Abstract
The aim of this paper is dual — to review the relevant literature on pathology of radiation induced heart disease (RIHD), and to present an illustrative case of our own. Therapeutic ionizing radiation, such as that used in the treatment of Hodgkin´s lymphoma and cancers of left breast, lungs, esophagus, and thymus, can cause cardiac damage that may take several years to manifest.
The spectrum of RIHD is broad and includes [1] pericarditis and pericardial effusion; [2] endocardial fibrosis and valvular dysfunction; [3] nonischemic myocardial fibrosis; [4] obstructive coronary artery disease with resultant myocardial ischemia; [5] damage to the great vessels; and [6] conduction system dysfunction. Pericardial disease, however, is the most common manifestation of mediastinal irradiation. A case is described of a typical RIHD in a 52-year-old female who died from heart failure with a history of mediastinal neuroblastoma operated and irradiated at the age of 9 years. Her autopsy heart lesions comprised chronic and acute pericarditis with constrictive features, myocardial fibrosis with features of restrictive cardiomyopathy and fibrosis with calcification of the left heart valves. As a unique lesion, there were small calcifications in the mural endocardium and in the large arterial intima. This finding seems to be diagnostic for RIHD.
Keywords: Radiation, Heart disease, Pathology
1. Introduction
Although the heart and great vessels were initially thought to be relatively resistant to radiation induced injury, by the 1930s evidence of cardiac damage resulting from thoracic irradiation was accumulating. By 1975, reports of patients treated with thoracic irradiation, mainly for Hodgkin`s lymphoma and cancers of the left breast, lungs, esophagus and thymus, firmly established the existence of radiation induced heart disease.1 The spectrum of heart involvement is broad and includes lesions of the pericardium, myocardium, endocardium, coronary arteries, conduction system, and great vessels.
This paper will present a survey of relevant literature on pathology of radiation induced heart disease and illustrate it by a case report.
2. Radiation induced heart disease (RIHD)
It is interesting that the heart was one of the first organs to be investigated for sensitivity to X-rays. Experiments on animals (frogs, dogs, rabbits, sheep, rats, guinea pigs) started already towards the end of the 19th century. The results, however, were inconsistent. The first to report cardiac changes in dogs subjected to thoracic irradiation by 135 kV X-rays was Davis in 1924.2 In the following decade, several such accounts appeared, with findings of hydropericardium, various forms of myocardial injury, intramyocardial hemorrhages, and narrowing of blood vessel lumina.3 It must be stressed, however, that in these experiments the doses localized to the heart were very high.
In human subjects, clinical reports of postirradiation cardiac disorders started to appear in the 1930s–1940s.
The most important factors affecting the risk of subsequent damage include total radiation dose, dose fractionation, adjuvant chemotherapeutic treatment, and the amount of heart exposed. The interval from radiation therapy to symptom development is variable and ranges between 2 months and many years, with most cardiovascular events occurring more than 10 years after completing radiotherapy.1
The author is aware of only three autopsy-based studies of larger groups of patients who received cardiac irradiation.1,4,5
In 1968, Fajardo et al.4 reported on 16 patients (7 surgical-pericardiectomy specimens, and 9 necropsies; average age 34.5 years). Organizing pericarditis with extensive fibrosis, and diffuse interstitial myocardial fibrosis appeared as the most common findings.
In 1981, Brosius et al.5 described certain clinical and necropsy findings in 16 young (aged 15–33 years) patients who had received >3500 rads to the heart 5 months to 12 years before death. All 16 had some radiation induced damage to the heart.
In 1996, Veinot and Edwards1 reported on 27 patients (15 surgical, 10 autopsy, and 2 both) aged from 22 to 76 years (mean 49) that exhibited radiation related heart injury.
Pathological findings from the above studies, and also from other relevant sources6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 are summarized further:
The heart has traditionally been considered a radioresistant organ that would be unaffected by cardiac doses below about 30 Gy. Later, however, evidence that RIHD can occur following lower doses has emerged. The risk of RIHD increases with higher radiotherapy dose, younger age at irradiation, and the presence of conventional risk factors.10, 11, 12 The majority of RIHD has been reported in patients previously treated for Hodgkin´s lymphoma. Because these patients tend to be young, they generally live long enough to develop delayed sequelae.
As patients with malignancies are nowadays surviving longer, RIHD may become more prevalent in the future. On the other hand, recent treatment protocols reduce the risk of cardiovascular involvement.
RIHD encompasses a range of deleterious effects on the heart, from subclinical histopathological findings to overt clinical disease.
2.1. Pericardium
Pericarditis is the best known and most common manifestation of RIHD. Acute fibrinous pericarditis, with or without effusion, may manifest early, usually a few weeks after treatment. Rapid accumulation of pericardial effusion can cause potentially fatal heart tamponade. The pericarditis is thought to result from necrosis or inflammation of the tumor, rather than directly from a toxic effect of mediastinal irradiation. The most common form of RIHD is that of late onset chronic pericarditis with extensive fibrous thickening of both lists of the pericardium; up to 20% of patients develop symptoms of constriction within 10 years following irradiation.11
Myocardium is relatively resistant to direct effects of radiation because of the lack of myocyte cell division. Radiation leads to reduced myocardial compliance through microvascular insufficiency and ischemia resulting in various degrees of diffuse and patchy interstitial fibrosis without an inflammatory reaction.11 At a microscopy level, collagen not only increases as a whole but the proportion of type I. collagen increases proportionally to type III. This change is thought to alter the compliance of the myocardium, and thus contribute to the diastolic dysfunction seen in this population.10
Myocardial fibrosis is usually seen at radiation doses >30 Gy and is often asymptomatic. A clinically significant myocardial disease as it is, radiation cardiomyopathy is relatively rare. It does occur, especially, in patients also exposed to chemotherapy (anthracyclines) or high radiation doses (>60 Gy). In general, a restrictive cardiomyopathy suggests prior high dose radiation exposure while a dilated cardiomyopathy is more likely to follow exposure to both anthracycline and radiation.11
Severity of fibrosis can be markedly different from one region to another. The right ventricle can also be affected, because of its anterior location in the chest.
Radiation induced myocardial fibrosis can be diagnosed by endomyocardial biopsy. Extreme adventitial fibrosis around arterioles is highly suggestive of radiation induced damage. The diagnosis can be facilitated by electron microscopy — thickening of the capillary basement membrane, with replication of basement membrane in the early stages, and fibrosis and thickening at the late stages.8
2.2. Endocardium
Both valvar and mural endocardium may be affected by irradiation. Radiation induced valvar injury is a late complication that is due to endocardial fibrosis initially manifested as a thickening and calcification of the valvar endocardium. Fusion of the comissures and other characteristics of postinflammatory valve disease are usually absent. The mean time to detection has been reported to be 11.5 years for asymptomatic valve disease, and 16.5 years for symptomatic disease. The disease more often involves the left heart valves. Aortic valve disease is more common than mitral or right sided valve disease and usually presents as combined aortic stenosis and regurgitation.11 Anthracycline adjuvant therapy appears to increase the risk of valvar fibrosis. A particular pattern of endocardial calcification has been noted in some patients, and experimental data suggest that radiation induction of an osteogenic phenotype may be responsible.17
The mural endocardial involvement appears as whitish endocardial plaques in one or more chambers from proliferation of fibrous tissue, containing some elastic fibers. The fibrous plaques sometimes contain microcalcifications and this pattern seems to be diagnostic for a postirradiation lesion.
2.3. Coronary arteries
Even as relatively recently as the 1980s, the issue of whether radiation exposure may lead to coronary artery disease was controversial and a relationship was not established until the mid to late 1990s.
The dose distribution in the heart is not homogenous and the highest doses are likely to be delivered to the anterior aspect of the heart, including the left anterior descending coronary artery.14
It is unclear to what degree the pathophysiology of coronary artery disease after irradiation is different from that of typical coronary artery disease. After irradiation, coronary artery endothelial cells are probably damaged with resultant intimal fibrous hyperplasia and plaque formation. A recent demonstration of local and sustained up-regulation of nuclear factor — kappa B (NF - κB) in irradiated human blood vessels suggests that NF – κB contributes to the pathology by inducing pro-inflammatory genes.13,16 Radiation induced coronary artery disease is accelerated by traditional risk factors for atherosclerosis.
The pathology of coronary artery disease after radiotherapy differs only slightly from that of coronary artery disease in general population and overlap with atherosclerosis is possible. Angiographically, the coronary artery radiation induced lesions are longer than classical atherosclerotic lesions, and the regions of maximal stenosis tend to be at the proximal end of the lesion, often with ostial stenosis. Histologically, the plaques are largely composed of fibrous tissue with little lipid, the media is frequently replaced by fibrous tissue, and the adventitia is often densely thickened by fibrous tissue.5,11,13
2.4. Conduction system
Serious arrhythmias and atrioventricular blocks can occur as a late (>10 years) complication of mediastinal irradiation. The histopathological finding of fibrosis adjacent to the conduction system myocardium may account for the disturbances.
2.5. Great vessels
Radiation injury is usually limited to the thoracic aorta with abdominal aorta being spared. In the acute stage, histologically, there is necrosis of all layers of the wall with fibrous hyperplasia of intima and adventicia and round cell infiltration (radiation aortitis). In the chronic stage, the affected segment shows a thickened wall and wrinkled intima with fibrous plaques.6 Aortic branches, e.g. carotids, and also the pulmonary artery trunk and main branches may be similarly affected. Like in the heart mural endocardium, there may be microcalcifications in the thickened arterial intima.
3. Case report
A 52-year-old woman had a history of mediastinal neuroblastoma operated and irradiated in 1966 at the age of 9 years (no particulars on the irradiation technique are available). The treatment led eventually to chest deformity and atrophy of the left breast. Her cardiological problems started 3 years before death in 2006, i.e. 40 years after the surgery plus irradiation. She was hospitalized for dyspnea on effort. Examinations revealed massive pericardial effusion and multivalve heart disease (mild mitral insufficiency, mild aortic stenosis + insufficiency, and marked tricuspid insufficiency). Coronarography was negative. Ejection fraction was 60%. Her last hospitalization in 2009 was for signs of heart failure (NYHA II–III). Again, there was pericardial effusion and multivalve heart disease (moderate mitral insufficiency, moderate to severe aortic stenosis, mild to moderate aortic insufficiency, and severe tricuspid insufficiency), with signs of heart restriction, and moderate to severe pulmonary hypertension. Transesophageal echo showed a thickening and mild calcification of both the aortic and mitral valve, and dilatation of the right atrium. The ejection fraction was normal. Repeated ECG recorded diffuse repolarization changes, in support of the diagnosis of pericarditis. The patient died from heart failure.
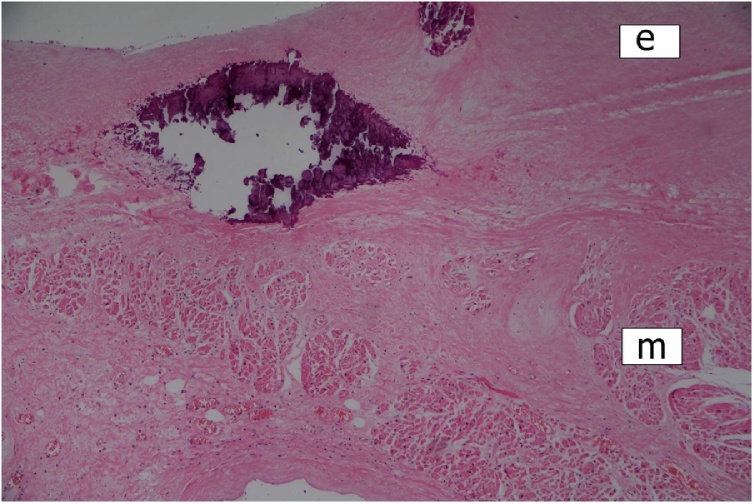
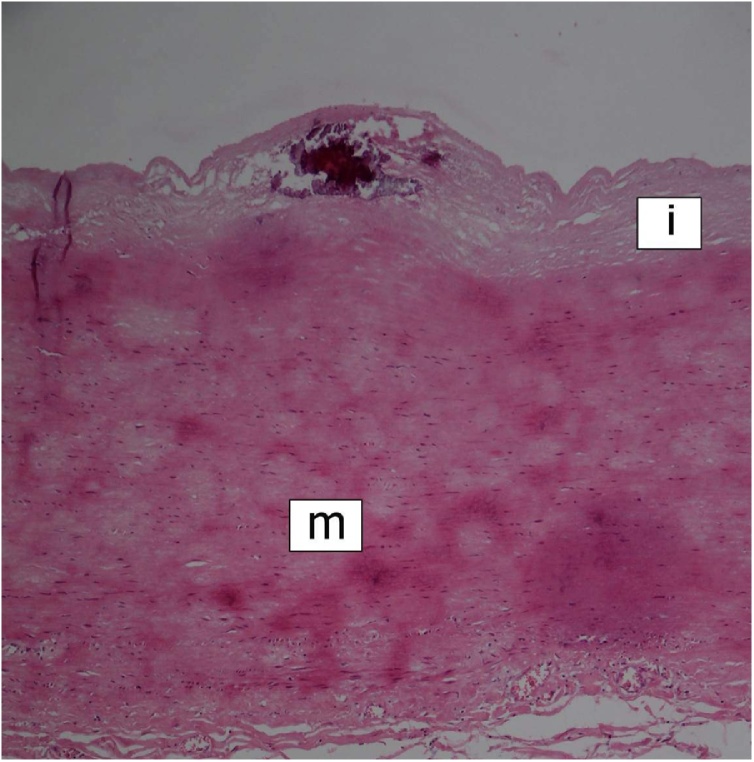
At autopsy, the heart was of normal size and weight (310 g). The main pathological finding appeared to be massive fibrofibrinous pericarditis with features of constriction. The aortic and mitral valves were thickened with small calcifications. There were no gross myocardial changes. The mural endocardium showed unusual findings of two types — multiple pin-head-sized calcifications in the left atrium (Fig. 1), and several whitish fibrous plaques in both ventricles. The main coronary arteries appeared hypoplastic, but with no sclerotic changes. Small calcifications were present in the intima of both ascending aorta and main pulmonary arteries.
Fig. 1.
Calcifications in the left atrial endocardium. Scarring of the underlying myocardium (e = endocardium; m = myocardium). HE stain, ×100.
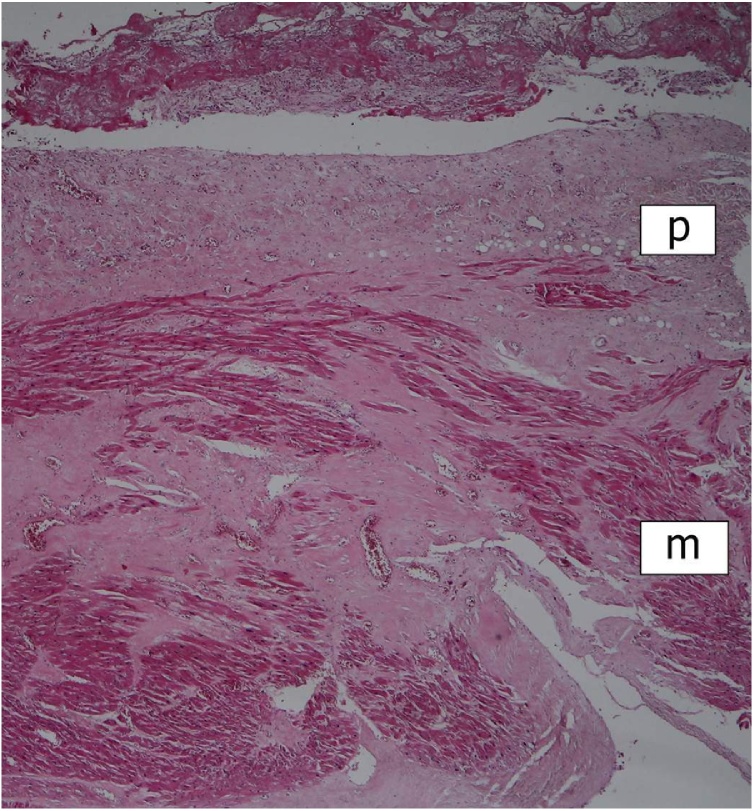
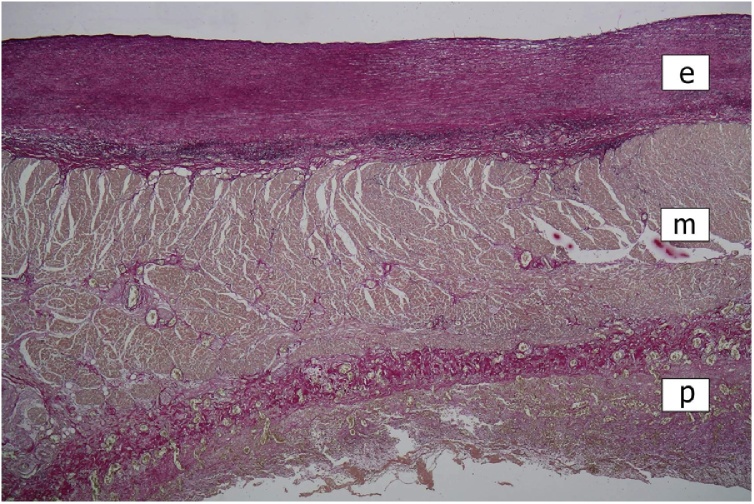
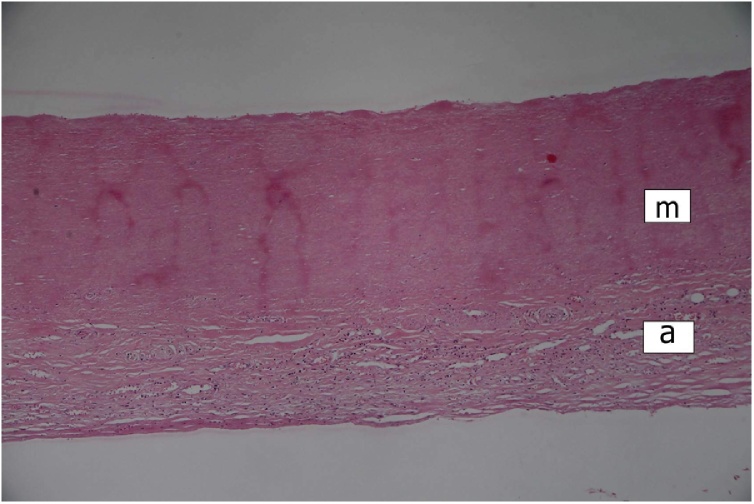
Histologically, the greatly thickened pericardium showed layers of tissue of various age – dense hyaline, cellular fibrosis, and fresh fibrin, suggesting repeated attacks of inflammation (Fig. 2). Myocardium of all four heart chambers was hypertrophic with diffuse interstitial fibrosis and small solid scars present in all layers of their walls (Fig. 2). Aortic and mitral valve were mildly thickened by dense avascular fibrous tissue. The mural endocardial plaques were composed of fibroelastic tissue (Fig. 3). Both ascending aorta and main pulmonary arteries showed the loss of smooth muscle cells in the media with total hyalinization in the pulmonary artery (Fig. 4), presence of small calcifications in the intima (Fig. 5), and adventitial fibrosis.
Fig. 2.
Right ventricular wall. Chronic (fibrous) and acute (fibrinous) pericarditis; myocardial scarring (p = pericardium; m = myocardium). HE stain, ×40.
Fig. 3.
Mural endocardial plaque in the left atrium. Focal thickening of the endocardium by fibroelastic tissue. Chronic pericarditis (e = endocardium; m = myocardium; p = pericardium). Elastica stain, ×40.
Fig. 4.
Pulmonary artery. Complete loss of smooth muscle cells (lack of nuclei)) of the media with hyalinization, and fibrous thickening of the adventicia (m = media; a = adventicia). HE stain, ×100.
Fig. 5.
Aortic root. Microcalcification in the intima. Hyalinization of the media with loss of smooth muscle cells (i = intima; m = media). HE stain, ×100.
To summarize, this is an example of a very late (cca 40 years) clinical presentation of radiation induced heart disease. All three layers of the heart were involved – chronic pericarditis, restrictive cardiomyopathy, and multivalve disease. A unique feature of RIHD seems to be the presence of small calcifications in the heart mural endocardium and the large arterial intima, probably at sites of previous tissue necrosis.
Financial disclosure statement
There are not any financial disclosure.
Declaration of interest
None.
References
- 1.Veinot J.P., Edwards W.D. Pathology of radiation – Induced heart disease: A surgical and autopsy study of 27 cases. Hum Pathol. 1996;27:766–773. doi: 10.1016/s0046-8177(96)90447-5. [DOI] [PubMed] [Google Scholar]
- 2.Davis K.S. Intrathoracic changes following X-ray treatment: A clinical and experimental study. Radiology. 1924;3:301–322. [Google Scholar]
- 3.Jones A., Wedgwood J. Effects of radiations on the heart. Brit J Radiol. 1960;33:138–158. doi: 10.1259/0007-1285-33-387-138. [DOI] [PubMed] [Google Scholar]
- 4.Fajardo L.F., Stewart J.R., Cohn K.E. Morphology of radiation – Induced heart disease. Arch Pathol. 1968;86:512–519. [PubMed] [Google Scholar]
- 5.Brosius F.C., Waller B.F., Roberts W.C. Radiation heart disease. Analysis of 16 young (aged 15 to 33 years) necropsy patients who received over 3,500 rads to the heart. Am J Med. 1981;70:519–530. doi: 10.1016/0002-9343(81)90574-x. [DOI] [PubMed] [Google Scholar]
- 6.Hudson R.E.B. Vol. 2. E. Arnold; London: 1965. pp. 1612–1614. (Cardiovascular pathology). [Google Scholar]
- 7.Ariela Pomerance, Davies M.J. Blackwell; Oxford: 1975. The pathology of the heart; pp. 472–473. [Google Scholar]
- 8.Virmani R., Atkinson J.B., Fenoglio J.J. WB Saunders; Philadelphia: 1991. Cardiovascular pathology; p. 231. [Google Scholar]
- 9.Silver M.D., Gotlieb A.I., Schoen F.J. 3rd ed. Churchill Livingstone; Philadelphia: 2001. Cardiovascular pathology; pp. 577–578. [Google Scholar]
- 10.Adams M.J., Hardenbergh P.H., Constine L.S., Lipshultz S.E. Radiation - associated cardiovascular disease. Crit Rev Oncol Hematol. 2003;45:55–75. doi: 10.1016/s1040-8428(01)00227-x. [DOI] [PubMed] [Google Scholar]
- 11.Heidenreich P.A., Kapoor J.R. Radiation induced heart disease. Heart. 2009;95:252–258. doi: 10.1136/hrt.2008.149088. [DOI] [PubMed] [Google Scholar]
- 12.Darby S.C., Cutter D.J., Boerma M. Radiation - related heart disease: Current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76:656–665. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weintraub N.L., Jones W.K., Manka D. Understanding radiation – Induced vascular disease. J Am Coll Cardiol. 2010;55:1237–1239. doi: 10.1016/j.jacc.2009.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aznar M.C., Korreman S.-S., Pedersen A.N., Persson G.F., Josipovic M., Specht L. Evaluation of dose to cardiac structures during breast irradiation. Brit J Radiol. 2011;84:743–746. doi: 10.1259/bjr/12497075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burke A., Tavora F. Wolters Kluwer; Philadelphia: 2011. Practical cardiovascular pathology; pp. 39–40. [Google Scholar]
- 16.Cheng R.K., Lee M.S., Seki A. Radiation coronary arteritis refractory to surgical and percutaneous revascularization culminating in orthotopic heart transplantation. Cardiovasc Pathol. 2013;22:303–308. doi: 10.1016/j.carpath.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Ong D.S., Aertker R.A., Clark A.N. Radiation – Associated valvular heart disease. J Heart Valve Dis. 2013;22:883–892. [PubMed] [Google Scholar]