Introduction
Necrobiosis lipoidica (NL) is an idiopathic granulomatous disorder that causes plaques on the lower part of the legs that commonly ulcerate. Histologically, NL shows palisaded granulomatous inflammation horizontally layered within degenerated collagen and an associated lymphoplasmacytic infiltrate. Little is known about the molecular pathogenesis of NL, and no reliably effective therapies exist.
In autoimmune granulomatous disorders, macrophage recruitment and activation appear to depend on T-cell–derived cytokines including interferon gamma. Interference with such cytokine signals via blockade of the downstream Janus kinase (JAK)–signal transducer and activator of transcription (STAT) signaling pathway is a promising new treatment approach in disorders characterized by excessive macrophage activation.1,2 We and others have recently shown that JAK inhibitors are effective in treating sarcoidosis and granuloma annulare (GA).3, 4, 5, 6
In 2018, Lee and English7 reported that a patient with ulcerative NL in the setting of polycythemia vera had marked improvement in the ulcerative component of her NL with ruxolitinib, a JAK1/2 inhibitor.7 Although provocative, it remains unclear how reproducible this effect is and whether JAK-STAT is activated in other cases of NL.
Histologic case series
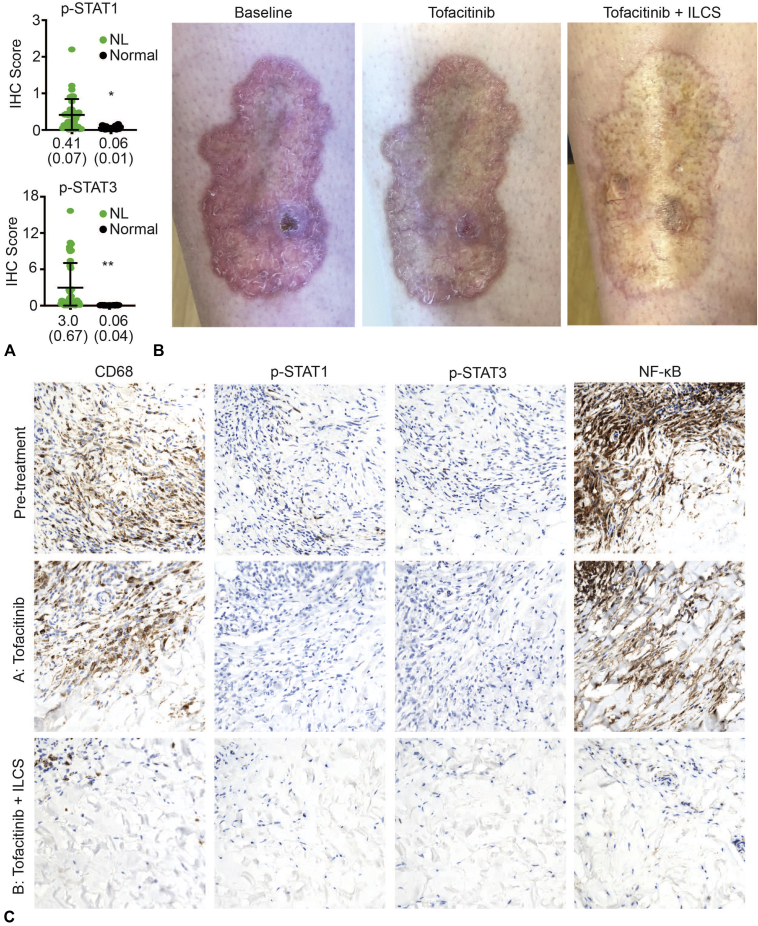
We evaluated 11 historical NL biopsy specimens using phosphorylated (p) STAT1 and p-STAT3 immunohistochemistry. p-STAT antibodies recognize STAT proteins only when the JAK-STAT pathway is activated. This approach has been described previously.3,6 We found constitutive activation of both STAT1 and STAT3 in all cases of NL examined (Fig 1, A). Very little STAT1 or STAT3 activation was observed in normal skin from 7 healthy control individuals (Fig 1, A). These data suggest that JAK-STAT signaling is constitutively activated at low levels in NL and that JAK inhibition might be an effective treatment approach in patients with this disease.
Fig 1.
Clinical and histologic responses of necrobiosis lipoidica to tofacitinib and intralesional corticosteroid. A, Quantification of p-STAT1 and p-STAT3 immunohistochemistry staining in 11 cases of NL from a histologic case series assembled from historical biopsy specimens. Three representative fields were scored for each case, and 7 cases of normal skin were included for comparison (as previously described3,6). *P = .0004, **P = .0027. Data are shown as mean (standard error of the mean). B, Clinical photographs from the patient described in the case report at baseline (left panel), after 6 weeks of tofacitinib (middle panel), and with tofacitinib (9 months' duration) plus concomitant ILCS; the photos in the right panel were taken 8 weeks after ILCS administration. C, Immunohistochemical analysis of biopsy specimens from the patient described in the case report taken at various intervals. Shown are CD68 (macrophage marker), p-STAT1 TYR701 (p-STAT1), p-STAT3 TYR705, and total NF-κB. (NF-κB is only transcriptionally active when present in the nucleus.) IHC, Immunohistochemistry; NF-κB, nuclear factor κB; NL, necrobiosis lipoidica; p, phosphorylated; STAT, signal transducer and activator of transcription.
Case report
With this molecular rationale in mind, we treated a patient with long-standing recalcitrant NL with tofacitinib, a JAK1/3 inhibitor. The patient is a 25-year-old woman with type I diabetes and a 9-year history of worsening NL on her shins. She reported frequent ulceration and slow wound healing. Treatment with topical triamcinolone, intralesional corticosteroids (ILCS) (triamcinolone 10 mg/mL), and pentoxifylline had not led to improvement. Physical examination showed pink-yellow plaques with focal ulceration (Fig 1, B).
Given that the patient's NL was refractory to standard treatment, off-label tofacitinib 5 mg twice daily was initiated. After 6 weeks of tofacitinib, the ulcer had healed. During the next 9 months of treatment, the patient noticed more rapid wound healing within the plaques after minor trauma, but the extent of the plaques did not diminish (Fig 1, B). Therefore, ILCS (triamcinolone 5 mg/mL, which was previously ineffective at higher doses) was begun, and tofacitinib was continued. In areas treated with ILCS, inflammation decreased, and the plaques flattened (Fig 1, B).
Noting that tofacitinib plus ILCS was superior to either monotherapy, we reasoned that the synergy may be due to the simultaneous blockade of JAK-dependent and JAK-independent cytokines, respectively. In particular, corticosteroids block nuclear factor κB (NF-κB) signaling, a pathway central to many non–JAK-dependent cytokines, such as tumor necrosis factor α.8
To better understand the response, biopsy specimens were obtained from an area of the plaque without ILCS treatment (tofacitinib alone, Area A) and an area of the plaque also treated with ILCS (representing tofacitinib + ILCS, Area B) (Fig 1, B). Area A showed a persistence of macrophages despite abrogation of JAK-STAT signaling (Fig 1, C); however, nuclear NF-κB staining (representing the activity of JAK-STAT–independent cytokines) was present. In area B, there was nearly complete resolution of inflammation, and p-STAT1/3 and NF-κB staining were essentially negative. Only dermal fibrosis remained.
After 11 months, the tofacitinib was reduced to 5 mg daily, which the patient has been taking for 3 months with stable control of disease. Treatment has been well tolerated.
Discussion
These data suggest that JAK inhibition plus ILCS may provide optimal disease control in refractory NL by simultaneously blocking JAK-dependent and JAK-independent inflammation. It is interesting that JAK inhibition alone normalized wound healing in both our single patient and the patient reported by Lee and English7 but did not remit the disease, suggesting that NL may be less susceptible to JAK inhibitor monotherapy than sarcoidosis and GA. More work is underway to better understand the role of JAK inhibition in the treatment of cutaneous granulomatous diseases and how differences in underlying biology among sarcoidosis, GA, NL, and other granulomatous disorders might explain differential responses to treatment.
Footnotes
Funding sources: Supported by a gift (to Dr King) from the Ranjini and Ajay Poddar Resource Fund for Dermatologic Diseases Research. Dr Damsky is supported by the Dermatology Foundation.
Disclosure: Dr Damsky has received research funding from Pfizer, but it did not support this work; he is a consultant for Eli Lilly. Dr King is an investigator for Concert Pharmaceuticals Inc, Eli Lilly and Company, and Pfizer Inc; is a consultant to and/or has served on advisory boards for Aclaris Therapeutics, Arena Pharmaceuticals, Concert Pharmaceuticals Inc, Dermavant Sciences, Eli Lilly and Company, and Pfizer Inc; and is on speakers bureaus for Pfizer Inc, Regeneron, and Sanofi Genzyme. Ms Singh and Dr Galan have no conflicts of interest to declare.
Contributor Information
William Damsky, Email: william.damsky@yale.edu.
Brett King, Email: brett.king@yale.edu.
References
- 1.De Vries L.C.S., Duarte J.M., De Krijger M. A JAK1 Selective kinase inhibitor and tofacitinib affect macrophage activation and function. Inflamm Bowel Dis. 2019;25:647–660. doi: 10.1093/ibd/izy364. [DOI] [PubMed] [Google Scholar]
- 2.Broglie L., Pommert L., Rao S. Ruxolitinib for treatment of refractory hemophagocytic lymphohistiocytosis. Blood Adv. 2017;1:1533–1536. doi: 10.1182/bloodadvances.2017007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damsky W., Thakral D., Emeagwali N., Galan A., King B. Tofacitinib treatment and molecular analysis of cutaneous sarcoidosis. N Engl J Med. 2018;379:2540–2546. doi: 10.1056/NEJMoa1805958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei J.J., Kallenbach L.R., Kreider M., Leung T.H., Rosenbach M. Resolution of cutaneous sarcoidosis after Janus kinase inhibitor therapy for concomitant polycythemia vera. JAAD Case Rep. 2019;5:360–361. doi: 10.1016/j.jdcr.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rotenberg C., Besnard V., Brillet P.Y., Giraudier S., Nunes H., Valeyre D. Dramatic response of refractory sarcoidosis under ruxolitinib in a patient with associated JAK2-mutated polycythemia. Eur Respir J. 2018;52(6):1–4. doi: 10.1183/13993003.01482-2018. [DOI] [PubMed] [Google Scholar]
- 6.Damsky W., Thakral D., McGeary M.K., Leventhal J., Galan A., King B. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2019 doi: 10.1016/j.jaad.2019.05.098. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J.J., English J.C., III Improvement in ulcerative necrobiosis lipoidica after Janus kinase-inhibitor therapy for polycythemia vera. JAMA Dermatol. 2018;154:733–734. doi: 10.1001/jamadermatol.2018.0756. [DOI] [PubMed] [Google Scholar]
- 8.Coutinho A.E., Chapman K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]