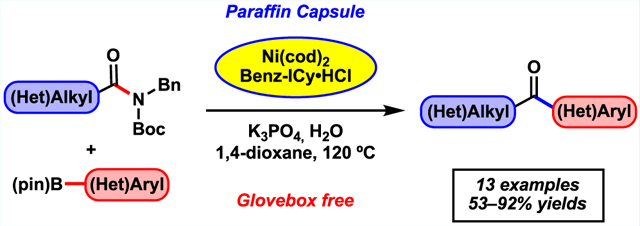
Abstract
Suzuki–Miyaura cross-couplings of amides offer an approach to the synthesis of ketones that avoids the use of basic or pyrophoric nucleophiles. However, these reactions require glovebox manipulations, thus limiting their practicality. We report a benchtop protocol for Suzuki–Miyaura cross-couplings of aliphatic amides that utilizes a paraffin capsule containing a Ni(0) precatalyst and NHC ligand. This methodology is broad in scope, is scalable, and provides a user-friendly approach to convert aliphatic amides to alkyl–aryl ketones.
Graphical Abstract
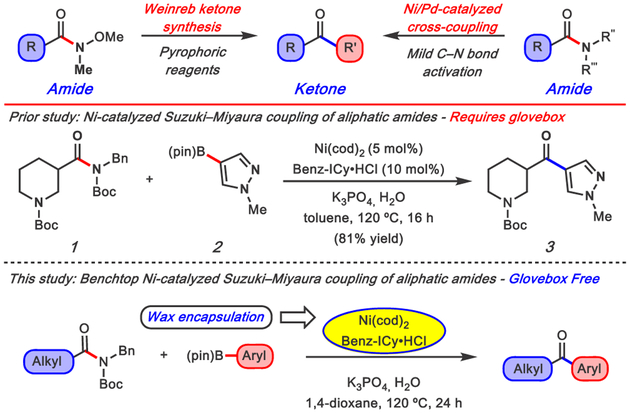
The conversion of carboxylic acid derivatives to ketones is a fundamental transformation in synthetic chemistry (Figure 1).1 A common strategy to achieve this conversion is the Weinreb ketone synthesis, in which a N-methoxy-N-methyl amide undergoes net substitution with an organometallic nucleophile.2 An alternative strategy lies in the development of transition-metal-catalyzed cross-couplings of acyl electrophiles,1c,3 which avoid the use of strongly basic and pyrophoric organometallic reagents. Our laboratory and others have shown that amides, which are well suited for multistep synthesis due to their pronounced stability, are particularly useful in this context.4 Specifically, Ni-5,6 and Pd-catalysis7 have enabled the mild activation of the amide C–N bond for cross-coupling with boronic acids and esters,8 as well as organozinc reagents.9
Figure 1.
Methods for the conversion of amides to ketones, prior studies of Ni-catalyzed Suzuki–Miyaura couplings that utilize a glovebox, and paraffin encapsulation strategy for benchtop delivery (present study).
We recently reported a Ni-catalyzed Suzuki–Miyaura coupling of aliphatic amides to generate alkyl–aryl ketones (Figure 1, e.g. 1 + 2 → 3).10,11 This methodology is broad in scope, but requires the use of a glovebox, thus limiting its practical utility.12 We questioned if a paraffin-encapsulation strategy, analogous to that pioneered by Buchwald, could prove useful.13 In this approach, air-sensitive reagents are stored in paraffin capsules, ultimately providing a user-friendly means to perform air-sensitive transition-metal-catalyzed reactions. Previously, we showed the promise of this strategy for the Suzuki–Miyaura cross-coupling of a single benzamide-derived substrate utilizing paraffin–Ni(cod)2/SIPr capsules.14 However, this precatalyst and ligand combination is ineffective in the coupling of amides derived from aliphatic carboxylic acids.10 Moreover, only a single example of a glovebox free arylation of an aliphatic amide derivative has been reported, which uses a bench-stable Pd(II) precatalyst.15 We report the realization of a paraffin encapsulation strategy to achieve the nickel-catalyzed Suzuki–Miyaura coupling of aliphatic amides on the benchtop.
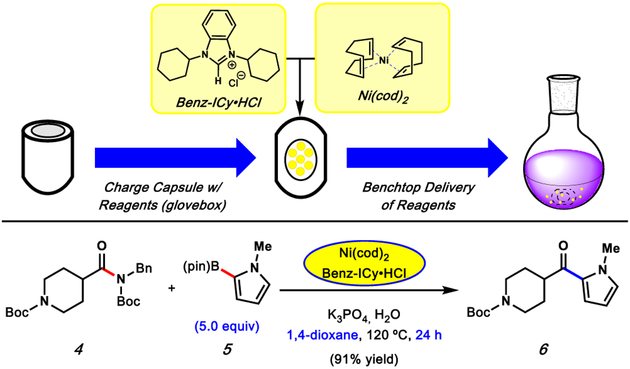
Our studies were initiated by preparing the desired paraffin capsules, using a molding process analogous to one we had previously reported (Figure 2).14 These capsules were charged with Ni(cod)2 and Benz-ICy·HCl, as this precatalyst/ligand combination had proven effective in our original studies on the Suzuki–Miyaura coupling of aliphatic amides using a glovebox.10 Next, we assessed the utility of these capsules in the benchtop Suzuki–Miyaura coupling of amide 4 with N-methylpyrrole-2-boronic acid pinacol ester (5), using 5 mol% Ni. Unfortunately, the use of our literature conditions resulted in a poor yield of ketone 6.16 Specifically, the coupling of 4 and 5 employing paraffin-encapsulated Ni(cod)2/Benz-ICy·HCl, 2.5 equiv of 5, toluene as the reaction solvent, and a stir rate of 400 rpm for 16 h at 120 °C provided ketone 9 in 28% 1H NMR yield.17 After extensive experimentation, it was found that employing higher equivalents of 5 (2.5 to 5.0), utilizing 1,4-dioxane as the reaction solvent, and extending the reaction time to 24 h proved beneficial. This provided ketone 6 in 91% yield on the benchtop. Additionally, these capsules displayed long-term air and moisture stability when stored outside of a glovebox. After two months of storage, a benchtop coupling of 4 and 5 generated 6 in comparable yield.16 These capsules are currently undergoing commercialization to enable their widespread use.18
Figure 2.
Preparation of Ni(cod)2/Benz-ICy·HCl–paraffin capsules and their use in the benchtop Suzuki–Miyaura coupling of piperidinyl amide 4 and pyrrole boronic ester 5 under optimized conditions. Yield was determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as an external standard.
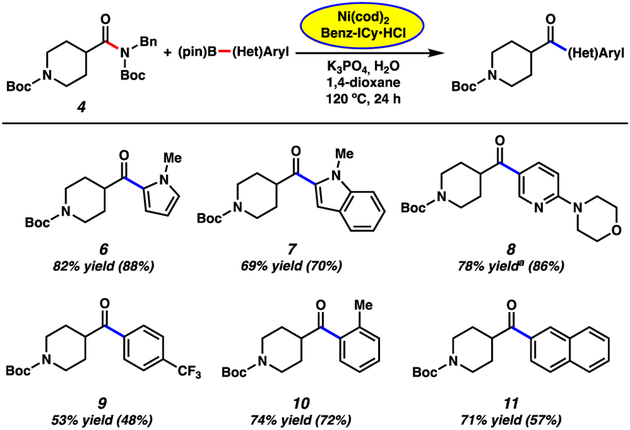
Having validated our encapsulation approach and arrived at optimized reaction conditions, we evaluated the scope of this transformation with respect to the boronate ester coupling partner. A variety of aryl boronate esters were assessed in couplings with piperidinyl amide 4 (Figure 3). The methodology was found to be tolerant of medicinally privileged N-heterocyclic aryl boronates,19 as evidenced by the formation of ketones 6–8, in good to excellent yields. Additionally, electron-poor p-CF3 and sterically encumbered o-CH3 substituted phenyl boronate esters could be employed in the coupling, providing ketones 9 and 10 in 53% and 74% yields, respectively. Boronate esters featuring extended aromatic ring systems were also competent nucleophiles in the methodology, as demonstrated by the formation of naphthyl ketone 11 in 71% yield. Of note, in all cases, benchtop yields of the desired ketone products were comparable to those obtained when using literature conditions requiring a glovebox (yields using the glovebox protocol are shown in parentheses in Figures 3 and 4).10
Figure 3.
Scope of the boronic ester coupling partners. General conditions unless otherwise stated: substrate 4 (1.0 equiv, 0.4 mmol), K3PO4 (4.0 equiv), boronic ester (5.0 equiv), Ni(cod)2 (5 mol%), Benz-ICy·HCl (10 mol%), and 1,4-dioxane (1.0 M) heated at 120 °C for 24 h in a sealed vial under an atmosphere of N2. Unless otherwise noted, yields reflect the average of two isolation experiments. Yields in parentheses were obtained by carrying out the reaction in a glovebox utilizing literature conditions without encapsulating Ni(cod)2 and Benz-ICy·HCl in paraffin. a Yield was determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as an external standard.
Figure 4.
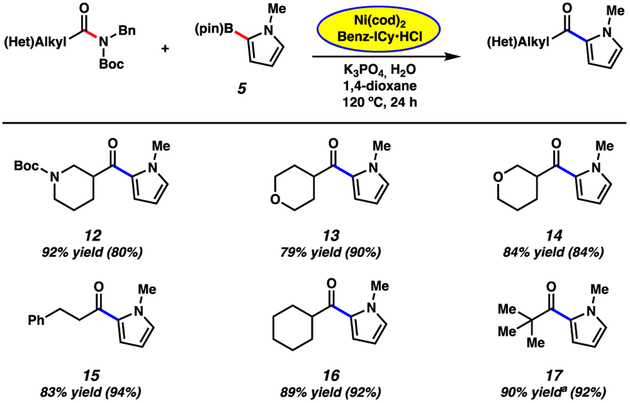
Scope of the amide substrate. General conditions unless otherwise stated: amide substrate (1.0 equiv, 0.4 mmol), K3PO4 (4.0 equiv), boronic ester 5 (5.0 equiv), Ni(cod)2 (5 mol%), Benz-ICy·HCl (10 mol%), and 1,4-dioxane (1.0 M) heated at 120 °C for 24 h in a sealed vial under an atmosphere of N2. Unless otherwise noted, yields reflect the average of two isolation experiments. Yields in parentheses were obtained by carrying out the reaction in a glovebox utilizing literature conditions without encapsulating Ni(cod)2 and Benz-ICy·HCl in paraffin. a Yield was determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as an external standard.
We next surveyed a range of amide substrates in the Suzuki–Miyaura coupling with pyrroloboronate 5 (Figure 4).20 An additional piperidine-derived amide substrate could be used in the coupling to furnish 12 in excellent yield. Furthermore, amides derived from isomeric 3- and 4-tetrahydropyran-carboxylic acids were competent substrates, giving rise to ketones 13 and 14 in 79% and 84% yields, respectively. We also evaluated the coupling of non-heterocyclic amides. Linear and carbocyclic amides underwent the reaction smoothly, as demonstrated by the formation of 15 and 16 in 83% and 89% yield, respectively. Notably, steric bulk adjacent to the amide carbonyl did not hinder the Suzuki–Miyaura coupling, as the use of a pivalamide substrate gave ketone 17 in 90% yield.
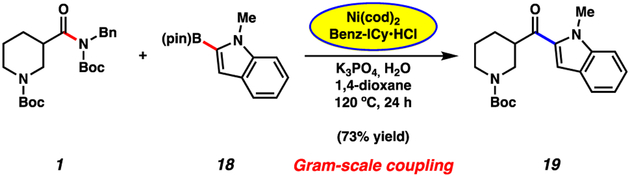
Finally, we assessed the Suzuki–Miyaura coupling of piperidine amide 1 with N-methylindole-2-boronic ester 18 on gram-scale as shown in Figure 5. Using 5 mol% Ni, the coupling proceeded smoothly to deliver ketone 19 in 73% yield. We view this result as promising in the context of the scalable construction of biologically relevant bis-heterocyclic ketones19 where the enolizable alkyl–aryl ketone provides a valuable synthetic handle for further manipulation.
Figure 5.
Gram-scale Suzuki–Miyaura coupling of amide 1 with boronate ester 18 to generate ketone 19.
We have developed a benchtop protocol for the Suzuki–Miyaura cross-coupling of aliphatic amides to access alkyl–aryl ketones. Our strategy leverages mild Ni-catalyzed C–N bond activation to avoid the use of strongly basic and pyrophoric reagents typically employed in amide to ketone conversions. Additionally, the Ni(cod)2/Benz-ICy·HCl–paraffin capsules, which are currently undergoing commercialization,18 obviate the need to set up the reactions in a glovebox. Notably, this methodology enables the coupling of heterocyclic and aliphatic amides with a variety of aryl boronic esters for the formation of C–C bonds. Moreover, this transformation is scalable and, further, provides a valuable approach to the synthesis of alkyl–aryl ketones from amides, which benefits further from the use of base-metal catalysis and commercially available boronic ester nucleophiles. Thus, we hope these studies promote the use of Ni-mediated Suzuki–Miyaura couplings of aliphatic amides as a complement to traditional synthetic strategies.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the NIH-NIGMS (R01-GM117016 to N.K.G.), the California Tobacco-Related Disease Research Program (28DT-0006 to T.B.B.), the NSF (DGE-1144087 to J.E.D.), the Trueblood Family (N.K.G.), the Foote Family (J.E.D.), and the University of California, Los Angeles for financial support. Hui-Qi Ni (Scripps Research Institute) and Angel Mendoza (University of California, Los Angeles) are acknowledged for experimental assistance. These studies were supported by shared instrumentation grants from the NSF (CHE-1048804) and the NIH-NCRR (S10RR025631). The cover was designed by the authors and created by Element26, Inc.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.9b03434.
Experimental details and compound characterization data (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).(a) Larock RC Comprehensive Organic Transformations: A Guide to Functional Group Preparations; VCH: New York, 1989; pp 685–702. [Google Scholar]; (b) Nicholson JW; Wilson AD The conversion of carboxylic acids to ketones: a repeated discovery. J. Chem. Educ 2004, 81, 1362–1366. [Google Scholar]; (c) Amani J; Molander GA Direct conversion of carboxylic acids to alkyl ketones. Org. Lett 2017, 19, 3612–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).(a) Nahm S; Weinreb SM N-Methoxy-N-methylamides as effective acylating agents. Tetrahedron Lett 1981, 22, 3815–3818. [Google Scholar]; (b) Balasubramaniam S; Aidhen IS The growing synthetic utility of the Weinreb amide. Synthesis 2008, 3707–3738. [Google Scholar]
- (3). For couplings of acyl halides, anhydrides, esters, and thioesters, see:; (a) Labadie JW; Tueting D; Stille JK Synthetic utility of the palladium-catalyzed coupling reaction of acid chlorides with organotins. J. Org. Chem 1983, 48, 4634–4642. [Google Scholar]; (b) Stille JK The palladium-catalyzed cross-coupling reactions of organotin reagents with organic electrophiles. Angew. Chem., Int. Ed 1986, 25, 508–524. [Google Scholar]; (c) Stephan MS; Teunissen AJJM; de Vries JG Heck reactions without salt formation: aromatic carboxylic anhydrides as arylating agents. Angew. Chem., Int. Ed 1998, 37, 662–664. [DOI] [PubMed] [Google Scholar]; (d) Gooßen LJ; Ghosh K Palladium-catalyzed synthesis of aryl ketones from boronic acids and carboxylic acids or anhydrides. Angew. Chem., Int. Ed 2001, 40, 3458–3460. [DOI] [PubMed] [Google Scholar]; (e) Kakino R; Narahashi H; Shimizu I; Yamamoto A Palladium-catalyzed direct conversion of carboxylic acids into ketones with organoboronic acids promoted by anhydride activators. Bull. Chem. Soc. Jpn 2002, 75, 1333–1345. [Google Scholar]; (f) Bykov VV; Korolev DN; Bumagin NA Palladium-catalyzed reactions of organoboron compounds with acyl chlorides. Russ. Chem. Bull 1997, 46, 1631–1632. [Google Scholar]; (g) Zhang L; Wu J; Shi L; Xia C; Li F Ionically tagged benzimidazole palladium(II) complex: preparation and catalytic application in cross-coupling reactions. Tetrahedron Lett 2011, 52, 3897–3901. [Google Scholar]; (h) Kakino R; Shimizu I; Yamamoto A Synthesis of trifluoromethyl ketones by palladium-catalyzed cross-coupling reaction of phenyl trifluoroacetate with organoboron compounds. Bull. Chem. Soc. Jpn 2001, 74, 371–376. [Google Scholar]; (i) Cherney AH; Reisman SE Pd-catalyzed Fukuyama cross-coupling of secondary organozinc reagents for the direct synthesis of unsymmetrical ketones. Tetrahedron 2014, 70, 3259–3265. [Google Scholar]; (j) Harada T; Kotani Y; Katsuhira T; Oku A Novel method for generation of secondary organozinc reagent: application to tandem carbon-carbon bond formation reaction of 1,1-dibromoalkane. Tetrahedron Lett 1991, 32, 1573–1576. [Google Scholar]; (k) Negishi E-I; Bagheri V; Chatterjee S; Luo F-T; Miller JA; Stoll AT Palladium-catalyzed acylation of organozincs and other organometallics as a convenient route to ketones. Tetrahedron Lett 1983, 24, 5181–5184. [Google Scholar]; (l) Iwai T; Nakai T; Mihara M; Ito T; Mizuno T; Ohno T Pd-catalyzed cross-coupling reactions of pyridine carboxylic acid chlorides with alkylzinc reagents. Synlett 2009, 1091–1094. [Google Scholar]; (m) Grey RA A palladium-catalyzed synthesis of ketones from acid chlorides and organozinc compounds. J. Org. Chem 1984, 49, 2288–2289. [Google Scholar]; (n) Bercot EA; Rovis T A mild and efficient catalytic alkylative monofunctionalization of cyclic anhydrides. J. Am. Chem. Soc 2002, 124, 174–175. [DOI] [PubMed] [Google Scholar]; (o) Bercot EA; Rovis T A palladium-catalyzed enantioselective alkylative desymmetrization of meso-succinic anhydrides. J. Am. Chem. Soc 2004, 126, 10248–10249. [DOI] [PubMed] [Google Scholar]; (p) Johnson JB; Yu RT; Fink P; Bercot EA; Rovis T Selective substituent transfer from mixed zinc reagents in Ni-catalyzed anhydride alkylation. Org. Lett 2006, 8, 4307–4310. [DOI] [PubMed] [Google Scholar]; (q) Tokuyama H; Yokoshima S; Yamashita T; Fukuyama T A novel ketone synthesis by a palladium-catalyzed reaction of thiol esters and organozinc reagents. Tetrahedron Lett 1998, 39, 3189–3192. [Google Scholar]; (r) Mori Y; Seki M A novel procedure for the synthesis of multifunctional ketones through the Fukuyama coupling reaction employing dialkylzincs. Tetrahedron Lett 2004, 45, 7343–7345. [Google Scholar]; (s) Miyazaki T; Han-ya Y; Tokuyama H; Fukuyama T New odorless protocols for the synthesis of aldehydes and ketones from thiol esters. Synlett 2004, 3, 477–480. [Google Scholar]; (t) Dieter RK Reaction of acyl chlorides with organometallic reagents: a banquet table of metals for ketone synthesis. Tetrahedron 1999, 55, 4177–4236. [Google Scholar]; (u) Shi S; Nolan SP; Szostak M Well-defined palladium(II)-NHC precatalysts for cross-coupling reactions of amides and esters by selective N–C/O–C cleavage. Acc. Chem. Res 2018, 51, 2589–2599. [DOI] [PubMed] [Google Scholar]; (v) Ben Halima T; Zhang W; Yalaoui T; Hong X; Yang Y-F; Houk KN; Newman SG Palladium-catalyzed Suzuki–Miyaura coupling of aryl esters. J. Am. Chem. Soc 2017, 139, 1311–1318. [DOI] [PubMed] [Google Scholar]
- (4). For recent reviews, see:; (a) Dander JE; Garg NK Breaking amides using nickel catalysis. ACS Catal 2017, 7, 1413–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Meng G; Shi S; Szostak M Cross-coupling of amides by N–C bond activation. Synlett 2016, 2530–2540. [Google Scholar]; (c) Takise R; Muto K; Yamaguchi J Cross-coupling of aromatic esters and amides. Chem. Soc. Rev 2017, 46, 5864–5888. [DOI] [PubMed] [Google Scholar]; (d) Kaiser D; Bauer A; Lemmerer M; Maulide N Chem. Soc. Rev 2018, 47, 7899–7925. [DOI] [PubMed] [Google Scholar]
- (5). For nickel-catalyzed reactions proceeding with cleavage of the amide C–N bond, see:; (a) Hie L; Fine Nathel NF; Shah TK; Baker EL; Hong X; Yang Y-F; Liu P; Houk KN; Garg NK Conversion of amides to esters by the nickel-catalysed activation of amide C-N bonds. Nature 2015, 524, 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Weires NA; Baker EL; Garg NK Nickel-catalysed Suzuki–Miyaura coupling of amides. Nat. Chem 2016, 8, 75–79. [DOI] [PubMed] [Google Scholar]; (c) Baker EL; Yamano MM; Zhou Y; Anthony SM; Garg NK A two-step approach to achieve secondary amide transamidation enabled by nickel catalysis. Nat. Commun 2016, 7, 11554. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Simmons BJ; Weires NA; Dander JE; Garg NK Nickel-catalyzed alkylation of amide derivatives. ACS Catal 2016, 6, 3176–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Shi S; Szostak M Nickel-catalyzed diaryl ketone synthesis by N–C bond cleavage: direct Negishi cross-coupling of primary amides by site-selective N,N-di-Boc activation. Org. Lett 2016, 18, 5872–5875. [DOI] [PubMed] [Google Scholar]; (f) Shi S; Szostak M Efficient synthesis of diaryl ketones by nickel-catalyzed Negishi cross-coupling of amides via carbon–nitrogen bond cleavage at room temperature accelerated by solvent effect. Chem. Eur. J 2016, 22, 10420–10424. [DOI] [PubMed] [Google Scholar]; (g) Hie L; Baker EL; Anthony SM; Desrosiers J-N; Senanayake C; Garg NK Nickel-catalyzed esterification of aliphatic amides. Angew. Chem., Int. Ed 2016, 55, 15129–15132. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Dey A; Sasmal S; Seth K; Lahiri GK; Maiti D Nickel-catalyzed deamidative step-down reduction of amides to aromatic hydrocarbons. ACS Catal 2017, 7, 433–437. [Google Scholar]; (i) Ni S; Zhang W; Mei H; Han J; Pan Y Ni-catalyzed reductive cross-coupling of amides with aryl iodide electrophiles via C–N bond activation. Org. Lett 2017, 19, 2536–2539. [DOI] [PubMed] [Google Scholar]; (j) Medina JM; Moreno J; Racine S; Du S; Garg NK Mizoroki–Heck cyclizations of amide derivatives for the introduction of quaternary centers. Angew. Chem., Int. Ed 2017, 56, 6567–6571. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Hu J; Wang M; Pu X; Shi Z Nickel-catalysed retro-hydroamidocarbonylation of aliphatic amides to olefins. Nat. Commun 2017, 8, 14993. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Weires NA; Caspi DD; Garg NK Kinetic modeling of the nickel-catalyzed esterification of amides. ACS Catal 2017, 7, 4381–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Shi S; Szostak M Nickel-catalyzed Negishi cross-coupling of N-acylsuccinimides: stable, amide-based, twist-controlled acyl transfer reagents via N–C activation. Synthesis 2017, 3602–3608. [Google Scholar]; (n) Dander JE; Baker EL; Garg NK Nickel-catalyzed transamidation of aliphatic amide derivatives. Chem. Sci 2017, 8, 6433–6438. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Huang P-Q; Chen H Ni-catalyzed cross-coupling reactions of N-acylpyrrole-type amides with organoboron reagents. Chem. Commun 2017, 53, 12584–12587. [DOI] [PubMed] [Google Scholar]; (p) Deguchi T; Xin H-L; Morimoto H; Ohshima T Direct catalytic alcoholysis of unactivated 8-aminoquinoline amides. ACS Catal 2017, 7, 3157–3161. [Google Scholar]; (q) Dander JE; Giroud M; Racine S; Darzi ER; Alvizo O; Entwistle D; Garg NK Chemoenzymatic conversion of amides to enantioenriched alcohols in aqueous medium. Commun. Chem 2019, 2, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6). For nickel-catalyzed decarbonylative coupling reactions of amides, see:; (a) Shi S; Meng G; Szostak M Synthesis of biaryls via nickel-catalyzed Suzuki-Miyaura coupling of amides by carbon–nitrogen cleavage. Angew. Chem., Int. Ed 2016, 55, 6959–6963. [DOI] [PubMed] [Google Scholar]; (b) Hu J; Zhao Y; Liu J; Zhang Y; Shi Z Nickel-catalyzed decarbonylative borylation of amides: evidence for acyl C–N bond activation. Angew. Chem., Int. Ed 2016, 55, 8718–8722. [DOI] [PubMed] [Google Scholar]; (c) Srimontree W; Chatupheeraphat A; Liao H-H; Rueping M Amide to alkyne interconversion via a nickel/copper-catalyzed deamidative cross-coupling of aryl and alkenyl amides. Org. Lett 2017, 19, 3091–3094. [DOI] [PubMed] [Google Scholar]; (d) Liu C; Szostak M Decarbonylative phosphorylation of amides by palladium and nickel catalysis: the Hirao cross-coupling of amide derivatives. Angew. Chem., Int. Ed 2017, 56, 12718–12722. [DOI] [PubMed] [Google Scholar]; (e) Yue H; Guo L; Liao H-H; Cai Y; Zhu C; Rueping M Catalytic ester and amide to amine interconversion: nickel-catalyzed decarbonylative amination of esters and amides by C–O and C–C bond activation. Angew. Chem., Int. Ed 2017, 56, 4282–4285. [DOI] [PubMed] [Google Scholar]; (f) Chatupheeraphat A; Liao H-H; Lee S-C; Rueping M Nickel-catalyzed C–CN bond formation via decarbonylative cyanation of esters, amides, and intramolecular recombination fragment coupling of acyl cyanides. Org. Lett 2017, 19, 4255–4258. [DOI] [PubMed] [Google Scholar]; (g) Yue H; Guo L; Lee S-C; Liu X; Rueping M Selective reductive removal of ester and amide groups from arenes and heteroarenes through nickel-catalyzed C–O and C–N bond activation. Angew. Chem., Int. Ed 2017, 56, 3972–3976. [DOI] [PubMed] [Google Scholar]; (h) Lee S-C; Guo L; Yue H; Liao H-H; Rueping M Nickel-catalyzed decarbonylative silylation, borylation, and amination of arylamides via a deamidative reaction pathway. Synlett 2017, 2594–2598. [Google Scholar]
- (7). For Pd-catalyzed amide C-N bond activations, see:; (a) Li X; Zou G Acylative Suzuki coupling of amides: acyl-nitrogen activation via synergy of independently modifiable activating groups. Chem. Commun 2015, 51, 5089–5092. [DOI] [PubMed] [Google Scholar]; (b) Yada A; Okajima S; Murakami M Palladium-catalyzed intramolecular insertion of alkenes into the carbon–nitrogen bond of β-lactams. J. Am. Chem. Soc 2015, 137, 8708–8711. [DOI] [PubMed] [Google Scholar]; (c) Meng G; Szostak M Palladium-catalyzed Suzuki–Miayura coupling of amides by carbon–nitrogen cleavage: general strategy for amide N–C bond activation. Org. Biomol. Chem 2016, 14, 5690–5705. [DOI] [PubMed] [Google Scholar]; (d) Meng G; Szostak M General olefin synthesis by the palladium-catalyzed Heck reaction of amides: sterically-controlled chemoselective N–C activation. Angew. Chem., Int. Ed 2015, 54, 14518–14522. [DOI] [PubMed] [Google Scholar]; (e) Meng G; Szostak M Sterically-controlled Pd-catalyzed chemoselective ketone synthesis via N–C cleavage in twisted amides. Org. Lett 2015, 17, 4364–4367. [DOI] [PubMed] [Google Scholar]; (f) Liu C; Meng G; Liu Y; Liu R; Lalancette R; Szostak R; Szostak M N-Acylsaccharins: stable electrophilic amide-based acyl transfer reagents in Pd-catalyzed Suzuki–Miyaura coupling via N–C cleavage. Org. Lett 2016, 18, 4194–4197. [DOI] [PubMed] [Google Scholar]; (g) Lei P; Meng G; Szostak M General method for the Suzuki–Miyaura cross-coupling of amides using commercially available, air- and moisture-stable palladium/NHC (NHC = N-heterocyclic carbene) complexes. ACS Catal 2017, 7, 1960–1965. [DOI] [PubMed] [Google Scholar]; (h) Liu C; Liu Y; Liu R; Lalancette R; Szostak R; Szostak M Palladium-catalyzed Suzuki–Miyaura cross-coupling of N-mesyl amides by N–C cleavage: electronic effect of the mesyl group. Org. Lett 2017, 19, 1434–1437. [DOI] [PubMed] [Google Scholar]; (i) Liu C; Meng G; Szostak M N-Acylsaccharins as amide-based arylating reagents via chemoselective N-C cleavage: Pd-catalyzed decarbonylative Heck reaction. J. Org. Chem 2016, 81, 12023–12030. [DOI] [PubMed] [Google Scholar]; (j) Meng G; Shi S; Szostak M Palladium-catalyzed Suzuki–Miyaura cross-coupling of amides via site-selective N–C bond cleavage by cooperative catalysis. ACS Catal 2016, 6, 7335–7339. [Google Scholar]; (k) Cui M; Wu H; Jian J; Wang H; Liu C; Stelck D; Zeng Z Palladium-catalyzed Sonogashira coupling of amides: access to ynones via C–N bond cleavage. Chem. Commun 2016, 52, 12076–12079. [DOI] [PubMed] [Google Scholar]; (l) Wu H; Li Y; Cui M; Jian J; Zeng Z Suzuki coupling of amides via palladium-catalyzed C–N cleavage of N-acylsaccharins. Adv. Synth. Catal 2016, 358, 3876–3880. [Google Scholar]; (m) Shi S; Szostak M Decarbonylative cyanation of amides by palladium catalysis. Org. Lett 2017, 19, 3095–3098. [DOI] [PubMed] [Google Scholar]; (n) Lei P; Meng G; Ling Y; An J; Szostak M Pd-PEPPSI: Pd-NHC precatalyst for Suzuki–Miyaura cross-coupling reactions of amides. J. Org. Chem 2017, 82, 6638–6646. [DOI] [PubMed] [Google Scholar]; (o) Meng G; Szostak R; Szostak M Suzuki–Miyaura cross-coupling of N-acylpyrroles and pyrazoles: planar, electronically activated amides in catalytic N–C cleavage. Org. Lett 2017, 19, 3596–3599. [DOI] [PubMed] [Google Scholar]; (p) Meng G; Lalancette R; Szostak R; Szostak M N-Methylamino pyrimidyl amides (MAPA): highly reactive, electronically-activated amides in catalytic N–C(O) cleavage. Org. Lett 2017, 19, 4656–4659. [DOI] [PubMed] [Google Scholar]; (q) Osumi Y; Szostak M N-Acylsuccinimides: twist-controlled, acyl transfer reagents in Suzuki-Miyaura cross-coupling by N–C amide bond activation. Org. Biomol. Chem 2017, 15, 8867–8871. [DOI] [PubMed] [Google Scholar]; (r) Lei P; Meng G; Ling Y; An J; Nolan SP; Szostak M General method for the Suzuki–Miyaura cross-coupling of primary amide-derived electrophiles enabled by [Pd(NHC)(cin)-Cl] at room temperature. Org. Lett 2017, 19, 6510–6513. [DOI] [PubMed] [Google Scholar]; (s) Li X; Zou G Palladium-catalyzed acylative cross-coupling of amides with diarylboronic acids and sodium tetraarylborates. J. Organomet. Chem 2015, 794, 136–145. [Google Scholar]; (t) Liu C; Li G; Shi S; Meng G; Lalancette R; Szostak R; Szostak M Acyl and decarbonylative Suzuki coupling of N-acetyl amides: Electronic tuning of twisted, acyclic amides in catalytic carbon-nitrogen bond cleavage. ACS Catal 2018, 8, 9131–9139. [Google Scholar]; (u) Meng G; Szostak M Palladium/NHC (NHC = N-heterocyclic carbene)-catalyzed B-alkyl Suzuki cross-coupling of amides by selective N–C bond cleavage. Org. Lett 2018, 20, 6789–6793. [DOI] [PubMed] [Google Scholar]; (v) Shi S; Szostak M Decarbonylative borylation of amides by palladium catalysis. ACS Omega 2019, 4, 4901–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]; (w) Zhou T; Li G; Nolan SP; Szostak M [Pd(NHC)(acac)Cl]: well-defined, air-stable, and readily available precatalysts for Suzuki and Buchwald–Hartwig cross-coupling (transamidation) of amides and esters by N-C/O-C activation. Org. Lett 2019, 21, 3304–3309. [DOI] [PubMed] [Google Scholar]
- (8). For Ni- and Pd-catalyzed cross-couplings of amides with aryl boronic esters or acids, see refs 5b, o, q, 6a, b, 7a, c, f–h, j, n, o, q–u, w.
- (9). For Ni- and Pd-catalyzed cross-couplings of amides with alkyl zinc reagents, see refs 5d–f, m.
- (10).Boit TB; Weires NA; Kim J; Garg NK Nickel-catalyzed Suzuki–Miyaura coupling of aliphatic amides. ACS Catal 2018, 8, 1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11). The Molander group has reported a dual-metal photoredox approach to the synthesis of alkyl–alkyl ketones using alkyltrifluoroborate salts and aliphatic acyl succinimides; see:; Amani J; Alam R; Badir S; Molander GA Synergistic visible-light photoredox/nickel-catalyzed synthesis of aliphatic ketones via N–C cleavage of imides. Org. Lett 2017, 19, 2426–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12). The use of air-stable Ni(II) precatalysts represents a complementary strategy for avoiding glovebox manipulations; see:; (a) Shields JD; Gray EE; Doyle AG A modular, air-stable nickel precatalyst. Org. Lett 2015, 17, 2166–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Magano J; Monfette S Development of an air-stable, broadly applicable nickel source for nickel-catalyzed cross-coupling. ACS Catal 2015, 5, 3120–3123. [Google Scholar]; (c) Standley EA; Jamison TF Simplifying nickel(0) catalysis: an air-stable nickel precatalyst for the internally selective benzylation of terminal alkenes. J. Am. Chem. Soc 2013, 135, 1585–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Park NH; Teverovskiy G; Buchwald SL Development of an air-stable nickel precatalyst for the amination of aryl chlorides, sulfamates, mesylates, and triflates. Org. Lett 2014, 16, 220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Ge S; Hartwig JF Highly reactive, single-component nickel catalyst precursor for Suzuki–Miyaura cross-coupling of heteroaryl boronic acids with heteroaryl halides. Angew. Chem., Int. Ed 2012, 51, 12837–12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).(a) Sather AC; Lee HG; Colombe JR; Zhang A; Buchwald SL Dosage delivery of sensitive reagents enables glovebox free synthesis. Nature 2015, 524, 208–211. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fang Y; Liu Y; Ke Y; Guo C; Zhu N; Mi X; Ma Z; Hu Y A new chromium-based catalyst coated with paraffin for ethylene oligomerization and the effect of chromium state on oligomerization selectivity. Appl. Catal., A 2002, 235, 33–38. [Google Scholar]; (c) Taber DF; Frankowski KJ Grubbs’ catalyst in paraffin: an air-stable preparation for alkene metathesis. J. Org. Chem 2003, 68, 6047–6048. [DOI] [PubMed] [Google Scholar]
- (14).(a) Dander JE; Weires NA; Garg NK Benchtop delivery of Ni(cod)2 using paraffin capsules. Org. Lett 2016, 18, 3934–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dander JE; Morrill LA; Nguyen MM; Chen S; Garg NK Breaking amide C–N bonds in an undergraduate organic chemistry laboratory. J. Chem. Educ 2019, 96, 776–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Lei P; Meng G; Shi S; Ling Y; An J; Szostak R; Szostak M Suzuki–Miyaura cross-coupling of amides and esters at room temperature: correlation with barriers to rotation around C–N and C–O bonds. Chem. Sci 2017, 8, 6525–6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16). See the Supporting Information (SI) for details.
- (17). See the Supporting Information for full optimization details.
- (18). TCI Chemicals is commercializing the capsules containing Ni(cod)2 and Benz-ICy·HCl.
- (19).(a) Vitaku E; Smith DT; Njardarson JT Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem 2014, 57, 10257–10274. [DOI] [PubMed] [Google Scholar]; (b) Hirata T; Funatsu T; Keto Y; Nakata M; Sasamata M Pharmacological profile of ramosetron, a novel therapeutic agent for IBS. Inflammopharmacology 2007, 15, 5–9. [DOI] [PubMed] [Google Scholar]
- (20). Amide derivatives featuring various N-substituents have been employed successfully in cross-coupling reactions (see ref 4). N-Bn-N-Boc derivatives were selected for the present study due to their utility in the glovebox methodology (see ref 10) and ease of preparation.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.