Abstract
Irradiating a tumor bed with boost dose after whole breast irradiation helps reducing the probability of local recurrence. However, the success of electron beam treatment with a small area aiming to cover a superficial lesion is a dual challenge as it requires an adequate dosimetry beside a double check for dose coverage with an estimation of various combined uncertainty of tumor location and losing lateral electron equilibrium within small field dimensions.
Aim of work
this work aims to measure the electron beam fluence within different field dimensions and the deviation from measurement performed in standard square electron applicator beam flatness and symmetry, then to calculate the average range of the correction factor required to overcome the loss of lateral electron equilibrium.
Material and method
the electron beam used in this work generated from the linear accelerator model ELEKTA Precise and dosimetry system used were a pair of PTW Pin Point ion chambers for electron beam dosimetry at standard conditions and assessment of beam quality at a reference depth of measurement, with an automatic water phantom, then a Roos ion chamber was used for absolute dose measurement, and PTW 2Darray to investigate the beam fluence of four applicators 6, 10, 14 and 20 cm2 and 4 rectangular cutouts 6 × 14, 8 × 14, 6 × 17 and 8 × 17 cm2, the second part was clinical application which was performed in a precise treatment planning system and examined boost dose after whole breast irradiation.
Results
revealed that lower energy (6MeV and 8MeV) showed the loss of lateral electron equilibrium and deviation from measurements of a standard applicator more than the high energy (15 MeV) which indicated that the treatment of superficial dose with 6MeV required higher monitor unit to allow for the loss of lateral electron equilibrium and higher margin as well.
Keywords: Electron beam, Boost dose, Small field, Whole breast irradiation
1. Introduction
Clinical need for electron beam therapy still exists even with the fast development of photon therapy, this is because of its ability to avoid exposing the deeper tissues while providing high dose to the superficial region. Clinical application of electron beam is very widespread in external beam radiotherapy for skull cancer injury in elderly patient1 boost or chest wall irradiation after breast surgery,2 And treatment of mycosis fungoid or skin cancer with total skin electron irradiation.3,4
Electron beam dose distribution was first calculated by an algorithm which sums the dose distribution of individual pencil beams as described by the Fermi-Eyges theory then with sector integration method (SIR)5 which was suggested for output calculations with irregular electron cutout considering changes in electron fluence, scatter from the edge of cutout shield and side scatter equilibrium.
Another dosimetric concern was the lateral build-up ratio (LBR) LBR data for a small circular field are utilized to extract radial spread of the pencil beam, depth and energy. The adapted method was more tested using a small triangular field, the maximum difference was 4.8 %.6
The quantity of scattered radiation was expected to be reliant on the design of applicator and also the electron beam formation in the treatment head. Therefore, the scattered radiation decreases slightly with increasing field size and spreads regularly over the aperture.7
The practical regards for electron beam treatment with small field size are specified to superficial lesions adjacent to critical structures, as the eye, which may need tight limits, nonstandard distances of treatment and small megavoltage fields. When the field size is smaller than the practical range of electron beam, dosimetric measurements should be performed to give a suitable beam flatness and penumbra. This is achieved by coning down the incident beam to a small field size, and adding a single lead sheet to the skin surface of patient. The space of sheet is required to be more than 2 × 2 cm2 in size and must be cut properly to adequately limit the treatment area.8 Experimental verification of (LBR) on 4-MeV electron beam reported the need for suitable matching within range9,10
Another method for calculating dose per monitor unit (dose /M.U.) calculated PDD and output factors from the measured electron beam PDD curves and output factors for circular fields. This method results were more accurate for the change in lateral scatter with a small field size. Therefore, it provides more accurate values of dose /M.U. at different depths for all types of field shapes and beam energies. For beam energies in the range of (6–21) MeV, differences between measured and calculated dose/M.U. values, at different depths, were ±1.0 %.11
The usual practical technique for breast cancer after tumor resection is to receive either chemotherapy or hormonal therapy and whole breast radiation therapy which classically includes two peripheral fields either with a total dose of 50 /25 Gy fraction or 40/15 in daily mode, then more boost dose which has a role to control the tumor bed.12,13
Various models of Monte Carlo simulation give further accurate calculations and allow for different atypical conditions which in turn decrease the uncertainty in the calculated dose to a few percent,14 combine those models to more accurate dosimetry and increase the treatment accuracy, too.
The irradiation technique for boost is not standard, whether electron, photon or remote after loading brachytherapy.15 Boost dose is used in brachytherapy either alone or combined with WBI in women with early-stage breast cancer treated with breast maintaining therapy. However, for early stage breast cancer, electron is preferred after 3D conformal radiotherapy (3DCRT) to avoid increasing dose to the heart and contralateral lung.16 Local recurrence is not found to be affected by the technique of direct electron compared to intensity modulated radiotherapy (IMRT)17 or even electron versus volumetric modulated radiotherapy (VMAT). Modification of electron beam is suggested to increase dose conformity with better sparing of healthy surrounding tissues.18 Better clinical and cosmetic outcomes were also observed in cases that received boost dose in electron beam within the 1st year and for twelve years of follow up.19
When high electron energies are selected, care should be taken as the coverage at depth is more difficult to uphold in the clinical setting.20 Monte Carlo (MC) evaluation of electron beam suggested the addition of electron degraders (ED) for breast boost irradiation to achieve uniformity in dose distribution in water using ED at 9 MeV.21
Another delivery mode of electron is intraoperative radiotherapy (IORT); in which boost dose is delivered prior to whole breast irradiation (WBI) and with single doses around 10 Gy. Compared to other boost methods, it has evident advantages of direct visualization of a tumor bed during surgery which guarantees a precise dose delivery with direct tissue exposure without expansion by hematoma/seroma.22,23 This technique is also used when further re-irradiation is needed.24
In this work, the target is to measure the electron beam fluence within different field dimensions and the deviation from measurement performed in standard square electron applicator beam flatness and symmetry, then to calculate the average range of the correction factor required to overcome the loss of lateral electron equilibrium.
2. Materials and methods
The study was performed in two separate steps; dosimetric measurements followed by clinical application on the electron boost.
2.1. Dosimetric section
The dosimetric section included the measurements of PDD curves and beam profiles at standard conditions, then the effect of different field dimensions on each energy was examined using PTW 2d array for beam profiles and PTW Roos ion chamber for absolute dose measurement.25 The clinical application of boost dose received by electron after whole breast irradiation included patients who first underwent sonography to define the perimeter of tumor bed localization on the breast skin with a marker and reported depth, then the CT scan simulation to receive adjuvant WBI therapy. The electron boost field was delineated with a 2 cm border perimeter of the demarcated area on the breast skin as the tumor bed. Then a lead wire marker compatible with CT scan was located on the borders. In the next step, the patient was taken under the CT scan simulation in the radiation treatment position on a breast board (CT scan model: Siemens, SOMATOM Sensation 14). The patient position was supine, with both arms up. The CT scan images were imported to ELEKTA precise treatment planning software (version 6). Localization of the tumor bed was performed with assistance of a radiopaque marker placed on the breast skin on the borders of the clinical delineation. Then, patients were subjected to CT simulation and the electron boost volume was made by contouring the tissue underlying the marker.
After importing CT images, treatment planning was performed for whole breast irradiation using two tangential photon fields with 3D-Conformal radiotherapy of 50 Gy in 25 fractions. Then, for boost radiotherapy, radiopaque markers (lead wire on the breast skin) in CT images was contoured as an electron boost field with assistance of clips, if present, and post-surgical seroma. Clinical target volume (CTV Boost) was contoured by adding 1 cm around it. Boost planning target volume (PTV) was obtained by adding 1 cm around the CTV. Electron treatment planning was made with a 2-cm border and the optimum energy was chosen for covering the depth of PTV boost depth by isodose 90 %. Then, isodose coverages were examined and the treatment planning data, including field size, energy, the dose covering 95 % of PTV and the volume receiving 90 % dose V90 %, and 50 % dose V50 % of PTV Boost, were recorded.
Electron beam dosimetry was performed with an automatic water phantom PTW 2d array 729 to measure beam fluence in rectangular fields and square fields.
PTV was generated using a 1-cm margin around the CTV. An electron beam treatment plan was generated for each technique using the FOCUS three-dimensional treatment planning system. Dose-volume histograms (DVH) were created to determine the fraction of the PTV getting 90 % of the specified dose if treatment was conveyed using the EBV. Furthermore, DVH analysis was done to limit the excess volume of normal tissue irradiated when using the EBV.
2.2. Clinical application
For clinical application, twenty breast cancer patients were planned to receive localized boost dose after whole breast irradiation. The tumor bed was defined and delineated with the guidance of preoperative mammography, surgical clips, if present, seroma and post-operative changes. The planning system was PRECISE ELEKTA and the electron beam was generated from ELEKTA linear accelerator and measured with PTW Markus ion camper for absolute electron dose measurement and PTW 2d Array for beam fluence and to evaluate flatness and symmetry.26
2.3. Time and place of study
This study was performed at the Radiation Oncology department, at the hospital for patients with breast cancer, from 2017 to 2018.
2.4. Inclusion criteria
Patients with breast cancer subjected to breast conserving surgery were eligible after twenty days of surgery.
2.5. Statistical method
The sample volume was determined for 24 patients based on former studies and formula for calculating the sample size. The obtained results were analyzed using the software (SPSS Vr20.0) and the hypothesis was tested using t-test.
2.6. Medical ethics
Use of the above method was approved depending on the previous dosimetric studies. Routine and standard treatment was done for all patients and no one was deprived of it.
3. Results
3.1. Dosimetric measurements
Table 1 illustrates the electron beam characteristics from the measured PDD curve, 10 × 10 applicate, SSD = 100 cm (at energies 6, 8, 10 & 15 MeV). The measurements showed that the depth of maximum dose, surface dose as well as the practical range increased with energy; however, the highest energy, 15 MeV, reached 50 % of incident dose at 8 cm depth, which made electron a suitable treatment option for superficial treatment sparing the deeper tissues. These findings agree with the study of Aghili et al. that examined electron at different energies at 100 cm SSD and 10 × 10 field size.26
Table 1.
Electron beam characteristics from the measured PDD curve, app 10, SSD = 100 cm.
| Parameter | 6 MeV | 8 MeV | 10 MeV | 15 MeV |
|---|---|---|---|---|
| Mean energy (MeV) | 6.01 | 7.86 | 9.63 | 15.22 |
| Ep0 (MeV) | 6.68 | 8.62 | 10.38 | 16.19 |
| R100 (mm) | 15.00 | 18 | 21 | 28 |
| R90 (mm) | 19.19 | 25.41 | 31.33 | 48.56 |
| R50 (mm) | 25.78 | 33.74 | 41.33 | 79.85 |
| Rt (mm) | 20.36 | 26.72 | 33.21 | 51.89 |
| Rp (mm) | 32.48 | 42.19 | 50.96 | 42.44 |
| Zref.(cm) | 1.4 | 1.9 | 2.4 | 3.8 |
| KQ | 0.934 | 0.927 | 0.920 | 0.9 |
Depending on Zref for each energy, with its beam quality factor, absolute dose was measured at different field dimensions and compared with dose calculated by the treatment planning system PRECISE ELEKTA as illustrated in Table 2.
Table 2.
Absolute dose at different field dimensions comparing with dose calculated by treatment planning system PRECISE ELEKTA.
| Energy factor | Field size | Calc. dose/100MU | Measured (ROOS IC) (mean ± SD) |
|---|---|---|---|
| 6 MeV | 6 × 6 | 78.9 | 85 |
| 8 × 8 | 99.7 | 99 | |
| 10 × 10 | 94.4 | 102 | |
| 14 × 14 | 94.4 | 100 ± 0.3 | |
| 6 × 14 | 94.4 | 100 ± 0.5 | |
| 8 × 14 | 95.62 | 102 | |
| 6 × 17 | 95.62 | 100 ± 0.2 | |
| 8 × 17 | 95.62 | 100 ± 0.3 | |
| 8 MeV | 6 × 6 | 86.3 | 85.5 |
| 8 × 8 | 99.4 | 100 | |
| 10 × 10 | 96.3 | 99.7 | |
| 14 × 14 | 96.3 | 99 ± 0.5 | |
| 6 × 14 | 96.3 | 100.6 ± 0.5 | |
| 8 × 14 | 96.5 | 100 | |
| 6 × 17 | 96.5 | 98 ± 0 | |
| 8 × 17 | 96.5 | 100 ± 0 | |
| 10 MeV | 6 × 6 | 84.1 | 93 |
| 8 × 8 | 99.9 | 100 | |
| 10 × 10 | 94.9 | 99 | |
| 14 × 14 | 94.9 | 96 | |
| 6 × 14 | 94.9 | 99 | |
| 8 × 14 | 94.2 | 98 | |
| 6 × 17 | 94.2 | 96 | |
| 8 × 17 | 94.2 | 99 | |
| 15 MeV | 6 × 6 | 95.6 | 98 |
| 8 × 8 | 99.8 | 100 | |
| 10 × 10 | 95.4 | 99 | |
| 14 × 14 | 95.4 | 98 | |
| 6 × 14 | 95.4 | 100 | |
| 8 × 14 | 94.7 | 98 | |
| 6 × 17 | 94.7 | 99 | |
| 8 × 17 | 94.7 | 100 |
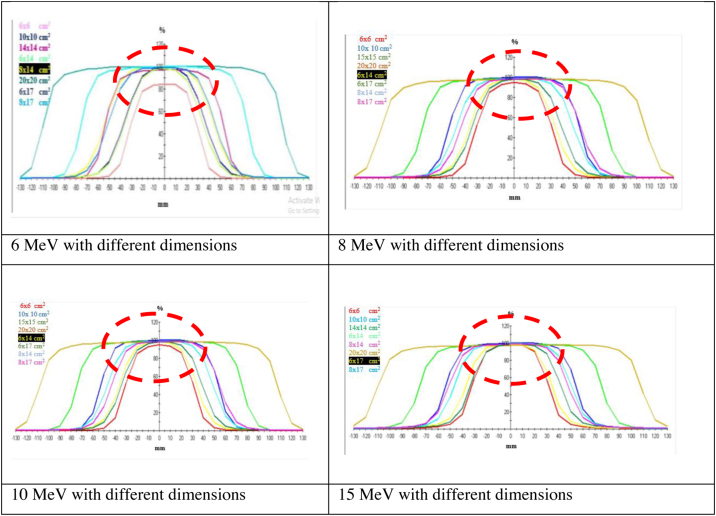
In addition, the electron beam fluence was measured at SSD 100 cm and at different field dimensions. Profiles of different beams were collected to be compared with the nominal standard profile in Fig. 1 showing the difference between fields due to minimized dimensions, which indicates the ability of high energy to keep lateral border equilibrium.
Fig. 1.
Collected profiles for each energy, the red dashed circle highlights the effect of field dimensions among the different energies (6, 8, 10 & 15 MeV).
Analysis of profiles, as illustrated in Table 3, revealed the most valuable differences in the measured parameters (central axis deviation (CAX Dev), point distance and target coverage factor (F80 | F90)) with the 4 different energies.
Table 3.
Electron beam Characteristics for different fields.
| Energy | Measured parameters | App 6 | App 10 | App 14 | App 14 | App 14 | App 20 | App 20 | App 20 |
|---|---|---|---|---|---|---|---|---|---|
| 6.0 × 6.0 cm² | 10 × 10 cm² | 14 × 14 cm² | 14 × 6 cm² | 14 × 8 cm² | 20 × 20 cm² | 20 × 6 cm² | 20 × 8 cm² | ||
| 6 MeV | CAX Dev. [mm] | −0.65 | −0.22 | −0.3 | −0.54 | −2.64 | −1.54 | 0.15 | −4.87 |
| Point Distance [mm] | 11.36 | 10.52 | 12.82 | 12.21 | 13.93 | 13.17 | 15.27 | 15.55 | 17.38 | 15.49 | 15.76 | 13.85 | 15.72 | 15.89 | 17.37 | 16.92 | |
| F80 | F90 [mm] | 9.44 | 9.72 | 9.19 | 9.60 | 8.57 | 9.13 | 9.85 | 10.76 | 709.62 | 710.16 | 7.42 | 8.06 | 8.56 | 9.08 | 11.94 | 12.89 | |
| 8 MeV | CAX Dev. [mm] | −0.79 | −0.38 | −0.43 | 0 | −2.12 | −1.48 | 0 | 0.43 |
| Point Distance [mm] | 11.80 | 10.66 | 12.92 | 12.25 | 14.26 | 13.48 | 15.91 | 15.91 | 16.51 | 15.11 | 15.79 | 14.30 | 16.18 | 16.18 | 16.20 | 16.30 | |
| F80 | F90 [mm] | 9.73 | 9.99 | 9.04 | 9.42 | 8.66 | 9.17 | 49.26 | 49.78 | 38.57 | 39.06 | 7.23 | 7.93 | 62.95 | 63.92 | 51.98 | 52.65 | |
| 10 MeV | CAX Dev. [mm] | 0.15 | 0.33 | 0.29 | −1.03 | 0.87 | −0.42 | 2.43 | 5.63 |
| Point Distance [mm] | 11.97 | 11.43 | 12.66 | 12.58 | 13.96 | 13.69 | 14.50 | 14.02 | 15.26 | 15.40 | 16.48 | 14.99 | 14.24 | 15.20 | 15.01 | 14.37 | |
| F80 | F90 [mm] | 9.83 | 10.63 | 9.29 | 9.69 | 8.88 | 9.41 | 49.24 | 49.65 | 37.49 | 38.09 | 7.61 | 8.51 | 64.25 | 64.67 | 55.10 | 56.45 | |
| 15 MeV | CAX Dev. [mm] | −0.13 | 0.26 | 0.17 | 0 | 2 | −0.97 | 0 | −1.79 |
| Point Distance [mm] | 11.76 | 11.55 | 13.03 | 12.73 | 14.48 | 13.77 | 13.89 | 13.89 | 14.53 | 14.30 | 16.73 | 14.66 | 15.17 | 15.17 | 14.91 | 14.25 | |
| F80 | F90 [mm] | 9.38 | 9.71 | 8.86 | 9.31 | 8.49 | 9.11 | 47.51 | 48.04 | 37.17 | 37.79 | 5.69 | 6.71 | 62.05 | 62.66 | 50.73 | 51.38 |
From Table 3, we noticed the following:
-
I
At 6 MeV, the central axis deviation of dose is higher with applicators 14 and 20; however, the measured dose, flatness and symmetry did not show such a significant variation, One explanation of such a lower effect is the design of Elekta applicators which was approved to cause less scatter effect when compared to other treatment machines.7
-
II
There is a small difference between the reference field size and the irregular lead cutout for low electron energy. Also, profile parameters for the small lead cutout differ from the reference field size when one of the field dimensions is smaller than the practical range of the electron energy used. The difference in the output factor for rectangular lead cutouts and its valent square field size decreases with the increase of field dimension.27
-
III
8 MeV is characterized by relatively higher surface dose and deeper practical range and dose symmetry, which make it more adequate to irradiate tumors within 2:2.5 cm depth. Dose distribution was examined for different applicators and 4 rectangular fields, as shown in Fig. 1; in irregular fields the flatness was lower because of the electron contamination from the cerrobend cutout. Penumbra is the middling distance which separate 80 % and 20 % of isodose lines. The electron field penumbra (20 %–80 % intensity) increased at lower energies and decreased for higher energies (inversely proportional to the energy).
-
IV
At 10 and 15 MeV, the beam is more forward dispersed, with smaller lateral dissipation, giving rise to a narrow penumbra. This is expected because high-energy electrons are subject to less scattering. These results show an agreement with another dosimetric study examined at electron of 6 and 20 MEV.28
-
V
Finally, Tumor bed irradiation in standard conditions applied within 2 cm margin was adequate within standard conditions of irradiation. Extra margin was required with a field width smaller than 0.8E. The same energy was taken when high electron energies were selected because of the higher stability of energy. However, other studies considered, the high electron energy coverage at depth is more difficult to maintain with breast curvature.21
3.2. Clinical application
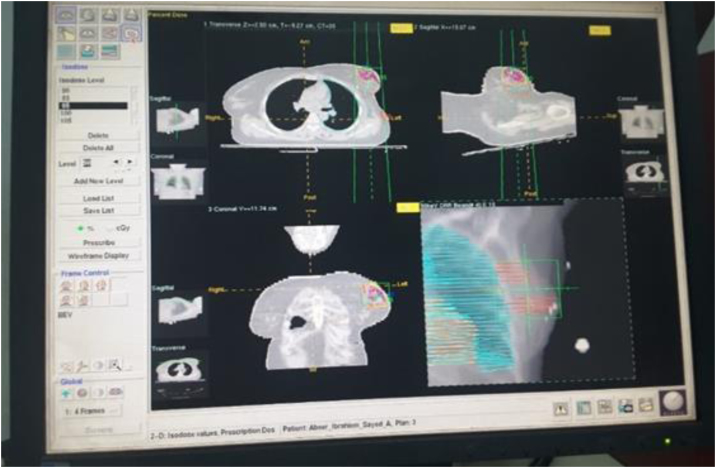
In the clinical part, twenty cases of breast cancer received conservative surgery and chemotherapy followed by whole breast irradiation. The regional tumor bed was delineated by an oncologist based on pathology report to receive 10 Gy in five fractions as a boost course, Fig. 2.
Fig. 2.
Breast case with tumor bed (dark orange), breast is defined for irradiation.
The average size of measured dose and the energy required for uniform radiation dose distribution are illustrated in Table 4.
Table 4.
Dose statistics for electron boost dose.
| Pt percentage | Stage | Boost field (cm2) | Prescribed depth (cm) | Energy | D100% (mm) | D95% (mm) | D50% (mm) |
|---|---|---|---|---|---|---|---|
| 10 | 1 | 6 × 6 | Dmax | 6 | 6 | 3.5 | 9.6 |
| 30 | 2 | 6 × 11 | Dmax | 8 and 10 | 15 and 23 | 25 and 34 | 12 and 50.8 |
| 8 × 11 | 3 | ||||||
| 40 | 3 | 8 × 14 | Dmax | 10 | 23 | 34 | 50.8 |
| 14 × 14 | |||||||
| 20 | 4 | 14 × 14 | Dmax | 10 | 23 | 34 | 50.8 |
From Table 4, the difference between the measured and calculated data of D95 % (the depth of 95 % dose coverage) indicated number of findings: The planning system did not include the corresponding variation due to the addition of block and external cutouts must be calculated and considered throughout the calculation. The addition of cutouts is most effective as field width decreases more than 6 cm, where electron beam loses lateral electron equilibrium. The difference remains within the same range for low and high energy, which means that correction factor must be added to allow for the difference. Each energy was examined with cone 10 × 10, and different field dimensions. The measured output factor was compared to the calculated one in the treatment planning.29 The calculated dose was under-estimated where the measured values found to be over-estimated. This clear evaluation of dose delivery in electron applicator help providing better cardiac sparing as well.26
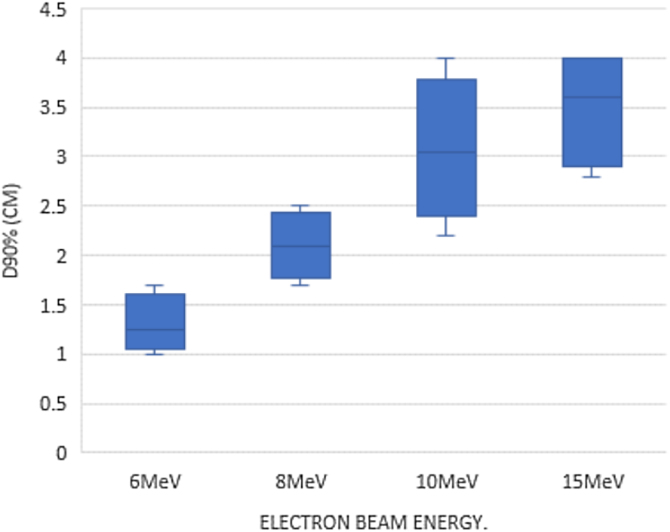
The relation between dose coverage and energy of electron beam is illustrated in Fig. 3. Selection of beam energy to irradiate boost dose depends mainly on the position and depth of it. Dose coverage per energy shown in Fig. 3 for the selected sample declared that 10 MeV is the energy that provides suitable coverage with minimal effect on the field borders as shown previously.
Fig. 3.
electron boost dose in breast cancer radiotherapy: relation between energy of electron beam and V90 %.
4. Conclusion
This study displayed the electron beam characteristics combined with inaccuracies to determine the accurate location tumor bed. The measurements showed that the low-energy electrons may be unable to provide adequate coverage while high-energy electrons may increase the dose received by organs at risk. In case of a field border lower than practical range, it’s recommended that either 10 or 15 MeV because they achieve the smallest deviation with narrow field borders. Limitation of electron boost treatment field within the practical range of energy will serve as a guide in the development of intensity modulated electron therapy.
Informed consent
Informed consent was obtained from all individual participants involved in the study.
Ethics committee approval
The reported measurements performed in this study on the patients were made based on the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Financial disclosure
None declared.
Conflict of interest
None declared.
References
- 1.Sankalp S., Niharika B., Abhishek P., Divya S., Priyanka B. Acantholytic squamous cell carcinoma of the scalp in an elderly patient treated with radical radiotherapy. J Cancer Res Pract. 2018;5(4):165–168. [Google Scholar]
- 2.Grellier Adedjouma N., Chevrier M., Fourquet A. Long-term results of a highly performing conformal electron therapy technique for chest wall irradiation after mastectomy. Int J Radiat Oncol Biol Phys. 2017;98(1):206–214. doi: 10.1016/j.ijrobp.2017.01.205. [DOI] [PubMed] [Google Scholar]
- 3.Petersen I.A., Haddock M.G., Donohue J.H. Use of intraoperative electron beam radiotherapy in the management of retroperitoneal soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 2002;52(2):469–475. doi: 10.1016/s0360-3016(01)02595-0. [DOI] [PubMed] [Google Scholar]
- 4.Diamantopoulos S., Platoni K., Kouloulias V. First treatment of mycosis fungoides by total skin electron beam (TSEB) therapy in Greece. Rep Pract Oncol Radiother. 2014;19(2):114–119. doi: 10.1016/j.rpor.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hogstrom K.R., Mills M.D., Almond P.R. Electron beam dose calculations. Phys Med Biol. 1981;26(3) doi: 10.1088/0031-9155/26/3/008. [DOI] [PubMed] [Google Scholar]
- 6.Jursinic P.A., Mueller R. A sector-integration method for calculating the output factors of irregularly shaped electron fields. Med Phys. 1997;24(11):1765–1769. doi: 10.1118/1.597962. [DOI] [PubMed] [Google Scholar]
- 7.Khan F.M., Higgins P.D., Gerbi B.J., Deibel F.C., Sethi A., Mihailidis D.N. Calculation of depth dose and dose per monitor unit for irregularly shaped electron fields. Phys Med Biol. 1998;43(10):2741–2754. doi: 10.1088/0031-9155/43/10/005. [DOI] [PubMed] [Google Scholar]
- 8.Battum L.J., Zee W., Huizenga H. Scattered radiation from applicators in clinical electron beams. Phys Med Biol. 2003;48(15):2493–2507. doi: 10.1088/0031-9155/48/15/316. [DOI] [PubMed] [Google Scholar]
- 9.McKenzie V., Balter P.A., Stingo F.C., Jones J., Followill D.S., Kry S.F. Toward optimizing patient-specific IMRT QA techniques in the accurate detection of dosimetrically acceptable and unacceptable patient plans. Med Phys. 2014;41(12):1–15. doi: 10.1118/1.4899177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow J.C.L., Newman S. Experimental verification of the application of lateral buildup ratio on the 4-MeV electron beam. J Appl Clin Med Phys. 2006;7(1):35–41. doi: 10.1120/jacmp.v7i1.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kehwar T.S., Huq M.S. The nth root percent depth dose method for calculating monitor units for irregularly shaped electron fields. Med Phys. 2008;35(4):1214–1222. doi: 10.1118/1.2868761. [DOI] [PubMed] [Google Scholar]
- 12.Jalali R., Singh S., Budrukkar A. Techniques of tumour bed boost irradiation in breast conserving therapy : Current evidence and suggested guidelines. Acta Oncol (Madr) 2007;46(879):892. doi: 10.1080/02841860701441798. [DOI] [PubMed] [Google Scholar]
- 13.Toulba The additional irradiation of the tumor bed "the boost" in the breast cancer conservative treatment: what techniques? J Nucl Med Radiat Ther. 2015;6(1):1–6. [Google Scholar]
- 14.Nedaie H.A., Mosleh-Shirazi M.A., Shariary M., Gharaati H., Allahverdi M. Monte Carlo study of electron dose distributions produced by the elekta precise linear accelerator. Rep Pract Oncol Radiother. 2006;11(6):287–292. [Google Scholar]
- 15.Alastuey I., Pardo J., Ariño A. Radiation-induced-cancer risk in breast cancer patients. Photon or electron boost? Rep Pract Oncol Radiother. 2013;18:S181. [Google Scholar]
- 16.Gliński B., Zą M., Mituś M. Brachytherapy boost in women with early-stage breast cancer treated with breast conserving therapy. Rep Pract Oncol Radiother. 2007;12(1):47–51. [Google Scholar]
- 17.Chelakkot G.P., Ravind R., Sruthi K. Adjuvant hypofractionated radiation in carcinoma breast – photon versus electron: comparison of treatment outcome. J Cancer Res Ther. 2017;13(2):262–267. doi: 10.4103/0973-1482.192851. [DOI] [PubMed] [Google Scholar]
- 18.Kovacs A., Hadjiev J., Lakosi F. Comparison of photon with electron boost in treatment of early stage breast cancer. Pathol Oncol Res. 2008;14(2):193–197. doi: 10.1007/s12253-008-9015-2. [DOI] [PubMed] [Google Scholar]
- 19.Alexander A., Soisson E., Hijal T., Sarfehnia A., Seuntjens J. Comparison of modulated electron radiotherapy to conventional electron boost irradiation and volumetric modulated photon arc therapy for treatment of tumour bed boost in breast cancer. Radiother Oncol. 2011;100(2):253–258. doi: 10.1016/j.radonc.2011.05.081. [DOI] [PubMed] [Google Scholar]
- 20.Hill-Kayser C.E., Chacko D., Hwang W.T., Vapiwala N., Solin L.J. Long-term clinical and cosmetic outcomes after breast conservation treatment for women with early-stage breast carcinoma according to the type of breast boost. Int J Radiat Oncol Biol Phys. 2011;79(4):1048–1054. doi: 10.1016/j.ijrobp.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Davidson S. Dosimetric impact of setup accuracy for an electron breast boost technique. Pract Radiat Oncol. 2015;5(5):e499–e504. doi: 10.1016/j.prro.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Park J.I., Ha S.W., Kim J., Lee H., Lee J., Kim I.H. Design and evaluation of electron beam energy degraders for breast boost irradiation. Radiat Oncol. 2016;11(1):112. doi: 10.1186/s13014-016-0686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sedlmayer F. Intraoperative radiotherapy (IORT) as boost in breast cancer. Radiat Oncol. 2017;12:23. doi: 10.1186/s13014-016-0749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falco M., Masojć B., Rolla M. Risk factors for seroma evacuation in breast cancer patients treated with intraoperative radiotherapy. Rep Pract Oncol Radiother. 2016;21(3):225–231. doi: 10.1016/j.rpor.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pathak P., Mishra P.K., Singh M., Mishra P.K. Analytical study of flatness and symmetry of electron beam with 2D array detectors. J Cancer Sci Ther. 2015;7(10):294–301. [Google Scholar]
- 26.Aghili M., Barzegartahamtan M., Alikhassi A., Mohammadpour R. Investigation of electron boost radiotherapy in patients with breast cancer: is a direct electron field optimal? Cancer/Radiother. 2017;22(1):52–56. doi: 10.1016/j.canrad.2017.08.109. [DOI] [PubMed] [Google Scholar]
- 27.Blandino G., Guenzi M., Belgioia L. Adjuvant intraoperative radiotherapy for selected breast cancers in previously irradiated women: Evidence for excellent feasibility and favorable outcomes. Rep Pract Oncol Radiother. 2017;22(4):277–283. doi: 10.1016/j.rpor.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deiab N., Kader S.Z.A., Tolba A.R., EL-Nagdy M., Mostafa M. Dosimetry for small, irregular and rectangular field size for electron beam therapy. Med J Cairo Univ. 2015;83(1):733–738. [Google Scholar]
- 29.Arunkumar T., Supe S., Ravikumar M., Sathiyan S., Ganesh M. Electron beam characteristics at extended source-to-surface distances for irregular cut-outs. J Med Phys. 2010;35(4):207–214. doi: 10.4103/0971-6203.71763. [DOI] [PMC free article] [PubMed] [Google Scholar]