Abstract
Antibody-cytokine fusion proteins represent a novel class of biopharmaceuticals, with the potential to increase the therapeutic index of cytokine payloads and to promote leukocyte infiltration at the site of disease. In this review, we present a survey of immunocytokines that have been used in preclinical models of cancer and in clinical trials. In particular, we highlight how antibody format, choice of target antigen, cytokine engineering as well as combination strategies may have a profound impact on therapeutic performance. Moreover, by using anti-inflammatory cytokines, antibody fusion strategies can conveniently be employed for the treatment of auto-immune and chronic inflammatory conditions.
From cytokines to immunocytokines
Cytokines are small immunoregulatory proteins, which are secreted by leukocytes and, in some cases, by endothelial cells, fibroblasts and stromal cells. Cytokines can induce important functional changes in tissues, such as an increase in vascular permeability or alteration of body temperature. Importantly, these proteins can also have inhibitory or activating effects on immune cells, depending on their features, on their concentration and on the environment in which they act [1]. Considering the important role in many physiological and pathological conditions, cytokines have been considered as products in their own right or as targets for the development of blocking agents.
The antibody-based blockade of pro-inflammatory cytokines [e.g., tumor necrosis factor (TNF), interleukin-2 (IL2), interleukin-12 (IL12), interleukin-17 (IL17)] or their cognate receptors [e.g., the interleukin-6 receptor (IL6R)] has led to the development of successful products for the treatment of chronic inflammatory conditions, such as rheumatoid arthritis, inflammatory bowel diseases and psoriasis. On the other hand, certain recombinant cytokines have received marketing authorization for the treatment of cancer (e.g., IL2, TNF), of viral infections [e.g., Interferon (IFN) α], and for few other biomedical applications.
The systemic administration of pro-inflammatory cytokines can lead to severe off-target related adverse effects, which may limit the dose and prevent escalation to therapeutically active regimens. Certain cytokine products (e.g., IL2, TNF, IL12) have exhibited recommended doses in the single-digit milligram range or even below [2–4]. Adverse effects associated with the intravenous administration of pro-inflammatory cytokines may include hypotension, fever, nausea or flu-like symptoms, but cytokines may occasionally also cause serious hematologic, endocrine, autoimmune or neurologic events [5]. In view of these considerations, there is a clear biomedical need for the development of next-generation cytokine products, which are better tolerated and which display a preferential action at the site of disease, helping spare normal tissues.
Antibodies specific to accessible markers, which are over-expressed at the site of disease, may represent ideal “vehicles” for the targeted delivery of therapeutic payloads, including cytokines. In mice, it has been clearly shown that certain tumor-homing antibody-cytokine fusion proteins (“immunocytokines”) can dramatically increase the therapeutic index of the corresponding cytokine payload [6–12]. Similarly, immunocytokines with anti-inflammatory properties, capable of selective accumulation at sites of tissue remodeling, have been considered for the treatment of chronic inflammatory conditions and of endometriosis [13, 14]. In this review, we survey some basic concepts associated with the development of immunocytokine biopharmaceuticals and discuss how engineered cytokine products may look like in the future.
Immunocytokine formats
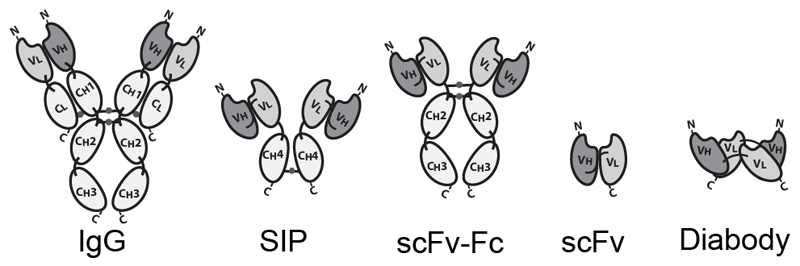
Antibodies can be used in full immunoglobulin G (IgG) format, in order to exploit the stability and long circulatory half-life of these products. Alternatively, antibody fragments [e.g., single-chain variable fragments (scFvs), diabodies, Fab fragments] may be considered, when a faster blood clearance and a more efficient extravasation is desired [15, 16]. Figure 1 illustrates some of the most popular antibody formats, which have been considered for immunocytokine development. The format may determine different pharmacokinetic and pharmacodynamic properties. These in vivo functional aspects may not be evident on the basis of simple in vitro assays, since the fusion of cytokine payloads with various types of antibodies often proceeds without loss of biological activity. On the other hand, the residence time of immunocytokines in blood and the ability of the product to extravasate and localize at the site of disease are crucial determinants of pharmaceutical performance [17].
Figure 1.
Schematic illustration of different antibody formats suitable for antibody-cytokine fusion proteins. From the left: full immunoglobulin (IgG), Small immunoprotein (SIP), scFv-FC, scFv, Diabody. N = N-terminus, C = C-terminus.
Intact IgG molecules can be fused to cytokines at various sites (N- or C-terminus of heavy or light chain), giving rise to large fusion proteins (e.g., 180 kDa for IL2 fusions). The size and recycling properties mediated by the interaction with the neonatal FcRn receptor may contribute to a longer circulatory half-life in vivo [18]. As a potential drawback, a larger molecular size may impair extravasation and penetration into the tumor mass [19]. In addition to the bioactivity of the cytokine moiety, an IgG-based immunocytokine would retain the ability to bind to Fc gamma receptors on leukocytes, with the potential to impair localization at the tumor site and to mediate undesired activation of white blood cells. Changing the amino acid composition, the isotype or the glycans of the Fc portion may reduce these off target effects [20, 21].
Immunocytokines based on small antibody fragments have a much shorter half-life in the circulation, compared to those based on the full IgG format. A rapid blood clearance may lead to a decreased uptake at the site of disease, since extravasation typically represents the rate-limiting step for ligand-based pharmacodelivery applications [15, 22]. Short linkers (less than 11 aminoacids) between the variable heavy (VH) and variable light (VL) chains form non-covalent homodimers (diabodies) [23]. Diabodies and other bivalent formats fused to the cytokine payloads retain a high binding avidity to the cognate antigen, thus potentially leading to a long residence time on the biological target [16, 24, 25]. Multivalent immunocytokines based on antibody fragments have exhibited tumor:organ ratios greater than 10:1 in biodistribution studies performed in mouse models of cancer, 24 hours after intravenous administration [11, 26].
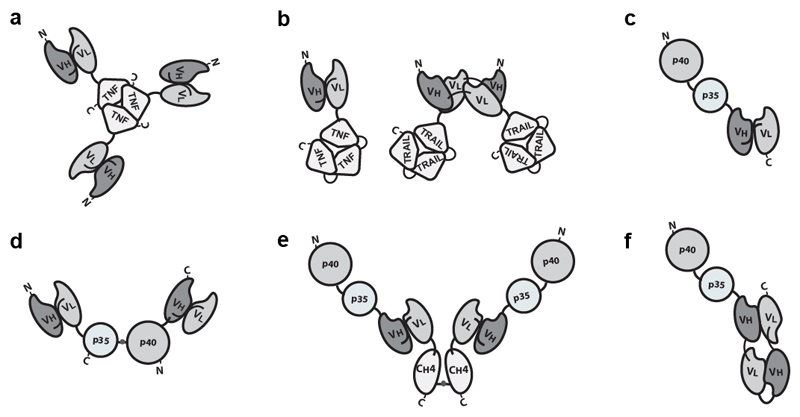
The antibody format and the position of the cytokine payload, as well as the amino acid composition of the linker connecting various protein domains, can substantially influence the in vivo performance of fusion proteins, thus offering rich opportunities for protein engineering applications [16, 27]. Design options further increase, when cytokines consisting of multiple subunits (e.g., components of the TNF or of the IL12 superfamily) are considered [7, 17, 26, 28–32] (Figure 2).
Figure 2.
Examples of immunocytokines built with multiple subunits. a) Homotrimeric scFv-TNF [26, 71]. b) Left: scFv fused to single chain TNF (scTNF). Right: Homodimeric immunocytokine formed by two diabody-scTRAIL subunits. Other TNF superfamily cytokines can be engineered with the same approach [28, 154]. c) The C-terminus of the p40 is connected to the N-Terminus of the p35 subunits of IL12 15 amino acid linker. The recombinant cytokine is linked to the N-terminus of a scFv [7]. d) IL12-based immunocytokine consisting of two polypeptides, scFv-p35 and p40-scFv, joined by a disulfide bond between the two cytokine subunits[32]. e) IL12-SIP with a C-terminal CH4 domain of human IgE, wich favor homodimerisation. f) The p40 and p35 subunits of IL12 sequentially fused to a “forced” diabody or tandem diabody [17].
Targets for oncological and non-oncological conditions
Ideal target antigens for the development of immunocytokine products should be abundantly expressed at the site of disease and absent in healthy tissues. In tumors and in chronic inflammatory conditions, tissue remodeling and neo-vascularization processes expose antigens, which are otherwise virtually undetectable in healthy organs [33–35]. One example is represented by splice isoforms of fibronectin, a glycoprotein of the extracellular matrix (ECM). The extra-domains A and B (EDA and EDB) of fibronectin are strongly expressed in tumors, at sites of tissue remodeling and during fetal development, but are otherwise not found in normal tissues, exception made for the female reproductive system [36, 37]. Similarly, splice variants of tenascin-C are specifically found in tissues and tumors undergoing neo-angiogenesis, in a process which is regulated by intracellular pH [38]. EDA, EDB and splice variants of tenascin-C represent suitable targets for the delivery of bioactive payloads, including cytokines.
In oncological malignancies molecular targets may include fibroblast activation protein (FAP) [39, 40], cellular antigens (e.g., CEA and PSMA) [41–43] or proteins, which become accessible in necrotic lesions, such as histones [44]. Antibodies which have been extensively characterized in the context of cytokine fusions include F8 (targeting EDA-fibronectin) [45], L19 (targeting EDB-fibronectin) [46], F16 (targeting the A1 domain of tenascin-C) [47], scFv36 (targeting FAP) [40], hu14.18 (targeting the GD2 ganglioside) [48], chCLL-1 (targeting CD20) [49] and anti-HER2/neu [50].
In tissues undergoing chronic inflammatory processes, specific cell subtypes that are present at higher local density than in normal tissues, may be targeted (e.g., neutrophils and macrophages) [51]. CD64 (i.e., the high-affinity Fcγ receptor 1) is expressed on the surface of macrophages, monocytes and their progenitor cells. Immunotoxins directed against CD64 were tested in animal models of inflammation [skin, rheumatoid arthritis and ischemia-induced kidney injury] and did not display adverse effects related to the presence of the antigen on other circulating leukocytes [52].
Cytokine payloads
Both for cancer therapy as well as for the treatment of chronic inflammation, several cytokine payloads have been developed and tested in preclinical trials. Pro-inflammatory cytokines such as IL2, TNF, and IL12 have been investigated for tumor therapy, as they have been found to increase and activate the local infiltrate of leukocytes at the tumor site [53–59]. By contrast, immunosuppressive cytokines (e.g., IL10) may be considered as payloads for the treatment of chronic inflammatory conditions [60, 61] or of other diseases (e.g., endometriosis) [62, 63].
The first antibody-cytokine fusions were reported by the groups of Reisfeld/Gillies, Alan Epstein and Sherie Morrison [48, 64, 65]. The authors described the fusions of IgG antibodies with various interleukins, interferons and members of the TNF superfamily [48–50, 64, 66–69]. Our group has mainly focused on the development of antibody-cytokine fusion proteins, which targeted extracellular matrix components (e.g, those based on the F8 and L19 antibodies). Quantitative biodistribution studies using radiolabelled immunocytokine preparations have allowed us to learn some lessons about payloads that can be efficiently delivered to solid tumors, as well as payloads which abrogate the targeting properties of the parental antibody. Payloads can broadly be grouped into five distinct categories in the mouse:
-
i)
Cytokines, which are specifically delivered to the tumor mass, by fusion to the antibody moiety (e.g., IL2, TNF, IL4, IL6, IL10, IFNα) [14, 53, 70–72].
-
ii)
Cytokines, which are specifically delivered to the tumor mass, by fusion to the antibody moiety in a specific format (IL12) [29].
-
iii)
Cytokines which can be efficiently delivered to the tumor site only at higher doses, after saturation of the cognate receptor (e.g., IFN γ, GM-CSF) [73, 74].
-
iv)
Payloads, which are too large or too negatively or positively charged to be able to extravasate and reach the target antigen in vivo (e.g., VEGF(120) vs. VEGF(164)) [75].
-
v)
Cytokines, which are heavily glycosylated and are captured by asialoglycoprotein receptor in the liver, which abrogates the tumor-homing performance of the antibody moiety (e.g., B7.2, IL9) [76, 77].
Our group has observed that glycosylation patterns may vary depending on the protein production protocol used (e.g., stable vs. transient gene expression), leading to striking differences in disease-targeting performance [77].
Immunocytokines for cancer therapy
The targeted delivery of cytokines to the tumor aims at inducing a local pro-inflammatory environment, which may activate and recruit immune cells. Here, we briefly present fusion proteins which have shown activity in preclinical models and which have progressed to clinical trials in patients with cancer. A comprehensive list of antibody-cytokine fusions described in the literature can be found in Ref. 78 [78]. Table 1 summarizes the clinical trials performed using immunocytokines for cancer treatment.
Table 1.
List of immunocytokines in clinical trials. NK: Natural Killer; NSCLC: Non-Small-cell Lung Carcinoma; EDB: extra domain B of Fibronectin; CEA: Carcinoembryonic Antigen; FAP: Fibroblast Activation Protein; PK: Pharmacokinetic; PD: Pharmacodynamic; RCC: Renal Cell Carcinoma; MTD: Maximum Tolerated Dose;
| Antibody | Antigen/Format | Clinical Trials | Indication | Status | ||
|---|---|---|---|---|---|---|
| Inteleukin-2 | ||||||
| Hu14.18-IL2 / EMD 273063; APN301 | GD2-ganglioside
|
NCT00003750 | Phase I: Efficacy study | Children with refractory or recurrent Neuroblastoma, or other Solid Tumors | Completed | [197] |
| NCT00109863 | Phase II: Efficacy study | Melanoma | Completed | |||
| NCT00082758 | Phase II: Efficacy study | Neuroblastoma | Completed | [91] | ||
| NCT01334515 | Phase II: Combination with GM-CSF and isotretinoin | Neuroblastoma | Completed | [92] | ||
| NCT00590824 | Phase II: Antitumor activity in minimal residual disease setting | Melanoma | Active, not recruiting | [90] | ||
| NCT03209869 | Phase I: Combination with expanded haploidentical NK cells | Neuroblastoma | Recruiting | |||
| NHS-IL2LT / Selectikine; EMD 521873; MSB0010445 | DNA/Histone complex
|
NCT00879866 | Phase I: Dose-escalation combined with local irradiation | Lung Cancer, NSCLC | Completed | [96] |
| NCT01032681 | Phase I: Safety and tolerability | Advanced Solid tumors or Solid B-cell non-Hodgkin lymphoma | Completed | [95] | ||
| NCT01973608 | Phase II: Dose-escalation combined with stereotactic body radiation therapy | Melanoma | Terminated | [98] | ||
| DI-Leu16-IL2 | CD20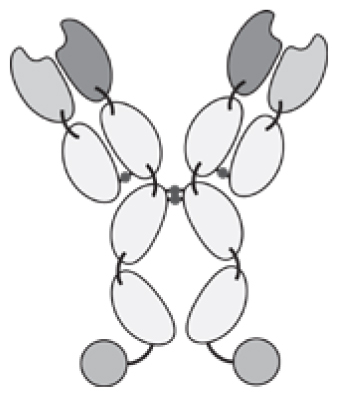
|
NCT00720135 | Phase I: Dose-escalation (i.v.) following Rituximab blood B cell depletion | B-cell non-Hodgkin lymphoma | Completed | |
| NCT01874288 | Phase I/II: Dose-escalation (s.c) following Rituximab blood B cell depletion | B-cell non-Hodgkin lymphoma | Completed | [106, 198, 199] | ||
| NCT02151903 | Phase I/II: Extension study | B-cell non-Hodgkin lymphoma | Completed | |||
| L19-IL2 / Darleukin | EDB
|
NCT01198522 | Phase I: Safety and dose-escalation in combination with Gemcitabine | Pancreatic cancer | Terminated | |
| NCT01058538 | Phase I/II: Dose-escalation and extension study on RCC | Advanced Solid Tumors | Completed | [116] | ||
| NCT02957019 | Phase I/II: Dose-finding combined with Rituximab and activity evaluation | Diffuse Large B-cell Lymphoma | Recruiting | |||
| NCT01055522 | Phase II: Dose finding (i.v.) in combination with Dacarbazine and efficacy study | Metastatic melanoma | Terminated | [115] | ||
| NCT02076646 | Phase I/II: Dose-finding (i.v.) in combination with Dacarbazine and efficacy study | Metastatic melanoma | Completed | |||
| NCT01253096 | Phase II: Safety and efficacy study (i.t.) | Metastatic melanoma | Completed | [118, 200, 201] | ||
| NCT02086721 | Phase I: Dose-escalation (i.v.) combined with Stereotactic Ablative Body Radiotherapy | Oligometastatic Solid Tumors | Completed | [120] | ||
| NCT03705403 | Phase II: Therapeutic efficacy combined with Stereotactic Ablative Body Radiotherapy | Metastatic Non-small cell lung cancer | Not yet recruiting | |||
| F16-IL2 / Teleukin | Tenascin C
|
NCT01131364 | Phase I/II: Dose-escalation combined with Doxorubicin and prospective therapy study | Solid tumors, Breast Cancer | Completed | [121] |
| NCT01134250 | Phase I/II: Dose-escalation combined with Paclitaxel and prospective therapy study | Solid Tumour, Breast Cancer, Metastatic melanoma, NSCLC | Completed | [202, 203] | ||
| NCT02054884 | Phase II: Therapeutic Efficacy combined with Paclitaxel | Merkel Cell Carcinoma | Terminated (lack of enrollment) | |||
| NCT02957032 | Phase I: Dose-escalation combined with Cytarabine | Acute Myeloid Leukemia | Recruiting | |||
| NCT03207191 | Phase I: Dose-escalation study in combination with an anti-CD33 antibody after allogeneic hematopoietic stem cell transplantation | Acute Myeloid Leukemia | Recruiting | [53, 122] | ||
| anti-CEA-IL2v / Cergutuzumab amunaleukin | CEA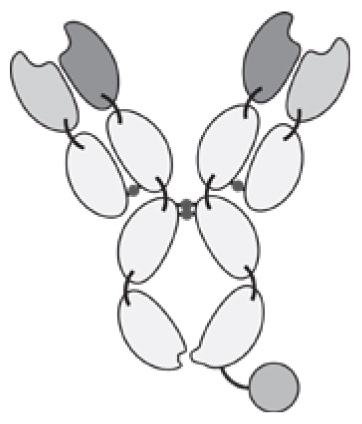
|
NCT02004106 | Phase I: Dose-escalation study | CEA-positive solid tumors | Completed | [99, 105, 204] |
| NCT02350673 | Phase I: Dose-escalation study and extension combined with Atezolizumab | CEA-positive solid tumors | Recruiting | |||
| anti-FAP-IL2v / RO6874281 | FAP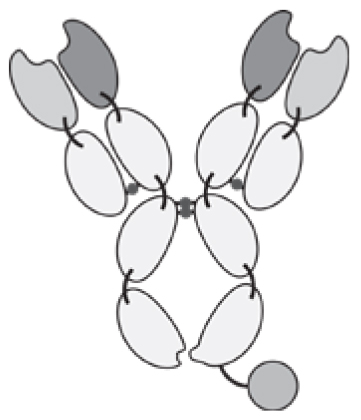
|
NCT03063762 | Phase I: Safety, Tolerability, PK, PD and therapeutic activity study combined with Atezolizumab with/without Bevacizumab | RCC | Recruiting | |
| NCT02627274 | Phase I: Dose-escalation as single agent or combined with Trastuzumab or Cetuximab | Solid Tumor, Breast Cancer, Head and Neck Cancer | Recruiting | [205] | ||
| NCT03386721 | Phase II: Therapeutic efficacy in combination with Atezolizumab | Solid and/or metastatic tumors | Recruiting | |||
| huKS-IL2 / EMD273066 | EpCAM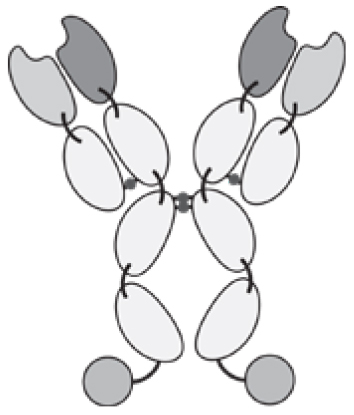
|
NCT00132522 | Phase I: Safety and Tolerability study in combination with Cyclophosphamide | Ovarian Cancer, Colorectal Cancer, NSCLC, Prostate Cancer | Completed | [206, 207] |
| Interleukin-12 | ||||||
| NHS-IL12 / M-9241 | DNA/Histone complex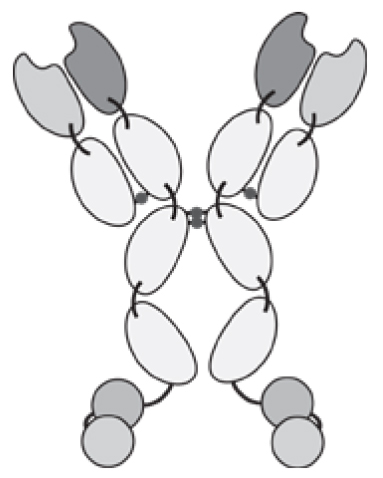
|
NCT01417546 | Phase I: Dose-escalation study | Solid tumors | Recruiting | [146] |
| NCT02994953 | Phase I: Dose-escalation and extension study combined with Avelumab | Solid tumors | Recruiting | [147] | ||
| BC1-IL12 / AS1409 | D7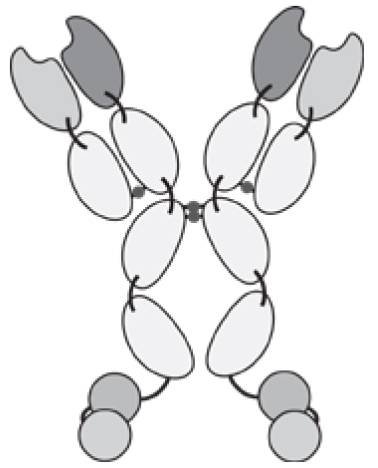
|
NCT00625768 | Phase I: Safety, tolerability and MTD determination | Metastatic RCC, Malignant Melanoma | Completed | [149, 208] |
| TNF superfamily members | ||||||
| L19-TNF / Fibromun | EDB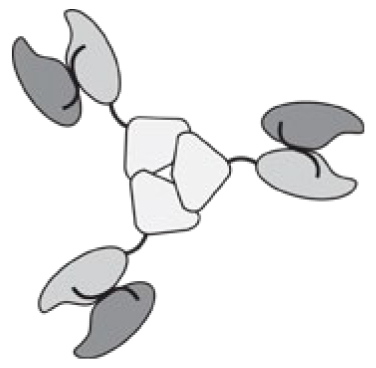
|
Phase I/II: safety and first clinical activity study | Metastatic solid cancer | Completed | [135] | |
| Phase I: Safety and Clinical Activity as isolated limb perfusion combined with melphalan | Metastatic melanoma | Completed | [136] | |||
| NCT03420014 | Phase II: Therapeutic efficacy combined with Doxorubicin | Soft Tissue Sarcoma | Recruiting | |||
| EudraCR number: 2016-003239-38 | Phase III: Efficacy of the combination with Doxorubicin against Doxorubicin monotherapy | Advanced or metastatic soft tissue sarcoma | Recruiting | |||
| NCT03779230 | Phase I/II: Dose-finding, safety and therapeutic activity study. | Glioma of the Brain | Recruiting | |||
| FAP-4-1BBL / RG7827 | FAP
|
Phase I: Single agent dose-escalation and combination with Atezolizumab | Solid Tumors | [155, 156, 209] | ||
| Combinations | ||||||
| L19-IL2 + L19-TNF / Daromun | EDB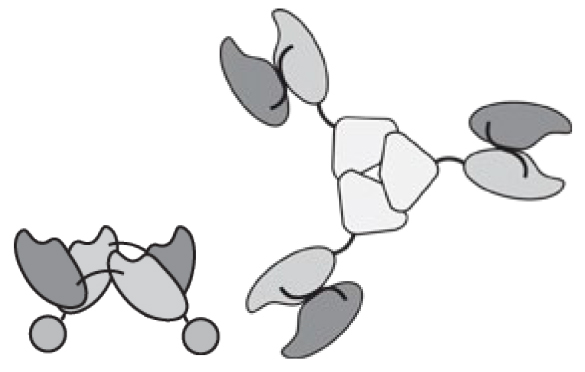
|
NCT02076633 | Phase II: Safety and efficacy of intratumoral administration | Malignant Melanoma | Completed | [117, 210] |
| NCT03567889 | Phase III: Efficacy as neoadjuvant intratumoral treatment followed by surgery | Melanoma Stage IIIB/C | Recruiting | |||
| NCT02938299 | Phase III: Pivotal study as neoadjuvant intratumoral treatment | Malignant Melanoma | Recruiting | |||
IL2-based immunocytokines
Interleukin-2 (IL2) is one of the best-characterized cytokines for tumor therapy applications, since the corresponding recombinant human protein is a product on the market (e.g., Proleukin™). IL2 is constitutively released by several immune cells, upon immune stimulation [1]. It leads to activation, differentiation and maintenance of T cells, that initially express a low-affinity dimeric receptor consisting of β- and γ-chain and (produced after T cell activation) the trimeric high affinity IL2 receptor (IL2R) containing also the α-chain at a later time point [79, 80]. As first immunotherapeutic agent in 1985, high dose IL2 monotherapy (600,000–720,000 IU/kg) led to tumor regression of patients with metastatic renal carcinoma and melanoma and was eventually approved for treatment of these malignancies in 1992 and 1998 respectively [81–83]. Nonetheless, the anticancer activity of untargeted IL2 is insufficient considering the severe adverse effects at these doses (e.g., capillary leak syndrome, hypotension, severe flu-like symptoms) [5, 83].
The first immunocytokines based on IL2 were developed more than two decades ago, featuring either full immunoglobulins or antibodies in scFv format [48, 84, 85]. A potent anti-cancer activity has been reported for animal models of murine colon carcinoma, human metastatic melanoma, prostate carcinoma and neuroblastoma, using antibodies specific to an epithelial cell adhesion protein (EpCAM G733-2) and the ganglioside GD2 [8, 86–88].
The groups of Reisfeld and Gillies investigated on the ch14.18-IL2 fusion protein, an anti-ganglioside GD2 IgG molecule with IL2 coupled to the C-terminus of the immunoglobulin heavy chain [48]. Strong anti-cancer activity was reported in several murine tumor models [8, 86, 88]. The humanized product Hu14.18-IL2 has been tested in Phase II clinical trials in melanoma and neuroblastoma [89–92]. Recently, Gillies has reported that positioning IL2 at the C-terminus of the light chain may lead to allosteric regulation of cytokine activity upon binding [93]. Additionally, the same research group reduced the toxicity of IL2 by introducing a single amino acid substitution (D20T). This IL2 mutant (IL2LT, standing for “low toxicity IL2”) was fused to the NHS antibody, which recognizes DNA-associated structures in the necrotic core of solid tumors. NHS-IL2LT dramatically decreased the metastasis load in mice injected intravenously with Lewis Lung Carcinoma (LLC) cells [94]. NHS-IL2LT (Selectikine) has been investigated as a single agent in a Phase I [95, 96] trial in patients with advanced solid tumors. Subsequent Phase II clinical studies in advanced melanoma patients have been discontinued [97, 98].
Scientists at Roche have recently described FAP- and CEA-targeted immunocytokines containing an IL2 variant, which does not bind the IL2R in its trimeric form. Three amino acid mutations were used to abolish CD25 binding in the interleukin-2 moiety [99, 100], in order to decrease an undesired activation of regulatory T cells (Tregs) and of the vascular endothelium [99–103]. The IL2 variant was fused at the C-terminus on the heavy chain of asymmetric IgG molecules, created using knob-in-the-hole technology [104]. In a Phase I clinical trial, CEA-IL2v showed initial evidence of tumor accumulation [100, 105]. The product has recently been tested in combination with atezolizumab in a Phase Ib clinical study (NCT02350673). Similarly, FAP-IL2v is being studied in early-stage clinical trials in combination with atezolizumab (anti PD-L1), trastuzumab (anti-Her2/Neu) or cetuximab (anti-EGFR).
The anti-CD20 immunocytokine Leu16-IL2, developed by Provenance Biopharmaceuticals, induced significantly greater inhibition of tumor growth in lymphoma xenografts, compared to the mixture of the isolated components (i.e., the intact anti-CD20 antibody moiety in IgG format and untargeted IL2) [12]. Even a deglycosylated form of Leu16-IL2 (DI-Leu16-IL2) improved survival of mice bearing Daudi metastases, with little difference compared to the glycosylated antibody, suggesting that ADCC may not play a relevant role in this model. The authors attributed most of the anti-tumoral activity to the targeted delivery of IL2, since a non-tumor specific immunocytokine displayed a substantially lower therapeutic effect [12]. In a Phase I/II clinical study in B cell lymphoma patients, partial responses and complete responses were observed in 5/13 and 3/13 patients, respectively [106].
Our group has mainly focused on the use of antibodies directed against extracellular matrix components (e.g., F8, L19 and F16, binding to alternatively spliced domains of fibronectin and tenascin C respectively) for the targeted delivery of cytokines. The three antibodies have been coupled to IL2 in the non-covalent homodimeric diabody format [11, 107, 108], leading to products with potent anticancer activity [11, 53, 107–114]. In preclinical studies L19-IL2 had shown a significantly stronger tumor growth inhibition, compared to untargeted IL2 [11, 111, 112]. Moreover, treatment with L19-IL2 increased the immune cell infiltrate (NK and T cells) in the neoplastic mass of mice [11, 111]. L19-IL2 (Darleukin) was tested in Phase I clinical studies as monotherapeutic against metastatic renal cell carcinoma (mRCC) and in combination with dacarbazine for the treatment of metastatic melanoma, showing encouraging anti-tumor activity [115, 116]. In an additional Phase II clinical study, the IL2 fusion protein was evaluated alone or in combination with another immunocytokine L19-TNF (Fibromun), with intratumoral injections in melanoma lesions [117, 118]. Based on the promising results, the IL2 conjugate has been brought to Phase III in combination with Fibromun. Additionally, a Phase I/II clinical trial assessing L19-IL2 in combination with Rituximab (anti-CD20) is currently on going in patients with B-cell lymphoma. A strong synergistic effect had been observed in B-cell lymphoma xenografts, driven by the increased number of immune effector cells in the tumor tissue capable of inducing antibody-dependent cell-mediated cytotoxicity (ADCC) [111].
In preclinical studies L19-IL2 has been combined with a small-molecule-drug conjugate (SMDC), selectively homing to tumor cells expressing carbonic anhydrase IX on their surface, delivering an anti-tubulin agent to the neoplastic mass. Combination of the two agents led to cancer cures in two mouse models of cancer, which do not respond to conventional chemotherapy [119]. Besides, when L19-IL2 was systemically administered after radiotherapy, a significant synergistic anti-tumor effect and a long-lasting immune protection were observed in mice bearing CT26 and C51 tumors [120]. Together, these results indicate that IL2-based immunocytokines may enhance the infiltration of primed immune effector cells in the tumor, after induction of immunogenic cell death.
F16-IL2 (Teleukin), a fusion protein with the same format used for L19-IL2, showed some promising anti-cancer activity in patients with different solid tumors and metastatic breast cancer in combination with Doxorubicin [121], as well as in relapsed acute myeloid leukemia (AML) after allogeneic hematopoietic stem cell transplantation [122]. Phase I clinical studies are now investigating Teleukin in combination with cytarabine or an anti-CD33 antibody.
TNF-based immunocytokines
TNF-α (TNF) is a homotrimeric pro-inlammatory cytokine mainly secreted by NK cells, macrophages and T cells. When released locally, it acts on endothelial cells triggering the containment of infections, by increasing vasculature permeability, blood clotting, attracting and activating adjacent immune cells [1]. In cancer therapy TNF is able to induce direct apoptosis of certain tumor cells, elicit hemorrhagic necrosis and increase the influx of immune effector cells [123–125]. Systemic administration of recombinant TNF (Beromun™) is approved for tumor size reduction before surgery or for locoregional treatments (i.e., isolated limb perfusion) of patients with non-resectable soft-tissue sarcoma, in combination with melphalan. The clinical application of recombinant TNF is limited, due to the substantial systemic adverse effects (e.g., shock, disseminated intravascular coagulation, organ failure) [126]. TNF has been coupled to various moieties for pharmacodelivery, in order to overcome limiting toxicities [127–130]. TNF-antibody fusion proteins have shown a potent anti-cancer activity in mouse models based on grafted human tumors or on syngeneic tumors [26, 71, 128, 131, 132]. However, cancer cures were only observed in immunocompetent mice bearing TNF-sensitive tumors, indicating that tumor infiltrating leukocytes may play a major role in eradication of TNF treated tumors [10].
L19-TNF is a clinical-stage product composed by the L19 antibody in scFv format, targeting the alternative spliced domain EDB in tumor neovasculature, coupled to TNF, which drives the formation of a homotrimeric structure (Figure 2). L19-TNF preferentially localized to tumor masses in mouse models of cancer, as shown in biodistribution experiments with radiolabeled protein preparations [26]. The immunocytokine showed an increased inhibition of tumor growth compared to untargeted TNF treatment in mouse models of fibrosarcoma and colorectal cancer [10]. Interestingly, most of the subcutaneous tumor mass became necrotic after a single intravenous administration of L19-TNF [26]. A synergistic effect was observed when L19-TNF was combined to mephalan [26]. Combination studies with L19-IL2 and L19-IL12 also showed strong additive antitumoral effects on models of murine teratocarcinoma and neuroblastoma [133, 134]. Phase III clinical studies are currently testing the combination of the fully-human L19-TNF product (Fibromun), administered intravenously, with doxorubicin in soft-tissue sarcoma patients
When used as monotherapeutic agent in a Phase I/II clinical study, L19-TNF did not induce any objective responses [135]. By contrast, combination with melphalan in patients with advanced extremity melanoma induced 89% objective responses with the agents given through isolated limb perfusion [136]. Immunohistochemical analyses of tumor sections showed accumulation of the L19-TNF at sites of tumor neo-angiogenesis [137]. A potent anticancer activity of L19-TNF was also observed upon intralesional administration in combination with L19-IL2 in patients with inoperable metastatic melanoma [117]. In a Phase II study, 5% complete responses (CR) and 50% partial responses were recorded, with tumor regression visible also in non-injected lesion, suggesting the activation of a protective immunity. The combination is currently tested in a Phase III clinical trial (NCT02938299, EudraCT number 2015-002549-72).
Other antibodies can also be considered for TNF delivery applications. The F8 antibody, specific to EDA fibronectin, was fused to murine TNF in scFv format and used as single agent or in combination with doxorubicin for the treatment of syngeneic murine tumor models of sarcoma. The combination regimen of doxorubicin and F8-TNF led to complete eradication of the tumor and long-lasting immunoprotection [55, 71]. Recent mechanistic studies revealed that the treatment with F8-TNF + doxorubicin increases the number of specific cytotoxic T cells, which recognize a common endogenous retroviral antigen AH1 (derived from an envelope protein of the murine leukemia virus) [55]. Generally, such retroviral sequences have been found also in the genome of vertebrate species and their expression has been associated with autoimmune diseases, chronic infections or cancer [138, 139]. In theory, a TNF-based immunocytokine could cause upregulation of a subset of cytotoxic T cells specific for a retroviral antigen found in human tumors, which would contribute to the antitumoral effect in patients.
Preclinical studies on a murine melanoma model revealed that the therapeutic activity of an anti-melanoma immunoglobulin IgG2a(TA99) was significantly potentiated when combined with the TNF antibody fusion protein TA99-mTNF. The increased density of leukocytes in the subcutaneous melanoma masses of mice treated with the combination treatment favored tumor cell killing through ADCC [140].
IL-12 based immunocytokines
Interleukin-12 (IL12) is a heterodimeric cytokine, composed by the p40 and p35 moieties linked by a disulfide bond, which provides a crucial connection between the innate and the adaptive immune system [141]. IL12 is mainly released by antigen presenting cells (APCs) as a response to infection, inducing the release of IFNγ and the promotion of TH1 differentiation of CD4+ T cells. IL12 additionally stimulates the proliferation of NK cells, T cells, the MHC1 antigen presentation process, and inhibits Treg activity [142].
The anti-tumor activity of recombinant IL12 has been assessed in mouse models of cancer [143, 144], but showed limited clinical efficacy and high toxicities at doses as low as low as 1 µg/Kg body weight in cancer patients [145]. Two immunocytokines based on IL12 are currently in clinical development, NHS-IL12 and BC1-IL12. NHS-IL12 specifically targets DNA-Histone H1 complex, released upon tumor cell apoptosis [44]. The product, developed by Merck is currently being tested in Phase I trials as monotherapy or in combination with avelumab (anti-PD-L1 antibody) [146, 147].
BC1-IL12 recognizes an epitope on domain 7 of fibronectin, which is cryptic (i.e., not exposed) in normal tissues, but becomes unmasked in the presence of the EDB domain [54]. The fusion protein consists of the full IgG1 linked to the IL12 heterodimer at the C-terminus of the heavy chain. Efficient in vivo localization [148] and anti-tumor activity have been confirmed in various mouse models of cancer [54]. When tested on patients with malignant melanoma in a Phase I clinical study, BC1-IL12 led to disease stabilization in 6 out of 11 patients for at least 4 months [149]. The therapeutic immunocytokine was well tolerated and increased IFNγ levels in the serum of all patients.
The heterodimeric nature of the IL12 and of the resulting fusion proteins allows the implementation of various types of immunocytokine formats (Figure 2) [7, 17, 29, 32], which however may represent a challenge for successful protein production. In the formats studied so far, the p35 and p40 subunits were typically connected by a peptide linker. Halin et al. linked the p40 domain of IL-12 to the N terminus of its p35 domain by a 15-amino acid linker and fused it by a 6-amino acid linker to the N terminus of the L19 antibody in the scFv format [7]. The fusion protein was compared to untargeted IL12 in therapy studies on a mouse model of lung metastasis and two subcutaneous tumor models. IL12-L19 displayed much stronger inhibition of tumor progression compared to the untargeted control and increased leukocyte infiltrate into the tumor masses. The therapeutic effect of IL12-L19 could be improved by combination with L19-TNF [134]. Other formats were investigated with the aim to increase tumor:organ ratios. IL12-SIP(L19) featuring a C-terminal CH4 domain of human IgE in order to favor the homodimerization of the fusion protein, accumulated mainly in the liver and exhibited modest tumor uptake compared to a heterotrimeric fusion protein having p40 linked to scFv(L19) forming a non covalent dimer with scFv(L19)-p35. This fusion protein showed an efficient tumor uptake in F9 teratocarcinoma mouse tumor models, with tumor:organ ratios of 10:1–20:1, 24 hours after intravenous administration [29].
The F8 antibody, specific to EDA fibronectin, was fused to murine IL12 in different formats [17, 32] and tested both in vitro and in vivo. The most promising version (named IL12-F8-F8) featured the IL12 moiety fused to the N-terminus of a “tandem” diabody fusion of two F8 moieties. IL12-F8-F8 efficiently inhibited tumor growth in three different immunocompetent syngeneic models of cancer. The effect was strongly potentiated by combination with paclitaxel [17].
IL12 has also been conjugated to a CD30 targeting antibody in the HSR(scFV)-IL12 construct. Binding and killing of CD30 positive cells, as well as increased IFNγ release by T cells were confirmed in vitro [150].
Other cytokine payloads
Other immunocytokines have been tested mainly in preclinical studies, but did not exhibit successful accumulation in the tumor tissue, had insufficient therapeutic effect and/or induced high transient body weight loss in mice. (e.g., F8-IL1β [72], F8-IL6 [72], F8-IL7-F8 [151], F8-IL17 [152]). Our group systematically explored the targeted delivery of cytokines from the TNF superfamily (e.g., CD40L, FasL, TRAIL, VEGI, LiGHT) in biodistribution studies. A lower accumulation (compared to TNF fusions) was observed in the tumor mass of various murine cancer models [153]. Recent work of the group of Roland Kontermann showed that better tumor homing properties might be achieved if the TNF superfamily cytokines are expressed as single chain polypeptides [30, 154] (Figure 2). Roche developed an anti-FAP-mu4-1BBL conjugate based on the murine single chain 4-1BBL able to potentiate the anti-tumor activity of anti-PD-L1 and Trastuzumab (anti-HER2) in different mouse models of cancer [155]. The humanized anti-FAP-4-1BBL is currently tested in Phase I clinical trials [156].
IL15 is a pro-inflammatory cytokine that features certain functional redundancies with IL2, as both cytokines signal through the beta and gamma subunits of the same receptor. A fusion protein consisting of a FAP binding moiety, IL15 and one domain of the IL15 receptor (IL15Rα), was generated by the group of Dafne Müller. The antibody-IL15/IL15Rα fusion protein was able to specifically activate intermediate affinity receptor expressing NK and T cells. Stronger tumor growth inhibition was observed in murine B16 metastasis model, compared to the untargeted and to the IL15Rα missing forms [157].
Granulocyte/macrophage-colony stimulating factor (GM-CSF) was coupled to antibodies in IgG, diabody or scFv format, targeting various tumor-associated antigens (e.g., HER2/Neu, CEA, EDB) [50, 74, 158]. Targeted delivery of GM-CSF to the tumors showed enhanced antitumor activity [50, 74], as well as increased antigen processing and presentation [158, 159].
Finally, it is conceivable to fuse two cytokines the same antibody, in order to exploit a synergistic benefit by the simultaneous presence of the two payloads at the site of disease. Dual-cytokine antibody fusions have been described for IL2 and IL12 [160–162], IL12 and GM-CSF [161], TNF and IL2 [57], as well as IL15 and 4-1BBL [40]. The group of Roland Kontermann has introduced the concept of Duokines, dual-acting cytokine fusion proteins generated by connecting two TNF superfamily members (combinations 4-1BBL, OX40L and CD27L) by a flexible peptide linker in different conformations [163].
Immunocytokines for the therapy of chronic inflammatory conditions
The in vivo activity of cytokines and of their derivatives depends on the immunological environment and on the concentration of the cytokine at the site of disease. Certain payloads may display a pro- or anti-inflammatory action depending on the immunological context [164–166]. For example, IL12 caused disease worsening and increased TNF production in the collagen-induced model of arthritis, when the product was given at low doses (5ng/day). By contrast, higher doses of (500ng/day) suppressed disease development inducing production of the anti-inflammatory cytokine IL10 and corticosterone [167]. More examples, reviewed by Cavaillon JM (e.g., IL6, IL4, IL10, TGFβ), indicate the dependence on the responding cell, the timing and even the experimental model [165].
Even though research has mainly focused on immunocytokines directed against oncological targets, potential applications in chronic inflammatory diseases have recently gained momentum. One product based on IL10 (F8-IL10) is currently being investigated in Phase II clinical trials in patients with rheumatoid arthritis or ulterative colitis, while other products have shown promising signs of efficacy in animal models [14, 168].
IL10 is a homodimeric, pleiotropic cytokine, with a potential to display an immunosuppressive activity at sites of inflammation, for example by reducing antigen presentation and the release of pro-inflammatory mediators [169]. Recombinant IL10 has been tested in various mouse models of inflammation showing encouraging results [60, 61]. Initial clinical studies have shown no significant differences in remission rates or disease improvements were reported [170, 171]. However, a pegylated version of murine IL10 induced rejection of solid and metastasizing tumors in mice by induction of cytotoxic T cells [172, 173]. In clinical trials the recombinant pegylated interleukin-10 (PEG-rIL-10) increased the density of activated intratumoral cytotoxic T cells in patients [174]. PEG-rIL-10 is currently being investigated in combination with immunecheckpoint inhibitors [anti-PD-1 antibodies Nivolumab (NCT03382912) and Permbrolizumab (NCT03382899)] or with chemotherapy (NCT02923921).
The antibody-cytokine fusion L19-IL10 selectively localized to sites of inflammation in mouse models [13]. L19-IL10 features the L19 antibody (specific to EDB fibronectin) in diabody format, fused to human IL10. In the mouse model of collagen induced arthritis (CIA), L19-IL10 treatment induced a significant inhibition of disease progression, compared to control mice treated with saline or with an IL10 fusion based on an antibody of irrelevant specificity [13]. Similarly, fusion of IL10 with the F8 antibody (specific to EDA fibronectin) [175] showed disease targeting and an encouraging activity in the murine CIA model [175, 176], both, alone or in combination with methotrexate or a TNF inhibitor. The product was also active in a mouse model of endometriosis [63]. The fully human F8-IL10 fusion protein (Dekavil) was tested in a Phase Ib clinical trial against rheumatoid arthritis in combination with methotrexate (NCT02076659). 15 out of 23 treated patients experienced therapeutic benefit, including 2 long-lasting remissions [177] and is now being investigated in multiple Phase II clinical trials (NCT02270632, EudraCT number 2013-005418-37). Additionally, F8-IL10 is tested in a Phase II clinical trial as add-on therapy to infliximab in patients with ulcerative colitis (EudraCT number 2017-002108-28).
In another approach, viral IL10, which is considered less immunostimulatory, was fused to an antibody fragment targeting ROS-CII (1-11E). Treatment of arthritic mice with 1-11E/IL10 inhibited disease progression and lowered pro-inflammatory cytokines serum levels [178, 179].
Immunomodulatory products can also be produced by antibody fusion with IL4, a so-called “prototypic immunoregulatory cytokine” [180], able to interact with various target cells. IL4 production is limited to a small subset of hematopoietic cells and the effects are mediated by a high affinity heterodimeric IL4 receptor. Recombinant IL4 reduced disease progression in preclinical models of rheumatoid arthritis [181], but failed to reproduce these results in human clinical studies[182]. By contrast, recombinant IL4 switched the immune environment to a TH2 pattern and markedly improved conditions in patients with moderate-to-severe TH1 mediated psoriasis [182]. The antibody-based delivery of IL4 at the site of chronic inflammation is thought to generate a more specific and focused immunomodulatory action. The F8-IL4 fusion protein (Tetravil), based on the EDA targeting antibody in diabody format, selectively accumulated in the proximity of newly formed blood vessels in arthritic paws and toes of mice with CIA. Therapy studies in CIA mice induced 100% complete remission of the disease when F8-IL4 was given together with dexamethasone [14]. The combination therapy rapidly resolved the inflammatory process in the paws, by recruiting TH2 cells and Tregs and by normalizing the concentration of pro-inflammatory cytokines [14, 168, 183]. In addition, treatment with F8-IL4 significantly inhibited endometriotic lesion growth in a mouse model, compared to untargeted IL4, which did not exhibit in vivo activity [62]. The therapeutic effect of F8-IL4 was also observed in imiquimod-induced and contact-hypersensitivity-induced mouse models of skin inflammation [184]. Motivated by these encouraging data, F8-IL4 is currently being considered for clinical testing for the treatment of rheumatoid arthritis and endometriosis [185].
Conclusions
A large variety of immunocytokines has been generated for multiple therapeutic applications in the past two decades. Even though only a few of those proceeded to clinical stage so far, the potential of antibody-cytokine fusion proteins is enormous. Immunocytokines may synergize with several combination partners, such as cytotoxic drugs [26, 44, 53, 71, 107–109, 186], radiation [44, 96, 120, 187], monoclonal antibodies [44, 99, 111], SMDCs [119], antibody drug conjugates [188, 189], cancer vaccines [159, 161], immunecheckpoint inhibitors [39, 56, 57, 134, 190, 191], bispecific antibodies [192–194], and other immunocytokines[17, 57, 114, 134, 160–162, 195, 196]. Over the next few years, the outcome of on-going clinical trials will shed light on the therapeutic benefit that can be achieved, while new protein engineering approaches will contribute to the development of second-generation products.
Acknowledgements
The authors would like to thank Jonathan Kiefer and Dr. Samuele Cazzamalli for proofreading the manuscript. Financial contributions from ETH Zürich, from the ERC Advanced Grant “Zauberkugel” (Grant Agreement 670603) and from the Swiss National Science Foundation (Projects Nr. 310030B_163479/1, SINERGIA CRSII2_160699/1) are gratefully acknowledged.
References
- 1.Murphy KP, et al., editors. Janeway Immunology. 8th Edition. New York: Garland Science; 2018. 1093 S. [Google Scholar]
- 2.Geertsen PF, et al. Safety and efficacy of subcutaneous and continuous intravenous infusion rIL-2 in patients with metastatic renal cell carcinoma. Br J Cancer. 2004;90(6):1156–62. doi: 10.1038/sj.bjc.6601709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lejeune F, et al. Rationale for using TNF alpha and chemotherapy in regional therapy of melanoma. J Cell Biochem. 1994;56(1):52–61. doi: 10.1002/jcb.240560110. [DOI] [PubMed] [Google Scholar]
- 4.Leonard JP, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997;90(7):2541–8. [PubMed] [Google Scholar]
- 5.Baldo BA. Side effects of cytokines approved for therapy. Drug Saf. 2014;37(11):921–43. doi: 10.1007/s40264-014-0226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lode HN, et al. Immunocytokines: a promising approach to cancer immunotherapy. Pharmacol Ther. 1998;80(3):277–92. doi: 10.1016/s0163-7258(98)00033-3. [DOI] [PubMed] [Google Scholar]
- 7.Halin C, et al. Enhancement of the antitumor activity of interleukin-12 by targeted delivery to neovasculature. Nat Biotechnol. 2002;20(3):264–9. doi: 10.1038/nbt0302-264. [DOI] [PubMed] [Google Scholar]
- 8.Pancook JD, et al. Eradication of established hepatic human neuroblastoma metastases in mice with severe combined immunodeficiency by antibody-targeted interleukin-2. Cancer Immunol Immunother. 1996;42(2):88–92. doi: 10.1007/s002620050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lode HN, et al. Targeted interleukin-2 therapy for spontaneous neuroblastoma metastases to bone marrow. J Natl Cancer Inst. 1997;89(21):1586–94. doi: 10.1093/jnci/89.21.1586. [DOI] [PubMed] [Google Scholar]
- 10.Balza E, et al. Targeted delivery of tumor necrosis factor-alpha to tumor vessels induces a therapeutic T cell-mediated immune response that protects the host against syngeneic tumors of different histologic origin. Clin Cancer Res. 2006;12(8):2575–82. doi: 10.1158/1078-0432.CCR-05-2448. [DOI] [PubMed] [Google Scholar]
- 11.Carnemolla B, et al. Enhancement of the antitumor properties of interleukin-2 by its targeted delivery to the tumor blood vessel extracellular matrix. Blood. 2002;99(5):1659–65. doi: 10.1182/blood.v99.5.1659. [DOI] [PubMed] [Google Scholar]
- 12.Gillies SD, et al. An anti-CD20-IL-2 immunocytokine is highly efficacious in a SCID mouse model of established human B lymphoma. Blood. 2005;105(10):3972–8. doi: 10.1182/blood-2004-09-3533. [DOI] [PubMed] [Google Scholar]
- 13.Trachsel E, et al. Antibody-mediated delivery of IL-10 inhibits the progression of established collagen-induced arthritis. Arthritis Res Ther. 2007;9(1):R9. doi: 10.1186/ar2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemmerle T, Doll F, Neri D. Antibody-based delivery of IL4 to the neovasculature cures mice with arthritis. Proc Natl Acad Sci U S A. 2014;111(33):12008–12. doi: 10.1073/pnas.1402783111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokota T, et al. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992;52(12):3402–8. [PubMed] [Google Scholar]
- 16.Borsi L, et al. Selective targeting of tumoral vasculature: comparison of different formats of an antibody (L19) to the ED-B domain of fibronectin. Int J Cancer. 2002;102(1):75–85. doi: 10.1002/ijc.10662. [DOI] [PubMed] [Google Scholar]
- 17.Pasche N, et al. The antibody-based delivery of interleukin-12 to the tumor neovasculature eradicates murine models of cancer in combination with paclitaxel. Clin Cancer Res. 2012;18(15):4092–103. doi: 10.1158/1078-0432.CCR-12-0282. [DOI] [PubMed] [Google Scholar]
- 18.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–25. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 19.Strohl WR. Fusion Proteins for Half-Life Extension of Biologics as a Strategy to Make Biobetters. BioDrugs. 2015;29(4):215–39. doi: 10.1007/s40259-015-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillies SD, et al. Improving the efficacy of antibody-interleukin 2 fusion proteins by reducing their interaction with Fc receptors. Cancer Res. 1999;59(9):2159–66. [PubMed] [Google Scholar]
- 21.Shinkawa T, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278(5):3466–73. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 22.Graff CP, Wittrup KD. Theoretical analysis of antibody targeting of tumor spheroids: importance of dosage for penetration, and affinity for retention. Cancer Res. 2003;63(6):1288–96. [PubMed] [Google Scholar]
- 23.Holliger P, Prospero T, Winter G. "Diabodies": small bivalent and bispecific antibody fragments. Proc Natl Acad Sci U S A. 1993;90(14):6444–8. doi: 10.1073/pnas.90.14.6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams GP, et al. Avidity-mediated enhancement of in vivo tumor targeting by single-chain Fv dimers. Clin Cancer Res. 2006;12(5):1599–605. doi: 10.1158/1078-0432.CCR-05-2217. [DOI] [PubMed] [Google Scholar]
- 25.Wu AM, et al. Tumor localization of anti-CEA single-chain Fvs: improved targeting by non-covalent dimers. Immunotechnology. 1996;2(1):21–36. doi: 10.1016/1380-2933(95)00027-5. [DOI] [PubMed] [Google Scholar]
- 26.Borsi L, et al. Selective targeted delivery of TNFalpha to tumor blood vessels. Blood. 2003;102(13):4384–92. doi: 10.1182/blood-2003-04-1039. [DOI] [PubMed] [Google Scholar]
- 27.Ongaro T, et al. A novel anti-cancer L19-interleukin-12 fusion protein with an optimized peptide linker efficiently localizes in vivo at the site of tumors. J Biotechnol. 2018;291:17–25. doi: 10.1016/j.jbiotec.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Fellermeier S, et al. Advancing targeted co-stimulation with antibody-fusion proteins by introducing TNF superfamily members in a single-chain format. Oncoimmunology. 2016;5(11):e1238540. doi: 10.1080/2162402X.2016.1238540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gafner V, Trachsel E, Neri D. An engineered antibody-interleukin-12 fusion protein with enhanced tumor vascular targeting properties. Int J Cancer. 2006;119(9):2205–12. doi: 10.1002/ijc.22101. [DOI] [PubMed] [Google Scholar]
- 30.Krippner-Heidenreich A, et al. Single-chain TNF a TNF derivative with enhanced stability and antitumoral activity. J Immunol. 2008;180(12):8176–83. doi: 10.4049/jimmunol.180.12.8176. [DOI] [PubMed] [Google Scholar]
- 31.Schneider B, et al. Potent antitumoral activity of TRAIL through generation of tumor-targeted single-chain fusion proteins. Cell Death Dis. 2010;1:e68. doi: 10.1038/cddis.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sommavilla R, et al. Expression, engineering and characterization of the tumor-targeting heterodimeric immunocytokine F8-IL12. Protein Eng Des Sel. 2010;23(8):653–61. doi: 10.1093/protein/gzq038. [DOI] [PubMed] [Google Scholar]
- 33.Neri D, Bicknell R. Tumour vascular targeting. Nat Rev Cancer. 2005;5(6):436–46. doi: 10.1038/nrc1627. [DOI] [PubMed] [Google Scholar]
- 34.Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10(10):767–77. doi: 10.1038/nrd3554. [DOI] [PubMed] [Google Scholar]
- 35.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Rybak JN, et al. The extra-domain A of fibronectin is a vascular marker of solid tumors and metastases. Cancer Res. 2007;67(22):10948–57. doi: 10.1158/0008-5472.CAN-07-1436. [DOI] [PubMed] [Google Scholar]
- 37.Zardi L, et al. Transformed human cells produce a new fibronectin isoform by preferential alternative splicing of a previously unobserved exon. EMBO J. 1987;6(8):2337–42. doi: 10.1002/j.1460-2075.1987.tb02509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borsi L, et al. Extracellular pH controls pre-mRNA alternative splicing of tenascin-C in normal, but not in malignantly transformed, cells. Int J Cancer. 1996;66(5):632–5. doi: 10.1002/(SICI)1097-0215(19960529)66:5<632::AID-IJC9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 39.Nicolini V, et al. Combining CEA-IL2v and FAP-IL2v immunocytokines with PD-L1 checkpoint blockade. Annual Meeting of the American Association for Cancer Research; New Orleans: 2016. [Google Scholar]
- 40.Muller D, Frey K, Kontermann RE. A novel antibody-4-1BBL fusion protein for targeted costimulation in cancer immunotherapy. J Immunother. 2008;31(8):714–22. doi: 10.1097/CJI.0b013e31818353e9. [DOI] [PubMed] [Google Scholar]
- 41.Mach JP, et al. Use of radiolabelled monoclonal anti-CEA antibodies for the detection of human carcinomas by external photoscanning and tomoscintigraphy. Immunol Today. 1981;2(12):239–49. doi: 10.1016/0167-5699(81)90011-6. [DOI] [PubMed] [Google Scholar]
- 42.Violet JA, et al. Fractionated 131I anti-CEA radioimmunotherapy: effects on xenograft tumour growth and haematological toxicity in mice. Br J Cancer. 2008;99(4):632–8. doi: 10.1038/sj.bjc.6604511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Brummelen EMJ, et al. (89)Zr-labeled CEA-targeted IL-2 variant immunocytokine in patients with solid tumors: CEA-mediated tumor accumulation and role of IL-2 receptor-binding. Oncotarget. 2018;9(37):24737–24749. doi: 10.18632/oncotarget.25343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fallon J, et al. The immunocytokine NHS-IL12 as a potential cancer therapeutic. Oncotarget. 2014;5(7):1869–84. doi: 10.18632/oncotarget.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villa A, et al. A high-affinity human monoclonal antibody specific to the alternatively spliced EDA domain of fibronectin efficiently targets tumor neo-vasculature in vivo. Int J Cancer. 2008;122(11):2405–13. doi: 10.1002/ijc.23408. [DOI] [PubMed] [Google Scholar]
- 46.Pini A, et al. Design and use of a phage display library. Human antibodies with subnanomolar affinity against a marker of angiogenesis eluted from a two-dimensional gel. J Biol Chem. 1998;273(34):21769–76. doi: 10.1074/jbc.273.34.21769. [DOI] [PubMed] [Google Scholar]
- 47.Brack SS, et al. Tumor-targeting properties of novel antibodies specific to the large isoform of tenascin-C. Clin Cancer Res. 2006;12(10):3200–8. doi: 10.1158/1078-0432.CCR-05-2804. [DOI] [PubMed] [Google Scholar]
- 48.Gillies SD, et al. Antibody-targeted interleukin 2 stimulates T-cell killing of autologous tumor cells. Proc Natl Acad Sci U S A. 1992;89(4):1428–32. doi: 10.1073/pnas.89.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hornick JL, et al. Chimeric CLL-1 antibody fusion proteins containing granulocyte-macrophage colony-stimulating factor or interleukin-2 with specificity for B-cell malignancies exhibit enhanced effector functions while retaining tumor targeting properties. Blood. 1997;89(12):4437–47. [PubMed] [Google Scholar]
- 50.Dela Cruz JS, et al. Recombinant anti-human HER2/neu IgG3-(GM-CSF) fusion protein retains antigen specificity and cytokine function and demonstrates antitumor activity. J Immunol. 2000;165(9):5112–21. doi: 10.4049/jimmunol.165.9.5112. [DOI] [PubMed] [Google Scholar]
- 51.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hristodorov D, et al. Macrophage-targeted therapy: CD64-based immunotoxins for treatment of chronic inflammatory diseases. Toxins (Basel) 2012;4(9):676–94. doi: 10.3390/toxins4090676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gutbrodt KL, et al. Antibody-based delivery of interleukin-2 to neovasculature has potent activity against acute myeloid leukemia. Sci Transl Med. 2013;5(201):201ra118. doi: 10.1126/scitranslmed.3006221. [DOI] [PubMed] [Google Scholar]
- 54.Lo KM, et al. huBC1-IL12, an immunocytokine which targets EDB-containing oncofetal fibronectin in tumors and tumor vasculature, shows potent anti-tumor activity in human tumor models. Cancer Immunol Immunother. 2007;56(4):447–57. doi: 10.1007/s00262-006-0203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Probst P, et al. Sarcoma Eradication by Doxorubicin and Targeted TNF Relies upon CD8(+) T-cell Recognition of a Retroviral Antigen. Cancer Res. 2017;77(13):3644–3654. doi: 10.1158/0008-5472.CAN-16-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwager K, et al. The immunocytokine L19-IL2 eradicates cancer when used in combination with CTLA-4 blockade or with L19-TNF. J Invest Dermatol. 2013;133(3):751–758. doi: 10.1038/jid.2012.376. [DOI] [PubMed] [Google Scholar]
- 57.De Luca R, et al. Potency-matched Dual Cytokine-Antibody Fusion Proteins for Cancer Therapy. Mol Cancer Ther. 2017;16(11):2442–2451. doi: 10.1158/1535-7163.MCT-17-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lode HN, et al. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood. 1998;91(5):1706–15. [PubMed] [Google Scholar]
- 59.Yang RK, et al. Intratumoral hu14.18-IL-2 (IC) induces local and systemic antitumor effects that involve both activated T and NK cells as well as enhanced IC retention. J Immunol. 2012;189(5):2656–64. doi: 10.4049/jimmunol.1200934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Powrie F, et al. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1(7):553–62. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 61.Walmsley M, et al. Interleukin-10 inhibition of the progression of established collagen-induced arthritis. Arthritis Rheum. 1996;39(3):495–503. doi: 10.1002/art.1780390318. [DOI] [PubMed] [Google Scholar]
- 62.Quattrone F, et al. The Targeted Delivery of Interleukin 4 Inhibits Development of Endometriotic Lesions in a Mouse Model. Reprod Sci. 2015;22(9):1143–52. doi: 10.1177/1933719115578930. [DOI] [PubMed] [Google Scholar]
- 63.Schwager K, et al. The antibody-mediated targeted delivery of interleukin-10 inhibits endometriosis in a syngeneic mouse model. Hum Reprod. 2011;26(9):2344–52. doi: 10.1093/humrep/der195. [DOI] [PubMed] [Google Scholar]
- 64.Harvill ET, Morrison SL. An IgG3-IL2 fusion protein activates complement, binds Fc gamma RI, generates LAK activity and shows enhanced binding to the high affinity IL-2R. Immunotechnology. 1995;1(2):95–105. doi: 10.1016/1380-2933(95)00009-7. [DOI] [PubMed] [Google Scholar]
- 65.Hu P, et al. A chimeric Lym-1/interleukin 2 fusion protein for increasing tumor vascular permeability and enhancing antibody uptake. Cancer Res. 1996;56(21):4998–5004. [PubMed] [Google Scholar]
- 66.Huang TH, Morrison SL. A trimeric anti-HER2/neu ScFv and tumor necrosis factor-alpha fusion protein induces HER2/neu signaling and facilitates repair of injured epithelia. J Pharmacol Exp Ther. 2006;316(3):983–91. doi: 10.1124/jpet.105.095513. [DOI] [PubMed] [Google Scholar]
- 67.Penichet ML, et al. A recombinant IgG3-(IL-2) fusion protein for the treatment of human HER2/neu expressing tumors. Hum Antibodies. 2001;10(1):43–9. [PubMed] [Google Scholar]
- 68.Vasuthasawat A, et al. Targeted immunotherapy using anti-CD138-interferon alpha fusion proteins and bortezomib results in synergistic protection against multiple myeloma. MAbs. 2016;8(7):1386–1397. doi: 10.1080/19420862.2016.1207030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xuan C, et al. Targeted delivery of interferon-alpha via fusion to anti-CD20 results in potent antitumor activity against B-cell lymphoma. Blood. 2010;115(14):2864–71. doi: 10.1182/blood-2009-10-250555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frey K, et al. Antibody-based targeting of interferon-alpha to the tumor neovasculature: a critical evaluation. Integr Biol (Camb) 2011;3(4):468–78. doi: 10.1039/c0ib00099j. [DOI] [PubMed] [Google Scholar]
- 71.Hemmerle T, et al. The antibody-based targeted delivery of TNF in combination with doxorubicin eradicates sarcomas in mice and confers protective immunity. Br J Cancer. 2013;109(5):1206–13. doi: 10.1038/bjc.2013.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hess C, Neri D. Tumor-targeting properties of novel immunocytokines based on murine IL1beta and IL6. Protein Eng Des Sel. 2014;27(6):207–13. doi: 10.1093/protein/gzu013. [DOI] [PubMed] [Google Scholar]
- 73.Hemmerle T, Neri D. The dose-dependent tumor targeting of antibody-IFNgamma fusion proteins reveals an unexpected receptor-trapping mechanism in vivo. Cancer Immunol Res. 2014;2(6):559–67. doi: 10.1158/2326-6066.CIR-13-0182. [DOI] [PubMed] [Google Scholar]
- 74.Kaspar M, Trachsel E, Neri D. The antibody-mediated targeted delivery of interleukin-15 and GM-CSF to the tumor neovasculature inhibits tumor growth and metastasis. Cancer Res. 2007;67(10):4940–8. doi: 10.1158/0008-5472.CAN-07-0283. [DOI] [PubMed] [Google Scholar]
- 75.Halin C, et al. Tumor-targeting properties of antibody-vascular endothelial growth factor fusion proteins. Int J Cancer. 2002;102(2):109–16. doi: 10.1002/ijc.10674. [DOI] [PubMed] [Google Scholar]
- 76.Hemmerle T, Wulhfard S, Neri D. A critical evaluation of the tumor-targeting properties of bispecific antibodies based on quantitative biodistribution data. Protein Eng Des Sel. 2012;25(12):851–4. doi: 10.1093/protein/gzs061. [DOI] [PubMed] [Google Scholar]
- 77.Venetz D, et al. Glycosylation profiles determine extravasation and disease-targeting properties of armed antibodies. Proc Natl Acad Sci U S A. 2015;112(7):2000–5. doi: 10.1073/pnas.1416694112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hutmacher C, Neri D. Antibody-cytokine fusion proteins: Biopharmaceuticals with immunomodulatory properties for cancer therapy. Adv Drug Deliv Rev. 2018 doi: 10.1016/j.addr.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 79.Arenas-Ramirez N, Woytschak J, Boyman O. Interleukin-2: Biology, Design and Application. Trends Immunol. 2015;36(12):763–777. doi: 10.1016/j.it.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 80.Benczik M, Gaffen SL. The interleukin (IL)-2 family cytokines: survival and proliferation signaling pathways in T lymphocytes. Immunol Invest. 2004;33(2):109–42. doi: 10.1081/imm-120030732. [DOI] [PubMed] [Google Scholar]
- 81.Atkins MB, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–16. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 82.Klapper JA, et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma : a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113(2):293–301. doi: 10.1002/cncr.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192(12):5451–8. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xiang J, et al. Single-chain antibody variable region-targeted interleukin-2 stimulates T cell killing of human colorectal carcinoma cells. Immunol Cell Biol. 1994;72(4):275–85. doi: 10.1038/icb.1994.42. [DOI] [PubMed] [Google Scholar]
- 85.Savage P, et al. A recombinant single-chain antibody interleukin-2 fusion protein. Cell Biophys. 1993;22(1–3):61–77. doi: 10.1007/BF03033867. [DOI] [PubMed] [Google Scholar]
- 86.Becker JC, et al. T cell-mediated eradication of murine metastatic melanoma induced by targeted interleukin 2 therapy. J Exp Med. 1996;183(5):2361–6. doi: 10.1084/jem.183.5.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dolman CS, et al. Suppression of human prostate carcinoma metastases in severe combined immunodeficient mice by interleukin 2 immunocytokine therapy. Clin Cancer Res. 1998;4(10):2551–7. [PubMed] [Google Scholar]
- 88.Sabzevari H, et al. A recombinant antibody-interleukin 2 fusion protein suppresses growth of hepatic human neuroblastoma metastases in severe combined immunodeficiency mice. Proc Natl Acad Sci U S A. 1994;91(20):9626–30. doi: 10.1073/pnas.91.20.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Albertini MR, et al. Phase II trial of hu14.18-IL2 for patients with metastatic melanoma. Cancer Immunol Immunother. 2012;61(12):2261–71. doi: 10.1007/s00262-012-1286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Albertini MR, et al. A pilot trial of hu14.18-IL2 in patients with completely resectable recurrent stage III or stage IV melanoma. Journal of Clinical Oncology. 2014;32(15_suppl):9044–9044. [Google Scholar]
- 91.Shusterman S, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children's Oncology Group (COG) phase II study. J Clin Oncol. 2010;28(33):4969–75. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shusterman S, et al. A feasibility and phase II study of the hu14.18-IL2 immunocytokine in combination with GM-CSF and isotretinoin in patients with recurrent or refractory neuroblastoma: A Children’s Oncology Group study. Journal of Clinical Oncology. 2015;33(15_suppl):10017–10017. [Google Scholar]
- 93.Gillies SD. A new platform for constructing antibody-cytokine fusion proteins (immunocytokines) with improved biological properties and adaptable cytokine activity. Protein Eng Des Sel. 2013;26(10):561–9. doi: 10.1093/protein/gzt045. [DOI] [PubMed] [Google Scholar]
- 94.Gillies SD, et al. A low-toxicity IL-2-based immunocytokine retains antitumor activity despite its high degree of IL-2 receptor selectivity. Clin Cancer Res. 2011;17(11):3673–85. doi: 10.1158/1078-0432.CCR-10-2921. [DOI] [PubMed] [Google Scholar]
- 95.Gillessen S, et al. A phase I dose-escalation study of the immunocytokine EMD 521873 (Selectikine) in patients with advanced solid tumours. Eur J Cancer. 2013;49(1):35–44. doi: 10.1016/j.ejca.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 96.van den Heuvel MM, et al. NHS-IL2 combined with radiotherapy: preclinical rationale and phase Ib trial results in metastatic non-small cell lung cancer following first-line chemotherapy. J Transl Med. 2015;13:32. doi: 10.1186/s12967-015-0397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Group M. Management Report. 2014 http://ar2014.merckgroup.com/management-report/fundamental-information-about-the-group/research-and-development-at-merck.
- 98.Kaufman HL, et al. Targeted modified IL-2 (NHS-IL2, MSB0010445) combined with stereotactic body radiation in advanced melanoma patients after progression on ipilimumab: Assessment of safety, clinical, and biologic activity in a phase 2a study. Journal of Clinical Oncology. 2014;32(15_suppl):TPS9107–TPS9107. [Google Scholar]
- 99.Klein C, et al. Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: Overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines. Oncoimmunology. 2017;6(3):e1277306. doi: 10.1080/2162402X.2016.1277306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Klein C, Waldhauer I, Nicolini V. Abstract 486: Tumor-targeted, engineered IL-2 variant (IL-2v)-based immunocytokines for the immunotherapy of cancer. Cancer Res; Proceedings of the 104th Annual Meeting of the American Association for Cancer Research; Philadelphia: 2013. [Google Scholar]
- 101.Krieg C, et al. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc Natl Acad Sci U S A. 2010;107(26):11906–11. doi: 10.1073/pnas.1002569107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carmenate T, et al. Human IL-2 mutein with higher antitumor efficacy than wild type IL-2. J Immunol. 2013;190(12):6230–8. doi: 10.4049/jimmunol.1201895. [DOI] [PubMed] [Google Scholar]
- 103.Levin AM, et al. Exploiting a natural conformational switch to engineer an interleukin-2 'superkine'. Nature. 2012;484(7395):529–33. doi: 10.1038/nature10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Merchant AM, et al. An efficient route to human bispecific IgG. Nat Biotechnol. 1998;16(7):677–81. doi: 10.1038/nbt0798-677. [DOI] [PubMed] [Google Scholar]
- 105.Van Brummelen E, et al. Pharmacokinetics (PK) and Pharmacodynamics (PD) of cergutuzumab amunaleukin (CA), a carcinoembryonic antigen (CEA)-targeted interleukin 2 variant (IL2v) with abolished binding to CD25. Ann Oncol; ESMO 2017 Congress; Madrid: 2017. [Google Scholar]
- 106.Bachanova V, et al. Remission Induction in a Phase I/II Study of an Anti-CD20-Interleukin-2 Immunocytokine DI-Leu16-IL2 in Patients with Relapsed B-Cell Lymphoma. Blood. 2015;126(23):1533–1533. [Google Scholar]
- 107.Frey K, et al. The immunocytokine F8-IL2 improves the therapeutic performance of sunitinib in a mouse model of renal cell carcinoma. J Urol. 2010;184(6):2540–8. doi: 10.1016/j.juro.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 108.Marlind J, et al. Antibody-mediated delivery of interleukin-2 to the stroma of breast cancer strongly enhances the potency of chemotherapy. Clin Cancer Res. 2008;14(20):6515–24. doi: 10.1158/1078-0432.CCR-07-5041. [DOI] [PubMed] [Google Scholar]
- 109.Moschetta M, et al. Paclitaxel enhances therapeutic efficacy of the F8-IL2 immunocytokine to EDA-fibronectin-positive metastatic human melanoma xenografts. Cancer Res. 2012;72(7):1814–24. doi: 10.1158/0008-5472.CAN-11-1919. [DOI] [PubMed] [Google Scholar]
- 110.Pretto F, et al. Preclinical evaluation of IL2-based immunocytokines supports their use in combination with dacarbazine, paclitaxel and TNF-based immunotherapy. Cancer Immunol Immunother. 2014;63(9):901–10. doi: 10.1007/s00262-014-1562-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schliemann C, et al. Complete eradication of human B-cell lymphoma xenografts using rituximab in combination with the immunocytokine L19-IL2. Blood. 2009;113(10):2275–83. doi: 10.1182/blood-2008-05-160747. [DOI] [PubMed] [Google Scholar]
- 112.Wagner K, et al. The targeted immunocytokine L19-IL2 efficiently inhibits the growth of orthotopic pancreatic cancer. Clin Cancer Res. 2008;14(15):4951–60. doi: 10.1158/1078-0432.CCR-08-0157. [DOI] [PubMed] [Google Scholar]
- 113.Wieckowski S, et al. Therapeutic efficacy of the F8-IL2 immunocytokine in a metastatic mouse model of lung adenocarcinoma. Lung Cancer. 2015;88(1):9–15. doi: 10.1016/j.lungcan.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 114.Ziffels B, Pretto F, Neri D. Intratumoral administration of IL2- and TNF-based fusion proteins cures cancer without establishing protective immunity. Immunotherapy. 2018;10(3):177–188. doi: 10.2217/imt-2017-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eigentler TK, et al. A dose-escalation and signal-generating study of the immunocytokine L19-IL2 in combination with dacarbazine for the therapy of patients with metastatic melanoma. Clin Cancer Res. 2011;17(24):7732–42. doi: 10.1158/1078-0432.CCR-11-1203. [DOI] [PubMed] [Google Scholar]
- 116.Johannsen M, et al. The tumour-targeting human L19-IL2 immunocytokine: preclinical safety studies, phase I clinical trial in patients with solid tumours and expansion into patients with advanced renal cell carcinoma. Eur J Cancer. 2010;46(16):2926–35. doi: 10.1016/j.ejca.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 117.Danielli R, et al. Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: results of a phase II study. Cancer Immunol Immunother. 2015;64(8):999–1009. doi: 10.1007/s00262-015-1704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weide B, et al. Limited efficacy of intratumoral IL-2 applied to large melanoma metastases. Cancer Immunol Immunother. 2014;63(11):1231–2. doi: 10.1007/s00262-014-1584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cazzamalli S, et al. Enhanced Therapeutic Activity of Non-Internalizing Small-Molecule-Drug Conjugates Targeting Carbonic Anhydrase IX in Combination with Targeted Interleukin-2. Clin Cancer Res. 2018;24(15):3656–3667. doi: 10.1158/1078-0432.CCR-17-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rekers NH, et al. The immunocytokine L19-IL2: An interplay between radiotherapy and long-lasting systemic anti-tumour immune responses. Oncoimmunology. 2018;7(4):e1414119. doi: 10.1080/2162402X.2017.1414119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Catania C, et al. The tumor-targeting immunocytokine F16-IL2 in combination with doxorubicin: dose escalation in patients with advanced solid tumors and expansion into patients with metastatic breast cancer. Cell Adh Migr. 2015;9(1–2):14–21. doi: 10.4161/19336918.2014.983785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schliemann C, et al. Targeting interleukin-2 to the bone marrow stroma for therapy of acute myeloid leukemia relapsing after allogeneic hematopoietic stem cell transplantation. Cancer Immunol Res. 2015;3(5):547–56. doi: 10.1158/2326-6066.CIR-14-0179. [DOI] [PubMed] [Google Scholar]
- 123.Old L. Tumor necrosis factor (TNF) Science. 1985;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- 124.van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11(4):397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
- 125.Watanabe N, et al. Toxic Effect of Tumor Necrosis Factor on Tumor Vasculature in Mice. Cancer Research. 1988;48(8):2179–2183. [PubMed] [Google Scholar]
- 126.Gamm H, et al. Phase I trial of recombinant human tumour necrosis factor alpha in patients with advanced malignancy. Eur J Cancer. 1991;27(7):856–63. doi: 10.1016/0277-5379(91)90134-y. [DOI] [PubMed] [Google Scholar]
- 127.Cooke SP, et al. In vivo tumor delivery of a recombinant single chain Fv::tumor necrosis factor-alpha fusion [correction of factor: a fusion] protein. Bioconjug Chem. 2002;13(1):7–15. doi: 10.1021/bc000178a. [DOI] [PubMed] [Google Scholar]
- 128.Liu Y, et al. The antimelanoma immunocytokine scFvMEL/TNF shows reduced toxicity and potent antitumor activity against human tumor xenografts. Neoplasia. 2006;8(5):384–93. doi: 10.1593/neo.06121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rosenblum MG, et al. An antimelanoma immunotoxin containing recombinant human tumor necrosis factor: tissue disposition, pharmacokinetic, and therapeutic studies in xenograft models. Cancer Immunol Immunother. 1995;40(5):322–8. doi: 10.1007/BF01519633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sharifi J, et al. Generation of human interferon gamma and tumor Necrosis factor alpha chimeric TNT-3 fusion proteins. Hybrid Hybridomics. 2002;21(6):421–32. doi: 10.1089/153685902321043954. [DOI] [PubMed] [Google Scholar]
- 131.Bauer S, et al. Targeted therapy of renal cell carcinoma: synergistic activity of cG250-TNF and IFNg. Int J Cancer. 2009;125(1):115–23. doi: 10.1002/ijc.24359. [DOI] [PubMed] [Google Scholar]
- 132.Scherf U, et al. Cytotoxic and antitumor activity of a recombinant tumor necrosis factor-B1(Fv) fusion protein on LeY antigen-expressing human cancer cells. Clin Cancer Res. 1996;2(9):1523–31. [PubMed] [Google Scholar]
- 133.Balza E, et al. Therapy-induced antitumor vaccination in neuroblastomas by the combined targeting of IL-2 and TNFalpha. Int J Cancer. 2010;127(1):101–10. doi: 10.1002/ijc.25018. [DOI] [PubMed] [Google Scholar]
- 134.Halin C, et al. Synergistic therapeutic effects of a tumor targeting antibody fragment, fused to interleukin 12 and to tumor necrosis factor alpha. Cancer Res. 2003;63(12):3202–10. [PubMed] [Google Scholar]
- 135.Spitaleri G, et al. Phase I/II study of the tumour-targeting human monoclonal antibody-cytokine fusion protein L19-TNF in patients with advanced solid tumours. J Cancer Res Clin Oncol. 2013;139(3):447–55. doi: 10.1007/s00432-012-1327-7. [DOI] [PubMed] [Google Scholar]
- 136.Papadia F, et al. Isolated limb perfusion with the tumor-targeting human monoclonal antibody-cytokine fusion protein L19-TNF plus melphalan and mild hyperthermia in patients with locally advanced extremity melanoma. J Surg Oncol. 2013;107(2):173–9. doi: 10.1002/jso.23168. [DOI] [PubMed] [Google Scholar]
- 137.Ventura E, et al. Selective targeted delivery of the TNF-alpha receptor p75 and uteroglobin to the vasculature of inflamed tissues: a preliminary report. BMC Biotechnol. 2011;11:104. doi: 10.1186/1472-6750-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Balada E, Ordi-Ros J, Vilardell-Tarres M. Molecular mechanisms mediated by human endogenous retroviruses (HERVs) in autoimmunity. Rev Med Virol. 2009;19(5):273–86. doi: 10.1002/rmv.622. [DOI] [PubMed] [Google Scholar]
- 139.Kassiotis G. Endogenous retroviruses and the development of cancer. J Immunol. 2014;192(4):1343–9. doi: 10.4049/jimmunol.1302972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Murer P, et al. Targeted delivery of TNF potentiates the antibody-dependent cell-mediated cytotoxicity of an anti-melanoma immunoglobulin. J Invest Dermatol. 2018 doi: 10.1016/j.jid.2018.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Trinchieri G, et al. Natural killer cell stimulatory factor (NKSF) or interleukin-12 is a key regulator of immune response and inflammation. Prog Growth Factor Res. 1992;4(4):355–68. doi: 10.1016/0955-2235(92)90016-b. [DOI] [PubMed] [Google Scholar]
- 142.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3(2):133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 143.Brunda MJ, et al. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993;178(4):1223–30. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nastala CL, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994;153(4):1697–706. [PubMed] [Google Scholar]
- 145.Portielje JE, et al. Phase I study of subcutaneously administered recombinant human interleukin 12 in patients with advanced renal cell cancer. Clin Cancer Res. 1999;5(12):3983–9. [PubMed] [Google Scholar]
- 146.Kim JW, et al. First-in-human phase I trial of NHS-IL12 in advanced solid tumors. Journal of Clinical Oncology. 2012;30(15_suppl):TPS2617–TPS2617. [Google Scholar]
- 147.Xu C, et al. Combination Therapy with NHS-muIL12 and Avelumab (anti-PD-L1) Enhances Antitumor Efficacy in Preclinical Cancer Models. Clin Cancer Res. 2017;23(19):5869–5880. doi: 10.1158/1078-0432.CCR-17-0483. [DOI] [PubMed] [Google Scholar]
- 148.Mariani G, et al. Tumor targeting potential of the monoclonal antibody BC-1 against oncofetal fibronectin in nude mice bearing human tumor implants. Cancer. 1997;80(12 Suppl):2378–84. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2378::aid-cncr7>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]
- 149.Rudman SM, et al. A phase 1 study of AS1409, a novel antibody-cytokine fusion protein, in patients with malignant melanoma or renal cell carcinoma. Clin Cancer Res. 2011;17(7):1998–2005. doi: 10.1158/1078-0432.CCR-10-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Heuser C, et al. Anti-CD30-IL-12 antibody-cytokine fusion protein that induces IFN-gamma secretion of T cells and NK cell-mediated lysis of Hodgkin's lymphoma-derived tumor cells. Int J Cancer. 2003;106(4):545–52. doi: 10.1002/ijc.11279. [DOI] [PubMed] [Google Scholar]
- 151.Pasche N, et al. Cloning and characterization of novel tumor-targeting immunocytokines based on murine IL7. J Biotechnol. 2011;154(1):84–92. doi: 10.1016/j.jbiotec.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 152.Pasche N, Frey K, Neri D. The targeted delivery of IL17 to the mouse tumor neo-vasculature enhances angiogenesis but does not reduce tumor growth rate. Angiogenesis. 2012;15(1):165–9. doi: 10.1007/s10456-011-9239-8. [DOI] [PubMed] [Google Scholar]
- 153.Hemmerle T, et al. Tumor targeting properties of antibody fusion proteins based on different members of the murine tumor necrosis superfamily. J Biotechnol. 2014;172:73–6. doi: 10.1016/j.jbiotec.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 154.Siegemund M, et al. An optimized antibody-single-chain TRAIL fusion protein for cancer therapy. MAbs. 2016;8(5):879–91. doi: 10.1080/19420862.2016.1172163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sam J, et al. Abstract 5621: FAP-4-1BBL: A novel versatile tumor-stroma targeted 4-1BB agonist for combination immunotherapy with checkpoint inhibitors, T-cell bispecific antibodies, and ADCC-mediating antibodies. Cancer Research. 2018;78(13 Supplement):5621–5621. [Google Scholar]
- 156.http://www.genentechclinicaltrials.com/. 2019 06.03.2019].
- 157.Kermer V, et al. An antibody fusion protein for cancer immunotherapy mimicking IL-15 trans-presentation at the tumor site. Mol Cancer Ther. 2012;11(6):1279–88. doi: 10.1158/1535-7163.MCT-12-0019. [DOI] [PubMed] [Google Scholar]
- 158.Schwegler C, et al. Monoclonal anti-idiotype antibody 6G6.C4 fused to GM-CSF is capable of breaking tolerance to carcinoembryonic antigen (CEA) in CEA-transgenic mice. Cancer Res. 2005;65(5):1925–33. doi: 10.1158/0008-5472.CAN-04-3591. [DOI] [PubMed] [Google Scholar]
- 159.Dela Cruz JS, et al. Anti-HER2/neu IgG3-(IL-2) and anti-HER2/neu IgG3-(GM-CSF) promote HER2/neu processing and presentation by dendritic cells: implications in immunotherapy and vaccination strategies. Mol Immunol. 2006;43(6):667–76. doi: 10.1016/j.molimm.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 160.Gillies SD, et al. Bi-functional cytokine fusion proteins for gene therapy and antibody-targeted treatment of cancer. Cancer Immunol Immunother. 2002;51(8):449–60. doi: 10.1007/s00262-002-0302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Helguera G, et al. Vaccination with novel combinations of anti-HER2/neu cytokines fusion proteins and soluble protein antigen elicits a protective immune response against HER2/neu expressing tumors. Vaccine. 2006;24(3):304–16. doi: 10.1016/j.vaccine.2005.07.073. [DOI] [PubMed] [Google Scholar]
- 162.Jahn T, et al. An IL12-IL2-antibody fusion protein targeting Hodgkin's lymphoma cells potentiates activation of NK and T cells for an anti-tumor attack. PLoS One. 2012;7(9):e44482. doi: 10.1371/journal.pone.0044482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fellermeier-Kopf S, et al. Duokines: a novel class of dual-acting co-stimulatory molecules acting in cis or trans. Oncoimmunology. 2018;7(9):e1471442. doi: 10.1080/2162402X.2018.1471442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Biedermann T, Rocken M. Pro- and anti-inflammatory effects of IL-4: from studies in mice to therapy of autoimmune diseases in humans. Ernst Schering Res Found Workshop. 2005(50):235–42. doi: 10.1007/3-540-26811-1_13. [DOI] [PubMed] [Google Scholar]
- 165.Cavaillon JM. Pro- versus anti-inflammatory cytokines: myth or reality. Cell Mol Biol (Noisy-le-grand) 2001;47(4):695–702. [PubMed] [Google Scholar]
- 166.Scheller J, et al. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–88. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 167.Kasama T, et al. Biphasic regulation of the development of murine type II collagen-induced arthritis by interleukin-12: possible involvement of endogenous interleukin-10 and tumor necrosis factor alpha. Arthritis Rheum. 1999;42(1):100–9. doi: 10.1002/1529-0131(199901)42:1<100::AID-ANR13>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 168.Schmid AS, et al. Antibody-based targeted delivery of interleukin-4 synergizes with dexamethasone for the reduction of inflammation in arthritis. Rheumatology (Oxford) 2018;57(4):748–755. doi: 10.1093/rheumatology/kex447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Moore KW, et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 170.Fedorak RN, et al. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn's disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology. 2000;119(6):1473–82. doi: 10.1053/gast.2000.20229. [DOI] [PubMed] [Google Scholar]
- 171.van Roon J, et al. Interleukin 10 treatment of patients with rheumatoid arthritis enhances Fc gamma receptor expression on monocytes and responsiveness to immune complex stimulation. J Rheumatol. 2003;30(4):648–51. [PubMed] [Google Scholar]
- 172.Emmerich J, et al. IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs. Cancer Res. 2012;72(14):3570–81. doi: 10.1158/0008-5472.CAN-12-0721. [DOI] [PubMed] [Google Scholar]
- 173.Mumm JB, et al. IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer Cell. 2011;20(6):781–96. doi: 10.1016/j.ccr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 174.Chan IH, et al. IL-10: Expanding the Immune Oncology Horizon. Receptors Clin Investig. 2015;2(4) [PMC free article] [PubMed] [Google Scholar]
- 175.Schwager K, et al. Preclinical characterization of DEKAVIL (F8-IL10), a novel clinical-stage immunocytokine which inhibits the progression of collagen-induced arthritis. Arthritis Res Ther. 2009;11(5):R142. doi: 10.1186/ar2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Doll F, et al. Murine analogues of etanercept and of F8-IL10 inhibit the progression of collagen-induced arthritis in the mouse. Arthritis Res Ther. 2013;15(5):R138. doi: 10.1186/ar4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Galeazzi M, et al. A phase IB clinical trial with Dekavil (F8-IL10), an immunoregulatory 'armed antibody' for the treatment of rheumatoid arthritis, used in combination wiIh methotrexate. Isr Med Assoc J. 2014;16(10):666. [PubMed] [Google Scholar]
- 178.Hughes C, et al. Human single-chain variable fragment that specifically targets arthritic cartilage. Arthritis Rheum. 2010;62(4):1007–16. doi: 10.1002/art.27346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hughes C, et al. Targeting of viral interleukin-10 with an antibody fragment specific to damaged arthritic cartilage improves its therapeutic potency. Arthritis Res Ther. 2014;16(4):R151. doi: 10.1186/ar4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Brown MA, Hural J. Functions of IL-4 and Control of Its Expression. Crit Rev Immunol. 2017;37(2–6):181–212. doi: 10.1615/CritRevImmunol.v37.i2-6.30. [DOI] [PubMed] [Google Scholar]
- 181.Joosten LA, et al. Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis. Arthritis Res. 1999;1(1):81–91. doi: 10.1186/ar14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ghoreschi K, et al. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat Med. 2003;9(1):40–6. doi: 10.1038/nm804. [DOI] [PubMed] [Google Scholar]
- 183.Kawalkowska JZ, et al. Targeted IL-4 therapy synergizes with dexamethasone to induce a state of tolerance by promoting Treg cells and macrophages in mice with arthritis. Eur J Immunol. 2016;46(5):1246–57. doi: 10.1002/eji.201546221. [DOI] [PubMed] [Google Scholar]
- 184.Hemmerle T, et al. Antibody-mediated delivery of interleukin 4 to the neo-vasculature reduces chronic skin inflammation. J Dermatol Sci. 2014;76(2):96–103. doi: 10.1016/j.jdermsci.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 185.Bootz F, Neri D. Immunocytokines: a novel class of products for the treatment of chronic inflammation and autoimmune conditions. Drug Discov Today. 2016;21(1):180–189. doi: 10.1016/j.drudis.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pedretti M, et al. Combination of temozolomide with immunocytokine F16-IL2 for the treatment of glioblastoma. Br J Cancer. 2010;103(6):827–36. doi: 10.1038/sj.bjc.6605832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Eckert F, et al. Enhanced binding of necrosis-targeting immunocytokine NHS-IL12 after local tumour irradiation in murine xenograft models. Cancer Immunol Immunother. 2016;65(8):1003–13. doi: 10.1007/s00262-016-1863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gutbrodt KL, Casi G, Neri D. Antibody-based delivery of IL2 and cytotoxics eradicates tumors in immunocompetent mice. Mol Cancer Ther. 2014;13(7):1772–6. doi: 10.1158/1535-7163.MCT-14-0105. [DOI] [PubMed] [Google Scholar]
- 189.List T, Casi G, Neri D. A chemically defined trifunctional antibody-cytokine-drug conjugate with potent antitumor activity. Mol Cancer Ther. 2014;13(11):2641–52. doi: 10.1158/1535-7163.MCT-14-0599. [DOI] [PubMed] [Google Scholar]
- 190.De Luca R, Neri D. Potentiation of PD-L1 blockade with a potency-matched dual cytokine-antibody fusion protein leads to cancer eradication in BALB/c-derived tumors but not in other mouse strains. Cancer Immunol Immunother. 2018;67(9):1381–1391. doi: 10.1007/s00262-018-2194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fallon JK, et al. Enhanced antitumor effects by combining an IL-12/anti-DNA fusion protein with avelumab, an anti-PD-L1 antibody. Oncotarget. 2017;8(13):20558–20571. doi: 10.18632/oncotarget.16137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hornig N, et al. Combination of a bispecific antibody and costimulatory antibody-ligand fusion proteins for targeted cancer immunotherapy. J Immunother. 2012;35(5):418–29. doi: 10.1097/CJI.0b013e3182594387. [DOI] [PubMed] [Google Scholar]
- 193.Hornig N, et al. Evaluating combinations of costimulatory antibody-ligand fusion proteins for targeted cancer immunotherapy. Cancer Immunol Immunother. 2013;62(8):1369–80. doi: 10.1007/s00262-013-1441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kiefer JD, Neri D. Immunocytokines and bispecific antibodies: two complementary strategies for the selective activation of immune cells at the tumor site. Immunol Rev. 2016;270(1):178–92. doi: 10.1111/imr.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hemmerle T, Neri D. The antibody-based targeted delivery of interleukin-4 and 12 to the tumor neovasculature eradicates tumors in three mouse models of cancer. Int J Cancer. 2014;134(2):467–77. doi: 10.1002/ijc.28359. [DOI] [PubMed] [Google Scholar]
- 196.Kermer V, et al. Combining antibody-directed presentation of IL-15 and 4-1BBL in a trifunctional fusion protein for cancer immunotherapy. Mol Cancer Ther. 2014;13(1):112–21. doi: 10.1158/1535-7163.MCT-13-0282. [DOI] [PubMed] [Google Scholar]
- 197.Osenga KL, et al. A phase I clinical trial of the hu14.18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children's Oncology Group. Clin Cancer Res. 2006;12(6):1750–9. doi: 10.1158/1078-0432.CCR-05-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lansigan F, et al. Phase I/II study of an anti-CD20-interleukin-2 immunocytokine DI-Leu16-IL2 in patients with relapsed b-cell lymphoma (NHL) Journal of Clinical Oncology. 2016;34(15_suppl):e19046–e19046. [Google Scholar]
- 199.Lansigan F, et al. DI-Leu16-IL2, an Anti-CD20-Interleukin-2 Immunocytokine, Is Safe and Active in Patients with Relapsed and Refractory B-Cell Lymphoma: A Report of Maximum Tolerated Dose, Optimal Biologic Dose, and Recommended Phase 2 Dose. Blood. 2016;128(22):620–620. [Google Scholar]
- 200.Neri D, et al. Intralesional treatment of stage III metastatic melanoma patients with L19-IL2: Clinical and systemic immunological responses. Journal of Clinical Oncology. 2014;32(15_suppl):9041–9041. doi: 10.1158/2326-6066.CIR-13-0206. [DOI] [PubMed] [Google Scholar]
- 201.Weide B, et al. Intralesional treatment of stage III metastatic melanoma patients with L19-IL2 results in sustained clinical and systemic immunologic responses. Cancer Immunol Res. 2014;2(7):668–78. doi: 10.1158/2326-6066.CIR-13-0206. [DOI] [PubMed] [Google Scholar]
- 202.de Braud F, et al. Combinations of the immunocytokine F16-IL2 with doxorubicin or with paclitaxel investigated in phase Ib studies in patients with advanced solid tumors. Journal of Clinical Oncology. 2010;28(15_suppl):e13017–e13017. [Google Scholar]
- 203.De Braud FG, et al. Combination of the immunocytokine F16-IL2 with doxorubicin or paclitaxel in patients with solid tumors: Results from two phase Ib trials. Journal of Clinical Oncology. 2011;29(15_suppl):2595–2595. [Google Scholar]
- 204.Schellens JHM, et al. CEA-targeted engineered IL2: Clinical confirmation of tumor targeting and evidence of intra-tumoral immune activation. Journal of Clinical Oncology. 2015;33(15_suppl):3016–3016. [Google Scholar]
- 205.Soerensen MM, et al. Safety, PK/PD, and anti-tumor activity of RO6874281, an engineered variant of interleukin-2 (IL-2v) targeted to tumor-associated fibroblasts via binding to fibroblast activation protein (FAP) Journal of Clinical Oncology. 2018;36(15_suppl):e15155–e15155. [Google Scholar]
- 206.Connor JP, et al. A phase 1b study of humanized KS-interleukin-2 (huKS-IL2) immunocytokine with cyclophosphamide in patients with EpCAM-positive advanced solid tumors. BMC Cancer. 2013;13:20. doi: 10.1186/1471-2407-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ko YJ, et al. Safety, pharmacokinetics, and biological pharmacodynamics of the immunocytokine EMD 273066 (huKS-IL2): results of a phase I trial in patients with prostate cancer. J Immunother. 2004;27(3):232–9. doi: 10.1097/00002371-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 208.Spicer JF, et al. A phase I study of AS1409, a novel antibody-cytokine fusion protein, in patients with malignant melanoma (MM) or renal cell carcinoma (RCC) Journal of Clinical Oncology. 2009;27(15S):3024–3024. doi: 10.1158/1078-0432.CCR-10-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ammann M, et al. Antigen binding molecules comprising a trimeric tnf family ligand. In: H-LR Inc, editor. Google patents. Hoffmann-La Roche Inc; USA: 2016. [Google Scholar]
- 210.Weide B, Neri D, Elia G. Intralesional treatment of metastatic melanoma: a review of therapeutic options. Cancer Immunol Immunother. 2017;66(5):647–656. doi: 10.1007/s00262-016-1952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]