Recent breakthroughs in cancer drugs have led to practice-changing paradigms in cancer care and new treatment options, yet patients, insurers, and policymakers struggle with determining how to afford them. Although cancer is currently among the fastest growing therapeutic areas for drug sales and the primary focus for industry research and development,1 rising cancer drug costs are unsustainable for the US health care system. Furthermore, financial toxicity compromises access to recommended therapies, reduces quality of life, and potentially shortens survival among patients with cancer.2-4 Averaging 12% to 15% growth each year, US health care spending on cancer drugs is projected to grow to $100 billion by 2022.5 In 2017, the chimeric antigen receptor (CAR) T-cell therapies, tisagenlecleucel (Kymriah; Novartis, Basel, Switzerland), and axicabtagene ciloleucel (Yescarta; Kite Pharma, Santa Monica, CA) broke the price record with one-time price tags of $475,000 and $373,000, respectively, far exceeding the estimated $150,000 average cost of cancer care and treatment.6,7
Nearly 80% of all first-in-class drugs in the pharmaceutical pipeline are indicated for cancer, and without effective policy intervention, launch prices are expected to increase with abandon given the dysfunction of the current pharmaceutical marketplace.8 This fragmented purchasing and reimbursement system has facilitated the rise in cancer drug prices, in many cases, at a rate that is disproportionate to the incremental clinical benefit.9,10 Efforts to curb prices and associated health care spending are complicated by the high willingness to pay for drugs with uncertain benefits and the low price elasticity of demand.11-13 Furthermore, efforts to counter costs by insurers has led to use management strategies that may limit patient access to cancer drugs.14,15 The American public’s top health care concern in 2019 is the rising price of drugs and, in response, lowering drug prices is a stated bipartisan policy priority, leading to continued congressional inquiries.16 This support has prompted regulatory and legislative developments with proposals aimed at controlling health care costs and alleviating patient out-of-pocket expenses.
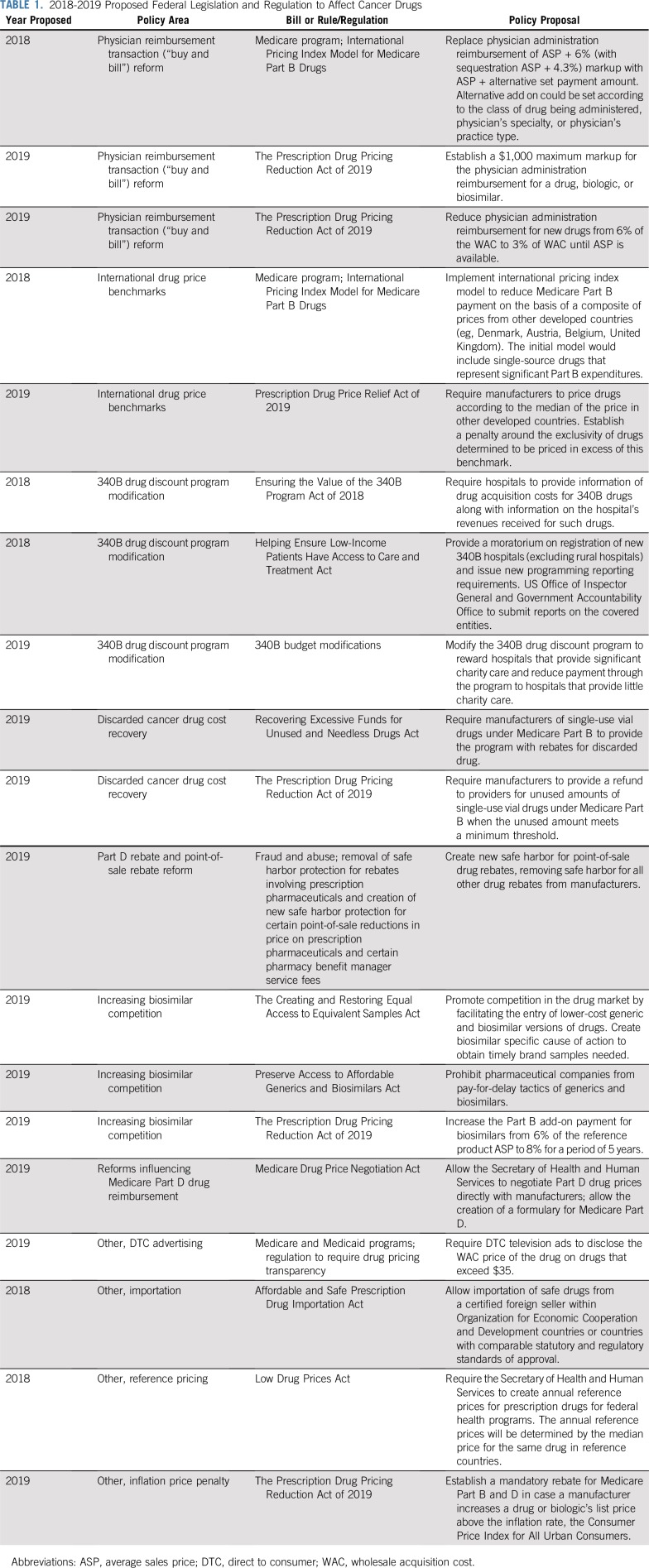
In 2018, the administration outlined proposals to reduce drug costs in key areas of Medicare reform.17 In addition, there are ongoing efforts to increase competition among biologics and encourage value-based pricing for drugs. The policies outlined here are the major recent federal proposals that affect drug pricing in oncology for both drugs administered under Medicare Part B and Part D (Table 1). The overarching goals of these policy measures include removing the financial incentives that favor prescribing higher-priced versus more affordable cancer drugs, encouraging free-market pressures to counteract the current price trajectory, implementing government regulations to prevent market exploitation and anticompetitive tactics, and realigning drug prices with their clinical value. Of note, this review does not address policy reforms that are directed at the commercial insurance sector, which has a complex regulatory and competitive landscape that differs from the Medicare system.
TABLE 1.
2018-2019 Proposed Federal Legislation and Regulation to Affect Cancer Drugs
REFORMS INFLUENCING MEDICARE PART B DRUG REIMBURSEMENT
Cancer drugs covered under Medicare Part B—intravenous (IV) and injectable drugs administered in the hospital or outpatient setting—represent a significant proportion of health care spending. These include most traditional chemotherapies, immunotherapies, and targeted monoclonal antibodies, as well as rare oral cancer drugs used interchangeably with IV counterparts, such as capecitabine. In 2017, rituximab and nivolumab, both Part B drugs, accounted for the greatest cancer drug–related spending by Medicare with average annual expenditures of $1.75 billion and $1.47 billion, respectively.19 Proposals affecting Part B drugs are put forth in 4 major policy areas and are listed in Table 1: physician reimbursement reform, a “buy and bill” system for distribution and reimbursement of provider-administered outpatient drugs20; international drug price benchmarking through implementation of a model based on pricing data from other countries20; a 340B drug discount system modification of reimbursement for Part B drugs to qualifying hospitals21; and cost reduction by recapturing costs of drug waste.22,23
Physician Reimbursement Transaction (buy and bill) Reform
The current buy and bill system for physician reimbursement of Part B drugs incentivizes the use of expensive drugs over lower-price alternatives. Buy and bill describes the mechanism by which physician practices or hospital outpatient departments purchase drugs and bill Medicare the cost of the drug plus a percentage markup.24,25 Current policy sets the reimbursement level as a 6% markup plus the average sales price (ASP), commonly referred to as ASP + 6. As a result, administration of expensive drugs is rewarded and physician behavior shifts accordingly.
In response, the Department of Health and Human Services (HHS) and the Centers for Medicare & Medicaid Services (CMS) propose reimbursement based on a flat fee addition and using third-party vendors to purchase drugs in lieu of the physician practices or hospitals.20 This is essentially a resurrection of the 2003 Medicare Modernization Act’s Competitive Acquisition Plan (CAP), which was largely unsuccessful, with few practices participating.26 The original 2003 program was voluntary, however, and imposing a mandate for CAP vendors, particularly for high-cost Part B drugs, is expected to help this proposal realize its goal for drug cost savings.27 This is controversial, however, because of concerns that a CAP model may result in use management policies—for example, step therapy, which could interfere with or delay treatment of patients with cancer, the implementation of which has been contested by oncologists.28 More recent legislative proposals included within the Prescription Drug Pricing Reduction Act of 2019 attempt to further refine reductions in reimbursement for Part B drugs (Table 1).
International Drug Price Benchmarks
Proposed international drug price models use the global price differences to institute price benchmarks. In an analysis by HHS,29 among the top drugs based on spending, prices in the United States are 1.8 times those of other developed countries, and as of 2017, the United States accounted for 46% of the total cancer drug spending worldwide according to IQVIA estimates.5 CMS proposes an international pricing framework for determining Medicare reimbursement for Part B drugs using a target price based on what other countries pay. The Advance Notice of Proposed Rulemaking intends to better align Medicare payment with that of other developed countries, which pay substantially less for the highest-cost drugs, using payment models based on international sales data provided by manufacturers. However, the details of this proposal remain uncertain and several issues require consideration before its implementation. These include clarifying the reference countries used to determine Medicare payments and how to account for the lag time in international sales data when new drugs enter the market. Additional information gathered through pilot testing would assist in predicting the impact of the proposed International Price Index model on the pharmaceutical marketplace in the United States and its potential influence on drug availability and access for Medicare beneficiaries.30
340B Drug Discount Program Modification
Proposals to modify the 340B drug discount program are aimed at the opaque implementation and misaligned distortions of the original intent for this program. Instituted in 1992 to reduce drug costs for health care facilities serving disadvantaged patients, 340B hospitals are able to purchase Part B drugs for substantial discounts from the manufacturer while receiving full reimbursement from Medicare. Steering from the original intention, 340B increasingly provides benefits to larger, well-financed hospitals.31,32 The number of hospitals that qualify for 340B now accounts for 48% of critical access hospitals, sole community hospitals, and general acute care hospitals.21 Within 340B-covered entities, there are few restrictions on qualifying patients who do not need to be uninsured. Unintended consequences of the 340B drug discount program, which increase costs for cancer care, include incentivizing the prescribing of expensive drugs in lieu of cheaper alternatives to increase profit margins, encouraging consolidation and the creation of more lucrative hospital-based outpatient infusion suites, and lastly, likely spurring higher list prices by manufacturers to account for revenue lost through 340B discounts.33 Although hospital systems have generated billions of dollars in revenue as a result, there is limited evidence of a direct benefit to the disadvantaged patient population that the policy was intended to serve.
In a measure contested by the American Hospital Association, CMS in 2017 decreased its Part B drug reimbursement rate for 340B hospitals to reflect the provided discounts and mitigate the distortions of the program. The reimbursement rate for Part B drugs under 340B was reduced to 22.5% below the ASP before the 340B discount, a notable difference from the current reimbursement of ASP + 6% for Part B drugs, effectively decreasing hospital profits. The impact on cancer drug spending by Medicare would depend on the extent to which prescribing at 340B hospitals shifts to more affordable alternatives. Legal issues, including a lawsuit filed against HHS by multiple hospital organizations, have threatened the feasibility of this measure and, as of May 2019, a federal court ruled that 340B cuts to hospitals imposed in 2018 and 2019 were unlawful.32,34,35
Discarded Cancer Drug Cost Recovery
Discarded IV drugs leftover from single-dose vials for weight-based dosing is a source of excess health care spending. Part B drugs are priced per unit and, as a result of limited vial sizes made available by the manufacturer, a substantial proportion of the drug often is wasted after a patient is treated. This generates additional profits for the pharmaceutical manufacturers, but expenses for the health care system. Bach and colleagues35a previously recommended policy strategies to counter this source of overspending, including requirements for manufacturers to offer a variety of size options to minimize waste or to refund the wasted amount. Legislators have recently introduced a bipartisan bill that would require manufacturers to refund the majority of these costs to providers. In 2019, legislators introduced the Recovering Excessive Funds for Unused and Needless Drugs Act.36 This measure would require that the drug manufacturer rebate 90% of the amount of discarded medication to CMS, which would in turn reimburse beneficiaries for the out-of-pocket costs attributed to the discarded drug. Consistent guidance from US regulators on vial combinations (example, using multiple sizes to minimize waste) and vial sharing (administering leftover drug to other patients while ensuring quality control) is still needed to develop effective methods to reduce spending associated with leftover drugs.
REFORMS INFLUENCING MEDICARE PART D DRUG REIMBURSEMENT
Cancer drugs covered under Medicare Part D, the prescription pharmacy benefit for Medicare beneficiaries enacted under the Medicare Modernization Act of 2003,37 include most oral cancer drugs dispensed through a pharmacy and self-administered, as for example, oral tyrosine kinase inhibitors and other small-molecule targeted drugs as well as hormonal agents. Medicare Part D, intended to assist beneficiaries in paying for prescription drugs, is a complex program with private insurers offering plans with varying formularies and an opaque intermediary system used for drug price negotiation. The Medicare Modernization Act explicitly prohibits the government from interfering in drug price negotiations between manufacturers and Part D plan sponsors, a clause originally intended to protect free-market principles and ensure drug access for Medicare beneficiaries. Intermediaries, called pharmacy benefit managers (PBMs), generally negotiate purchases for the Medicare Part D plan sponsors by offering preferred formulary placement. Unintended consequences of the Medicare Part D design include the lack of price transparency and consistency, the formation of a perverse and secretive rebate system governed by PBMs, and the inappropriate linkage of patient out-of-pocket expenses and government spending to the list price of a drug as opposed to net price.38 These have allowed drug costs to rise steeply at the expense of patients and taxpayers and to the benefit of insurers, intermediaries, and drug manufacturers. An additional barrier to lowering drug costs for cancer specifically is that cancer drugs are considered a protected class. Under the protected class status, Medicare Part D plans are required to cover all US Food and Drug Administration (FDA)–approved drugs, thereby limiting the power of formulary exclusion as a negotiating tactic to lower cancer drug prices.
Part D Rebate and Point-of-Sale Rebate Reform
PBMs obtain rebates from the manufacturer, instead of upfront discounted prices, in exchange for formulary preference. Yet out-of-pocket costs for Medicare beneficiaries are calculated on the basis of the pre-rebate price rather than the net price. This leads to higher patient cost sharing, which has drawn scrutiny to the Part D rebate system. Historically, manufacturer rebates for Part D drugs are used as a defense against high list prices, but patients have not benefited from the resulting discounts. As a result, earlier this year HHS proposed a rule, now abandoned by the administration,39 to eliminate rebates paid by manufacturers to PBMs by removing protections under the Federal antikickback statute. The rule also proposed that manufacturers compensate PBMs on the basis of a flat fee, instead of by a percentage of the drug’s list price, to delink intermediary profits from drug list prices. Although eliminating rebates was applauded as a measure to improve price transparency and promote lower costs for patients, there remained uncertainty regarding the response of stakeholders to the regulation and manufacturers’ willingness to discount prices upfront in lieu of postsale rebates. Whereas analyses predicted that total cost-sharing savings for patients would offset an increase in premiums, the overall impact on Medicare health care spending varied widely because of its dependence on the behavior of manufacturers.40 For cancer specifically, rebate reform was expected to have little effect on lowering costs for patients as drugs in the protected classes experience few price concessions at baseline regardless of the mechanism.
Other Part D Reforms
Ultimately, an extensive restructuring of the Part D system beyond rebate reform is likely necessary to lower cancer drug costs for the health care system and Medicare beneficiaries, as previously suggested by Bach and Dusetzina.38 As mentioned, particularly problematic is the lack of formulary flexibility in the protected drug classes, which has allowed manufacturers to essentially dictate the prices for oral cancer drugs. In November 2018, CMS proposed a rule to allow for formulary flexibility within the protected drug classes to allow Part D plans to exclude drugs with price increases greater than inflation, exclude drugs that lack sufficient innovation over available agents, and allow plans to require step therapy and/or prior authorization while maintaining patient protections.41 CMS has previously attempted to propose limits to the protected classes in 2014 but, because of concerns regarding drug access from stakeholders, it did not finalize this policy. In a separate measure to improve price transparency for Part D drugs, as of 2019, HHS and CMS passed and finalized a rule that requires manufacturers to disclose the list price for drugs in direct-to-consumer advertisements for drugs with a list price greater than $35 for a 1-month supply.42 Yet after pharmaceutical manufacturers filed a lawsuit against the rule in June 2019, the federal justice system ruled that HHS does not have the authority to require pharmaceutical companies to disclose drug prices in advertisements, blocking the rule from taking effect.43
Capping out-of-pocket spending at the catastrophic threshold for Part D drugs is another recommended policy change to decrease the direct cost burden among Medicare beneficiaries. According to data from the Henry Kaiser Family Foundation, median out-of-pocket costs for Medicare patients in 2019 for 14 specialty cancer drugs—drugs that cost $670 or more per month—exceeds $8,000 annually, with most spending occurring in the catastrophic phase.44 Patients reach the catastrophic phase after paying $5,100 annually in 2019 for Part D medications and then are responsible for 5% coinsurance on drugs for the remainder of the year. Setting an out-of-pocket maximum to this amount would relieve financial toxicity and ideally improve accessibility for patients who require high-cost cancer drugs. In addition, the Medicare Drug Price Negotiation Act, introduced earlier in 2019 by Congress, proposes to allow CMS to negotiate covered part D drug prices with pharmaceutical companies on behalf of Medicare beneficiaries.45,46 The effect of such legislation on drug costs is controversial. According to the Congressional Budget Office, to have effective leverage to drive down cancer costs, Medicare must be able to refuse coverage, similar to the Veterans Administration model, which in consequence has raised concerns regarding drug access for beneficiaries.47
COMPETITION AMONG BIOLOGIC DRUGS
Biologics play a key role in the treatment of a variety of cancers and account for 70% of the growth in drug spending from 2010 to 2015.48,49 FDA-approved biologics for cancer indications include monoclonal antibodies with a range of targets (eg, rituximab, trastuzumab, bevacizumab, and pembrolizumab), immune modulators, oncolytic viruses, and adaptive cell therapy, including CAR T-cell therapy. Under the Hatch Waxman Act of 1984, small-molecule brand name drugs are granted 5 years of market exclusivity, until generics, chemical copies of small-molecule drugs, are able to enter. These generic drugs have generated billions of dollars in health care savings and account for 90% of all prescriptions in the United States.48,50 Given the success of generic drugs in reducing health care costs, Congress created a parallel pathway for the introduction of biosimilar products under the Biologics Price Competition and Innovation Act of 2009 as the follow-on agents for biologics, which have a 12-year period of market exclusivity.51
Unfortunately, the market entrance of biosimilars, molecules that are highly similar to the reference product, has been limited by multiple barriers to entry. Biologics are complex large molecules derived from living organisms often using recombinant DNA technology and relying on extensive purification to ensure quality control for human use. Thus, biosimilars are far more difficult and costly to recreate from the reference product compared with chemically synthesized generics. In addition, because of concerns regarding variability in the biosimilar product, the approval process for biosimilars is onerous and requires the sponsor to demonstrate safety and clinical equivalency to the reference product with phase I and III clinical trials.
Even after successful FDA approval, the marketing launch and clinical use of biosimilars in the United States has been delayed by patent infringement lawsuits and other anticompetitive tactics by brand name manufacturers.52-54 Legislation has been proposed to prohibit brand name manufacturers from such delay tactics for the introduction of biosimilar drugs and to encourage biosimilar competition. As of August 2019, the FDA has approved 9 biosimilars for the treatment of cancer and yet, 7 remain unmarketed to date. In addition, physicians raise concerns about perceptions of the efficacy and safety of biosimilars, adding potential negative impact in uptake in clinical practice.55 In turn, biosimilars are expected to have a meager effect on cancer-related drug costs, which has even led to calls to abandon the use of biosimilars in the United States in favor of postexclusivity price regulations.56
VALUE-BASED PRICING FOR CANCER DRUGS
The current system of pricing based on what the market will bear has resulted in launch prices for drugs that are often disproportionately high for the benefit that they provide to patients. Value-based pricing is defined as setting the price of a drug at market entry on the basis of its clinical benefits and harms, although the concept is often applied more broadly to include other nontraditional payment models.57 A component of value-based pricing, indication-based pricing, allows for varying prices of a drug for different approved indications when the clinical benefits differ. In contrast, outcomes-based pricing contracts, which have been pursued for CAR T-cell therapy reimbursement, do not base price on benefit but instead offer money back in case a prespecified outcome of disease response is not met. For example, Novartis introduced an outcomes-based pricing mechanism for its CAR T-cell therapy tisagenlecleucel for its indication in pediatric or young adult acute lymphoblastic leukemia.58 Under this contract, insurers would only pay the company for the drug if a patient achieves a disease response 30 days after therapy. However, innovative outcomes-based contracts pose logistical challenges to implement, require a more comprehensive discussion of meaningful disease outcomes for payment decisions, do not address the primary issue of too high launch prices, and, in their current state, are expected to produce only minimal drug cost savings overall.59-61
Value-based frameworks that take into account the clinical benefit and cost of drugs are commonly used in Europe for coverage and reimbursement purposes. In the United States, the Institute for Clinical and Economic Review is focused on clarifying the value-based price for expensive drugs to inform a value-driven pricing system. With this knowledge, insurers could provide incentives to manufacturers for drugs that meet value-driven price thresholds, such as guaranteed formulary inclusion or placement in preferred tiers.62 In contrast, drugs priced above the value-driven price could be subject to higher tier placement, larger coinsurance burdens, and use strategies for cost containment. The goal of such strategies is to induce the appropriate market pressures to drive prices for cancer drugs to be in line with the benefit that they provide to patients with cancer. ASCO, which publishes its own value framework to facilitate shared decision making with patients, has also expressed support for a value-based pricing system as an ideal method to manage cancer drug prices without compromising patient access to cancer therapy.63 The unsustainable trajectory of cancer drug costs reveals the underlying dysfunction of the current system and has led to the re-examination of government policies that have facilitated this trend. Public outcry has motivated bipartisan scrutiny of industry price-gouging tactics and has encouraged greater transparency within the pharmaceutical supply chain. By implementing effective policy measures, legislators have the power to minimize the incentives for prescribing high-cost drugs, reduce Medicare reimbursement rates to control spending, and introduce leverage within the pharmaceutical marketplace. Although more work is needed, the recent flurry of policy measures is promising for influencing real change in cancer drug costs that can improve the affordability and quality of care that patients with cancer deserve.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Administrative support: Jennifer A. Ohn
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Review of Current Policy Strategies to Reduce US Cancer Drug Costs
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Peter B. Bach
Honoraria: Gilead Sciences, WebMD, Goldman Sachs, Defined Health, Vizient, Anthem, Excellus Health Plan, Hematology Oncology Pharmacy Association, Novartis, Janssen, Third Rock Ventures, JMP Securities, American Society of Health-System Pharmacists, Genentech
Consulting or Advisory Role: Foundation Medicine, GRAIL
Research Funding: Kaiser Permanente, Laura and John Arnold Foundation, National Institutes of Health Core Grant P30-CA008748
Travel, Accommodations, Expenses: Gilead Sciences, WebMD, Goldman Sachs, Defined Health, Vizient, Anthem, Excellus Health Plan, Hematology Oncology Pharmacy Association, Novartis, Janssen, Third Rock Ventures, American Society of Health-System Pharmacists, Mercer, United Rheumatology, Genentech
No other potential conflicts of interest were reported.
REFERENCES
- 1.EvaluatePharma World Preview 2019, Outlook to 2024. https://info.evaluate.com/rs/607-YGS-364/images/EvaluatePharma_World_Preview_2019.pdf
- 2.Lathan CS, Cronin A, Tucker-Seeley R, et al. Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. J Clin Oncol. 2016;34:1732–1740. doi: 10.1200/JCO.2015.63.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsey SD, Bansal A, Fedorenko CR, et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol. 2016;34:980–986. doi: 10.1200/JCO.2015.64.6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119:3710–3717. doi: 10.1002/cncr.28262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.IQVIA Institute Global oncology trends 2018. https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/global-oncology-trends-2018.pdf?_=1536696423000
- 6.Moore P. The high cost of cancer treatment. https://www.aarp.org/money/credit-loans-debt/info-2018/the-high-cost-of-cancer-treatment.html
- 7.Milliman A multi-year look at the cost burden of cancer care. http://us.milliman.com/uploadedFiles/insight/2017/cost-burden-cancer-care.pdf
- 8.Long G, Analysis Group The biopharmaceutical pipeline: Innovative therapies in clinical development. http://www.analysisgroup.com/uploadedfiles/content/insights/publishing/the_biopharmaceutical_pipeline_report_2017.pdf
- 9.Kantarjian HM, Fojo T, Mathisen M, et al. Cancer drugs in the United States: Justum Pretium—The just price. J Clin Oncol. 2013;31:3600–3604. doi: 10.1200/JCO.2013.49.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hillner BE, Smith TJ. Efficacy does not necessarily translate to cost effectiveness: A case study in the challenges associated with 21st-century cancer drug pricing. J Clin Oncol. 2009;27:2111–2113. doi: 10.1200/JCO.2008.21.0534. [DOI] [PubMed] [Google Scholar]
- 11.Howard DH, Bach PB, Berndt ER, et al. Pricing in the market for anticancer drugs. J Econ Perspect. 2015;29:139–162. doi: 10.1257/jep.29.1.139. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqui M, Rajkumar SV. The high cost of cancer drugs and what we can do about it. Mayo Clin Proc. 2012;87:935–943. doi: 10.1016/j.mayocp.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chino F, Peppercorn JM, Rushing C, et al. Going for broke: A longitudinal study of patient-reported financial sacrifice in cancer care. J Oncol Pract. 2018;14:e533–e546. doi: 10.1200/JOP.18.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: Associations with medication and medical utilization and spending and health. JAMA. 2007;298:61–69. doi: 10.1001/jama.298.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham PJ. Medicaid cost containment and access to prescription drugs. Health Aff (Millwood) 2005;24:780–789. doi: 10.1377/hlthaff.24.3.780. [DOI] [PubMed] [Google Scholar]
- 16.Kirzinger A, Minana C, Brodie M. KFF health tracking poll – January 2019: The public on next steps for the ACA And proposals to expand coverage. https://www.kff.org/health-reform/poll-finding/kff-health-tracking-poll-january-2019/
- 17.US Department of Health and Human Services American patients first: The Trump administration blueprint to lower drug prices and reduce out-of-pocket costs. https://www.hhs.gov/sites/default/files/AmericanPatientsFirst.pdf
- 18.Reference deleted.
- 19.Centers for Medicare & Medicaid Services Medicare Part B drug spending dashboard. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Information-on-Prescription-Drugs/MedicarePartB.html
- 20. Centers for Medicare & Medicaid Services: Medicare program; International Pricing Index Model for Medicare Part B drugs. CMS-5528-ANPRM. 42 C.F.R.
- 21.Centers for Medicare & Medicaid Services Medicare program: Changes to hospital outpatient prospective payment and ambulatory surgical center payment systems and quality reporting programs. https://s3.amazonaws.com/public-inspection.federalregister.gov/2018-24243.pdf [PubMed]
- 22.116th US Congress S.551: Recovering Excessive Funds for Unused and Needless Drugs (REFUND) Act. https://www.congress.gov/bill/116th-congress/senate-bill/551/text?format=txt [Google Scholar]
- 23.US Senate Committee on Finance The Prescription Drug Pricing Reduction Act (PDPRA) of 2019. https://www.finance.senate.gov/imo/media/doc/FINAL%20Description%20of%20the%20Chairman's%20Mark%20for%20the%20Prescription%20Drug%20Pricing%20Reduction%20Act%20of%202019.pdf
- 24.Bach PB, Ohn J. Does the 6% in Medicare Part B drug reimbursement affect prescribing? Drug Pricing Lab; https://drugpricinglab.org/wp-content/uploads/2018/05/Part-B-Reimbursement-and-Prescribing.pdf [Google Scholar]
- 25.Mitchell AP, Rotter JS, Patel E, et al. Association between reimbursement incentives and physician practice in oncology: A systematic review. JAMA Oncol. 2019;5:893–899. doi: 10.1001/jamaoncol.2018.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Medicare & Medicaid Services Evaluation of the Competitive Acquisition Program for Part B Drugs: Final Report. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Reports/downloads/CAPPartB_Final_2010.pdf
- 27.Bach PB. Competitive acquisition program. Drug Pricing Lab; https://drugpricinglab.org/wp-content/uploads/2018/05/DPL-Policy-Brief_Competitive-Acquisition-Program_051418_v2.pdf [Google Scholar]
- 28.American Society of Clinical Oncology ASCO weighs in on CAP demo in drug pricing proposal. https://www.asco.org/advocacy-policy/asco-in-action/asco-weighs-cap-demo-drug-pricing-proposal
- 29.US Department of Health and Human Services Comparison of U.S. and international prices for top Medicare Part B drugs by total expenditures. https://aspe.hhs.gov/system/files/pdf/259996/ComparisonUSInternationalPricesTopSpendingPartBDrugs.pdf
- 30.Centers for Medicare & Medicaid Services Medicare program: International Pricing Index Model for Medicare Part B. https://www.federalregister.gov/documents/2018/10/30/2018-23688/medicare-program-international-pricing-index-model-for-medicare-part-b-drugs
- 31.Conti RM, Bach PB. The 340B drug discount program: Hospitals generate profits by expanding to reach more affluent communities. Health Aff (Millwood) 2014;33:1786–1792. doi: 10.1377/hlthaff.2014.0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bach PB, Sachs RE. Expansion of the Medicare 340B payment program: Hospital participation, prescribing patterns and reimbursement, and legal challenges. JAMA. 2018;320:2311–2312. doi: 10.1001/jama.2018.15667. [DOI] [PubMed] [Google Scholar]
- 33.Conti RM, Bach PB. Cost consequences of the 340B drug discount program. JAMA. 2013;309:1995–1996. doi: 10.1001/jama.2013.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Civil Action No: 18-2084 (RC). Document 25. American Hospital Association v. Alex Azar II. https://images.magnetmail.net/images/clients/AHA_MR1/attach/2019/May/MemorandumOpinionGrantingPartMotionforPermanentInjunction_Remanding2018and2019OPPSRules_HHS_05062019.PDF
- 35.Civil Action No: 18-2084 (RC). Document 50. American Hospital Association v. Alex Azar II. https://images.magnetmail.net/images/clients/AHA_MR1/attach/2019/May/MemorandumOpinionGrantingPartMotionforPermanentInjunction_Remanding2018and2019OPPSRules_HHS_05062019.PDF
- 35a.Bach PB, Conti RM, Muller RJ, et al. Overspending driven by oversized single dose vials of cancer drugs BMJ 352i788doi: 10.1136/bmj.i788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.US Senate Committee on Finance Recovering Excessive Funds for Unused and Needless Drugs (REFUND) Act of 2019. https://www.congress.gov/bill/116th-congress/senate-bill/551?q=%7B%22search%22%3A%5B%22Recovering+Excessive+Funds+for+Unused+and+Needless+Drugs+%28REFUND%29+Act%22%5D%7D&s=1&r=1
- 37.Bach PB, McClellan MB. The first months of the prescription-drug benefit: A CMS update. N Engl J Med. 2006;354:2312–2314. doi: 10.1056/NEJMp068108. [DOI] [PubMed] [Google Scholar]
- 38.Dusetzina SB, Conti RM, Yu NL, et al. Association of prescription drug price rebates in Medicare Part D with patient out-of-pocket and federal spending. JAMA Intern Med. 2017;177:1185–1188. doi: 10.1001/jamainternmed.2017.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owens C. White House kills major drug pricing proposal. Axios; https://www.axios.com/trump-drug-prices-plan-pharma-ec527a14-0287-492b-937d-a7144c47b734.html [Google Scholar]
- 40.Sachs R. Trump administration releases long-awaited drug rebate proposal. Health Affairs; https://www.healthaffairs.org/do/10.1377/hblog20190201.545950/full/ [Google Scholar]
- 41.Centers for Medicare & Medicaid Services Modernizing Part D and Medicare Advantage to lower drug prices and reduce out-of-pocket expenses [proposed rule] https://s3.amazonaws.com/public-inspection.federalregister.gov/2018-25945.pdf
- 42.Centers for Medicare & Medicaid Services Medicare and Medicaid programs; regulation to require drug pricing transparency. https://www.federalregister.gov/documents/2019/05/10/2019-09655/medicare-and-medicaid-programs-regulation-to-require-drug-pricing-transparency
- 43.Bellon T, Raymond N. U.S. judge strikes down Trump administration rule requiring drug prices in TV ads. Reuters; https://www.reuters.com/article/us-usa-drugpricing-lawsuit/us-judge-strikes-down-trump-administration-rule-requiring-drug-prices-in-tv-ads-idUSKCN1U32L2 [Google Scholar]
- 44.Cubanski J, Koma W, Neuman T. The out-of-pocket cost burden for specialty drugs in Medicare Part D in 2019. Kaiser Family Foundation; https://www.kff.org/medicare/issue-brief/the-out-of-pocket-cost-burden-for-specialty-drugs-in-medicare-part-d-in-2019/ [Google Scholar]
- 45.Doshi JA, Pettit AR, Pengxiang L. Addressing out-of-pocket specialty drug costs in Medicare Part D: The good, the bad, the ugly, and the ignored. Health Affairs; https://www.healthaffairs.org/do/10.1377/hblog20180724.734269/full/ [Google Scholar]
- 46.Doshi JA, Li P, Pettit AR, et al. Reducing out-of-pocket cost barriers to specialty drug use under Medicare Part D: Addressing the problem of “too much too soon”. Am J Manag Care. 2017;23(suppl):S39–S45. [PubMed] [Google Scholar]
- 47.US Congressional Budget Office Medicare Prescription Drug Price Negotiation Act of 2007 cost estimate. https://www.cbo.gov/sites/default/files/110th-congress-2007-2008/costestimate/s30.pdf
- 48.US Food and Drug Administration Biosimilars action plan: Balancing innovation and competition. https://www.fda.gov/media/114574/download
- 49.Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the United States. Rand; https://www.rand.org/content/dam/rand/pubs/perspectives/PE200/PE264/RAND_PE264.pdf [PMC free article] [PubMed] [Google Scholar]
- 50.Association for Accessible Medicines Generic drug access and savings in the U.S. https://accessiblemeds.org/sites/default/files/2018_aam_generic_drug_access_and_savings_report.pdf
- 51. The Public Health and Welfare. 42 U.S.C. § 262(k). https://uscode.house.gov/view.xhtml?path=/prelim@title42&edition=prelim.
- 52.IQVIA Institute Advancing biosimilar sustainability in Europe. https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/advancing-biosimilar-sustainability-in-europe.pdf?_=1558678774430
- 53.Congressional Research Service Biologics and biosimilars: Background and key issues. https://fas.org/sgp/crs/misc/R44620.pdf
- 54.Atteberry PJ, Bach PB, Ohn JA, et al. Biologics are natural monopolies (Part 1): Why biosimilars do not create effective competition. Health Affairs; https://www.healthaffairs.org/do/10.1377/hblog20190405.396631/full/ [Google Scholar]
- 55.Lawrence LK. Oncologic biologic biosimilars are coming to market: Are you ready? IASLC Lung Cancer News; https://www.lungcancernews.org/2018/06/01/oncologic-biologic-biosimilars-are-coming-to-market-are-you-ready/ [Google Scholar]
- 56.Trusheim MR, Atteberry PJ, Ohn JA, et al. Biologics are natural monopolies (Part 2): A proposal for post-exclusivity price regulation of biologics. Health Affairs; https://www.healthaffairs.org/do/10.1377/hblog20190405.839549/full/ [Google Scholar]
- 57.Bach PB, Pearson SD. Payer and policy maker steps to support value-based pricing for drugs. JAMA. 2015;314:2503–2504. doi: 10.1001/jama.2015.16843. [DOI] [PubMed] [Google Scholar]
- 58.Bach PB, Giralt SA, Saltz LB. FDA approval of tisagenlecleucel: Promise and complexities of a $475 000 cancer drug. JAMA. 2017;318:1861–1862. doi: 10.1001/jama.2017.15218. [DOI] [PubMed] [Google Scholar]
- 59.Drug Pricing Lab Value-based pricing vs. outcomes-based contracting. https://drugpricinglab.org/our-work/value-based-pricing-vs-outcomes-based-contracting/
- 60.Navarria A, Drago V, Gozzo L, et al. Do the current performance-based schemes in Italy really work? “Success fee”: A novel measure for cost-containment of drug expenditure. Value Health. 2015;18:131–136. doi: 10.1016/j.jval.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 61.Garrison LP, Jr, Carlson JJ, Bajaj PS, et al. Private sector risk-sharing agreements in the United States: Trends, barriers, and prospects. Am J Manag Care. 2015;21:632–640. [PubMed] [Google Scholar]
- 62.Kaltenboeck A, Bach PB. Value-based pricing for drugs: Theme and variations. JAMA. 2018;319:2165–2166. doi: 10.1001/jama.2018.4871. [DOI] [PubMed] [Google Scholar]
- 63.American Society of Clinical Oncology Value-based approaches are focus of ASCO comments on administration’s blueprint to lower drug prices. https://www.asco.org/advocacy-policy/asco-in-action/value-based-approaches-are-focus-asco-comments-administrations