Abstract
Tubingensin B is an indole diterpenoid that bears a daunting chemical structure featuring a disubstituted carbazole unit, five stereogenic centres—three of which are quaternary—and a decorated [3.2.2]-bridged bicycle. We describe our synthetic design toward a concise and enantiospecific total synthesis of tubingensin B, which hinges on the strategic use of a transient aryne intermediate. Although initial studies led to unexpected reaction outcomes, we ultimately implemented a sequence of carbazolyne cyclization followed by Rh-catalysed fragmentation to install the seven-membered ring and vicinal quaternary stereocentres of the natural product. Coupled with a late-stage radical cyclization to construct the [3.2.2]-bridged bicycle, these efforts have enabled the total synthesis of tubingensin B. The design and evolution of our succinct total synthesis underscores the utility of long-avoided aryne intermediates for the introduction of structural motifs that have conventionally been viewed as challenging.
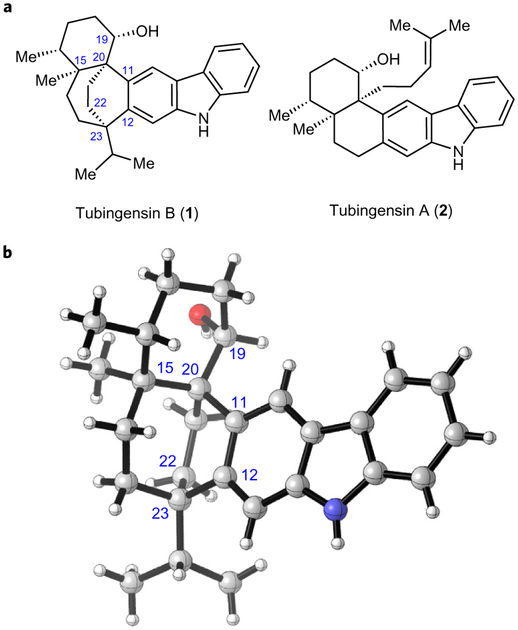
Indole alkaloids continue to inspire the development of new chemical transformations and innovative synthetic strategies. The focus of the present study is tubingensin B (1), an indole diterpenoid that was isolated from the fungus Aspergillus tubingensis in 1989 (Fig. 1.)1. Its absolute structure was determined via X-ray crystallography the same year2. Tubingensin B (1) is a secondary metabolite that is believed to help protect the producing fungus from natural predators. It exhibits activity against the widespread crop pest Heliothis zea, causing 10% mortality in a dietary assay at 125 ppm. In addition, tubingensin B (1) displays cytotoxicity against cervical cancer cells (HeLa) with an IC50 (half maximal inhibitory concentration) of 4 μg ml−1. Lastly, the natural product demonstrates in vitro antiviral activity against herpes simplex virus type 1 (HSV-1) with an IC50 of 9 μg ml−1. From a structural standpoint, the sheer complexity of tubingensin B (1) captured our attention. The eastern portion of the natural product possesses a carbazole unit, which, at glance, is deceptively simple. However, the presence of two adjacent sp2–sp3 carbon–carbon (C–C) bond linkages, with quaternary stereocentres stemming from the carbazole, presents a significant hurdle. Further compounding the challenge is the bicyclo[3.2.2]nonane core, which is fused to the carbazole and a densely functionalized six-membered ring. In total, the natural product possesses five stereogenic centres. Four of these stereocentres are contiguous and three are quaternary, including two vicinal quaternary centres. Although two total syntheses of the related natural product tubingensin A (2) have been reported (by Li3 and our own laboratory4), a total synthesis of the more daunting family member, tubingensin B (1), has remained elusive. Herein, we report our synthetic forays toward (−)-1, which have culminated in a succinct total synthesis of the targeted natural product. Critical to the success of this endeavour is the strategic use of a highly reactive aryne intermediate to rapidly build molecular complexity.
Figure 1 |. The structures of tubingensins B and A.
a, Tubingensin B (1) possesses an intricate hexacyclic ring system bearing a bicyclo[3.2.2]nonane core and five stereogenic centres, three of which are quaternary. Although its simpler counterpart tubingensin A (2) has been synthesized previously, there is no reported total synthesis of 1. b, Crystal structure oftubingensin B (1)2.
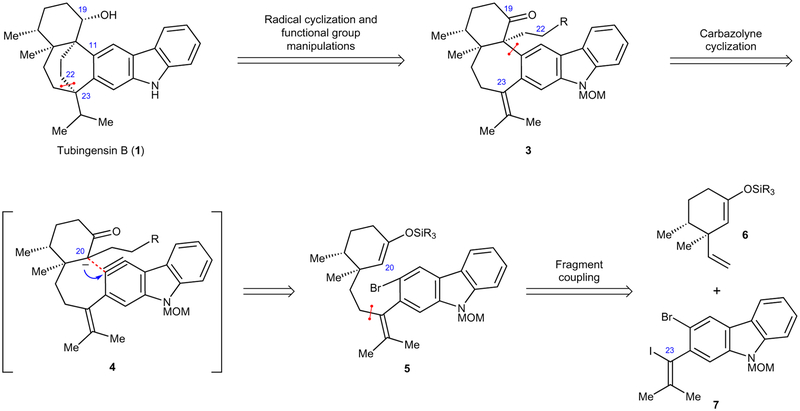
Our initial retrosynthetic analysis of tubingensin B (1) is depicted in Fig. 2. We envisioned that the natural product could arise from pentacycle 3 via a series of functional group manipulations and a late-stage radical cyclization. In the forward sense, the formation of the C22–C23 linkage using a radical cyclization would provide a suitable entryway to assemble the natural product’s bicyclo[3.2.2]nonane core and the C23 quaternary stereocentre. Next, in a critical disconnection, we thought pentacycle 3 to be accessible by cyclization of an in situ-generated aryne derivative of a carbazole, known as a carbazolyne (see transition structure 4). In turn, the carbazolyne would ultimately be derived from silyl enol ether 5 by C20 functionalization, followed by aryne formation and enolate trapping. Although potentially challenging due to the short-lived nature of the highly strained carbazolyne, the success of this step would facilitate installation of the necessary seven-membered ring bearing vicinal quaternary stereocentres. It should be noted that arynes, despite once being controversial and overlooked by the synthetic community, have recently gained traction in chemical synthesis5,6. However, their use as building blocks for the formation of vicinal quaternary stereocentres is limited to one example from our laboratory involving the assembly of a cyclohexyl ring present in tubingensin A4. Moreover, aryne cyclizations to form seven-membered rings are kinetically disfavoured and rare6. For example, the formation of a seven-membered ring via C–O bond construction was observed to be a minor reaction pathway (20% yield) in a synthesis of oxocularine7. Likewise, we have previously investigated seven-membered ring formation via enolate–aryne cyclizations in the context of the bridged bicyclic welwitindolinone alkaloids8. These efforts led to only modest yields of seven-membered ring-containing products, with competition between C- and O-arylation pathways (33 and 13%, respectively), without the complication of quaternary stereocentre formation as in the present endeavour. As such, the desired carbazolyne cyclization to form a seven-membered ring and access vicinal quaternary stereocentres (for example, 4) challenges the limits of modern aryne chemistry. Nonetheless, to complete our retrosynthesis, we envisioned that enol ether 5 could be derived from two simpler fragments, cyclohexyl derivative 6 and carbazole 7.
Figure 2 |. Retrosynthetic analysis of tubingensin B.
The natural product 1 was envisioned to arise through a series of disconnections that would enable the rapid generation of molecular complexity. Key strategies highlight: a radical cyclization to forge the bicyclo[3.2.2]nonane core, a carbazolyne cyclization for the formation of the seven-membered ring containing vicinal quaternary centres, and the union of two fragments via Suzuki–Miyaura coupling. MOM, methoxymethyl.
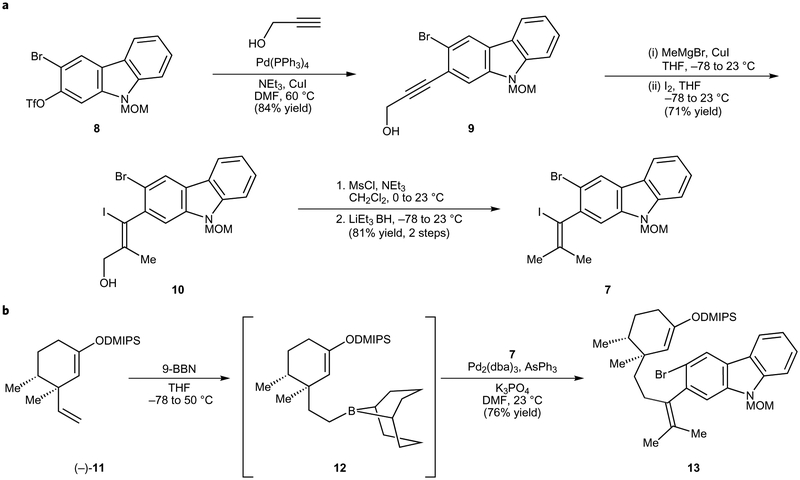
We first sought to prepare the carbazole fragment, 7 (Fig. 3a). The known bromotriflate 8 (prepared in three steps4) was treated with propargyl alcohol in the presence of catalytic Pd(PPh3)4 and CuI to effect Sonogashira coupling9. The resulting product, alkynol 9, was obtained in 84% yield. Of note, this transformation could be performed on a greater than ten-gram scale and provided an efficient means to homologate the carbazole fragment without disturbing the aryl bromide. Alkynol 9 was then subjected to a carbometallation/iodination sequence10, which delivered iodoalcohol 10 in 71% yield. Lastly, the desired vinyl iodide 7 was obtained following a two-step deoxygenation protocol11,12.
Figure 3 |. Synthesis of coupling fragment 7 and assembly of tetrasubstituted alkene 13.
a, Vinyl iodide 7 was synthesized on gram scale from known aryl triflate 8 in four steps. b, Hydroboration of known olefin 11 and subsequent palladium-catalysed Suzuki–Miyaura coupling with vinyl iodide 7 gave 13. MOM, methoxymethyl; DMF, dimethylformamide; THF, tetrahydrofuran; MsCl, methanesulfonyl chloride; DMIPS, dimethyl(isopropyl)silyl; 9-BBN, 9-borabicyclo[3.3.1]nonane; dba, dibenzylideneacetone.
With vinyl iodide 7 available in multigram quantities, we pursued the union of this fragment with an appropriate cyclohexyl building block (Fig. 3b). Olefin (−)-11, which is readily accessible from (+)-dihydrocarvone in four steps4,13, underwent hydroboration to furnish alkyl boron derivative 12. This adduct was directly subjected to Pd-catalysed Suzuki–Miyaura coupling with dihalocarbazole 7 to afford desired product 13 in 76% yield. Several features of this transformation should be emphasized: (a) the use of AsPh3 as the ligand14 proved critical for catalytic activity, (b) competitive cross-coupling of the aryl bromide was not observed, (c) the dimethyl(isopropyl)silyl (DMIPS) enol ether readily withstood the reaction conditions, and (d) the formation of 13 provides an uncommon example of a C(sp2)–C(sp3) cross coupling to access a tetrasubstituted olefin.
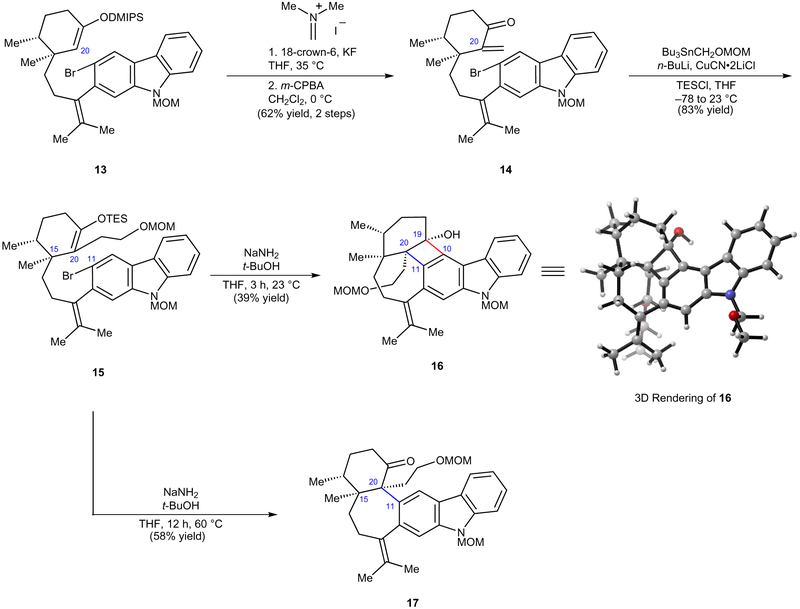
Having prepared silyl enol ether 13, we turned our attention to the critical aryne cyclization, with the hope of accessing the seven-membered ring and vicinal quaternary centres of the natural product (Fig. 4). However, it was first necessary to functionalize C20 with a two-carbon unit adorned with a functional group handle. This goal was achieved using a three-step sequence involving Eschenmoser methenylation15 of the labile dimethyl(isopropyl)silyl enol ether (13 → 14), followed by conjugate addition using the cuprate prepared in situ from [(methoxymethoxy) methyl]tributylstannane (MOMOCH2SnBu3) (ref. 16). Direct trapping of the resulting enolate with chlorotriethylsilane (TESCl) delivered C20-functionalized silyl enol ether 15. Given our prior success in forging a 6,6-fused ring system in our total synthesis of tubingensin A (ref. 4), we were hopeful that treatment of 15 with sodium amide/t-butanol17 at ambient temperature would lead to initial enolate formation, followed by dehydrohalogenation. Subsequent carbazolyne cyclization would forge the medium-sized ring. However, in this crucial reaction, the desired pentacyclic ketone was not observed. Rather, the major product isolated was cyclobutenol 16 as a single diastereomer, which presumably arises from formal [2+2] cycloaddition18. Although not the desired reaction outcome, the formation of strained hexacycle 16 underscores the power of aryne chemistry for the assembly of intricate molecular scaffolds (see 3D rendering19 in Fig. 4) through the formation of two C–C bonds and two fully substituted stereocentres. Not to be deterred, we explored the possibility of converting cyclobutenol 16 to the intended carbazolyne cyclization product18. Ultimately, it was found that such a ring fragmentation could be achieved using basic reaction conditions at elevated temperatures. Thus, we attempted the carbazolyne cyclization of 15 at 60 °C and were delighted to obtain the desired pentacyclic product 17, bearing the key C20 quaternary stereocentre, in 58% yield. Of note, this reaction proceeded with complete diastereoselectivity, presumably guided by the adjacent C15 stereocentre, and allowed us to access the challenging seven-membered ring and vicinal quaternary stereocentres of the natural product scaffold.
Figure 4 |. The key aryne cyclization forges the C20–C11 linkage.
Enol ether 15 was synthesized in three steps from 13 and assessed in the critical carbazolyne cyclization. Although the use of conventional aryne cyclization conditions delivered the undesired cyclobutenol 16, switching to longer reaction times and higher temperatures furnished the desired pentacyclic ketone 17. 18-crown-6, 1,4,7,10,13,16-hexaoxacyclooctadecane; MOM, methoxymethyl; DMIPS, dimethyl(isopropyl)silyl; THF, tetrahydrofuran; m-CPBA, meta-chloroperbenzoic acid; TESCl, triethylsilyl chloride.
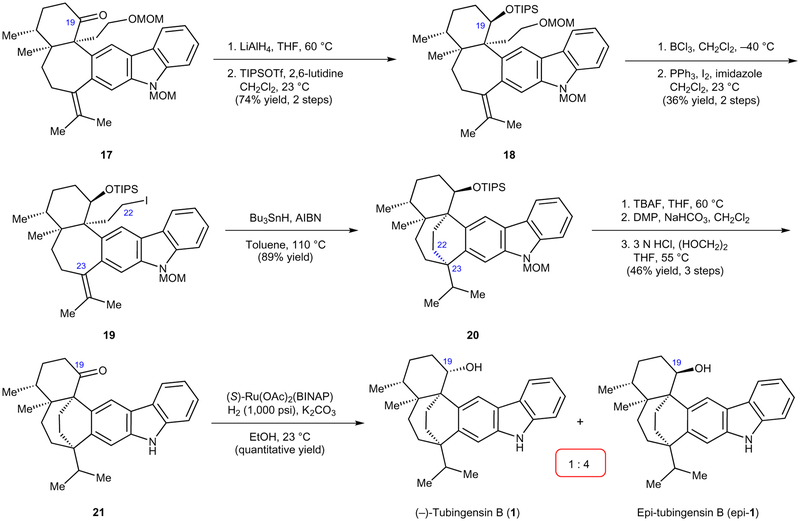
With pentacycle 17 in hand, we focused on construction of the bicyclo[3.2.2]nonane core (Fig. 5). Initial efforts to directly elaborate 17 to a suitable radical cyclization precursor were thwarted due to the incompatibility of the C19 ketone. For example, cleavage of the methoxymethyl (MOM) group from ether 17 led to the formation of an unreactive hemiketal. Thus, a more circuitous route was pursued, which involved initial reduction of ketone 17, followed by silyl protection of the resulting alcohol to give 18. Unfortunately, reduction of the C19 ketone occurred exclusively from the α-face of 17, regardless of the reducing agent employed, and exhaustive attempts to invert the stereochemical configuration failed. As such, the C19 stereochemistry would require correction at a later point in our synthesis. Nonetheless, removal of the MOM protecting group of 1820, followed by treatment of the resulting alcohol under Appel conditions21, delivered iodide 19. Gratifyingly, exposure of iodide 19 to tributyltin hydride and azobisisobutyronitrile (AIBN) in toluene at 110 °C delivered bridged bicycle 20 in 89% yield22. This radical cyclization constructs the final C–C bond needed for the total synthesis (that is, C22–C23), in addition to the final quaternary stereocentre at C23. Of note, the conversion of 19 to 20 provides a rare example of a radical cyclization being used to forge a quaternary stereocentre from a primary alkyl halide and an electronrich olefin22,23. From bicycle 20, all that remained to complete the total synthesis was the removal of protecting groups and inversion of the C19 stereocentre. Although triisopropylsilyl (TIPS) cleavage proceeded readily using tetrabutylammonium fluoride (TBAF), exhaustive attempts to achieve the critical stereochemical inversion proved fruitless. Thus, following TIPS cleavage, we performed a straightforward alcohol oxidation, followed by acid-mediated cleavage of the MOM protecting group, to furnish the penultimate ketone intermediate 21. A myriad of reducing agents, such as hydride donors, single-electron donors, and heterogeneous hydrogenation catalysts were evaluated for the final step of the total synthesis (see Supplementary Fig. 1 for details). However, all efforts to reduce the hindered ketone of 21 led exclusively to the undesired C19 epimer of the natural product, epi-tubingensin B epi-1). Similarly unfortunate, exhaustive attempts to invert the C19 stereochemistry were unsuccessful (for example, Mitsunobu reaction). With few options remaining, we evaluated catalyst-controlled reduction conditions and ultimately found that treatment of ketone 21 with diacetato[(S)-2,2′-bis(diphenylphosphino)-1,1′-binaphthyl]ruthenium(II) ((S)-Ru(OAc)2(BINAP)) under Noyori reduction conditions24,25 delivered a 1:4 ratio of tubingensin B (1) and epi-tubingensin B (epi-1) in quantitative yield. Although the yield of 1 was modest, this effort provided our first synthetic sample of the natural product.
Figure 5 |. Radical cyclization and first generation total synthesis of tubingensin B.
Radical cyclization precursor 19 was prepared using several functional group manipulations. In turn, the radical cyclization led to the efficient assembly of the [3.2.2]nonane core and provided bicycle 20. Following further manipulations, 1 was obtained, albeit with its C19 epimer forming predominantly in the final step. MOM, methoxymethyl; THF, tetrahydrofuran; TIPSOTf, triisopropylsilyl trifluoromethanesulfonate; AIBN, azobisisobutyronitrile; TBAF, tetrabutylammonium fluoride; DMP, Dess–Martin Periodinane; BINAP, 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl.
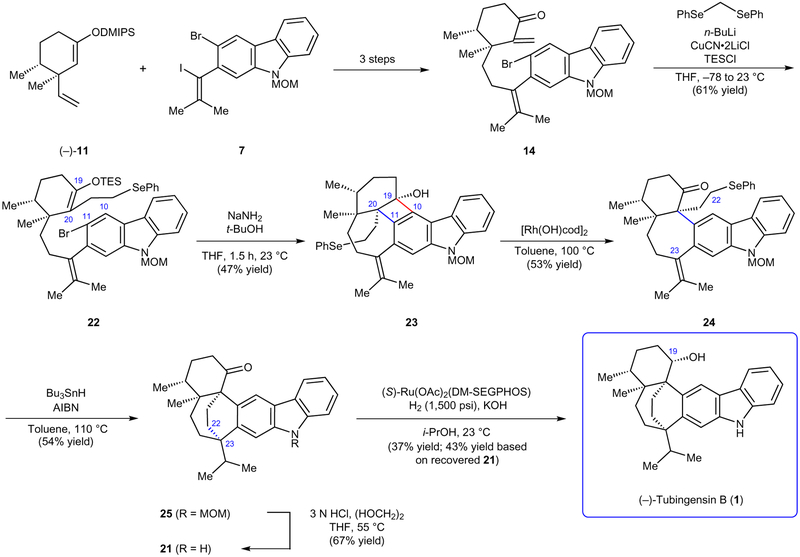
Having accessed tubingensin B (1) and validated several critical elements of our synthetic design, such as the carbazolyne and radical cyclizations, we sought to streamline our total synthesis. We reasoned that the ideal endgame would avoid lengthy manipulations pertaining to the C19 ketone and the elaboration of the MOM-protected alcohol to a suitable radical precursor (see Fig. 5). After pursuing several unsuccessful strategies toward this end, we uncovered the concise route to tubingensin B (1) depicted in Fig. 6. The early stages of our total synthesis remain unchanged, which allowed access to gram quantities of enone 14 in three steps from (−)-11 and 7. Enone 14 was treated with phenylselenomethyl lithium26 in the presence of CuCN•2LiCl and TESCl in tetrahydrofuran (THF) to generate phenylselenide 22 in 61% yield. This seemingly simple tactic was intended to serve as a synthetic lynch-pin, as we envisioned the phenylselenide motif would be sufficiently stable to be carried through subsequent steps and later used directly as a radical precursor. We next tested the carbazolyne cyclization to access the seven-membered ring and vicinal quaternary stereocentres. Unfortunately, when this key step was carried out at elevated temperatures, which we had previously deemed necessary to surpass cyclobutenol formation (see Fig. 4), decomposition of the phenylselenide was observed. We thus had to perform the cyclization at ambient temperature, which provided cyclobutenol 23 as a single diastereomer, without disturbing the phenylselenide. In order to fragment the unnecessary C10–C19 bond of 23, we turned to Murakami’s mild rhodium-catalysed ring opening methodology27. To our delight, exposure of cyclobutenol 23 to catalytic hydroxy (cyclooctadiene)rhodium(I) dimer ([Rh(OH)cod]2) in toluene at 100 °C led to rupture of the desired C–C bond to supply ketone 24. To forge the last carbon–carbon bond and final quaternary stereocentre of the natural product, ketone 24 was treated with tributyltin hydride and AIBN in toluene at 110 °C to furnish bridged bicycle 25 in 54% yield. This complexity-generating step provides a rare example of a phenylselenide radical precursor being used in total synthesis, particularly to form a quaternary centre28. Following cleavage of the MOM protecting group under acidic conditions (25 → 21), the only challenge that remained was the diastereoselective reduction of the ketone. After evaluating a variety of chiral ligands, it was found that exposure of ketone 21 to diacetato[(S)-5,5′-bis[di(3,5-xylyl)phosphino]-4,4′-bi-1,3-benzodioxole]ruthenium(II) (S)-Ru(OAc)2(DM-SEGPHOS)24, KOH, and H2 (1,500 psi) led to a notably improved 1:1.3 ratio of tubingensin B (1) to epi-1 (see Fig. 5 for previous reduction, which gave 1:4 ratio of 1 to epi-1). Given that the undesired epimer could be recycled to 21 using a straightforward Dess–Martin oxidation, while also considering the brevity of our synthesis, we viewed this final transformation as a viable solution to introducing the troublesome C19 stereocentre. Spectroscopic analysis of synthetic material and comparisons to an authentic sample validated our successful total synthesis of tubingensin B (1).
Figure 6 |. Concise total synthesis of tubingensin B.
To overcome arduous functional group manipulations, alkyl selenide 22 was prepared and employed in the key aryne cyclization. To complete the synthesis, several key steps including rhodium-catalysed cyclobutenol ring-opening, radical cyclization, and diastereoselective reduction were executed. This second-generation total synthesis affords (−)-tubingensin B (1) in 9 steps from fragments 11 and 7. MOM, methoxymethyl; DMIPS, dimethyl(isopropyl)silyl; TESCl, triethylsilyl chloride; cod, 1,5-cyclooctadiene; AIBN, azobisisobutyronitrile; DM-SEGPHOS,(R)-(+)-5,5′−Bis[di(3,5-xylyl)phosphino]-4,4′-bi-1,3-benzodioxole,[(4R)-(4,4′-bi-1,3-benzodioxole)-5,5′diyl]bis[bis(3,5-dimethylphenyl)phosphine].
We have achieved the total synthesis of the structurally complex indole diterpenoid tubingensin B (1). Following validation of our initial approach, we uncovered a concise enantiospecific route that features a number of key steps, including: (a) a B-alkyl Suzuki–Miyaura coupling to access a tetrasubstituted olefin and unite readily available cyclohexyl and carbazole fragments, (b) a carbazolyne cyclization to constructtheseven-membered ring and access vicinal quaternary stereocentres, (c) a Rh-catalysed fragmentation of a fortuitously formed cyclobutenol ring, (d) and a late-stage radical cyclization stemming from a phenylselenide intermediate to install a final quaternary stereocentre and the [3.2.2]-bridged bicyclic framework of the natural product.
Of the strategic advances that enabled our synthesis, the carbazolyne cyclization is worthy of further emphasis and reflection. Arynes have a rich history, as alluded to earlier, including decades where even the existence of such species was questioned. Additionally, their high reactivity has seemingly steered chemists away from using them to assemble stereochemically complex scaffolds. The present study, on the other hand, provides an opposing perspective. Our results demonstrate that arynes can and should be used strategically to enable the synthesis of complex molecules with motifs that have conventionally been viewed as challenging, such as medium-sized rings and vicinal quaternary stereocentres.
Supplementary Material
Acknowledgements
The authors are grateful to the NIH-NIGMS (R01 GM090007 to N.K.G.), the Dreyfus Foundation, the UCLA Gold Shield Alumnae, the National Science Foundation Graduate Research Fellowship Program (DGE-1144087 to M.A.C.) and the University of California, Los Angeles for financial support. We thank Y. Tang (University of California, Los Angeles) for an authentic sample of tubingensin B. These studies were also supported by shared instrumentation grants from the NSF (no. CHE-1048804) and the National Center for Research Resources (no. S10RR025631).
Footnotes
Supplementary information and chemical compound information are available in the online version of the paper.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.TePaske MR, Gloer JB, Wicklow DT & Dowd PF The structure of tubingensin B: a cytotoxic carbazole alkaloid from the sclerotia of Aspergillus tubingensis. Tetrahedron Lett. 30, 5965–5968 (1989). [Google Scholar]
- 2.Swenson DC, TePaske MR, Baenziger NC & Gloer JB Tubingensin B, a cytotoxic carbazole alkaloid from the sclerotia of Aspergillus tubingensis. Acta Cryst. C 53, 1447–1449 (1997). [Google Scholar]
- 3.Bian M et al. Total synthesis of anominine and tubingensin A. J. Am. Chem. Soc 134, 8078–8081 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Goetz AE, Silberstein AL, Corsello MA & Garg NK Concise enantiospecific total synthesis of tubingensin A. J. Am. Chem. Soc 136, 3036–3039 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goetz AE, Shah TK & Garg NK Pyridynes and indolynes as building blocks for functionalized heterocycles and natural products. Chem. Commun 51, 34–45 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Tadross PM & Stoltz BM A comprehensive history of arynes in natural product total synthesis. Chem. Rev 112, 3550–3577 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Boente JM et al. Oxocompostelline and oxocularine, structure and synthesis. Tetrahedron Lett. 24, 2295–2298 (1983). [Google Scholar]
- 8.Huters AD, Quasdorf KW, Styduhar ED & Garg NK Total synthesis of (−)-N-methylwelwitindolinone C isothiocyanate. J. Am. Chem. Soc 133, 15797–15799 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skorobogatyi MV et al. 5-Arylethynyl-2´-deoxyuridines, compounds active against HSV-1. Org. Biomol. Chem 4, 1091–1096 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Tummatorn J & Dudley GB Generation of medium-ring cycloalkynes by ring expansion of vinylogous acyl triflates. Org. Lett 13, 1572–1575 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Gulías M, Durán J, López F, Castedo L & Mascareñas JL Palladium-catalyzed [4+3] intramolecular cycloaddition of alkylidenecyclopropanes and dienes. J. Am. Chem. Soc 129, 11026–11027 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Freeman F & Robarge KD Stereoselectivity in electrophile-mediated intramolecular cyclizations of hept-2-enitols. J. Org. Chem 54, 346–359 (1989). [Google Scholar]
- 13.White JD, Grether UM & Lee C-S (R)-(+)-3,4-Dimethylcyclohex-2-en-1-one. Org. Synth 82, 108–114 (2005). [Google Scholar]
- 14.Kojima A, Honzawa S, Boden CDJ & Shibasaki M Tandem Suzuki cross-coupling-Heck reactions. Tetrahedron Lett. 38, 3455–3458 (1997). [Google Scholar]
- 15.Dudley GB, Tan DS, Kim G, Tanski JM & Danishefsky SJ Remarkable stereoselectivity in the alkylation of a hydroazulenone: progress towards the total synthesis of guanacastepene. Tetrahedron Lett. 42, 6789–6791 (2001). [Google Scholar]
- 16.Schwartz BD, Denton JR, Lian Y, Davies HWL & Williams CM Asymmetric [4+3] cycloadditions between vinylcarbenoids and dienes: application to the total synthesis of the natural product (−)-5-epi-vibsanin E.J. Am. Chem. Soc 131, 8329–8332 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caubere P Applications of sodamide-containing complex bases in organic synthesis. Acc. Chem. Res 7, 301–308 (1974). [Google Scholar]
- 18.Gregoire B, Carre M-C & Caubere P Arynic condensation of ketone enolates.17. New general access to benzocyclobutene derivatives. J. Org. Chem 51, 1419–1427 (1986). [Google Scholar]
- 19.Legault CY CYLview v. 1.0b (Université de Sherbrooke, 2009); http://www.cylview.org [Google Scholar]
- 20.Lipomi DJ, Langille MF & Panek JS Total synthesis of basiliskamides A and B. Org. Lett 6, 3533–3536 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Szpilman AM, Cereghetti DM, Wurtz NR, Manthorpe JM & Carreira EM Synthesis of 35-deoxy amphotericinB methyl ester: a strategy for molecular editing. Angew. Chem. Int. Ed 47, 4335–4338 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Uyehara T, Murayama T, Sakai K, Ueno M & Sato T Formal substitution at both bridgeheads of a bicyclo[2.2.2]oct-5-en-2-one and its application to a synthesis of (±)-modhephene. Tetrahedron Lett. 37, 7295–7298 (1996). [Google Scholar]
- 23.Uyehara T et al. Rearrangement approaches to cyclic skeletons. XIII. Total synthesis of triquinane sesquiterpenes, (±)-modhephene and (±)-isocomene, on the basis of formal substitution at both bridgeheads of a bicyclo[2.2.2]-oct-5-en-2-one. Bull. Chem. Soc. Jpn 71, 231–242 (1998). [Google Scholar]
- 24.Tang W & Zhang X New chiral phosphorus ligands for enantioselective hydrogenation. Chem. Rev 103, 3029–3069 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Tranchier J-P, Ratovelomanana-Vidal V, Genêt J-P, Tong S & Cohen T Asymmetric hydrogenation of phenylthio ketones with chiral Ru(II) catalysts. Tetrahedron Lett. 38, 2951–2954 (1997). [Google Scholar]
- 26.Reich HJ, Chow F & Shah SK Selenium stabilized carbanions. Preparation of α-lithio selenides and applications to the synthesis of olefins by reductive elimination of β-hydroxy selenides and selenoxide syn elimination. J. Am. Chem. Soc 101, 6638–6648 (1979). [Google Scholar]
- 27.Ishida N, Sawano S, Masuda Y & Murakami M Rhodium-catalyzed ring opening of benzocyclobutenols with site-selectivity complementary to thermal ring opening. J. Am. Chem. Soc 134, 17502–17504 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Clive DLJ, Cole DC & Tao Y Formation of angularly-fused triquinanes by successive use of the Pauson–Khand reaction and radical closure. J. Org. Chem 59, 1396–1406 (1994). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.