Abstract
Adjuvant radiotherapy after breast cancer surgery is an important part of breast cancer treatment improving local control and overall survival. However, a higher risk of cardiac mortality was observed when conventional radiotherapy techniques were used. Cardiac morbidity and mortality after radiation therapy have been studied in many meta-analyses. In those focused on modern radiotherapy techniques, cardiac morbidity and mortality were no longer presented. However, an extremely long follow-up period is required. Importantly, the cardiac morbidity rates vary depending not only on the dose delivered to the heart, but also on the systemic therapies administrated and the pre-existing cardiac disease. Systematic heart dose monitoring is of great importance, as are efforts to constantly decrease doses, using advanced radiotherapy techniques. Nowadays, it is essential to individualize treatment according to tumor characteristics and anatomical predispositions, and to consider the cost and benefits.
Keywords: Cardiotoxicity, Radiotherapy, Breast cancer
The importance of adjuvant radiotherapy (RT) following breast cancer surgery as part of the treatment protocol for improving local control and overall survival has been confirmed in several meta-analyses. Radiotherapy after breast-conserving surgery reduces the risk of local recurrence by 50% and contributes to the improvement of overall survival among patients with node-positive and node-negative disease.1 In patients with lymph node positivity, adjuvant radiotherapy after mastectomy improves the 10-year locoregional control rate by 60–80% and reduces the 10-year risk of overall recurrence by 25–33% and the 15-year risk of death from breast cancer by 13–22%.2
Radiotherapy influences surrounding healthy tissues and can lead to persistent edema, hyperpigmentation, fibrosis and pneumonitis and more severe late effect like cardiac toxicity and second primary cancer. The probability of radiotherapy long-term side effects generally depends on the dose per fraction, time interval between fractions, total radiation dose, irradiated volume, dosimetric parametres, cardiotoxicity of chemotherapy and anti Her 2 therapy and patient-specific risk factors. For instance, a synergistic effect was observed between smoking and RT on the risk of heart attack. The presence of hypertension was associated with a higher risk of coronary artery disease after left-sided breast radiotherapy.3,4
1. Physiopathology of long term side effects induced by radiotherapy
Heart irradiation, resp. the dose to the cardiac structures from left tangential fields, can cause many changes manifesting themselves as radiotherapy-induced heart disease (RIHD). Cardiac dysfunction due to radiation involves not only coronary heart disease (CHD) and pericardial disease (acute pericarditis, delayed and constrictive pericarditis) but, also, congestive heart failure, valvular damage, cardiomyopathies and arrhythmias. The main pathology is radiation-induced fibrosis and microvascular damage leading to ischemia. The symptoms of RIHD may include chest pain (angina), dyspnea, swollen feet, fatigue and irregular heartbeat. The volume of heart irradiated is a major factor in the development of chronic cardiac toxicity. Gagliari retrospectively analyzed 100 patients irradiated for left-sided breast cancer.5 The calculated risk of cardiac mortality increased with dose escalation. A Swedish study included 960 breast cancer patients enrolled between 1971 and 1976 who were allocated to three groups: low, intermediate, and high cardiac dose-volumes. Mortality due to ischemic heart disease was significantly increased in the group with the highest proportion of irradiated heart volume.6 In the other groups, the mortality due to ischemic heart disease was similar to that of surgical controls. To date, the cardiotoxicity of radiotherapy has been studied in many randomized trials and meta-analyses. In the earlier ones, conventional left-sided radiotherapy increased ischemic cardiac disease morbidity and mortality.7, 8, 9, 10 The first meta-analysis by Cuzcik published in 1987 reported significantly worse survival rates of irradiated patients after the first 10 years.7
2. Patient-specific risk factors
A large study using data from the SEER (Surveillance, Epidemiology, and End Results) cancer registries analyzed 1555 women irradiated for left-sided breast cancer and 1451 women with right-sided breast cancer.11 Among the irradiated patients, 2% of women with left-sided radiotherapy experienced fatal myocardial infarction compared to 1% of women with right-sided cases. The risk was significantly increased among women under the age of 60 years. Another large study analyzed almost 50,000 irradiated women reported to the Swedish Cancer Registry from 1970 to 1985 and observed an association between left-sided radiotherapy and late cardiac morbidity.12,13 Myocardial infarction mortality was significantly higher among women with left-sided breast cancer radiotherapy. An EBCTCG (Early Breast Cancer Trialists’ Collaborative Group) meta-analysis of 40 randomized trials with around 20,000 women showed a decrease in tumor-specific mortality; however, non-breast cancer mortality was increased.14
All the above trials have in common a relatively long follow-up time of patients irradiated in the 1970s and 1980s; however, older conventional radiotherapy techniques were used.
Recent clinical trials using modern treatment techniques have not documented an association between radiotherapy and cardiac morbidity or mortality. An analysis of a large Danish study with a 12-year follow-up comparing women with versus without adjuvant radiotherapy (irradiated from 1982 to 1990) did not show an increased risk of ischemic heart disease among women in the radiotherapy group.15 A meta-analysis of 36 EBCTCG studies confirmed significant improvement of overall survival after adjuvant radiotherapy as long as the current techniques and standard fractionation are used.16 Doyle´s analysis of data from the SEER registry, published in 2007, assessed the benefits of adjuvant radiotherapy, cardiac toxicity and impact of pre-existing risk factors. A total of 48,353 women over 65 years were involved and the median follow-up was 13 years. The analysis concluded that radiotherapy does not increase the risk of myocardial infarction over at least 10 years regardless of pre-existing risk factors.17
Of great importance was a paper by Sarah Darby published in 2013. This population study was based on data from the Sweden and Norway registers including patients irradiated from 1958 to 2001. For each woman, the mean radiation doses to the heart were estimated. The rates of major coronary artery events were rising linearly by 7.4% for each 1 Gy increase in mean heart dose, starting within the first 5 years after radiotherapy and continued for another three decades. An important role is played by pre-existing cardiac risk factors, too.18 Similarly, French authors proved that each Gy added to the mean heart dose would increase the risk of cardiotoxicity by 4%.19
Radiotherapy has made impressive advances in the second half of the 20th century with technological development continuing in the 21st century. The target volume doses remain the same. However, modern radiotherapy techniques have significantly reduced the doses delivered to the heart and major coronary vessels.
Over the last two years, several meta-analyses estimating the long-term risks of radiotherapy have been published. A paper from the Early Breast Cancer Trialists’ Collaborative Group published in 2017 focused on lung cancer risks. The estimated absolute risk of lung cancer development was 4% for smokers and 0.3% for non-smokers. Cardiac mortality was approximately 1% for smokers and 0.3% for non-smokers. Regarding long-term smokers, the absolute risk of modern radiotherapy may outweigh the benefits, but for most non-smokers (and ex-smokers), the benefits of radiotherapy significantly outweigh the risks.20
The German PASSOS-Heart Study analysing 11,982 patients treated for breast cancer between 1998 and 2008 showed that modern radiotherapy techniques do not increase cardiac mortality. However, it is important to identify pre-existing cardiac disease and ensure an extended follow-up period.21 Self-administered questionnaire surveys on cardiovascular events were used and validated by obtained medical records.22
Pre-existing cardiac disease is probably very important for developing radiotherapy-induced cardiac morbidity. A population-based study of patients treated between 2000 and 2010 using modern radiotherapy techniques showed higher risk of coronary interventions among patients with left-sided tumors and higher risk of cardiac mortality. However, the risk was limited to women with previous cardiac disease.23
Sardara's meta-analysis published in 2017 included 289,109 patients from 13 observational studies. Women who had received left-sided radiotherapy had an increased risk of cardiovascular death compared to those receiving right-sided radiotherapy. The difference in cardiovascular mortality was apparent after 15 years of follow-up. The highest risk was observed among women diagnosed before 1982. There was no difference in cardiovascular mortality between left-sided or right-sided radiotherapy in cases where the diagnosis was established after 1992; however, the follow-up was shorter (<10 years).24 The largest meta-analysis to date, published by Cheng in 2017, examined data of 1.191,371 women from 39 studies and 11 randomized trials between 1966 and 2015. The risk of coronary artery disease and cardiac mortality was increased among patients with left-sided radiotherapy compared to those undergoing right-sided radiotherapy and, also, among patients with radiation therapy compared to those without radiotherapy. The risk started to increase within the first decade for coronary artery disease and in the second decade for cardiac mortality. While current modern radiotherapy techniques reduce the risks, they cannot completely eliminate cardiotoxicity.25
A recently published German analysis from the SEER database with 347,476 patients focused on late radiotherapy- and chemotherapy-related cardiac mortality. Heart-specific mortality among breast cancer survivors was not increased compared to the general population. The cardiac specific mortality risk could be increased by the antiHer2 therapy, not because of the tumor biology.26
A review of studies and meta-analyses evaluating the impact of modern RT on cardiac morbidity and mortality is listed in the Table 1.
Table 1.
Studies evaluating the impact of modern breast cancer radiotherapy on cardiac morbidity and mortality.
| Author | Type of study | Number of patients | Period covered | Impact on cardiac morbidity and mortality |
|---|---|---|---|---|
| Doyle, 200717 | SEER | 48,353 | 1992–2000 | No impact on myocardial infarction occurrence |
| Darby, 201318 | Population-based case-control | 2168 | 1958–2001 | Higher risk (subsequent rate) of IHD |
| Taylor, 201720 | Meta-analysis | 40,781 | 2008–2011 | No impact on cardiac mortality amog non-smokers |
| Merzenich, 201721 | Retrospective | 1982 | 1998–2008 | No impact on mortality |
| Boero, 201623 | SEER | 29,102 | 2000–2009 | Higher risk of PCI among patients with a cardiac history |
| Sardar, 201724 | Meta-analysis | 289,109 | 1954–2008 | No increase in morbidity among patients treated after 1992 |
| Cheng, 201725 | Meta-analysis | 1,191,371 | 1949–2008 | Increased CV morbidity in the first decade, mortality in the second one |
| Weberpals, 201826 | SEER | 347,476 | 2000–2011 | No impact on mortality |
Abbreviations: SEER, Surveillance, Epidemiology, and End-Results cancer registry; IHD, ischemic heart disease; PCI, percutaneous coronary intervention; CV, cardiovascular.
3. New radiotherapy techniques
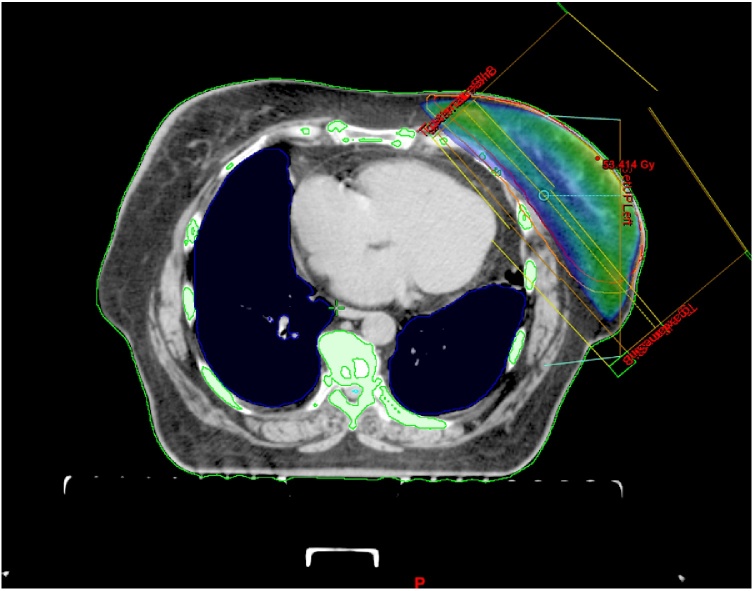
Currently, the use of conformal 3D radiotherapy is an absolute must, as it is able to reduce the dose delivered to the heart and decrease cardiac mortality.17,27,28 The key point is a precise delineation of target volumes and organs at risk. An important part of treatment planning is accurate and reproducible delineation of the heart and all its components.29 The most common radiation technique used today for breast or chest wall radiotherapy is called two tangential photon fields (Fig. 1).
Fig. 1.
Three-dimensional conformal adjuvant breast cancer radi.
Taking into account individual patients’ risks, the aim is to deliver the lowest dose not only to the heart but, also, to the left ventricle and left coronary artery.30 The relationship between cardiac dose and induced toxicity has not been well defined due to the variation in substructure delineation: whole heart pericardium, chambers, vessels and valves. According to several studies, cardiac mortality reflect a risk of radiation – induced atheroma in the anterior descending coronary artery function of total dose rather than the length of the artery exposed.
A study from Michigan, USA, proved that prospective systematic heart dose monitoring can lead to gradual dose reduction. A total of 4,688 patients with breast cancer from 20 centers treated between 2012 and 2015 were enrolled in the registry. For left-sided breast cancer treated with conventional fractionation, the median heart dose (MHD) in 2012 was 2.19 Gy versus 1.65 Gy in 2015; the results are statistically significant. Similar statistically significant results were achieved with hypofractionated radiotherapy (MHD in 2012, 1.70 Gy vs. 1.22 Gy in 2015).31
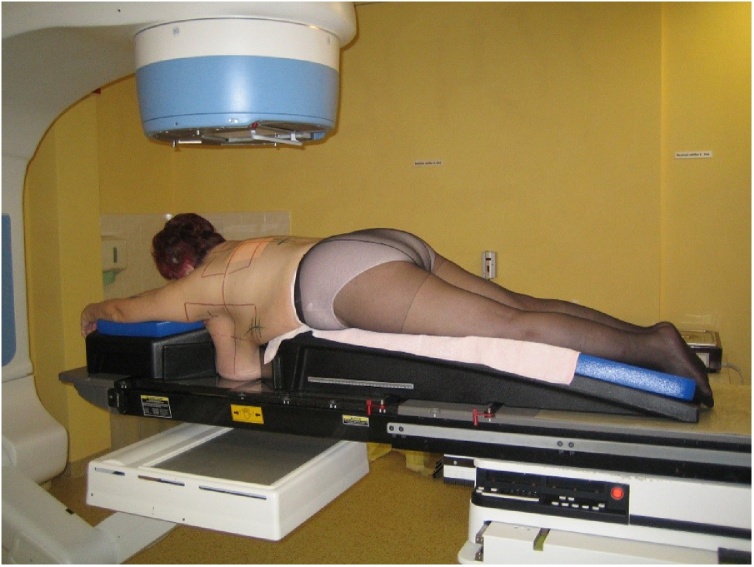
Patients’ individual anatomical predispositions also need to be taken into consideration while planning appropriate radiotherapy techniques. For women with large and pendulous breasts, it is appropriate to position the body face down (prone position), using a breast board (Fig. 2). In the prone position, the dose delivered to the heart is significantly reduced. However, the main issue is to ensure an appropriate, reproducible position of the patient.32 Many helpful positioning devices are available including the arm positioner, chest fixation thermoplastic mask, etc.
Fig. 2.
Prone position.
4. Maximizing heart protection – technical issues
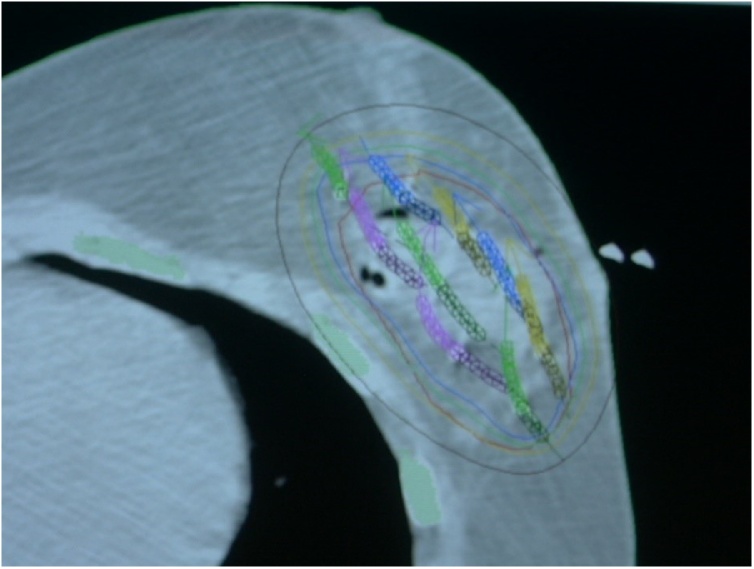
Many other studies have suggested a significant advance toward heart dose reduction by using techniques monitoring chest wall respiratory movements. Conformal radiotherapy is static, while 4D radiotherapy follows the movement of the organs using 4D CT scans during the whole respiratory cycle. Results from clinical trials suggesting an increase in cardiovascular morbidity and mortality, especially after left-sided radiotherapy, led to the development of a new technique called deep inspiration breath holding. The aim of this technique is to deliver a dose only during a particular phase of the respiratory cycle when the breast and chest wall are as far as possible from the heart.33, 34, 35 This technique is very effective for a significant heart dose reduction.36 Another technique considerably reducing the dose delivered to the heart is accelerated partial breast irradiation (APBI), which focuses radiation on the tumor bed (Fig. 3). In selected (low risk) patients, it is currently considered the standard technique replacing whole breast irradiation.37 The use of partial breast irradiation results in lower 5-year non-breast cancer and overall mortality rates compared to whole-breast irradiation.38
Fig. 3.
Dose distribution in perioperative interstitial brachyt.
A systematic review of heart doses from radiation therapy, published in 2015, showed a wide variation among studies, and even similar regimens. The lowest doses are achieved by using the deep inspiration breath holding technique and proton therapy.39 Reducing heart doses and decreasing radiation cardiac morbidity are the two key considerations favoring the use of proton beam for left-sided breast cancer. The advantages – based on theoretical physics axioms – of proton beam dose distribution have been repeatedly described. Even though breast cancer radiotherapy has gained widespread acceptance, there are relatively few clinical data supporting proton radiotherapy. Dosimetric studies and plan comparisons showed that the doses delivered to the heart by a proton beam might be lower if compared even with the deep inspiration breast hold (DIBH) technique.40 A comparison of dosimetric plans has documented better target volume coverage, and reduced heart and lung doses in cases where proton therapy was used for treatment.41 It is essential to take into consideration the cost-effectiveness of the treatment. A systematic review published in 2016 concluded that the cost of proton radiation therapy is double that of photon therapy. The value of proton therapy stands out particularly among highly selected patients at increased risk of cardiac morbidity. However, the analysis did not include modern radiotherapy techniques (breath holding). There are cost savings associated with proton radiotherapy delivered to the tumor bed (APBI) when compared with the use of the MammoSite system.42 A systematic review of 13 studies analyzing proton therapy published this year has again shown that the mean doses delivered to the heart and lungs are reduced with proton therapy compared to photon therapy. However, large well-designed controlled clinical trials are needed to confirm a clinical benefit of proton therapy. Several studies are currently underway including the phase III RADCOMP study comparing photon versus proton radiation therapy after partial mastectomy with lymph node involvement. The study was launched in 2016 and is due to be completed by 2020 after enrollment of 1720 patients. The results are expected in 2030.44
5. Conclusion
The results analyzing the impact of left-sided radiotherapy on cardiac morbidity and mortality are not unequivocal but it is essential to evaluate cardiovascular toxicity after an extremely long follow-up period. The effort aimed at reducing heart doses is a well founded.
Potential radiotherapy cardiac risk factors include a large volume of heart irradiated, younger age at the time of radiotherapy concomitant cardiotoxic chemotherapy and trastuzumab, pre-existing cardiac disease, and conventional cardiac risk factors, such as diabetes, hypertension, hyperlipidemia, obesity, and smoking. The radiation dose delivered to the heart can be reduced by using modern radiotherapy techniques, treatment individualization regarding the choice of target volume, prescribed dose, the adequacy of target volume coverage and protection of normal structure. And it is necessary consideration of the risk of recurrence versus cardiac toxicity risk. The individual decision between sufficient protection of cardiac structures versus optimal target volume coverage remains in the physician’s hand.
Conflict of interest
I have not any conflict of interest.
Financial disclosure
I have not any financial disclosure.
References
- 1.Clarke M., Collins R., Darby S. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomized trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 2.McGale P., Taylor C., Correa C. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(June 21 (9935)):2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hooning M.J., Botma A., Aleman B.M. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365–375. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 4.Harris E.E., Correa C., Hwang W.T. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast conservation treatment. J Clin Oncol. 2006;24:4100–4106. doi: 10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 5.Gagliardi G., Lax I., Soderstorm S. Prediction of excess risk of long-term cardiac mortality after radiotherapy of Stage I breast cancer. Radiat Oncol. 1998;46:63–71. doi: 10.1016/s0167-8140(97)00167-9. [DOI] [PubMed] [Google Scholar]
- 6.Gyenes G., Gagliardi G., Lax I., Fornander T., Rutqvist L.E. Evaluation of irradiated heart volumes in stage I breast cancer patients treated with postoperative adjuvant radiotherapy. J Clin Oncol. 1997;15:1348–1353. doi: 10.1200/JCO.1997.15.4.1348. [DOI] [PubMed] [Google Scholar]
- 7.Cuzick J., Stewart H., Peto R. Overview of randomized trials comparing radical mastectomy without radiotherapy against simple mastectomy with radiotherapy in breast cancer. Cancer Treat Rep. 1987;71:7–14. [PubMed] [Google Scholar]
- 8.Cuzick J., Stewart H., Rutqvist L. Cause-specific mortality in longterm survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994;12:447–453. doi: 10.1200/JCO.1994.12.3.447. [DOI] [PubMed] [Google Scholar]
- 9.Gyenes G., Rutqvist L.E., Liedberg A., Fornander T. Long-term cardiac morbidity and mortality in a randomized trial of pre- and postoperative radiation therapy versus surgery alone in primary breast cancer. Radiother Oncol. 1998;48:185–190. doi: 10.1016/s0167-8140(98)00062-0. [DOI] [PubMed] [Google Scholar]
- 10.Correa C.R., Litt H.I., Hwang W.T. Coronary artery findings after left sided compared with right-sided radiation treatment for early-stage breast cancer. J Clin Oncol. 2007;25:3031–3037. doi: 10.1200/JCO.2006.08.6595. [DOI] [PubMed] [Google Scholar]
- 11.Paszat L.F., Mackillop W.J., Groome P.A., Boyd C., Schulze K., Holowaty E. Mortality from myocardial infarction after adjuvant radiotherapy for breast cancer in the Surveillance, Epidemiology, and End-Results cancer registries. J Clin Oncol. 1998;16:2625–2631. doi: 10.1200/JCO.1998.16.8.2625. [DOI] [PubMed] [Google Scholar]
- 12.Rutqvist L.E., Johansson H. Mortality by laterality of the primary tumour among 55,000 breast cancer patients from the Swedish Cancer registry. Br J Cancer. 1990;61:866–868. doi: 10.1038/bjc.1990.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutqvist L.E., Lax I., Fornander T., Johansson H. Cardiovascular mortality in a randomized trial of adjuvant radiation therapy versus surgery alone in primary breast cancer. Int J Radiat Oncol Biol Phys. 1992;22:887–896. doi: 10.1016/0360-3016(92)90784-f. [DOI] [PubMed] [Google Scholar]
- 14.Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: An overview of the randomised trials. Early Breast Cancer trialists’ Collaborative Group. Lancet. 2000;355:1757–1770. [PubMed] [Google Scholar]
- 15.Hojris I., Overgaard M., Christensen J.J., Overgaard J. Morbidity and mortality of ischaemic heart disease in high-risk breast-cancer patients after adjuvant postmastectomy systemic treatment with or without radiotherapy: Analysis of DBCG 82b and 82c randomised trials. Radiotherapy Committee of the Danish Breast Cancer Cooperative Group. Lancet. 1999;354:1425–1430. doi: 10.1016/s0140-6736(99)02245-x. [DOI] [PubMed] [Google Scholar]
- 16.Van de Steene J., Soete G., Storme G. Adjuvant radiotherapy for breast cancer significantly improves overall survival: The missing link. Radiother Oncol. 2000;55:263–272. doi: 10.1016/s0167-8140(00)00204-8. [DOI] [PubMed] [Google Scholar]
- 17.Doyle J.J., Neugut A.I., Jacobson J.S. Radiation therapy, cardiac risk factors, and cardiac toxicity in early-stage breast cancer patients. Int J Radiat Oncol Biol Phys. 2007;68(1):82–93. doi: 10.1016/j.ijrobp.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Darby S.C., Ewertz M., McGale P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(14 (11)):987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 19.Mège A., Ziouèche A., Pourel N., Chauvet B. Radiation-related heart toxicity. Cancer Radiother. 2011;15(6-7):495–503. doi: 10.1016/j.canrad.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Taylor C., Correa C., Duane F.K. Estimating the risks of breast Cancer radiotherapy: Evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol. 2017;35(May 20 (15)):1641–1649. doi: 10.1200/JCO.2016.72.0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merzenich H., Bartkowiak D., Schmidberger H. 3D conformal radiotherapy is not associated with the long-term cardiac mortality in breast cancer patients: A retrospective cohort study in Germany (PASSOS-Heart Study) Breast Cancer Res Treat. 2017;161(January (1)):143–152. doi: 10.1007/s10549-016-4042-2. [DOI] [PubMed] [Google Scholar]
- 22.Merzenich H., Blettner M., Niehoff D. Cardiac late events in German breast cancer patients: A validation study on the agreement between patient self-reports and information from physicians. BMC Cardiovasc Disord. 2018;18(November 29 (1)):218. doi: 10.1186/s12872-018-0961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boero I.J., Paravati A.J., Triplett D.P. Modern radiation therapy and cardiac outcomes in breast Cancer. Int J Radiat Oncol Biol Phys. 2016;94(March 15 (4)):700–708. doi: 10.1016/j.ijrobp.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 24.Sardar P., Kundu A., Chatterjee S. Long-term cardiovascular mortality after radiotherapy for breast cancer: A systematic review and meta-analysis. Clin Cardiol. 2017;40(February (2)):73–81. doi: 10.1002/clc.22631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng Y.J., Nie X.Y., Ji C.C. Long-term cardiovascular risk after radiotherapy in women with breast Cancer. J Am Heart Assoc. 2017;6(May 21 (5)) doi: 10.1161/JAHA.117.005633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weberpals J., Jansen L., Müller O.J., Brenner H. Long-term heart-specific mortality among 347 476 breast cancer patients treated with radiotherapy or chemotherapy: A registry-based cohort study. Eur Heart J. 2018;39(November 14 (43)):3896–3903. doi: 10.1093/eurheartj/ehy167. [DOI] [PubMed] [Google Scholar]
- 27.Giordano S.H., Kuo Y.F., Freeman J.L. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97:419–424. doi: 10.1093/jnci/dji067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris E.E., Correa C., Hwang W.T. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast conservation treatment. J Clin Oncol. 2006;24:4100–4106. doi: 10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 29.Duane F., Aznar M.C., Bartlett F. A cardiac contouring atlas for radiotherapy. Radiother Oncol. 2017;122(March (3)):416–422. doi: 10.1016/j.radonc.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piroth M.D., Baumann R., Budach W. Heart toxicity from breast cancer radiotherapy : Current findings, assessment, and prevention. Strahlenther Onkol. 2018;(October 11) doi: 10.1007/s00066-018-1378-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierce L.J., Feng M., Griffith K.A. Recent time trends and predictors of heart dose from breast radiation therapy in a large quality consortium of radiation oncology practices. Int J Radiat Oncol Biol Phys. 2017;99(December 1 (5)):1154–1161. doi: 10.1016/j.ijrobp.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 32.Formenti S.C., DeWyngaert J.K., Jozsef G., Goldberg J.D. Prone vs supine positioning for breast cancer radiotherapy. JAMA. 2012;308:861–863. doi: 10.1001/2012.jama.10759. [DOI] [PubMed] [Google Scholar]
- 33.Smyth L.M., Knight K.A., Aarons Y.K., Wasiak J. The cardiac dose-sparing benefits of deep inspiration breath-hold in left breast irradiation: A systematic review. J Med Radiat Sci. 2015;62(March (1)):66–73. doi: 10.1002/jmrs.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin A., Sharieff W., Juhasz J., Whelan T., Kim D.H. The benefit of deep inspiration breath hold: Evaluating cardiac radiation exposure in patients after mastectomy and after breast-conserving surgery. Breast Cancer. 2017;24(January (1)):86–91. doi: 10.1007/s12282-016-0676-5. [DOI] [PubMed] [Google Scholar]
- 35.Al-Hammadi N., Caparrotti P., Naim C. Voluntary deep inspiration breath-hold reduces the heart dose without compromising the target volume coverage during radiotherapy for left-sided breast Cancer. Radiol Oncol. 2018;52(February 23 (1)):112–120. doi: 10.1515/raon-2018-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rice L., Goldsmith C., Green M.M., Cleator S., Price P.M. An effective deep-inspiration breath-hold radiotherapy technique for left-breast cancer: Impact of post mastectomy treatment, nodal coverage, and dose schedule on organs at risk. Breast Cancer (Dove Med Press) 2017;9:437–446. doi: 10.2147/BCTT.S130090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Correa C., Harris E.E., Leonardi M.C. Accelerated partial breast irradiation: Executive summary for the update of an ASTRO evidence-based consensus statement. Pract Radiat Oncol. 2017;7(March-April (2)):73–79. doi: 10.1016/j.prro.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Vaidya J.S., Bulsara M., Wenz F. Reduced mortality with partial-breast irradiation for early breast Cancer: A meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys. 2016;96(October 1 (2)):259–265. doi: 10.1016/j.ijrobp.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Taylor C.W., Wang Z., Macaulay E., Jagsi R., Duane F., Darby S.C. Exposure of the heart in breast Cancer radiation therapy: A systematic review of heart doses published during 2003 to 2013. Int J Radiat Oncol Biol Phys. 2015;93(4):845–853. doi: 10.1016/j.ijrobp.2015.07.2292. [DOI] [PubMed] [Google Scholar]
- 40.Mohan R., Grosshans D. Proton therapy - Present and future. Adv Drug Deliv Rev. 2017;109:26–44. doi: 10.1016/j.addr.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bradley J.A., Dagan R., Ho M.W. Initial report of a prospective dosimetric and clinical feasibility trial demonstrates the potential of protons to increase the therapeutic ratio in breast Cancer Compared with photons. Int J Radiat Oncol Biol Phys. 2016;95(1):411–421. doi: 10.1016/j.ijrobp.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 42.Verma V., Mishra M.V., Mehta M.P. A systematic review of the cost and cost effectiveness studies of proton radiotherapy. Cancer. 2016;122(10):1483–1501. doi: 10.1002/cncr.29882. [DOI] [PubMed] [Google Scholar]
- 44.NCT02603341 - Pragmatic Randomized Trial of Proton vs. Photon Therapy for Patients With Non-Metastatic Breast Cancer: A Radiotherapy Comparative Effectiveness (RADCOMP) Consortium Trial. [DOI] [PMC free article] [PubMed]