Abstract
The remarkable clinical success of immune checkpoint inhibitors for the treatment of a growing number of cancer types has sparked interest in the discovery of novel forms of immunotherapy, which may be used alone or in combination. In this context, cytokine-based therapeutics are well poised to play a role in modern cancer therapy. This article focuses on antibody-cytokine fusion proteins (also called “immunocytokines”) as one class of biopharmaceuticals which can substantially improve the therapeutic index and, thus, the applicability of cytokine products. In many preclinical settings, antibodies can be used to preferentially deliver many (but not all) types of cytokines to primary and metastatic tumor lesions. The antibody-based delivery of certain pro-inflammatory payloads (such as IL2, IL12, and TNF) to the tumor microenvironment can lead to a dramatic potentiation of their anti-cancer activity. However, although some fusion proteins have advanced to late-stage clinical trials, much work remains to be done in order to fully characterize the mechanism of action and the pharmaceutical potential of immunocytokines in the clinical setting. Various factors contribute to in vivo performance, including the target antigen, the antibody properties, the nature of the payload, the format of the fusion protein, the dose and schedule, as well as their use in combination with other therapeutic modalities. Protein engineering opportunities and insights in cancer immunology are contributing to the development of next-generation immunocytokine products and of novel therapeutic concepts, with the goal to increase antitumor activity and reduce systemic toxicity (a common problem for cytokine-based biopharmaceuticals).
Keywords: Antibody-cytokine fusions, immunotherapy, tumor targeting, combination therapy, pharmaceutical biotechnology
From cytokines to immunocytokines
Cytokines are a broad and loosely-defined class of relatively small proteins that play a role in regulating the function of the immune system [1]. Many cytokines are present in blood at very low concentrations [e.g., single-digit picomolar concentrations for IL6 and IL12], but these values can grow more than 1,000-fold in certain pathological conditions. Some cytokines stimulate the activity of leukocytes that are important for fighting cancer, and recombinant preparations of these proteins have been considered as biopharmaceutical products. Cytokine-based products which have received marketing approval for cancer therapy include recombinant human IL2 (e.g., Proleukin™ as a T-cell and NK cell stimulator), IFNα (e.g., Intron A™ and Roferon-A™ as proteins with tumor cell biocidal properties and immunostimulatory action), and TNF (e.g., Beromun™, a biopharmaceutical which may induce hemorrhagic necrosis of certain tumors and increase inflammation). Cytokines that stimulate the bone marrow to produce granulocytes (e.g., G-CSF and GM-CSF) are routinely used to accelerate white blood cell recovery after bone marrow transplantation or chemotherapy.
Most cytokine products on the market are used at low doses (sometimes less than 1 mg) because these proteins bind to the cognate receptors with low dissociation constants and are, therefore, very active. Pro-inflammatory cytokines may cause adverse events (e.g., hypotension and leakage of fluids in the extravascular space, flu-like symptoms, nausea, and vomiting), preventing dose escalation to therapeutically active regimens. It is, therefore, generally accepted that recombinant cytokine products (e.g., IL2) would benefit from an increase in therapeutic index [2]. One general avenue for increasing the therapeutic index of cytokines with anti-tumoral properties consists of the antibody-based delivery of these immunomodulatory payloads to the neoplastic site. The earliest reports on antibody-cytokine fusions include the work of the groups of Reisfeld and Gillies [3–5], Morrison [6–8] and Epstein [9–11]. These authors described and characterized antibody fusions with many payloads, including IL2, IL4, IL12, IL21, as well as interferons and members of the TNF superfamily. Those proteins are described in detail in dedicated reviews [12–16] and will not be repeated here. In most cases, antibodies were directed against tumor-associated antigens expressed on the surface of tumor cells. Over the last two decades, our group (in collaboration with Luciano Zardi and with Philogen) has systematically developed a large number of antibody-cytokine fusions directed against components of the modified extracellular matrix of the tumor [12–16]. These included the human F8 and L19 antibodies, which are specific to the alternatively-spliced EDA and EDB domains of fibronectin, respectively [17,18]. F8 and L19 recognize their cognate antigen with identical affinity in mouse and man. The tumor targeting performance of these antibodies has been well established in tumor-bearing mice. More than 100 patients with solid tumors [19] or lymphomas [20] have been imaged using radiolabeled preparations of L19, validating the tumor-homing potential of this antibody for clinical applications. Most of the general statements presented in this article stem from work with L19 and F8 derivatives, as these antibodies have been fused to the largest number of cytokine payloads so far [12–16]. At the preclinical level, the anti-cancer activity of antibody-cytokine fusions directed against cellular surface antigens or against extracellular matrix components can be of comparable magnitude [21]. The biomedical and pharmaceutical potential of the immunocytokine strategy is well illustrated by the following example. When treating immunocompetent mice bearing subcutaneous C51 tumors either with murine IL12 or with the same payload fused to the L19 antibody in single-chain Fv (scFv) format, a dose-dependent therapeutic effect was observed, alongside a >20-fold potentiation of therapeutic activity for the L19 fusion protein [22].
Most observations published indicate that the targeted delivery of immunomodulatory payloads may lead to a potentiation of cytokine activity. A high local concentration of pro-inflammatory factors may lead to a preferential expansion and activation of NK cells and of tumor-specific T cells, which are found at higher relative frequencies in the neoplastic mass compared to secondary lymphoid organs [23]. The surprising observation of a similar therapeutic activity for IL2 fusions based on full immunoglobulins specific to a tumor-associated antigen or to an irrelevant target protein used as negative control has been reported [24]. It is possible that the systemic effect of a persistently high concentration of IL2 in blood may drive therapeutic activity in certain animal models of cancer. However, numerous other studies have reported a clear correlation between the use of tumor-specific cytokine fusion proteins and the observation of a therapeutic index gain compared to non-targeted cytokine fusions [3–16].
When small antibody fragments are fused to pro-inflammatory cytokines, the circulatory half-life is typically short, and the toxicity profile is similar to the one of the unconjugated cytokines. The therapeutic activity of payloads such as IL2, IL12, and TNF increases (at least in mice) as a result of a preferential localization of the fusion protein at the tumor site. More than 30 cytokine payloads have been fused to antibodies, and the resulting products have been characterized, both in terms of pharmacokinetic properties and of biological activity in mouse models of disease [reviewed in 12–16].
Which molecular formats can be used for the design of immunocytokine products?
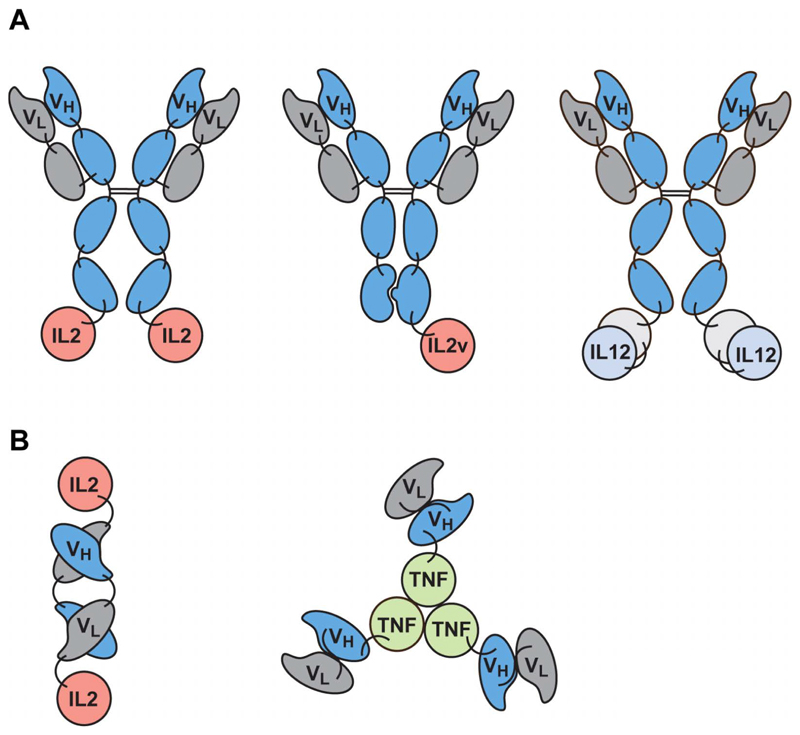
Antibody-cytokine fusions can be grouped into two broad categories: those based on immunoglobulins in IgG format and those featuring antibody fragments. Fig. 1A shows a schematic domain representation of immunocytokines which have been moved to clinical trials. The first products to be developed industrially feature IgGs bearing a human IL2 moiety at the C-terminus of each heavy chain [3,12–16]. Scientists have developed similar products, bearing only one IL2 moiety, due to an asymmetric arrangement of heavy chains achieved using “knob-into-hole” technology [25]. IL12, a heterodimeric cytokine, has been fused as a single polypeptide (with a linker connecting the p40 and p35 subunits) to the extremity of an antibody by others [26,27]. Interestingly, in spite of these IgG derivatives retaining FcRn binding, they typically exhibit a much faster clearance rate compared to intact human immunoglobulins [25–27].
Figure 1.
Formats of immunocytokine products in clinical trials. (A) IgG-based biopharmaceuticals bearing IL2, an IL2 variant (IL2v) with reduced CD25 binding, or IL12 (in single-chain format) as payloads. In order to achieve the IgG display of a single-cytokine moiety, researchers adopted “knob-into-hole” technology for the heterodimerization of different antibody heavy chains. (B) Immunocytokines based on antibody fragments (a diabody structure in case of IL2 and scFv fragments for TNF fusions).
Our group and others have typically preferred to use antibody fragments for the generation of immunocytokines [reviewed in 12–16]. Fig. 1B illustrates the domain organization of antibody fusions with IL2 (featuring the use of diabody structure) and TNF (featuring the antibody in scFv format), which have been moved to advanced clinical trials for the treatment of patients with cancer [28–34]. Biodistribution studies in mouse models of cancer have revealed that tumor:organ ratios greater than 10:1 can be achieved with the best fragment-based immunocytokines 24 hours after intravenous administration [28,30,35].
More complex cytokines (e.g., members of the IL12 family) allow various domain arrangements, which may substantially differ in terms of tumor-homing and therapy performance [36,37]. Protein engineering opportunities also exist for members of the TNF superfamily because some of these payloads can efficiently be delivered to tumors, whereas others cannot [38]. One group has pioneered the use of TNF-related proteins as single-chain polypeptides, with stabilizing mutations when necessary, leading to a substantial improvement in performance [39].
Which cytokines cannot be delivered to the tumor and why?
The use of the L19 and F8 antibodies, targeting fibronectin splice variants located in the sub-endothelial aspect of tumor blood vessels [17,18], has allowed a systematic evaluation of the tumor targeting performance of dozens of antibody-cytokine fusions in various immunocompetent cancer animal models [reviewed in 12–16]. The results of biodistribution studies with radiolabeled protein preparations have allowed grouping of cytokine payloads into three main categories: (i) those efficiently delivered to the tumor at ultra-low doses (e.g., IL2, IL4, IL6, IL9, IL10, IL12, IFNα, TNF) [12–16]; (ii) those trapped by cognate receptors at low doses but can efficiently accumulate in the tumor when the receptor has been saturated (e.g., IFNγ and IL15) [12–16,40–42]; and (iii) those which abrogate the tumor-homing performance of the parental antibody (e.g., payloads which are too large, bear too many positive or negative charges, or are glycosylated with an insufficient number of terminal sialic acid moieties, leading to efficient removal by the asialoglycoprotein receptor). Antibody-based pharmaco-delivery problems have been reported for fusions with large splice isoforms of murine vascular endothelial growth factor A (e.g., VEGF-A164), calmodulin, TAT peptides, CD86, as well as IL9 (depending on the glycoforms that were used in biodistribution experiments) [12–16,36,43–46].
What is the mechanism of action and main side-effects of anti-cancer immunocytokines?
Different payloads display different modes of action, and some cytokines do not display a potent anti-cancer activity (at least in the mouse and other models tested so far), in spite of an efficient accumulation at the tumor site. For example, IL6 [47] and IL10 [48] can be selectively delivered to solid tumors without mediating a substantial tumor growth retardation. The most striking antitumor activity in mice has been observed with fusion proteins based on murine versions of IL2, IL4, IL12, and TNF [3–16,22,23,28,30,34–37,49]. Human IL2 can also be used in mice, as this cytokine cross-reacts with the murine receptor [28]. In most cases, products based on these payloads can substantially reduce tumor growth rates upon intravenous administration and tumor clearance can be observed in some (but not all) immunocompetent mouse models.
Depletion experiments have evidenced a crucial role of CD8+ T cells and NK cells for IL2- and IL12-based immunocytokines. Tumor-homing fusions based on these payloads mediate a substantial and rapid increase of the density of T and NK cells at the site of disease both in mice [22,28,34] and in humans [50]. However, it is unclear whether these cells migrate through a barrier of cytokines located at high density in the abluminal aspects of tumor blood vessels or proliferate in situ.
The biology of TNF-based immunocytokines differs. The targeted delivery of this payload can trigger a rapid increase in vascular permeability of tumor blood vessels, leading to hemorrhagic necrosis of a vast portion of the neoplastic mass both in mice [30,31,35] and humans [32]. In rodents, it has been formally proven that the immune system plays an important role for the eradication of surviving tumor cells, in a process which depends on CD8+ T cells and NK cells [23,51]. Interestingly, mice cleared of soft-tissue sarcoma reject subsequent challenges with different tumors from the same mouse strain. In the case of BALB/c tumors, the AH1 peptide (derived from the envelope protein of the murine leukemia virus, endogenous in the mouse genome) represents the dominant tumor rejection antigen [23,51,52]. A complete understanding of the mechanism of action of anti-cancer immunocytokines is still missing. It is also reasonable to assume, for example, that some products may play an important role in driving Treg fragility [53].
Pro-inflammatory immunocytokines are vasoactive and can trigger further cytokine production. The main side-effects observed in the clinic are hypotension, flu-like symptoms, nausea, and vomiting. Leukopenia has also been reported for IL12 fusions [54]. Dose, schedule, and infusion time strongly impact on the tolerability of the product. Although high-dose IL2 regimens feature the administration of up to 800 million international units (IU) of this payload for one week (with substantial toxicity) [55], it is common to administer IL2-based immunocytokines at much lower doses (e.g., 20-60 million IU) once a week, with a procedure that can be continued for more than six months [25,29].
Which combination opportunities can be considered for antibody-cytokine fusions?
A strong potentiation of certain immunocytokines has been observed with some (but not all) chemotherapeutic agents [30,31,37,40,42,51,56,57], radiation [58,59] and immunostimulatory drugs (including immune checkpoint inhibitors and combinations of immunocytokines) [49,60–63]. Combination studies for immunocytokine products remains an intense area of research, as many factors can influence additive or synergistic benefits. For example, it makes a difference if chemotherapeutic drugs are given before or after immunocytokines [56]. From a theoretical viewpoint, chemotherapy could mediate immunogenic cell death and make tumors more responsive to the action of pro-inflammatory biopharmaceuticals [64–66]. Killed tumor cells release necrotic components that can activate dendritic cells capable of cross-antigen presentation [65], and stressed tumor cells overexpress surface proteins that can be recognized by NK cells [66]. However, an excessive dose of cytotoxic agents could be immunosuppressive.
Products based on IL2 and IL12 can substantially increase NK cell density within the tumor mass [50,57,67], and, as a consequence, these agents would be expected to potentiate the action of IgG therapeutics, working through antibody-dependent cell cytotoxicity. A potentiation of the anti-cancer activity of clinical-stage intact antibodies has been reported [67]. Similarly, a potentiation of vaccination strategies [23,68] or of bispecific antibodies [69] could be expected, with a judicious choice of target antigens, cytokine payload, and biopharmaceutical format.
Which immunocytokines are being investigated in trials for oncological indications?
The protein formats depicted in Fig. 1 (featuring IL2, IL12, or TNF as payloads) are described thoroughly in the respective studies (12-18) and details will not be repeated here. A fusion protein consisting of the F8 antibody fused to human IL10 has been moved to phase II clinical trials in patients with rheumatoid arthritis and ulcerative colitis, indicating that the immunocytokine concept can also be applied to indications beyond cancer [48].
One issue in the field relates to the format (e.g., full IgGs vs. antibody fragments) and the nature of the payload (e.g., wild-type IL2 vs. mutants with reduced CD25 binding), which should be chosen for product development [16,25,28,29]. Clinical trials have not yet provided a definitive answer on this matter. The therapeutic activity of antibody-cytokine fusions is likely to be a composite of systemic effects (which may be greater for cytokine derivatives with long circulatory half-lives) and of immunostimulation at the tumor site (which is likely to correlate with the product uptake in the neoplastic mass). The combination of L19-TNF (given by intravenous infusion) and doxorubicin is being compared to doxorubicin alone in pivotal trials for the treatment of first-line metastatic soft-tissue sarcoma (EudraCT 2016-003239-38, NCT03420014). The intra-lesional administration of combination L19-IL2 and L19-TNF as neoadjuvant therapy prior to surgery is being tested in phase III clinical trials for the treatment of fully resectable stage IIIB/C melanoma (NCT02938299, NCT03567889).
The future of immunocytokine products. Can systemic toxicity be reduced?
An important (and largely unexplored) area of immunocytokine research relates to the use of cytokine mutants with decreased affinity towards the cognate receptor. The lower biological activity allows the use of higher doses and may provide an avenue to create in vivo selectivity. Scientists have described preclinical results using an anti-CD38 antibody fused to a de-potentiated version of IFNα [70].
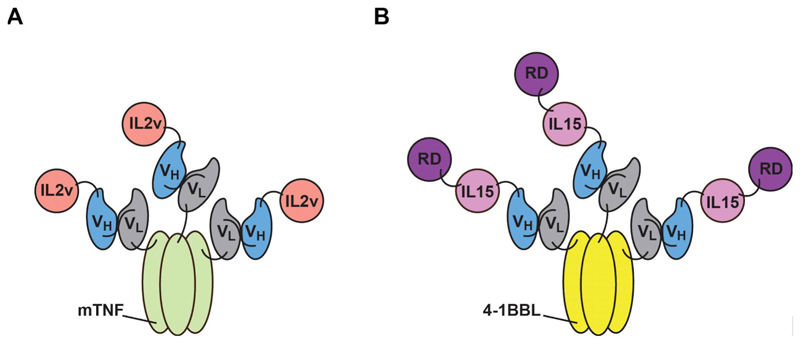
Most large pharmaceutical companies have started immunocytokine programs and, for this reason, the real potential of this class of biopharmaceuticals will become clearer in the next few years. Although immunocytokines may provide a benefit to patients when used as single agent, it is likely that anti-cancer therapeutic activity will best be displayed when suitable combination strategies are implemented. The development of two products to be used in combination can be cumbersome from a pharmaceutical perspective. For this reason, it would be tempting to incorporate cytokine moieties into other biopharmaceuticals, thus, creating multifunctional products. Examples of potency-matched antibody-cytokine-drug conjugates [71] and of dual-cytokine antibody fusions [35,36,72,73] have been reported [Fig. 2], some of which have shown encouraging biodistribution and therapeutic results.
Figure 2.
Domain arrangement for dual-cytokine fusion proteins. These homotrimeric fusion proteins feature antibodies in scFv fused to members of the TNF superfamily and to other immunomodulatory payloads. (A) Dual-cytokine fusion protein based on IL2 and murine (m) TNF. (B) Fusion protein featuring 4-1BBL, IL15, and the alpha-subunit of the IL15 receptor (RD). The VH and VL domains of antibodies in scFv format are indicated.
The majority of toxicities of pro-inflammatory immunocytokines are observed in concomitance with the peak serum concentration of the product. Antibody-cytokine fusions can be more efficacious than the unmodified cytokines because of preferential uptake in the tumor. However, the toxicity profile is often similar to the one of the parental cytokine (in absolute terms) because only a small fraction of the product is taken up by the neoplastic lesion (in the best cases, 0.01-0.1% injected dose/gram of tumor). In other words, the tumor can be targeted more efficiently compared to normal organs but will not be a “sponge” that absorbs most of the product.
Although the use of cytokine-based therapeutics has traditionally been limited by systemic toxicity, it may be possible to circumvent this problem in a number of ways. On one hand, tumor-targeting antibody-cytokine fusions may allow for administering less product, thus, improving tolerability. A second aspect relates to the non-linearity of cytokine-mediated toxicity and to the possibility of progressively increasing payload concentration in the tumor. At the clinical level, undesired side effects are often seen when the peak concentration of cytokine activity grows above a certain threshold. In most cases, adverse events disappear when cytokines are cleared from circulation. It should be possible to implement repeated administrations of small doses of antibody-cytokine products (or use very slow infusion procedures) to progressively build up cytokine concentrations within the neoplastic mass, while keeping blood concentrations sufficiently low. In this context, the use of antibodies with efficient tumor-homing properties and the targeting of stable antigens that do not internalize (e.g., extracellular matrix components) should facilitate the creation, over time, of a concentration differential between the cancer site and normal organs. In the clinical trial NCT02076646, the administration of L19-IL2 with a 3-hour infusion procedure allowed for increasing the maximal tolerated dose (MTD) of the product by at least threefold, compared to the MTD which had previously been reported for a 1-hour infusion procedure [29,74]. The use of very slow infusion procedures for cancer immunotherapy is not uncommon. For example, blinatumomab was found to be efficacious when given as a 30-day infusion but not as bolus injection [75].
Future research programs will likely aim at the generation of immunocytokine products with “activity-on-demand”, which may display their therapeutic potential at the tumor site, while sparing normal organs. We have described “split cytokine fusions”, a biopharmaceutical strategy that may be particularly suitable for immunomodulatory payloads consisting of multiple subunits [76]. In principle, it should be possible to sequentially deliver cytokine subunits to the site of disease, allowing a reconstitution of biological activity within the tumor mass. These approaches, however, may be challenging if individual cytokine subunits retain potent biological activity [77]. Selectivity may also be created if antigen binding by immunocytokine products would allosterically enhance biological activity. Gillies and collaborators have reported that the fusion of IL2 at the light chain C-terminus of antibodies in IgG format with judiciously chosen linkers could lead to a product activation upon antigen binding, possibly as a result of steric hindrance and conformational changes of the hinge region [77]. Alternatively, the transient inhibition of immunocytokines with kinetically tuned blocking moieties, which progressively dissociate and clear from blood, may represent an avenue for building up activity at the tumor site, while decreasing systemic toxicity.
In summary, immunocytokines are modular biopharmaceutical agents, which have shown exceptional activity in preclinical models of cancer but whose pharmaceutical potential is still largely unexplored. The opportunities provided by protein engineering technologies and by the implementation of judicious combination modalities suggest that antibody-cytokine fusions will play an important role for the immunotherapy of cancer in a not too distant future.
Acknowledgments
This work was supported by the ETH Zürich, the Swiss National Science Foundation (Grant Nr. 310030B_163479/1) and the ERC Advanced Grant “ZAUBERKUGEL” (Grant Nr. 670603). Help from Cornelia Hutmacher and Anja Schmid for the preparation of Figures 1 and 2 and for a critical reading of the manuscript (together with Giuliano Elia and Teresa Hemmerle) is gratefully acknowledged.
Funding: This work was supported by the ETH Zürich, the Swiss National Science Foundation (Grant Nr. 310030B_163479/1) and the ERC Advanced Grant “ZAUBERKUGEL” (Grant Nr. 670603).
References
- [1].Murphy K. Janeway’s Immunobiology. 8th Edition. New York, NY: Garland Science; 2017. [Google Scholar]
- [2].Charych DH, Hoch U, Langowski JL, Lee SR, Addepalli MK, Kirk PB, et al. NKTR-214, an Engineered Cytokine with Biased IL2 Receptor Binding, Increased Tumor Exposure, and Marked Efficacy in Mouse Tumor Models. Clin Cancer Res. 2016;22:680–90. doi: 10.1158/1078-0432.CCR-15-1631. [DOI] [PubMed] [Google Scholar]
- [3].Lode HN, Xiang R, Becker JC, Gillies SD, Reisfeld RA. Immunocytokines: a promising approach to cancer immunotherapy. Pharmacol Ther. 1998;80:277–92. doi: 10.1016/s0163-7258(98)00033-3. [DOI] [PubMed] [Google Scholar]
- [4].Sabzevari H, Gillies SD, Mueller BM, Pancook JD, Reisfeld RA. A recombinant antibody-interleukin 2 fusion protein suppresses growth of hepatic human neuroblastoma metastases in severe combined immunodeficiency mice. Proc Natl Acad Sci U S A. 1994;91:9626–30. doi: 10.1073/pnas.91.20.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Becker JC, Varki N, Gillies SD, Furukawa K, Reisfeld RA. Longlived and transferable tumor immunity in mice after targeted interleukin-2 therapy. J Clin Invest. 1996;98:2801–4. doi: 10.1172/JCI119107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Penichet ML, Morrison SL. Antibody-cytokine fusion proteins for the therapy of cancer. J Immunol Methods. 2001;248:91–101. doi: 10.1016/s0022-1759(00)00345-8. [DOI] [PubMed] [Google Scholar]
- [7].Dela Cruz JS, Trinh KR, Chen HW, Ribas A, Morrison SL, Penichet ML. Anti-HER2/neu IgG3-(IL-2) and anti-HER2/neu IgG3-(GM-CSF) promote HER2/neu processing and presentation by dendritic cells: Implications in immunotherapy and vaccination strategies. Mol Immunol. 2006;43:667–76. doi: 10.1016/j.molimm.2005.04.007. [DOI] [PubMed] [Google Scholar]
- [8].Dela Cruz JS, Trinh KR, Morrison SL, Penichet ML. Recombinant anti-human HER2/neu IgG3-(GM-CSF) fusion protein retains antigen specificity and cytokine function and demonstrates antitumor activity. J Immunol. 2000;165:5112–21. doi: 10.4049/jimmunol.165.9.5112. [DOI] [PubMed] [Google Scholar]
- [9].Hornick JL, Khawli LA, Hu P, Khanna C, Epstein AL. Clin Cancer Res. 1999;5:51–60. [PubMed] [Google Scholar]
- [10].Zhang N, Sadun RE, Arias RS, Flanagan ML, Sachsman SM, Nien YC, Khawli LA, Hu P, Epstein AL. Clin Cancer Res. 2007;13:2758–67. doi: 10.1158/1078-0432.CCR-06-2343. [DOI] [PubMed] [Google Scholar]
- [11].Hu P, Hornick JL, Glasky MS, Yun A, Milkie MN, Khawli LA, Anderson PM, Epstein AL. A chimeric Lym-1/interleukin 2 fusion protein for increasing tumor vascular permeability and enhancing antibody uptake. Cancer Res. 1996;56:4998–5004. [PubMed] [Google Scholar]
- [12].Schrama D, Reisfeld RA, Becker JC. Antibody targeted drugs as cancer therapeutics. Nat Rev Drug Discov. 2006;5:147–59. doi: 10.1038/nrd1957. [DOI] [PubMed] [Google Scholar]
- [13].Müller D. Antibody fusions with immunomodulatory proteins for cancer therapy. Pharmacol Ther. 2015;154:57–66. doi: 10.1016/j.pharmthera.2015.07.001. [DOI] [PubMed] [Google Scholar]
- [14].Young PA, Morrison SL, Timmerman JM. Antibody-cytokine fusion proteins for treatment of cancer: engineering cytokines for improved efficacy and safety. Semin Oncol. 2014;41:623–36. doi: 10.1053/j.seminoncol.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Neri D, Sondel PM. Immunocytokines for cancer treatment: past, present and future. Curr Opin Immunol. 2016;40:96–102. doi: 10.1016/j.coi.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hutmacher C, Neri D. Antibody-cytokine fusion proteins: biopharmaceuticals with immunomodulatory properties for cancer therapy. Adv Drug Deliv Rev. 2018 doi: 10.1016/j.addr.2018.09.002. in press. [DOI] [PubMed] [Google Scholar]
- [17].Villa A, Trachsel E, Kaspar M, Schliemann C, Sommavilla R, Rybak JN, Rösli C, Borsi L, Neri D. A high-affinity human monoclonal antibody specific to the alternatively spliced EDA domain of fibronectin efficiently targets tumor neo-vasculature in vivo. Int J Cancer. 2008;122:2405–13. doi: 10.1002/ijc.23408. [DOI] [PubMed] [Google Scholar]
- [18].Pini A, Viti F, Santucci A, Carnemolla B, Zardi L, Neri P, Neri D. Design and use of a phage display library. Human antibodies with subnanomolar affinity against a marker of angiogenesis eluted from a two-dimensional gel. J Biol Chem. 1998;273:21769–76. doi: 10.1074/jbc.273.34.21769. [DOI] [PubMed] [Google Scholar]
- [19].Poli GL, Bianchi C, Virotta G, Bettini A, Moretti R, Trachsel E, Elia G, Giovannoni L, Neri D, Bruno A. Radretumab radioimmunotherapy in patients with brain metastasis: a 124I-L19SIP dosimetric PET study. Cancer Immunol Res. 2013;1:134–43. doi: 10.1158/2326-6066.CIR-13-0007. [DOI] [PubMed] [Google Scholar]
- [20].Erba PA, Sollini M, Orciuolo E, Traino C, Petrini M, Paganelli G, Bombardieri E, Grana C, Giovannoni L, Neri D, Menssen HD, et al. Radioimmunotherapy with radretumab in patients with relapsed hematologic malignancies. J Nucl Med. 2012;53:922–7. doi: 10.2967/jnumed.111.101006. [DOI] [PubMed] [Google Scholar]
- [21].Epstein AL, Khawli LA, Hornick JL, Taylor CR. Identification of a monoclonal antibody, TV-1, directed against the basement membrane of tumor vessels, and its use to enhance the delivery of macromolecules to tumors after conjugation with interleukin 2. Cancer Res. 1995;55:2673–80. [PubMed] [Google Scholar]
- [22].Halin C, Rondini S, Nilsson F, Berndt A, Kosmehl H, Zardi L, Neri D. Enhancement of the antitumor activity of interleukin-12 by targeted delivery to neovasculature. Nat Biotechnol. 2002;20:264–9. doi: 10.1038/nbt0302-264. [DOI] [PubMed] [Google Scholar]
- [23].Probst P, Stringhini M, Ritz D, Fugmann T, Neri D. Antibody-based delivery of TNF to the tumor neo-vasculature potentiates the therapeutic activity of a peptide anti-cancer vaccine. Clin Cancer Res. 2018 doi: 10.1158/1078-0432.CCR-18-1728. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tzeng A, Kwan BH, Opel CF, Navaratna T, Wittrup KD. Antigen specificity can be irrelevant to immunocytokine efficacy and biodistribution. Proc Natl Acad Sci U S A. 2015;112:3320–5. doi: 10.1073/pnas.1416159112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ribba B, Boetsch C, Nayak T, Grimm HP, Charo J, Evers S, Klein C, Tessier J, Charoin JE, Phipps A, Pisa P, et al. Prediction of the optimal dosing regimen using a mathematical model of tumor uptake for immunocytokine-based cancer immunotherapy. Clin Cancer Res. 2018;24:3325–3333. doi: 10.1158/1078-0432.CCR-17-2953. [DOI] [PubMed] [Google Scholar]
- [26].Strauss J, Heery CR, Kim JW, Jochems C, Donahue RN, Montgomery AS, McMahon S, Lamping E, Marte JL, Madan RA, Bilusic M, et al. First-In-Human Phase I Trial of a Tumor-Targeted Cytokine (NHS-IL12) in Subjects with Metastatic Solid Tumors. Clin Cancer Res. 2018 doi: 10.1158/1078-0432.CCR-18-1512. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rudman SM, Jameson MB, McKeage MJ, Savage P, Jodrell DI, Harries M, Acton G, Erlandsson F, Spicer JF. A phase 1 study of AS1409, a novel antibody-cytokine fusion protein, in patients with malignant melanoma or renal cell carcinoma. Clin Cancer Res. 2011;17:1998–2005. doi: 10.1158/1078-0432.CCR-10-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Carnemolla B, Borsi L, Balza E, Castellani P, Meazza R, Berndt A, Ferrini S, Kosmehl H, Neri D, Zardi L. Enhancement of the antitumor properties of interleukin-2 by its targeted delivery to the tumor blood vessel extracellular matrix. Blood. 2002;99:1659–65. doi: 10.1182/blood.v99.5.1659. [DOI] [PubMed] [Google Scholar]
- [29].Eigentler TK, Weide B, de Braud F, Spitaleri G, Romanini A, Pflugfelder A, González-Iglesias R, Tasciotti A, Giovannoni L, Schwager K, Lovato V, et al. A dose-escalation and signal-generating study of the immunocytokine L19-IL2 in combination with dacarbazine for the therapy of patients with metastatic melanoma. Clin Cancer Res. 2011;17:7732–42. doi: 10.1158/1078-0432.CCR-11-1203. [DOI] [PubMed] [Google Scholar]
- [30].Borsi L, Balza E, Carnemolla B, Sassi F, Castellani P, Berndt A, Kosmehl H, Biro A, Siri A, Orecchia P, Grassi J, et al. Selective targeted delivery of TNFalpha to tumor blood vessels. Blood. 2003;102:4384–92. doi: 10.1182/blood-2003-04-1039. [DOI] [PubMed] [Google Scholar]
- [31].Hemmerle T, Probst P, Giovannoni L, Green AJ, Meyer T, Neri D. The antibody-based targeted delivery of TNF in combination with doxorubicin eradicates sarcomas in mice and confers protective immunity. Br J Cancer. 2013;109:1206–13. doi: 10.1038/bjc.2013.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Papadia F, Basso V, Patuzzo R, Maurichi A, Di Florio A, Zardi L, Ventura E, González-Iglesias R, Lovato V, Giovannoni L, Tasciotti A, et al. Isolated limb perfusion with the tumor-targeting human monoclonal antibody-cytokine fusion protein L19-TNF plus melphalan and mild hyperthermia in patients with locally advanced extremity melanoma. J Surg Oncol. 2013;107:173–9. doi: 10.1002/jso.23168. [DOI] [PubMed] [Google Scholar]
- [33].Danielli R, Patuzzo R, Di Giacomo AM, Gallino G, Maurichi A, Di Florio A, Cutaia O, Lazzeri A, Fazio C, Miracco C, Giovannoni L, et al. Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: results of a phase II study. Cancer Immunol Immunother. 2015;64:999–1009. doi: 10.1007/s00262-015-1704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gutbrodt KL, Schliemann C, Giovannoni L, Frey K, Pabst T, Klapper W, Berdel WE, Neri D. Antibody-based delivery of interleukin-2 to neovasculature has potent activity against acute myeloid leukemia. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3006221. 201rla118. [DOI] [PubMed] [Google Scholar]
- [35].De Luca R, Soltermann A, Pretto F, Pemberton-Ross C, Pellegrini G, Wulhfard S, Neri D. Potency-matched Dual Cytokine-Antibody Fusion Proteins for Cancer Therapy. Mol Cancer Ther. 2017;16:2442–2451. doi: 10.1158/1535-7163.MCT-17-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gafner V, Trachsel E, Neri D. An engineered antibody-interleukin-12 fusion protein with enhanced tumor vascular targeting properties. Int J Cancer. 2006;119:2205–12. doi: 10.1002/ijc.22101. [DOI] [PubMed] [Google Scholar]
- [37].Pasche N, Wulhfard S, Pretto F, Carugati E, Neri D. The antibody-based delivery of interleukin-12 to the tumor neovasculature eradicates murine models of cancer in combination with paclitaxel. Clin Cancer Res. 2012;18:4092–103. doi: 10.1158/1078-0432.CCR-12-0282. [DOI] [PubMed] [Google Scholar]
- [38].Hemmerle T, Hess C, Venetz D, Neri D. Tumor targeting properties of antibody fusion proteins based on different members of the murine tumor necrosis superfamily. J Biotechnol. 2014;172:73–6. doi: 10.1016/j.jbiotec.2013.12.010. [DOI] [PubMed] [Google Scholar]
- [39].Hutt M, Marquardt L, Seifert O, Siegemund M, Müller I, Kulms D, Pfizenmaier K, Kontermann RE. Superior Properties of Fc-comprising scTRAIL Fusion Proteins. Mol Cancer Ther. 2017;16:2792–2802. doi: 10.1158/1535-7163.MCT-17-0551. [DOI] [PubMed] [Google Scholar]
- [40].Ebbinghaus C, Ronca R, Kaspar M, Grabulovski D, Berndt A, Kosmehl H, Zardi L, Neri D. Engineered vascular-targeting antibody-interferon-gamma fusion protein for cancer therapy. Int J Cancer. 2005;116:304–13. doi: 10.1002/ijc.20952. [DOI] [PubMed] [Google Scholar]
- [41].Hemmerle T, Neri D. The dose-dependent tumor targeting of antibody-IFNγ fusion proteins reveals an unexpected receptor-trapping mechanism in vivo. Cancer Immunol Res. 2014;2:559–67. doi: 10.1158/2326-6066.CIR-13-0182. [DOI] [PubMed] [Google Scholar]
- [42].Kaspar M, Trachsel E, Neri D. The antibody-mediated targeted delivery of interleukin-15 and GM-CSF to the tumor neovasculature inhibits tumor growth and metastasis. Cancer Res. 2007;67:4940–8. doi: 10.1158/0008-5472.CAN-07-0283. [DOI] [PubMed] [Google Scholar]
- [43].Melkko S, Halin C, Borsi L, Zardi L, Neri D. An antibody-calmodulin fusion protein reveals a functional dependence between macromolecular isoelectric point and tumor targeting performance. Int J Radiat Oncol Biol Phys. 2002;54:1485–90. doi: 10.1016/s0360-3016(02)03927-5. [DOI] [PubMed] [Google Scholar]
- [44].Halin C, Niesner U, Villani ME, Zardi L, Neri D. Tumor-targeting properties of antibody-vascular endothelial growth factor fusion proteins. Int J Cancer. 2002;102:109–16. doi: 10.1002/ijc.10674. [DOI] [PubMed] [Google Scholar]
- [45].Niesner U, Halin C, Lozzi L, Günthert M, Neri P, Wunderli-Allenspach H, Zardi L, Neri D. Quantitation of the tumor-targeting properties of antibody fragments conjugated to cell-permeating HIV-1 TAT peptides. Bioconjug Chem. 2002;13:729–36. doi: 10.1021/bc025517+. [DOI] [PubMed] [Google Scholar]
- [46].Venetz D, Hess C, Lin CW, Aebi M, Neri D. Glycosylation profiles determine extravasation and disease-targeting properties of armed antibodies. Proc Natl Acad Sci U S A. 2015;112:2000–5. doi: 10.1073/pnas.1416694112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hess C, Neri D. Tumor-targeting properties of novel immunocytokines based on murine IL1β and IL6. Protein Eng Des Sel. 2014 Jun;27(6):207–13. doi: 10.1093/protein/gzu013. [DOI] [PubMed] [Google Scholar]
- [48].Schwager K, Kaspar M, Bootz F, Marcolongo R, Paresce E, Neri D, Trachsel E. Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis. Arthritis Res Ther. 2009;11:R142. doi: 10.1186/ar2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hemmerle T, Neri D. The antibody-based targeted delivery of interleukin-4 and 12 to the tumor neovasculature eradicates tumors in three mouse models of cancer. Int J Cancer. 2014;134:467–77. doi: 10.1002/ijc.28359. [DOI] [PubMed] [Google Scholar]
- [50].Schliemann C, Gutbrodt KL, Kerkhoff A, Pohlen M, Wiebe S, Silling G, Angenendt L, Kessler T, Mesters RM, Giovannoni L, Schäfers M, et al. Targeting interleukin-2 to the bone marrow stroma for therapy of acute myeloid leukemia relapsing after allogeneic hematopoietic stem cell transplantation. Cancer Immunol Res. 2015;3:547–56. doi: 10.1158/2326-6066.CIR-14-0179. [DOI] [PubMed] [Google Scholar]
- [51].Probst P, Kopp J, Oxenius A, Colombo MP, Ritz D, Fugmann T, Neri D. Sarcoma Eradication by Doxorubicin and Targeted TNF Relies upon CD8+ T-cell Recognition of a Retroviral Antigen. Cancer Res. 2017;77:3644–3654. doi: 10.1158/0008-5472.CAN-16-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Huang AY, Gulden PH, Woods AS, Thomas MC, Tong CD, Wang W, Engelhard VH, Pasternack G, Cotter R, Hunt D, Pardoll DM, et al. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc Natl Acad Sci U S A. 1996;93:9730–5. doi: 10.1073/pnas.93.18.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Overacre-Delgoffe AE, Vignali DAA. Treg Fragility: A Prerequisite for Effective Antitumor Immunity? Cancer Immunol Res. 2018;6:882–887. doi: 10.1158/2326-6066.CIR-18-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Car BD, Eng VM, Lipman JM, Anderson TD. The toxicology of interleukin-12: a review. Toxicol Pathol. 1999;27:58–63. doi: 10.1177/019262339902700112. [DOI] [PubMed] [Google Scholar]
- [55].Smith FO, Downey SG, Klapper JA, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Restifo NP, Levy CL, White DE, et al. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res. 2008;14:5610–8. doi: 10.1158/1078-0432.CCR-08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Moschetta M, Pretto F, Berndt A, Galler K, Richter P, Bassi A, Oliva P, Micotti E, Valbusa G, Schwager K, Kaspar M, et al. Paclitaxel enhances therapeutic efficacy of the F8-IL2 immunocytokine to EDA-fibronectin-positive metastatic human melanoma xenografts. Cancer Res. 2012 Apr 1;72(7):1814–24. doi: 10.1158/0008-5472.CAN-11-1919. [DOI] [PubMed] [Google Scholar]
- [57].Cazzamalli S, Ziffels B, Widmayer F, Murer P, Pellegrini G, Pretto F, Wulhfard S, Neri D. Enhanced Therapeutic Activity of Non-Internalizing Small-Molecule-Drug Conjugates Targeting Carbonic Anhydrase IX in Combination with Targeted Interleukin-2. Clin Cancer Res. 2018;24:3656–3667. doi: 10.1158/1078-0432.CCR-17-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zegers CM, Rekers NH, Quaden DH, Lieuwes NG, Yaromina A, Germeraad WT, Wieten L, Biessen EA, Boon L, Neri D, Troost EG, et al. Radiotherapy combined with the immunocytokine L19-IL2 provides long-lasting antitumor effects. Clin Cancer Res. 2015;21:1151–60. doi: 10.1158/1078-0432.CCR-14-2676. [DOI] [PubMed] [Google Scholar]
- [59].Eckert F, Jelas I, Oehme M, Huber SM, Sonntag K, Welker C, Gillies SD, Strittmatter W, Zips D, Handgretinger R, Schilbach K, et al. Tumor-targeted IL-12 combined with local irradiation leads to systemic tumor control via abscopal effects in vivo. Oncoimmunology. 2017;6:e1323161. doi: 10.1080/2162402X.2017.1323161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Xu C, Zhang Y, Rolfe PA, Hernández VM, Guzman W, Kradjian G, Marelli B, Qin G, Qi J, Wang H, Yu H, et al. Combination Therapy with NHS-muIL12 and Avelumab (anti-PD-L1) Enhances Antitumor Efficacy in Preclinical Cancer Models. Clin Cancer Res. 2017;23:5869–5880. doi: 10.1158/1078-0432.CCR-17-0483. [DOI] [PubMed] [Google Scholar]
- [61].Schwager K, Hemmerle T, Aebischer D, Neri D. The immunocytokine L19-IL2 eradicates cancer when used in combination with CTLA-4 blockade or with L19-TNF. J Invest Dermatol. 2013;133:751–758. doi: 10.1038/jid.2012.376. [DOI] [PubMed] [Google Scholar]
- [62].De Luca R, Neri D. Potentiation of PD-L1 blockade with a potency-matched dual cytokine-antibody fusion protein leads to cancer eradication in BALB/c-derived tumors but not in other mouse strains. Cancer Immunol Immunother. 2018;67:1381–91. doi: 10.1007/s00262-018-2194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Balza E, Carnemolla B, Mortara L, Castellani P, Soncini D, Accolla RS, Borsi L. Therapy-induced antitumor vaccination in neuroblastomas by the combined targeting of IL-2 and TNFalpha. Int J Cancer. 2010;127:101–10. doi: 10.1002/ijc.25018. [DOI] [PubMed] [Google Scholar]
- [64].Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3:436–43. doi: 10.1158/2326-6066.CIR-15-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Martin K, Schreiner J, Zippelius A. Modulation of APC function and anti-tumor activity by anti-cancer drugs. Front Immunol. 2015;6:501. doi: 10.3389/fimmu.2015.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol. 2013;31:413–41. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Schliemann C, Palumbo A, Zuberbühler K, Villa A, Kaspar M, Trachsel E, Klapper W, Menssen HD, Neri D. Complete eradication of human B-cell lymphoma xenografts using rituximab in combination with the immunocytokine L19-IL2. Blood. 2009;113:2275–83. doi: 10.1182/blood-2008-05-160747. [DOI] [PubMed] [Google Scholar]
- [68].Moynihan KD, Opel CF, Szeto GL, Tzeng A, Zhu EF, Engreitz JM, Williams RT, Rakhra K, Zhang MH, Rothschilds AM, Kumari S, et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat Med. 2016;22:1402–1410. doi: 10.1038/nm.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kiefer JD, Neri D. Immunocytokines and bispecific antibodies: two complementary strategies for the selective activation if immune cells at the tumor site. Immunol Rev. 2016;270:178–92. doi: 10.1111/imr.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Pogue SL, Taura T, Bi M, Yun Y, Sho A, Mikesell G, Behrens C, Sokolovsky M, Hallak H, Rosenstock M, Sanchez E, et al. Targeting attenuated interferon-α to myeloma cells with an a CD38 antibody induces potent tumor regression with reduced off-target activity. PLoS One. 2016;11:e0162472. doi: 10.1371/journal.pone.0162472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].List T, Casi G, Neri D. A chemically defined trifunctional antibody-cytokine-drug conjugated with potent antitumor activity. Mol Cancer Ther. 2014;13:2641–52. doi: 10.1158/1535-7163.MCT-14-0599. [DOI] [PubMed] [Google Scholar]
- [72].Gillies SD, Lan Y, Brunkhorst B, Wong WK, Li Y, Lo KM. Bi-functional cytokine fusion proteins for gene therapy and antibody-targeted treatment of cancer. Cancer Immunol Immunother. 2002;51:449–60. doi: 10.1007/s00262-002-0302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kermer V, Hornig N, Harder M, Bondarieva A, Kontermann RE, Müller D. Combining antibody-directed presentation of IL-15 and 4-1BBL in a trifunctional fusion protein for cancer immunotherapy. Mol Cancer Ther. 2013;13:112–21. doi: 10.1158/1535-7163.MCT-13-0282. [DOI] [PubMed] [Google Scholar]
- [74].Johannsen M, Spitaleri G, Curigliano G, Roigas J, Weikert S, Kempkensteffen C, Roemer A, Kloeters C, Rogalla P, Pecher G, Miller K, et al. The tumor-targeting human L19-IL2 immunocytokine: preclinical safety studies, phase I clinical trial in patients with solid tumors and expansion into patients with advanced renal cell carcinoma. Eur J Cancer. 2010;46:2926–35. doi: 10.1016/j.ejca.2010.07.033. [DOI] [PubMed] [Google Scholar]
- [75].Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M, Einsele H, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–77. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- [76].Venetz D, Koovely D, Weder B, Neri D. Targeted Reconstitution of Cytokine Activity upon Antigen Binding using Split Cytokine Antibody Fusion Proteins. J Biol Chem. 2016;291:18139–47. doi: 10.1074/jbc.M116.737734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Gillies SD. A new platform for constructing antibody-cytokine fusion proteins (immunocytokines) with improved biological properties and adaptable cytokine activity. Protein Eng Des Sel. 2013;26:561–9. doi: 10.1093/protein/gzt045. [DOI] [PubMed] [Google Scholar]