1. INTRODUCTION
Dendrites are the primary site of synaptic activity in the nervous system, occupying a large amount of the total volume of the brain, with differences in the complexity of the dendritic arbor being correlated to overall neuronal function (McAllister, 2000; Purves and Hume, 1981; Rubin, 1985). Abnormal changes in synapses are the first pathological event in neurodegenerative diseases. These are evidenced as changes in the number of dendrites per neuron and loss of the complexity of the dendritic spine (Wishart, Parson, and Gillingwater, 2006). Changes in synapses are reflected as deficits in neuron-neuron communication and loss of neuronal function. Examples of these cases are seen throughout the aging process of those with dementia (Buell and Coleman, 1981) and as a result of traumatic brain injuries (Ito, Kuroiwa, Nagasao, Kawakami, and Oyanagi, 2006). Important research regarding changes in dendritic growth in these diseases and understanding the mechanisms of regeneration after injury requires investigation of neuron morphology, which is usually achieved by evaluating soma size, number of dendrites, and length of dendrites per cell (Chandrasekaran, Lea, Sosa, Higgins, and Lein, 2015; Kleene, Yang, Kutsche, and Schachner, 2001; Liu, Sang, Lu, Wang, Yu, and Zhong, 2017).
As research on preventing or reversing synaptic degeneration develops, the demands increase for user-friendly neurite analyzers without undermining accuracy and reproducibility. Successful programs have been developed for accurate, efficient methods of tracing 3D confocal microscopy image stacks (Hines and Carnevale, 2001; Parekh and Ascoli, 2013; Pool, Thiemann, Bar-Or, and Fournier, 2008; Wearne, Rodriguez, Ehlenberger, Rocher, Henderson, and Hof, 2005). On the other hand, 2-D neurite analysis, which is routinely used for evaluating neuritogenesis, contains more ambiguity than 3-D analysis with some neurites being out of focus and branching or crossing over being uncertain (Kim, Son, and Palmore, 2015; Meijering, Jacob, Sarria, Steiner, Hirling, and Unser, 2004). Neurite measurements of 2-D images are usually taken manually, which is a tedious and time-consuming process, especially when considering multiple morphological aspects (number of dendrites, length of dendrites, number of branches, and size of soma) as well as the large number of neurons needed to be analyzed.
Of the 2-D analyses software tools available, ImageJ (Schneider, Rasband, and Eliceiri, 2012) is one of the commonly used software and uses a series of connected straight lines to trace neurites (Pool et al., 2008). Although fairly easy to use, this method completely relies on the user’s ability to distinguish the shape, size, and branching of the neurite, opening the door for unintentional operator errors and subjective biases. Additional plug-ins for ImageJ such as NeuronJ have been developed that facilitate the measurement process on ImageJ (Liu, Sang, Lu, Wang, Yu, and Zhong, 2017; Meijering, 2010; Meijering, Jacob, Sarria, Steiner, Hirling, and Unser, 2004). These programs trace the shape of the neurites semi-automatically with a smooth line, providing measurements of the total length of dendrites per cell. However, the accuracy of the measurements depends on the user’s ability to distinguish the start and end of the neurite. Furthermore, there are also image processing software, such as ImagePro, that can be used as neurite analyzers (Bhaskar, Miller, Chludzinski, Herrup, Zagorski, and Lamb, 2009; Liu, Sang, Lu, Wang, Yu, and Zhong, 2017) and may reduce processing time and user interaction by creating macros for automatic quantification (Francisco, Moraes, and Dias, 2004; Nielsen and Hansen, 2010). However, the consistency and accuracy in the dendritic growth measurements acquired using these methods has been mostly evaluated in hippocampal neurons, but not in other neuronal models. Therefore, in this study, dendritic growth in primary cultures of embryonic rat sympathetic neurons was assessed using each of these methods to determine the best methods for 2-D analyses of dendritic outgrowth in these neurons.
Although non-proliferating and limited in supply, primary neurons are biologically more relevant than established neuronal cell lines, possessing characteristics that more closely resemble cells in vivo (Gordon, Amini, and White, 2013). Neuronal cell lines are easier to work with, but they differ from primary neuron cultures by vital characteristics, suggesting use of cell lines may not accurately model the response of primary neurons (LePage, Dickey, Gerwick, Jester, and Murray, 2005). Primary cultures of embryonic rat sympathetic neurons have been used as a well-characterized model for studying the initiation and early stages dendritic growth in vitro (Lein, Johnson, Guo, Rueger, and Higgins, 1995). These neurons do not extend dendrites in cell culture when grown in the absence of serum or glial cells and can be induced to extend a complex dendritic arbor upon exposure to bone morphogenetic protein-7, resembling the dendritic arbor observed in vivo (Bruckenstein and Higgins, 1988; Ghogha, Bruun, and Lein, 2012; Lein, Johnson, Guo, Rueger, and Higgins, 1995). Therefore, images of primary sympathetic neurons with BMP-7-induced dendritic growth were used to evaluate the consistency and accuracy of dendritic growth measurement methods.
Our data show that similar results were obtained when using ImageJ and NeuronJ methods and their results were observed to be reproducible through multiple trials, but significant differences and inconsistencies across images were obtained by ImagePro measurements. Through application of an automated macro for ImagePro, this study demonstrates that the expertise and training of the operator are key in accurate tracing of neurites. Although use of automatic neurite analysis allows the user to produce measurements at a much faster rate than manual methods, the results are not nearly as reliable and should not be used in place of manual or semi-automatic methods.
2. MATERIALS AND METHODS
2.1. Chemicals and Biochemicals
Recombinant human bone morphogenetic proteins (BMPs) were generously provided by Dr. Pamela Lein at the University of California Davis. Cytosine-β-D arabinoside (Ara-C) was purchased from Sigma Aldrich Corporation (St. Louis, MO). Other tissue culture media components, gel electrophoresis supplies were purchased from Life Technologies (Grand Island, NY). All chemicals and biochemicals were of analytical grade or higher.
2.2. Acquisition of Images Used for Dendritic Growth Measurement and Analysis
Primary cultures of sympathetic neurons were isolated from perinatal rat superior cervical ganglia neurons, treated with BMP-7 (50 ng/ml) for 5 days, immunostained with antibody against microtubule associated protein-2 (MAP-2), a dendrite specific marker, and visualized by immunofluorescence according to previously described protocols (Ghogha, Bruun, and Lein, 2012). 2-D images of the dendritic arbor of individual neurons were acquired using the Nikon Eclipse E400 fluorescent microscope and SPOT camera.
2.3. ImageJ Manual Measurements
Using the “segmented lines selection” tool on ImageJ freeware (Schneider, Rasband, and Eliceiri, 2012), the total length of the dendritic arbors of sympathetic neurons were manually measured (Figure 1A). This tool requires the user to indicate the start and end of each dendrite, using a crosshair pointer, and map the length and shape of each dendrite with a series of connected straight lines. Soma size was measured using the “selection brush” tool on ImageJ freeware to carefully outline the shape of the soma (Figure 1B). To verify the reproducibility of the tool, length and soma measurements were taken a total of 5 times for each neuron with at least 24 hours between trials. The number of dendrites per cell was determined by visually counting projections from the soma. Requirements for counting a projection as a dendrite included: (i) Neurons being analyzed were at least one cell body away from another neuron, (ii) Projections were counted as dendrites if the length was at least the diameter of the soma.
Figure 1. Results from three methods of morphological analysis.
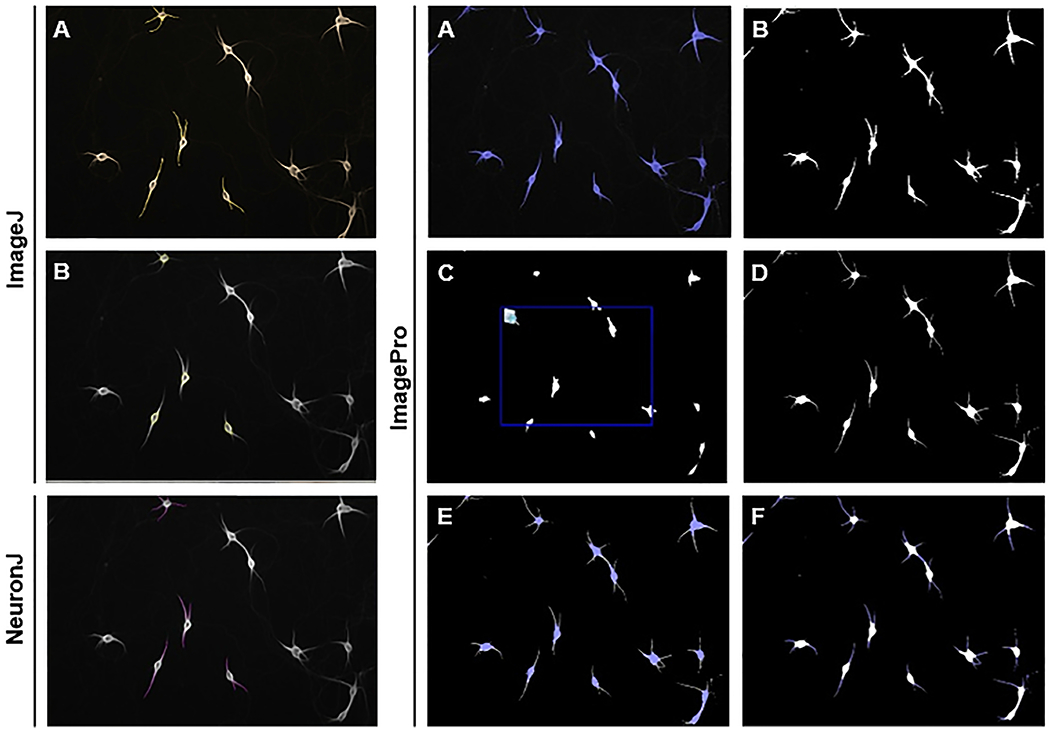
ImageJ: (A) “Segmented lines selection” tool for dendrite length measurements, (B) “Selection brush” tool for soma measurements.
NeuronJ: “Add tracings” tool for dendrite length measurements.
ImagePro: (A) Object selection, (B) Create a mask of objects, (C) Dilate eroded mask, (D) “Image compare” to overlay original mask and dilated-eroded mask, (E) Select somas as objects and dendrites as background for soma measurements, (F) Select dendrites as objects and soma as background for dendrite measurements.
2.4. NeuronJ Semi-Automatic Dendrite Measurement
Similar to manual measuring, the use of NeuronJ plug-in for ImageJ (Meijering, 2010; Meijering, Jacob, Sarria, Steiner, Hirling, and Unser, 2004) requires the user to indicate the start and end of each dendrite. However, this plug-in facilitates tracing of these neurites, resolving any uncertainties generated by use of the “segmented lines selection” tool, by automatically tracing the exact morphology of the dendrites with a single smooth line (Figure 1). In order to consider reproducibility of the tool, measurements were made a total of 5 times for each neuron with at least 24 hours between trials. NeuronJ plug-in does not have the ability to count the number of dendrites or measure the area of the soma; these measurements were taken by visually counting the number of dendrites and manually measuring the soma area using ImageJ.
2.5. ImagePro Automatic Measurement
Since measurements taken by ImagePro were automatic, they were not tested for reproducibility. The following steps were used to define dendrites and measure their length using ImagePro. The steps were recorded as an automatic macro for “batch processing”:
Using the “smart” segmentation tool, the areas that occupied the background and object were identified (Figure 1A) and a mask of the neurons was created (Figure 1B).
The mask was eroded until the soma was the only visible area (for these images, 9 passes). The eroded image was then dilated so that the mask matched the size and shape of the soma in the original image (for these images, 8 passes; Figure 1C).
The eroded-dilated mask was overlaid with the original mask using the “image compare” tool, creating a new image (Figure 1D).
Using the “smart” segmentation tool, the dendrites and black background of the new image were identified as background, and the somas were identified as objects (Figure 1E).
Each neuron to be measured was selected as a separate region.
Having selected the type of measurement as “area”, the selected regions of interest were counted for the area of each soma.
Using the “smart” segmentation tool, the soma and black background of the image were identified as background, and the dendrites were identified as objects (Figure 1F).
Having selected the type of measurement as “region: number of dendrites”, the selected regions of interest were counted for the total number of dendrites per cell.
The count options were then identified as “vectorized”. Having selected the type of measurement as “length”, regions of interest were again counted for length measurements and summed for each neuron.
2.6. Statistical Analysis
A total of 341 neurons were analyzed—number of dendrites, length of dendrites, and size of soma—using each macro or software as indicated in the text. Statistical significance of data across the three methods—ImageJ, NeuronJ, and ImagePro—and reproducibility of data across trials was determined by ANOVA followed by Bonferroni’s post-hoc test. Statistical significance of data across the two methods—ImageJ and ImagePro—was determined by using the Student’s t-test with equal or unequal variance depending on the result from the F-test. Significance was set at p = 0.05 or lower.
3. RESULTS
3.1. Reproducibility of methods
One hundred-fifty images of neurons were imported into each software that was used to quantify dendrite length, number of dendrites per neuron, and soma size, namely ImageJ, NeuronJ, and ImagePro. Total of 341 neurons were analyzed by each method. In order to test for reproducibility of each method, each of the 341 neurons analyzed were measured for dendrite length and soma size in 5 separate trials by one single operator. Each trial was separated by at least 24 hours. Due to the fact that ImagePro is automated, only ImageJ and NeuronJ were tested for reproducibility.
Compared to trial 1, measurements of dendritic length were significantly larger in the last 3 trials (p = 0.0053, p < 0.001, p < 0.001) for ImageJ and last 2 trials (p = 0.0005, p = 0.001) for NeuronJ (Figure 2A). No statistically significant differences were observed between ImageJ and NeuronJ throughout all 5 trials, representing comparable reproducibility between methods.
Figure 2. Effect of operator training on neuron measurements.
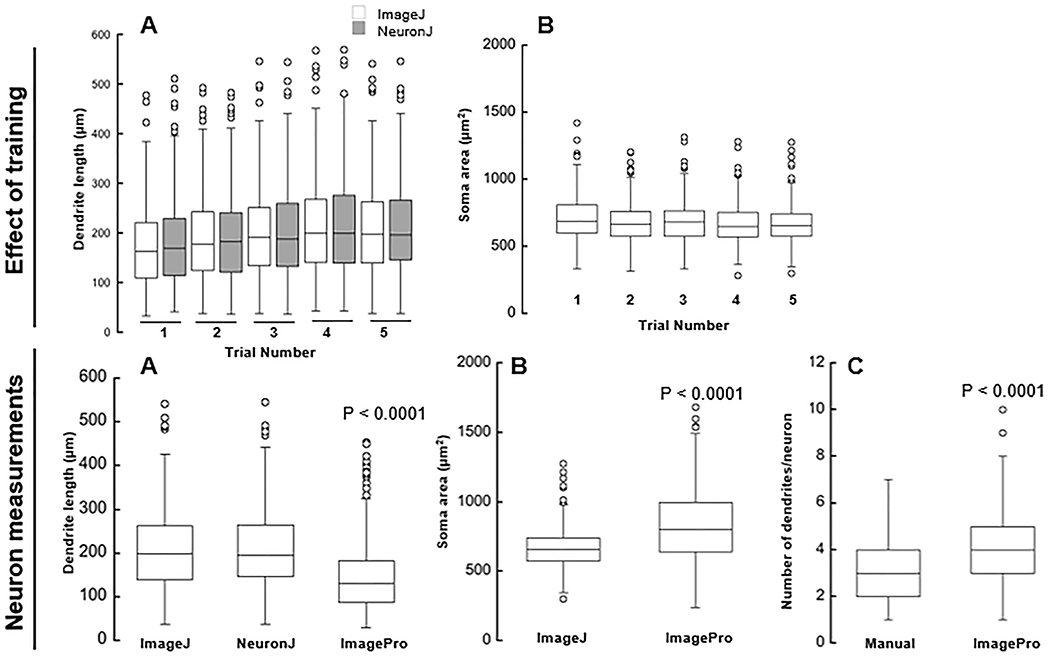
Effect of training: (A) Total length of dendrites per neuron (μm); Measurements taken by ImageJ and NeuronJ across 5 trials of measurements. (B) Area of soma (μm2); Measurements taken by ImageJ across 5 trials of measurements.
Neuron measurements: (A) Total length of dendrites per neuron (μm); Measurements taken by ImageJ, NeuronJ, and ImagePro from trial 5. (B) Area of soma (μm2); Measurements taken by NeuronJ and ImagePro from trial 5. (C) Number of dendrites per neuron; Measurements taken manually and by ImagePro.
Data trials regarding soma size indicate significantly smaller measurements in trials 2, 4, and 5 compared to trial 1 (p = 0.0486, p = 0.04, p = 0.0029) (Figure 2B). No statistically significant differences were observed between trials 2, 3, 4, and 5.
All neuron measurements—number of dendrites, length of dendrites, and soma size—used for method comparison are from trial 5.
3.2. Evaluation of dendrite length
The manual feature of ImageJ resulted in an average neurite length of 209 ± 96 μm (mean ± SD) with minimum and maximum values of 38 and 542 μm. By using NeuronJ, the average was 211 ± 96 μm (minimum and maximum values of 38 and 547 μm) and ImagePro (minimum and maximum values of 30 and 455 μm) resulted in lower mean value of 148 ± 82 μm. Analysis of the data regarding measurements of the average length of dendrites per neuron (Figure 2A) indicated no statistically significant difference between manual measurements by ImageJ and semi-automatic measurements by NeuronJ (p = 1). But, automatic measurements performed with ImagePro were significantly smaller (by 29% and 30% compared to ImageJ and NeuronJ, respectively) than those taken manually or semi-automatically (p < 0.0001).
3.3. Evaluation of dendrite number
Manual counting of dendrites resulted in an average number of dendrites per neuron of 3.17 ± 1.2 dendrites (minimum and maximum values of 1 and 5 dendrites), whereas automatic counting by ImagePro resulted in a higher average value of 4.15 ± 1.9 dendrites per neuron (minimum and maximum values of 2 and 7 dendrites). Data regarding the number of dendrites per neuron (Figure 2B) indicated that ImagePro counted significantly more dendrites per cell (by 31%) than those counted manually (p < 0.0001).
3.4. Evaluation of soma size
Manual measurements of soma size taken with ImageJ resulted in an average area of 666 ±140 μm2 (minimum and maximum values of 300 and 1279 μm2) compared to the larger mean area value, 828 ± 250 μm2 (minimum and maximum values of 237 and 1683 μm2), measured automatically using ImagePro (Figure 2C). Measurements of soma size were significantly greater when taken automatically using ImagePro (by 24%) than manually with ImageJ (p < 0.0001).
4. DISCUSSION
The central goal of many studies regarding changes in dendritic growth in the fields of neurodegenerative diseases or brain injury is to elucidate the pathways regulating synaptic connections. This requires accurate, quantitative methods for measurement of the soma size, number of dendrites, and length of dendrites per cell. Neurite analysis relies on taking measurements from neurons of many batches of images from multiple experimental conditions, resulting in a long, arduous process. As these areas of research continue to grow, the demand increases for a 2D neurite analyzer that is cost effective, efficient, and requires minimal user interaction.
In this study, we investigated the validity of three 2-D neurite analysis methods—manual, semi-automatic, and automatic—providing insight into the inconsistencies in measurements across different platforms. Since primary cultures of sympathetic neurons are a well-characterized model system to study initiation and early stages of dendritic growth (Lein, Johnson, Guo, Rueger, and Higgins, 1995), our study was focused on optimizing the methods for analyzing the measurement of dendritic growth during the initial phases of dendritic growth, i.e. following 5 days of BMP-7 exposure. A previous study compared the reliability of manual tracing and NeuronJ for measuring neurite length in primary hippocampal neurons and demonstrated the reliability and reproducibility of NeuronJ measurements (Cottrell, Ahmed, James, Hodson, McDonnell, Rauz, and Williams, 2014). The findings from our study have extended the applicability of NeuronJ tracing to dendritic growth measurements in sympathetic neurons.
In comparison to manual measurements with ImageJ, NeuronJ limited unintentional user-bias is achieved by requiring less user interaction and processing time (Cottrell, Ahmed, James, Hodson, McDonnell, Rauz, and Williams, 2014). Unlike ImagePro, NeuronJ can be used on a wide variety of photo qualities, and the results are unchanged by differences in background noise and various levels of staining (Meijering, Jacob, Sarria, Steiner, Hirling, and Unser, 2004). Although NeuronJ does require the user to indicate the start and end of each dendrite, it automatically fills in the shape between those points, whereas the accuracy of tracing each dendrite with ImageJ relies completely on the user’s expertise. Nonetheless, reproducing measurements of length indicated that, although variations exist between trials due to operator error, neither method is more equipped than the other for controlling differences between trials.
Our study also found ImagePro (following the procedure utilized in this study) to be the least reliable method of dendrite analysis tested here, whereas ImageJ and NeuronJ performed similarly in measuring the total length of dendrites per neuron. Our data showed that the manual and semi-automatic tracings of dendritic length were very similar, whereas ImagePro underestimated the length of the dendrites. The differences between the methods stemmed from abilities of NeuronJ and ImageJ to accurately adhere to the shape of each dendrite and the lack of ability of ImagePro to mask and measure the complete dendritic arbor (Figure 3) or the inability to detect either the tip of the dendrite or size of the cell body (Figure 3; Neuron B). These changes decreased the length of dendrites compared to ImageJ and NeuronJ by around 130 μm to 140 μm and increased cell body area by around 230 μm2 (Figure 3; Neuron B). Although our results demonstrate that automated ImagePro (with the sequence limiting user interaction and employing the “batch processing” feature) is not a reliable option for neuron analysis, other studies have utilized ImagePro for certain aspects of neurite analysis through different sequences of tools with varying levels of user interaction (Bhaskar, Miller, Chludzinski, Herrup, Zagorski, and Lamb, 2009; Liu, Sang, Lu, Wang, Yu, and Zhong, 2017). Similarly, ImagePro has also been found to be useful for counting labeled nuclei (Francisco, Moraes, and Dias, 2004) and measurement of fluorescent foci location in bacterial cells (Nielsen and Hansen, 2010). Slight variations in individual photo quality—intensity of staining, density of cells, level of background noise—leads to drastic changes in the detection of each part of the neuron by ImagePro. The “batch processing” feature of ImagePro has potential to be an efficient option, processing many photos at once with limited user interaction. However, additional studies are required to determine parameters that can improve the accuracy of the masking of soma and detection of dendrite length to increase its utility for dendritic growth measurements.
Figure 3. Visual comparison of measurement methods.
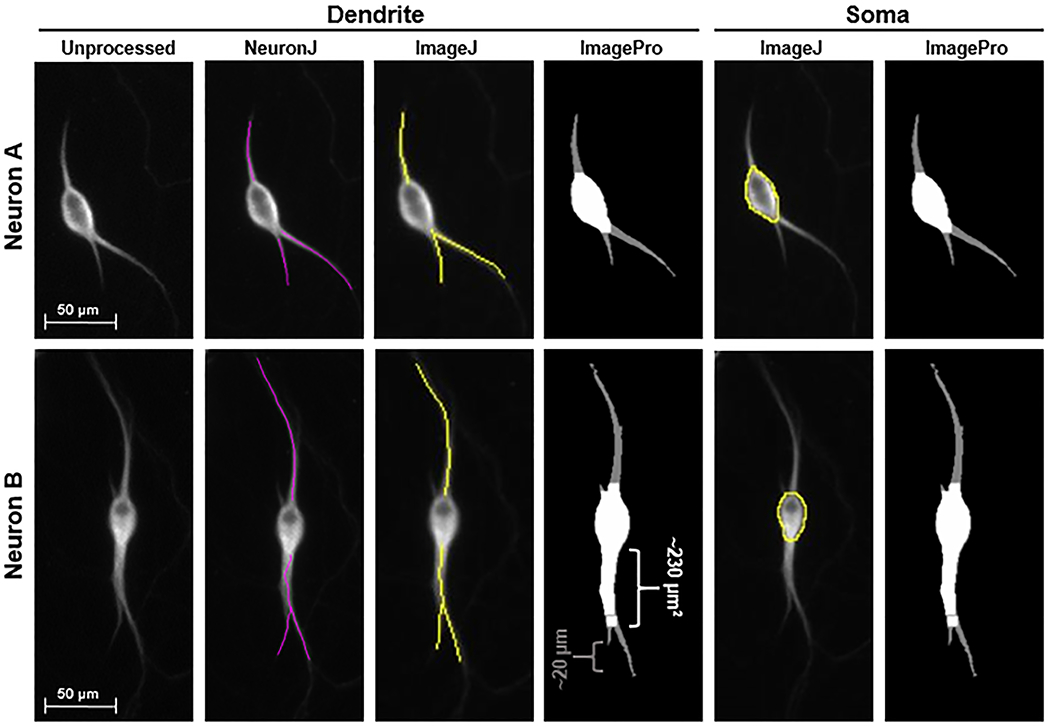
Comparison of automatic mask by ImagePro to ImageJ and NeuronJ tracing of dendrites and soma indicate the regions that cause the differences in measurements, specifically the base of the dendrite counted as soma and the tip of some dendrites not detected by ImagePro. Visual comparison of the two soma measurement methods to the original image indicate more consistency with ImageJ and NeuronJ measurements than those done using ImagePro. Neuron A displays an instance in which ImagePro is mostly adherent to cell structure. Neuron B displays an instance in which ImagePro incorrectly masks the base of a dendrite as the soma.
While other studies have displayed many options of neuron modeling (Parekh and Ascoli, 2013) and compared automatic or semi-automatic methods to manual (Pool, Thiemann, Bar-Or, and Fournier, 2008; Wearne, Rodriguez, Ehlenberger, Rocher, Henderson, and Hof, 2005), our study, by considering three methods of measurement, showed that the operator’s expertise and training are important in avoiding computational mistakes, placing more importance on accuracy of measurements than efficiency. Performing multiple trials develops the operator’s expertise, as is evident by the consistency in measurement of later trials. Through reproduction of measurements, our data show that accuracy and precision of measurements are commensurate with experience, as the operator’s measurements stabilize with more trials. One possible limitation of the study is that the increased reproducibility following multiple trials using the same images was due to unconscious learning of the images. However, this is unlikely due to the large number of neuronal images processed by the operator and the 24-hour waiting period between the repeat trials.
In summary, while additional studies are necessary to examine the accuracy and reliability of these methods to dendritic growth measurements in long-term cultures of sympathetic neurons which have a more complex dendritic arbor, our study demonstrates that use of NeuronJ in conjunction with ImageJ, resulted in objective, user-friendly, and efficient dendrite analysis. Furthermore, our data demonstrated that there was a trade-off between faster analysis and reliability when using ImagePro for analysis of dendritic growth in sympathetic neurons.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Pamela Lein for providing laboratory space and resources for setting up the rat sympathetic neurons. This work was supported by Saint Mary’s College of California Summer Research Program.
FUNDING
This work was supported by Saint Mary’s College of California Summer Research Program.
References
- Bhaskar K, Miller M, Chludzinski A, Herrup K, Zagorski M, & Lamb BT The PI3K-Akt-mTOR pathway regulates Abeta oligomer induced neuronal cell cycle events. Mol Neurodegener, 4 (2009), pp. 14. doi: 10.1186/1750-1326-4-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckenstein DA, & Higgins D Morphological differentiation of embryonic rat sympathetic neurons in tissue culture. II. Serum promotes dendritic growth. Dev Biol, 128 (1988), pp. 337–348. [DOI] [PubMed] [Google Scholar]
- Buell SJ, & Coleman PD Quantitative evidence for selective dendritic growth in normal human aging but not in senile dementia. Brain Research, 214 (1981), pp. 23–41. doi: 10.1016/0006-8993(81)90436-4 [DOI] [PubMed] [Google Scholar]
- Chandrasekaran V, Lea C, Sosa JC, Higgins D, & Lein PJ Reactive oxygen species are involved in BMP-induced dendritic growth in cultured rat sympathetic neurons. Mol Cell Neurosci, 67 (2015), pp. 116–125. doi: 10.1016/j.mcn.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell P, Ahmed S, James C, Hodson J, McDonnell PJ, Rauz S, & Williams GP Neuron J is a rapid and reliable open source tool for evaluating corneal nerve density in herpes simplex keratitis. Invest Ophthalmol Vis Sci, 55 (2014), pp. 7312–7320. doi: 10.1167/iovs.14-15140 [DOI] [PubMed] [Google Scholar]
- Francisco JS, Moraes HP, & Dias EP Evaluation of the Image-Pro Plus 4.5 software for automatic counting of labeled nuclei by PCNA immunohistochemistry. Braz Oral Res, 18 (2004), pp. 100–104. doi:/S1517-74912004000200002 [DOI] [PubMed] [Google Scholar]
- Ghogha A, Bruun DA, & Lein PJ Inducing dendritic growth in cultured sympathetic neurons. J Vis Exp (2012), pp. doi: 10.3791/3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J, Amini S, & White MK General overview of neuronal cell culture. Methods Mol Biol, 1078 (2013), pp. 1–8. doi: 10.1007/978-1-62703-640-5_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines ML, & Carnevale NT NEURON: a tool for neuroscientists. Neuroscientist, 7 (2001), pp. 123–135. doi: 10.1177/107385840100700207 [DOI] [PubMed] [Google Scholar]
- Ito U, Kuroiwa T, Nagasao J, Kawakami E, & Oyanagi K Temporal profiles of axon terminals, synapses and spines in the ischemic penumbra of the cerebral cortex: ultrastructure of neuronal remodeling. Stroke, 37 (2006), pp. 2134–2139. doi: 10.1161/01.STR.0000231875.96714.b1 [DOI] [PubMed] [Google Scholar]
- Kim KM, Son K, & Palmore GT Neuron Image Analyzer: Automated and Accurate Extraction of Neuronal Data from Low Quality Images. Sci Rep, 5 (2015), pp. 17062. doi: 10.1038/srep17062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene R, Yang H, Kutsche M, & Schachner M The neural recognition molecule L1 is a sialic acidbinding lectin for CD24, which induces promotion and inhibition of neurite outgrowth. J Biol Chem, 276 (2001), pp. 21656–21663. doi: 10.1074/jbc.M101790200 [DOI] [PubMed] [Google Scholar]
- Lein P, Johnson M, Guo X, Rueger D, & Higgins D Osteogenic protein-1 induces dendritic growth in rat sympathetic neurons. Neuron, 15 (1995), pp. 597–605. [DOI] [PubMed] [Google Scholar]
- LePage KT, Dickey RW, Gerwick WH, Jester EL, & Murray TF On the use of neuro-2a neuroblastoma cells versus intact neurons in primary culture for neurotoxicity studies. Crit Rev Neurobiol, 17 (2005), pp. 27–50. [DOI] [PubMed] [Google Scholar]
- Liu H, Sang S, Lu Y, Wang Z, Yu X, & Zhong C Thiamine metabolism is critical for regulating correlated growth of dendrite arbors and neuronal somata. Sci Rep, 7 (2017), pp. 5342. doi: 10.1038/s41598-017-05476-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK Cellular and molecular mechanisms of dendrite growth. Cereb Cortex, 10 (2000), pp. 963–973. [DOI] [PubMed] [Google Scholar]
- Meijering E Neuron tracing in perspective. Cytometry A, 77 (2010), pp. 693–704. doi: 10.1002/cyto.a.20895 [DOI] [PubMed] [Google Scholar]
- Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, & Unser M Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A, 58 (2004), pp. 167–176. doi: 10.1002/cyto.a.20022 [DOI] [PubMed] [Google Scholar]
- Nielsen HJ, & Hansen FG An automated and highly efficient method for counting and measuring fluorescent foci in rod-shaped bacteria. J Microsc, 239 (2010), pp. 194–199. doi: 10.1111/j.1365-2818.2010.03374.x [DOI] [PubMed] [Google Scholar]
- Parekh R, & Ascoli GA Neuronal morphology goes digital: a research hub for cellular and system neuroscience. Neuron, 77 (2013), pp. 1017–1038. doi: 10.1016/j.neuron.2013.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool M, Thiemann J, Bar-Or A, & Fournier AE NeuriteTracer: a novel ImageJ plugin for automated quantification of neurite outgrowth. J Neurosci Methods, 168 (2008), pp. 134–139. doi: 10.1016/j.jneumeth.2007.08.029 [DOI] [PubMed] [Google Scholar]
- Purves D, & Hume RI The relation of postsynaptic geometry to the number of presynaptic axons that innervate autonomic ganglion cells. J Neurosci, 1 (1981), pp. 441–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin E Development of the rat superior cervical ganglion: initial stages of synapse formation. J Neurosci, 5 (1985), pp. 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, & Eliceiri KW NIH Image to ImageJ: 25 years of image analysis. Nat Methods, 9 (2012), pp. 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wearne SL, Rodriguez A, Ehlenberger DB, Rocher AB, Henderson SC, & Hof PR New techniques for imaging, digitization and analysis of three-dimensional neural morphology on multiple scales. Neuroscience, 136 (2005), pp. 661–680. doi: 10.1016/j.neuroscience.2005.05.053 [DOI] [PubMed] [Google Scholar]
- Wishart TM, Parson SH, & Gillingwater TH Synaptic vulnerability in neurodegenerative disease. J Neuropathol Exp Neurol, 65 (2006), pp. 733–739. doi: 10.1097/01.jnen.0000228202.35163.c4 [DOI] [PubMed] [Google Scholar]


