Abstract
Introduction
Ablation remains a modality of choice in select patients with Atrial fibrillation (AF). Which is done via a surgical or catheter-based approach.
Objective
This meta-analysis aimed to compare the efficacy of Surgical and Catheter ablation in the management of AF.
Methods
Electronic search on PubMed (MEDLINE), EBSCO, EuropePMC, Clinicaltrials.gov, and Google Scholar was done. Studies comparing the use of surgical or catheter ablation in patients with AF were included. The Primary outcome of interest was Arrhythmia free patients at 12 months post-ablation.
Results
Eight studies (744 patients) reported a statistically significant difference in Arrhythmia recurrence rate between surgical and catheter-based ablation. The pooled hazard ratio was chosen to compare the risk of AF recurrence between these groups with pooled Hazard ratio comparing surgical to catheter approach of 0.40 [0.35,0.45], p < 0.001 favoring surgical approach; low heterogeneity I2 22%, p = 0.25. Meta-analyses were also performed on procedural time, length of stay and major adverse events.
Conclusion
The increased rate of adverse effects and length of hospitalization impedes the implementation of surgical ablation as primary ablation method of AF in general. However, the result of our meta-analysis shows the promising result of surgical ablation compared to catheter-based ablation.
Keywords: Atrial fibrillation, Arrhythmia, Catheter ablation, Surgical ablation
1. Introduction
Atrial fibrillation (AF) remains a burden in the field of cardiology. AF can present adverse consequences in relation to a reduction in cardiac output and possible embolization from the formation of thrombus at the atrial appendage. Furthermore, patients with AF are at increased risk of mortality [1,2].
Catheter ablation has lead to symptom improvement in many AF patients, however, it has not been shown to decrease risks of embolization or death. The principal efficacy outcome is through symptom reduction by reducing AF burden. Even then trials such as MANTRA and RAAFT-2 showed freedom of AF to be decreasing as time progresses [3,4].
Treatment of AF is generally divided between rate and rhythm control. A rate control strategy employs the use of drugs that slows conduction across the atrioventricular node. While rhythm control strategy uses either antiarrhythmic drug therapy, percutaneous catheter ablation, or a surgical ablation procedure.
This meta-analysis aims to compare surgical and catheter-based ablation approaches in the management of atrial fibrillation.
2. Methods
We performed a comprehensive search on studies that assess the comparison between surgical and catheter-based ablation in AF patients from inception up until February 2019. We searched [Surgical versus catheter ablation for atrial fibrillation] and its synonyms using PubMed, EuropePMC, EBSCOhost, Cochrane Central Database, ClinicalTrials.gov, and snowballing from potential articles cited by other studies. The records were then systematically evaluated using inclusion and exclusion criteria. Two researchers (E.Y and R.P) independently performed an initial search, discrepancies were resolved by discussion. (A preferred reporting items for systematic reviews and Meta-Analysis flowchart of the literature search strategy of studies).
2.1. Selection criteria
The inclusion criteria for this study are all studies that assess the comparison between surgical and catheter ablation of AF and its relation to AF recurrence. Cross-sectional and case-control studies were excluded as of those studies with insufficient data to assess the outcome of interest. The primary outcome measured was the Arrhythmia free period between patients who underwent surgical ablation compared to catheter ablation. Secondary outcomes were procedural time, Major complications, and length of stay. We include all clinical researches/original articles and exclude case reports, review articles, and non-English language articles.
2.2. Data extraction
Data extraction and risk of bias assessment were done by two independent authors (E.Y and R.P) using standardized extraction form with includes authors, year of publication, study design, sample size, type of ablation, and length of follow up.
2.3. Statistical analysis
Meta-analysis was done using Review Manager (RevMan) version 5.3 Software (Cochrane Collaboration). We used the Hazard ratio (HR) and 95% CI as a pooled measure for dichotomous data. We used the odds ratio (OR) and a 95% CI as a pooled measure for dichotomous data. We used Mean Difference (MD) and its standard deviation (SD) as a pooled measure for the continuous data. Inconsistency index (I2) test which ranges from 0 to 100% was used to assess heterogeneity across studies. A value above 50% or p < 0.05 indicates statistically significant heterogeneity. We used the Mantel-Haenszel method (for OR) and the generic inverse variance method (for HR and MD) with a fixed-effect model for meta-analysis and a random-effect model in case of significant heterogeneity. All P values were two-tailed with a statistical significance set at 0.05 or below.
3. Results
The search result for studies that assess the comparison between surgical and catheter ablation of atrial ablation yielded a total of potential 1811 articles. We removed 1040 duplicates. We excluded 740 articles after screening the titles and abstracts. There were 36 potentially relevant articles. We screened the full-articles and abstracts and after applying the inclusion and exclusion criteria, 26 studies were excluded due to studies comparing between hybrid and catheter ablation (n=3), studies involving surgical cut and sew (n=2), studies involving surgery of mitral valve (n=3), studies being meta-analysis (n=2), studies did not include the outcome of interest (n=19). We included eight studies for qualitative synthesis and eight studies were available for meta-analysis. There were 744 patients with AF who underwent ablation from eight cohort studies. The subjects had either paroxysmal or persistent AF whom generally had already failed a single regiment of an antiarrhythmic drug. The follow-up ranges from a mean of 12–67 months (see Tables 1 and 2) (see Fig. 1).
Table 1.
Result of the studies included in the qualitative synthesis.
| Author | Year | Study Design | Sample Size | Patients | Left Atrial Size (mm) (SA vs CA) |
Valvular AF (%) | Paroxysmal/Persistent AF (%) | Intervention (Surgical/Catheter Ablation) | Arrhythmia recurrence at 12 months (SA vs CA) | Procedural Time (SA vs CA) | Major Complications (SA vs CA) | Length of Stay (SA vs CA) | Follow up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adiyaman et al. | 2018 | RCT | 52 | Symptomatic Paroxysmal or early persistent AF with failure of atleast 1 AAD SEE NOTE ON PDF |
LA = 39(37–42) vs 40(38–44) mm | Excluded | N/A | 26/26 | 19% vs 53% | (176 ± 78.88 vs 168 ± 52.59) | 5/26 (19.2%) vs 0/26 (0%) | N/A | >24 |
| Boersma et al. | 2011 | RCT | 124 | Persistent AF | LA 42.5 ± 6.5 vs 43.2 ± 4.8 mm | Excluded | 67%vs33% | 61/63 | 34.4% vs 63.5% | (188 ± 59 vs 163 ± 55) | 14/61 (23%) vs 4/63 (6.3%) | N/A | 12 |
| Castella et al. | 2017 | RCT | 124 | NA | Excluded | 66% vs 33% | 61/63 | 56% vs 87% (At The end of observation) | NA | 9/61 (15%) vs 10/63 (16%) | N/A | 84 ± 15 | |
| Demaat et al. | 2013 | Retrospective Observational study | 99 | Paroxysmal and Persistent AF with atleast 1 AAD failure | LA = 41.7 + 5.4 vs 40.8 + 5.4 mm | Excluded | 77%vs23% | 33/66 | 13% vs 58% | N/A | 7/33(21%) vs 3/66(5%) | 8.4 ± 4 vs 2.4 ± 2 | 12.6 ± 2 |
| Elesin et al. | 2015 | Prospective Randomized Study | 64 | Persistent AF after failed initial PVI | N/A | Excluded | 59vs41% | 32/32 | 19% vs 53% | N/A | 7/32(21%) vs 1/32 (3%) | N/A | 12 |
| Haldar et al. | 2017 | Prospective Cohort Study | 51 | Long Standing persistent atrial fibrillation. No prior CA | LA Vol Index = 58.8 ± 14.1 vs 60.0 ± 15.7 mL/m2 | Excluded | Paroxsymal Excluded | 25/26 SA done using thoracoscopic approac |
27% vs 68% | N/A | 8/26(30.7%) vs 2/25 (8%) | 7.4 ± 3 vs 4.1 ± 3 | 24 |
| Pokushalov et al. | 2013 | RCT | 64 | Symptomatic Paroxysmal and Persistent AF with Prior Failed PVI | LA = 46 + 5 vs 45 + 7 mm | Excluded | 59vs41% | 32/32 | 19% vs 53% | 181 ± 21 Vs 142 ± 28 | 8/32 vs 6/32 | 5.2 ± 1.3 vs 2.4 ± 0.7 | 24 |
| Wang et al. | 2011 | Retrospective Cohort | 166 | Longstanding Persistent AF | 51 + 12 vs 53 + 11 | excluded | All Persistent | 83 vs 83 | 25.3% vs 41% | 143.4 + 26.2 vs 231 + 27 | 2/83(2.4%) vs 1/83(1.2%) | NA | 26.4 |
Table 2.
Procedural characteristics of studies.
| Author | Year | Ablation method | Ablation Device | Ablation endpoint | Contact force catheter used? |
|---|---|---|---|---|---|
| Adiyaman et al. | 2018 | SA = Minimal Invasive PVI (MIPI) CA = PVI Ganglionated Plexi (GP) Ablation were not performed on both CA and SA |
SA =Irrigated Bipolar Clamp, (Cardioblate, Medtronic.) CA = Thermocool 3.5 mm irrigated tip ablation catheter, Quadripolar cath (Bard), 3D CARTO Mapping, LASSO catheter. |
SA = Isolation of PVs confirmed with pacing maneuvers at the LA-PV junction CA = absence or dissociation of PV potentials with circular decapolar mapping cath. |
No |
| Boersma et al. | 2011 | SA = PVI CA = Wide-area linear antrum ablation, PVI |
SA = Bipolar RF ablation Clamp (Atricure), Cool Rail (Atricure) CA = RF Catheter (Biosense-webster), 3D NavX, CARTO. |
SA = PV block during pacing CA = PV Block during pacing |
No |
| Castella et al. | 2017 | SA = thoracoscopic PVI CA = PVI |
N/A | N/A | N/A |
| Demaat | 2013 | SA = PVI, with ablation of Ganglionated Plexi CA = Wide circumferential PVI, with ablation of ganglionated plexi. |
SA = Bipolar Clamp (atricure), Monopolar Isolator Pen (Atricure) CA = Decapolar Catheter (Cordis-webster), CARTO FAM 3D, EZ Steer (Biosense-Webster) |
SA = Exit block on PV during pacing, no Vagal response on GP. CA = Exit block on pacing |
No |
| Elesin et al. | 2015 | N/A | N/A | N/A | N/A |
| Haldar et al. | 2017 | CA =Circumferential PVI, ablation of roof and isthmus lines, ablation of sites. SA= Bilateral PVI epicardial ablation, epicardial ganglionated plexi (GP) ablation. |
CA= Thermocool Ablation Catheter, EnSite Velocity 3D guide (St Jude Medical, MN), Afocus II. SA= Bipolar RF ablation clamp and sensing unit (Atricure) |
CA = After restoration of sinus rhythm, PV and linear lesion assessed for bidirectional block. SA = circular catheter positioned on ablation “box” to test entrance and exit block. |
Yes |
| Pokushalov et al. | 2013 | SA = Bilateral PVI (VATS), ablation of bilateral epicardial ganglia. Ablation to create box lesion. LAA stapled and cut CA = Isolation of all PV using mapping catheter |
SA = Bipolar RF ablation Clamp (Atricure) CA = LASSO catheter, NaviStar Thermocool mapping catheter |
SA = PVI confirmed by exit block on pacing. Ganglia ablation confirmed by absence of vagal response. CA = PVI confirmed using LASSO catheter showing absence of PV potentials |
No |
| Wang et al. | 2011 | SA = Bilateral PVI (VATS), with Ganglionated Plexi detection CA = Bilateral PVI, with Ganglionated Plexi detection. Both SA and CA uses OSCOR temporary pacemaker. |
SA = Atricure RF clamp CA = Navistar ablation catheter guided by CARTO mapping system, and LASSO catheter. |
SA = Negative sensing result using temporary pacemaker at bilateral PV antrum. CA = Absence of any PV spike recorded on Lasso catheters on bilateral PV |
No |
Fig. 1.
Study flow diagram.
3.1. Risk of bias analysis
We performed a risk of bias analysis using tools provided in Review Manager (RevMan) version 5.3. Assessment of random sequence generation was done on 5 randomized studies with results of low risk of selection bias, this assessment was not done on other studies due to the fact that these studies were not randomized. Studies by demaat et al. posed a significant risk of selection bias due to the absence of allocation concealment. Studies by demaat et al. and Haldar et al. posed a significant risk of performance bias due to the absence of blinding. All of the studies included in this meta-analysis poses minimal risk for detection bias, attrition bias, reporting bias, and other bias. (Fig. 2A&B).
Fig. 2.
A. Risk of bias graph. B. Risk of bias summary.
3.2. Arrhythmia recurrence rate at 12 months
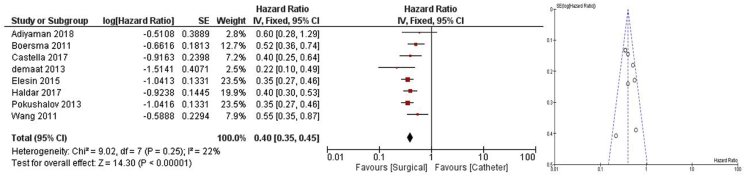
Eight studies reported a statistically significant difference between Arrhythmia recurrence rates between surgical and catheter-based ablation. The pooled hazard ratio was chosen to compare the risk of AF recurrence between these groups with pooled Hazard ratio comparing surgical to catheter approach of 0.40 [0.35,0.45], p < 0.001 favoring surgical approach; low heterogeneity I2 22%, p = 0.25 [[5], [6], [7], [8], [9], [10], [11]].(Fig. 3).
Fig. 3.
Meta-analysis, AF Recurrence rate at 12 Months, Pooled HR Favoring surgical ablation.
3.3. Procedure time surgical vs catheter ablation
Four studies reported a statistically significant difference between procedure time in minutes between surgical and catheter ablation groups with a pooled MD of 41.17 min [4.14,78.20], p = 0.03 favoring catheter ablation; High heterogeneity I296% p < 0.001. Removal of study by Wang et al. resulted in a decrease in heterogeneity to I2 56% p = 0.10, with a pooled MD of 27.60 min [10.85,44.35] p = 0.001. Removal of studies by Wang et al. and Pokushalov et al. resulted in a decrease in heterogeneity to I2 0% p = 0.34, with a pooled MD of 19.26 [2.91,35.62], p = 0.02. Removal of studies by Adiyaman et al. and Wang et al., resulted in a decrease in heterogeneity to I2 27% p = 0.24, with a pooled MD of 34.39 [21.49,47.28], p < 0.001 [5,6,9,11] (Fig. 4).
Fig. 4.
Meta-analysis, Procedure time, Pooled mean difference (minutes) favoring catheter.
3.4. Major adverse events in surgical vs catheter ablation
Six studies reported a statistically significant difference between Major adverse events occurring between surgical and catheter ablation groups. The pooled odds ratio was chosen to compare the risk of major adverse events occurring between these groups with pooled OR of 4.11 [2.26,7.50] p < 0.001 favoring catheter ablation; low heterogeneity I [2] 0%, p = 0.51. Major adverse events were defined as MACCE which consist of death, myocardial infarction, coronary artery bypass surgery, and stroke [5,6,[8], [9], [10],12] (Fig. 5).
Fig. 5.
Meta-Analysis, Major Adverse events, Pooled OR Favoring Catheter.
3.5. Length of hospital stay
Three studies reported a statistically significant difference between Length of hospital stay between surgical and catheter ablation groups. Pooled MD was chosen to compare the length of hospitalization between the groups with MD result of 3.97 days [2.00,5.95] p < 0.001 favoring catheter ablation; high heterogeneity I2 88%, p < 0.001. Removal of studies by deMaat et al. Reduces heterogeneity to I2 0% p = 0.57, pooled MD of 2.84 days [2.36,3.33],p < 0.001 [[8], [9], [10]] (Fig. 6).
Fig. 6.
Meta-Analysis, Length of Hospitalization, pooled mean difference (days) favoring catheter.
4. Discussion
In our study, we proved that surgical approach based ablation has a significant advantage over catheter ablation in the terms of AF recurrence at 12 months of follow up. Our study utilized pooled Hazard Ratio to better compare and comprehend the probability of having an AF recurrence.
Several factors across several studies might explain the heterogeneity of this analysis. A study by Adiyaman et al. includes rather a small sample size and abnormal distribution of data, which is shown by presentation of data in range, not in mean with standard deviation. Studies by demaat et al., despite being matched in a 1:2 ratio, was not randomized, it is not explained as to why matching was performed in a 1:2 ratio. It is interesting to note that study by Wang et al. also contributed to heterogeneity on analysis of procedure time, even though that the authors of this study stated that they performed a matched case-control procedure in the selection of study subjects [5,8,11].
On analysis of procedure length, a study by Adiyaman et al. showed a statistically insignificant confidence interval which crosses 0. This might arises due to the abnormality of data distribution in the study which necessitates mean difference calculation using interquartile range formulas [5].
A high heterogeneity can also be observed in the Analysis of the length of hospitalization which includes 3 studies. On sensitivity analysis this heterogeneity can be tracked to study by demaat et al. One of the reasons why a study by demaat et al. has significant heterogeneity is that choice of ablation methods were given to patients [8].
Several clinical considerations must be taken due to the result of meta-analyses of secondary outcomes that showed a longer procedural time in surgical ablation, an increased risk of developing adverse events, and a longer length of hospitalization.
These drawbacks of Surgical ablations must be weighed in on deciding whether a patient will undergo surgical or catheter-based ablation. Keeping in mind the lower risk of AF recurrence as opposed to the higher risk of developing adverse events, and lengthier procedure time and duration of admission.
In the light of recent advances in the ablation of AF, our study included one study which implements the use of contact force ablation catheter (Ensite Velocity 3D). Due to the unavailability of other studies that compare the use between contact force catheter versus surgical based ablation, we were unable to perform subgroup analysis on this subject. However, the result of this study also showed that more patients that underwent catheter ablation experienced AF recurrence compared to surgical ablation [10] (Table 2).
With the more established atrial remodeling both structurally and electrically in patients with longstanding persistent AF, a more thorough method of ablation is warranted. Surgical ablation is advantageous in this perspective compared to catheter due to the fact that it ensures the creation of robust and continuous lesions compared to catheter ablation, as the creation of transmural lesions is the mainstay of any ablation strategy [13].
Currently, as per the ACC/AHA/HRS 2014 guidelines on the management of AF, surgical ablation is only recommended on: (a). selected patients with AF undergoing cardiac surgery for other indications (Recommendation Class IIa, level of evidence C) and (b). selected patients with highly symptomatic AF not well managed with other approaches [14].
With this existing recommendation, surgical ablation might be useful for patients with longstanding persistent AF as initial therapy. Given the superior efficacy of surgical ablation compared to catheter-based ablation in this population of patients.
Furthermore, safer and less-invasive modalities have been developed for surgical epicardial ablation, such as; off-bypass thoracostomy, mini-thoracotomy, and video-assisted thoracoscopic surgery [14].
We also performed subgroup analysis specific studies that include each paroxysmal and persistent AF, however, results of these subgroup analysis still carry a significant heterogeneity and this subgroup analysis failed to decrease heterogeneity.
Arrhythmia recurrence at 12 months as studied within this meta-analysis might be influenced by several studies that include population of patients with left atrial enlargement. As left atrial structural remodeling, which includes enlargement, is a predisposing factor to the development and maintenance of AF. This structural remodeling facilitates an underlying electrical remodeling of the atria [15,16]. However it is prudent to also consider that presence of AF results in remodeling of the atrium over time, hence the “AF begets AF” norm [17].
This might also explain the tendency relapse in patients who underwent catheter-based ablation, as the more invasive approach is required for patients with pre-existing left atrial remodeling, which is believed to be achieved with surgical epicardial ablation. A multicenter study by Iribarne et al. showed that not only surgical ablation improved long term survival or AF patients, but also this “protective effect” of surgical ablation was seen across patients with or without valvular pathologies. This survival benefit was also not associated with an increase in postoperative morbidity or mortality [18].
The result of this study is in accordance with several other studies which states the superior efficacy of surgical based ablation approach in patients with AF. A study by Lall et al. which includes 242 patients who underwent surgical based ablation approach using COX-MAZE III and COX-MAZE IV showed a 96% freedom of AF at 12 months for COX III and 93% for COX-MAZE IV group [19]. This is a rather steep value compared to the average success rate of catheter-based ablation, even with the use of contact force-sensing catheter, the success rate of SMART AF study was lower (66%–81%) [20].
A major advantage of surgical ablation as stated by Ramlawi et al. is the robust and continuous nature of ablated lesions compared to results obtained from catheter-based ablation, it is also important to note that regardless of modality, reliable transmural lesions are of paramount importance in AF ablation [13].
The authors acknowledged several limitations in this study, a significant increase in heterogeneity were observed on the inclusion of studies by demaat et al. and Adiyaman et al. We performed a sensitivity analysis by excluding these studies and comparing the results with their inclusion. There are no data regarding the surgical risk of patients included in this study, and thus it’s difficult to assess the benefits of each modality based on the surgical risk of patients. There is only one study by Haldar et al. which compares the use of contact force catheter versus surgical ablation, we hope that more similar studies can be done in the future. Finally, as with all systematic reviews and meta-analysis, the risk of publication bias exists and cannot be dismissed. This is further complicated by the fact that negative studies are even less likely to be published. We put our best endeavors to search from various libraries and databases to minimalize this bias.
5. Conclusion
With the results of this meta-analyses, surgical ablation for AF was shown to be superior in efficacy compared to catheter-based ablation. However, the increased rate of adverse effects and length of hospitalization impedes the implementation of surgical ablation as an initial modality in the treatment of AF in general. This is further solidified by the apparent drawbacks of surgical ablation which includes increased risk of periprocedural adverse events and a longer hospital stay which must be weighed in on deciding ablation method. Currently, it is uncommon for patients without any indication for open-heart surgery to be referred for surgical ablation. However, the result of subgroup analysis on longstanding persistent AF patients shows the promising result of surgical ablation compared to catheter-based ablation, and thus referring these specific group of patients for surgical ablation will be beneficial.
Financial support
This paper received no specific grant from any funding agency, commercial or not-for-profit sectors.
Ethical clearance
Not Applicable.
Publication consent
All authors read the final manuscript and consented for publication.
Declaration of competing interest
None.
Acknowledgments
None.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
Contributor Information
Emir Yonas, Email: e_yonas@windowslive.com.
Raymond Pranata, Email: Raymond_pranata@hotmail.com.
Bambang Budi Siswanto, Email: bambbs@gmail.com.
Hafil Budianto Abdulgani, Email: hafil48@gmail.com, hafil@rsmph.co.id.
References
- 1.Atrial fibrillation: current understandings and research imperatives. The National heart, lung, and blood institute working group on atrial fibrillation. J Am Coll Cardiol. 1993;22(7):1830–1834. http://www.ncbi.nlm.nih.gov/pubmed/7902370 [PubMed] [Google Scholar]
- 2.Lip G.Y., Metcalfe M.J., Rae A.P. Management of paroxysmal atrial fibrillation. Q J Med. 1993;86(8):467–472. doi: 10.1093/qjmed/86.8.467. http://www.ncbi.nlm.nih.gov/pubmed/8210304 [DOI] [PubMed] [Google Scholar]
- 3.Englund A., Walfridsson H., Hansen P.S. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. Surv Anesthesiol. 2014;58(1):53–54. [Google Scholar]
- 4.Morillo C.A., Verma A., Connolly S.J. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2) a randomized trial. JAMA, J Am Med Assoc. 2014;311(7):692–699. doi: 10.1001/jama.2014.467. [DOI] [PubMed] [Google Scholar]
- 5.Adiyaman A., Buist T.J., Beukema R.J. Randomized controlled trial of surgical versus catheter ablation for paroxysmal and early persistent atrial fibrillation. Circ Arrhythmia Electrophysiol. 2018;11(10) doi: 10.1161/CIRCEP.118.006182. [DOI] [PubMed] [Google Scholar]
- 6.Boersma L.V.A., Castella M., van Boven W. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST) Circulation. 2012;125(1):23–30. doi: 10.1161/CIRCULATIONAHA.111.074047. [DOI] [PubMed] [Google Scholar]
- 7.Castellá M., Kotecha D., van Laar C. Thoracoscopic vs. catheter ablation for atrial fibrillation: long-term follow-up of the FAST randomized trial. EPP Eur. 2019;21(5):746–753. doi: 10.1093/europace/euy325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Maat G.E., Van Gelder I.C., Rienstra M. Surgical vs. transcatheter pulmonary vein isolation as first invasive treatment in patients with atrial fibrillation: a matched group comparison. Europace. 2014;16(1):33–39. doi: 10.1093/europace/eut208. [DOI] [PubMed] [Google Scholar]
- 9.Pokushalov E, Romanov A, Elesin D. Catheter versus surgical ablation of atrial fibrillation after a failed initial pulmonary vein isolation procedure: a randomized controlled trial. J Cardiovasc Electrophysiol. 2013;24(12):1338–1343. doi: 10.1111/jce.12245. [DOI] [PubMed] [Google Scholar]
- 10.Haldar S.K., Jones D.G., Bahrami T. Catheter ablation vs electrophysiologically guided thoracoscopic surgical ablation in long-standing persistent atrial fibrillation: the CASA-AF study. Heart Rhythm. 2017;14(11):1596–1603. doi: 10.1016/j.hrthm.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Wang J., Li Y., Shi J. Minimally invasive surgical versus catheter ablation for the long-lasting persistent atrial fibrillation. Akhter SA, ed. PLoS One. 2011;6(7) doi: 10.1371/journal.pone.0022122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yelesin D.A., Romanov A.B., Bogachev-prokofev A.V. Catheter versus surgical ablation of atrial fibrillation after failed initial pulmonary vein isolation. Patol krovoobrashcheniya i kardiokhirurgiya. 2015;18(4):123. [Google Scholar]
- 13.Ramlawi B., Abu Saleh W.K. Surgical ablation of atrial fibrillation. Methodist Debakey Cardiovasc J. 2015;11(2):104–108. doi: 10.14797/mdcj-11-2-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.January C.T., Wann L.S., Alpert J.S. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2014;130(23) doi: 10.1161/CIR.0000000000000040. 2014. [DOI] [PubMed] [Google Scholar]
- 15.Thijssen V.L., Ausma J., Liu G.S., Allessie M.A., van Eys G.J., Borgers M. Structural changes of atrial myocardium during chronic atrial fibrillation. http://www.ncbi.nlm.nih.gov/pubmed/10739903 Cardiovasc Pathol. 9(1):17-28. [DOI] [PubMed]
- 16.Allessie M.A. Atrial fibrillation-induced electrical remodeling in humans: what is the next step? Cardiovasc Res. 1999;44(1):10–12. doi: 10.1016/s0008-6363(99)00244-8. http://www.ncbi.nlm.nih.gov/pubmed/10615384 [DOI] [PubMed] [Google Scholar]
- 17.Allessie M.A., Konings K., Kirchhof C.J., Wijffels M. Electrophysiologic mechanisms of perpetuation of atrial fibrillation. Am J Cardiol. 1996;77(3):10A–23A. doi: 10.1016/s0002-9149(97)89114-x. http://www.ncbi.nlm.nih.gov/pubmed/8607387 [DOI] [PubMed] [Google Scholar]
- 18.Iribarne A., DiScipio A.W., McCullough J.N. Surgical atrial fibrillation ablation improves long-term survival: a multicenter analysis. Ann Thorac Surg. 2019;107(1):135–142. doi: 10.1016/j.athoracsur.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Lall S.C., Melby S.J., Voeller R.K. The effect of ablation technology on surgical outcomes after the Cox-maze procedure: a propensity analysis. J Thorac Cardiovasc Surg. 2007;133(2):389–396. doi: 10.1016/j.jtcvs.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Natale A., Reddy V.Y., Monir G. Paroxysmal AF catheter ablation with a contact force sensing catheter. J Am Coll Cardiol. 2014;64(7):647–656. doi: 10.1016/j.jacc.2014.04.072. [DOI] [PubMed] [Google Scholar]