane JS, Mumtaz S et al. Prevalence of, and predictors of, bile acid malabsorption in outpatients with chronic diarrhea. Neurogastroenterol Motil 2012;24:983–e538. [DOI] [PubMed] [Google Scholar]
- 35.Bajor A, Tornblom H, Rudling M et al. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut 2014; doi: 10.1136/gutjnl-2013-305965 (e-pub ahead of print). [DOI] [PubMed] [Google Scholar]
- 36.Camilleri M, Shin A, Busciglio I et al. Validating a biomarker for irritable bowel syndrome. Gastroenterology 2014;146 (Suppl1): S119–20. [Google Scholar]
- 37.Kolar GJ, Camilleri M, Burton D et al. Prevalence of colonic motor or evacuation disorders in patients presenting with chronic nausea and vomiting evaluated by a single gastroenterologist in a tertiary referral practice. Neurogastroenterol Motil 2014;26:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao AS, Wong BS, Camilleri M et al. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology 2010;139:1549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bampton PA, Dinning PG, Kennedy ML et al. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol 2002. ; 282 : G443–9. [DOI] [PubMed] [Google Scholar]
- 40.Alemi F, Poole DP, Chiu J et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology 2013;144:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammer HF, Santa Ana CA, Schiller LR et al. Studies of osmotic diarrhea induced in normal subjects by ingestion of polyethylene glycol and lactulose. J Clin Invest 1989;84:1056–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly OB, Mroz MS, Ward JB et al. Ursodeoxycholic acid attenuates colonic epithelial secretory function. J Physiol 2013;591:2307–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Kok TM, Van Faassen A, Glinghammar B et al. Bile acid concentrations, cytotoxicity, and pH of fecal water from patients with colorectal adenomas. Dig Dis Sci 1999;44:2218–25. [DOI] [PubMed] [Google Scholar]
- 44.van Gorkom B, van der Meer R, Boersma-van Ek W et al. Changes in bile acid composition and effect on cytolytic activity of fecal water by ursodeoxycholic acid administration: a placebo-controlled cross-over intervention trial in healthy volunteers. Scand J Gastroenterol 2002;37:965–71. [DOI] [PubMed] [Google Scholar]
- 45.Hess LM, Krutzsch MF, Guillen J et al. Results of a phase I multiple-dose clinical study of ursodeoxycholic acid. Cancer Epidemiol Biomarkers Prev 2004;13:861–7. [PubMed] [Google Scholar]
- 46.Roda A, Pellicciari R, Polimeni C et al. New 6-substituted bile acids: physico-chemical and biological properties of 6 a-methyl ursodeoxycholic acid and 6 a-methyl-7-epicholic acid. J Lipid Res 1994;35:2268–79. [PubMed] [Google Scholar]
- 47.Erickson RA, Epsten RM Jr. Oral chenodeoxycholic acid increases small intestinal permeability to lactulose in humans. Am J Gastroenterol 1988;83:541–4. [PubMed] [Google Scholar]
- 48.Fihn BM, Sjoqvist A, Jodal M. Involvement of enteric nerves in permeability changes due to deoxycholic acid in rat jejunum in vivo. Acta Physiol Scand 2003;178:241–50. [DOI] [PubMed] [Google Scholar]
- 49.Zhao DL, Hirst BH. Bile salt-induced increases in duodenal brush-border membrane proton permeability, fluidity, and fragility. Dig Dis Sci 1990;35:589–95. [DOI] [PubMed] [Google Scholar]
- 50.Stenman LK, Holma R, Eggert A et al. A novel mechanism for gut barrier dysfunction by dietary fat: epithelial disruption by hydrophobic bile acids. Am J Physiol Gastrointest Liver Physiol 2013;304:G227–34. [DOI] [PubMed] [Google Scholar]
- 51.Rezvani M, Wilde J, Vitt P et al. Association of a FGFR-4 gene polymorphism with bronchopulmonary dysplasia and neonatal respiratory distress. Dis Markers 2013;35:633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riemsma R, Al M, Corro Ramos I et al. SeHCAT [tauroselcholic (selenium-75) acid] for the investigation of bile acid malabsorption and measurement of bile acid pool loss: a systematic review and cost-effectiveness analysis. Health Technol Assess 2013;17:1–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnston IM, Nolan JD, Dew T et al. A new therapy for chronic diarrhea? A proof of concept study of the FXR agonist obeticholic acid in patients with primary bile acid diarrhea. Gastroenterology 2013;144 (Suppl 1): S60. [Google Scholar]