Abstract
Childhood trauma is associated with many long‐term negative outcomes, and is not limited to the individual experiencing the trauma, but extends to subsequent generations. However, mechanisms underlying the association between maternal childhood trauma and child psychopathology are not well understood. Here, we targeted frontal alpha asymmetry (FAA) as a potential underlying factor of the relationship between maternal childhood trauma and child behavioral problems. Electroencephalography (EEG) was recorded from (N = 45) children (Mean age = 57.9 months, SD = 3.13) during an eyes‐closed paradigm in order to evaluate FAA. Mothers reported on their childhood trauma experiences using the Childhood Trauma Questionnaire (CTQ), and on their child's behavior using the child behavior checklist (CBCL). We found that maternal childhood trauma significantly predicted child total, internalizing, and externalizing behavior at age 5 years. We also observed a role for FAA such that it acted as a moderator, but not mediator, for behavioral problems. We found that children with relative more right/less left frontal activity were more at risk to develop behavioral problems when their mother had been exposed to trauma in her childhood. These results indicate that child frontal asymmetry may serve as a susceptibility marker for child behavioral problems.
Keywords: asymmetry, EEG, externalizing, internalizing, trauma
1. INTRODUCTION
Childhood trauma is one of the most pervasive stressors in society and affects the lives of millions of children all over the world (Sandberg et al., 1993; World Health Organization, 2016). Numerous studies have shown that childhood trauma, including emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect, have devastating short‐ and long‐term consequences. These include structural and functional changes in brain development, decreased academic performance, emotional problems, substance abuse, criminal behavior, suicidal behaviors, and decreased overall health (Hussey, Chang, & Kotch, 2006; Malinosky‐Rummell & Hansen, 1993; Teicher et al., 2003). Furthermore, childhood trauma increases the risk for many psychiatric disorders in both childhood and adulthood (Cloitre et al., 2009; Etain et al., 2013; Heim & Nemeroff, 2001; Kong & Bernstein, 2009; Read, Os, Morrison, & Ross, 2005; Simeon, Guralnik, Schmeidler, Sirof, & Knutelska, 2001). Importantly, recent research suggests that the negative effects of childhood trauma are not limited to the individual, but are transmitted across generations.
Children of mothers who have been exposed to childhood trauma show increased risk for behavioral problems (Choi et al., 2017; Collishaw, Dunn, O'conner, & Golding, 2007; Dubowitz et al., 2001; Min, Singer, Minnes, Kim, & Short, 2013; Miranda, de la Osa, Granero, & Ezpeleta, 2013; Myhre, Dyb, Wentzel‐Larsen, Grøgaard, & Thoresen, 2014; Rijlaarsdam et al., 2014; Roberts, O'conner, Dunn, Golding, & ALSPAC Study Team, 2004; Thompson, 2007) and psychiatric disorders (Miranda, Osa, Granero, & Ezpeleta, 2011; Plant, Jones, Pariante, & Pawlby, 2017; Roberts, Lyall, Rich‐Edwards, Ascherio, & Weisskopf, 2013). Until recently, explanations for this relationship focused mainly on parenting skills, such as maternal hostility and harsh discipline toward the child (Berlin, Appleyard, & Dodge, 2011; Lang, Gartstein, Rodgers, & Lebeck, 2010; Rijlaarsdam et al., 2014; Thompson, 2007).
However, a recent study by Moog et al. (2018) demonstrated that effects of maternal childhood trauma already exist in newborns and therefore cannot be solely explained by parenting behavior. They found that newborns of mothers with a history of childhood trauma had a smaller overall brain size and less gray matter volume. These effects held even when controlling for socioeconomic status (SES), maternal exposure to interpersonal violence within the last year, maternal pre‐and early postpartum stress, maternal obesity, infant gestational age, infant sex, and obstetric complications. This raises the possibility that maternal experiences have bearing on the developing brain even when those experiences occur prior to conception. The work by Moog and colleagues confirms the need for a broader investigation of potential mechanisms, beyond parenting, that explains the transmission of risk across generations.
An important objective for improving outcomes in children is to increase understanding as to why some children develop behavioral problems in response to maternal childhood trauma and others do not. Indeed, prior research shows that not all children are equally susceptible to environmental influences (Belsky, 2005; Belsky & Pluess, 2009), also referred to as differential susceptibility. It has been suggested that differences in sensitivity to environmental influences are grounded in, and subserved by, neurobiological variation (Ellis, Boyce, Belsky, Bakermans‐Kranenburg, & Van Ijzendoorn, 2011). If this is the case, then discovery of early brain alterations associated with maternal history of trauma may not only assist in understanding of underlying etiology, but may serve as an early susceptibility marker, differentiating children at greatest risk for subsequent emergence of developmental psychopathology.
A candidate mechanism in the search for neural signatures that underlie the association between maternal childhood trauma may be frontal alpha asymmetry (FAA)—the difference between left and right alpha activity over the frontal regions as measured with electroencephalography (EEG; Marshall & Fox, 2013). A guiding assumption underlying the interpretation of FAA is that greater alpha power is indicative of less cortical activity in the underlying brain regions (Goldman, Stern, Engel, & Cohen, 2002; Laufs et al., 2003; Lindsley & Wicke, 1974; Pfurtscheller, Stancak, & Neuper, 1996). A score of zero reflects frontal symmetry, positive asymmetry scores reflect relatively greater left frontal activity, and lower asymmetry scores reflect relatively greater right frontal activity. The term activity refers to data recorded across a period of restful time, whereas activation refers to a change in activity due to some task or state change (Smith, Reznik, Stewart, & Allen, 2017).
FAA has been studied previously as both a state and a trait. Studies reporting frontal asymmetry as a state, show that asymmetry changes in response to various types of stimuli and different types of emotional expressions (Davidson & Fox, 1982; Fox & Davidson, 1986, 1988). In contrast, different studies have regarded frontal asymmetry as a predisposition to express positive or negative affect that varies between individuals (Coan & Allen, 2003; Tomarken, Davidson, Wheeler, & Kinney, 1992). Hageman, Naumann, Thayer, and Bartussek (2002) carried out a state‐trait analysis of FAA in adults and showed that for most scalp regions, 60% of the variance was due to individual differences and 40% was due to state‐specific fluctuations.
Relative more left frontal activity has been associated with the expression and experience of positive affect and approach‐directed behavior, whereas relative more right frontal activity has been associated with the expression and experience of negative affect and withdrawal behavior (Buss et al., 2003; Marshall & Fox, 2013; Silberman & Weingartner, 1986). Furthermore, relative more right frontal activity has been associated with internalizing as well as externalizing behavior (Baving, Laucht, & Schmidt, 2002; Santesso, Dana, Schmidt, & Segalowitz, 2006), and an increased psychiatric risk across development (Gabard‐Durnam, Tierney, Vogel‐Farley, Tager‐Flusberg, & Nelson, 2015; Harmon‐Jones & Allen, 1997; Kemp et al., 2010; Mathersul, Williams, Hopkinson, & Kemp, 2008).
In addition to affect and behavior, FAA has also been associated with stressful environments. Research by Miskovic, Schmidt, Georgiades, Boyle, and Macmillan (2009) demonstrated that maltreated children exhibit greater relative right frontal EEG activity than non‐maltreated children. This is in line with other studies showing a relation between right frontal asymmetry and stressful environments (Hane & Fox, 2006; Hane, Henderson, Reeb‐Sutherland, & Fox, 2010; Lewis, Weekes, & Wang, 2007; McLaughlin, Fox, Zeanah, & Nelson, 2011; Peltola et al., 2014). Relatively greater right frontal activity has thus been associated with both childhood trauma and child behavior.
Some studies suggest that FAA might act as a moderator between early adversity and behavioral problems. Peltola et al. (2014) raise this possibility in their meta‐analyses on the role of FAA in relation to psychosocial risk (parental depression or maltreatment) and child behavior. Examples of this come from studies reporting that more relative left frontal activity both mitigates the effects of stress in children with familial‐risk for depression (Lopez‐Duran, Nusslock, George, & Kovacs, 2012), and buffers against psychopathology in maltreated female adolescents (Tang et al., 2018). However, whether FAA serves as a mediator and/or moderator remains an open question as these are difficult relationships to parse and variation in human subjects samples and approaches influence study outcomes (Coan & Allen, 2003).
The distinction between mediation and moderation is important because it tells us more about the potential role of FAA. One possibility is that maternal childhood trauma has an effect on the child's brain development during pregnancy, affecting FAA, and this, in turn, leads to an increased risk in child behavioral problems (mediation by FAA). Another possibility is that FAA is not affected by maternal childhood trauma but might make certain children more vulnerable for the effects of maternal childhood trauma (moderation by FAA). In addition, it has yet to be investigated if changes in FAA can partially explain the cross‐generation linkage between maternal childhood trauma and child behavioral problems.
In this study, we examine the role of FAA on the relationship between maternal childhood trauma and child behavior in a low resource, minority sample with high risk for childhood trauma. We hypothesize that in accordance with previous literature, maternal childhood trauma is associated with behavioral problems in the child (H1). We examine whether frontal alpha asymmetry acts as a mediator and/or moderator of this relationship and test the hypothesis that maternal childhood trauma is associated with more negative frontal alpha asymmetry scores (e.g. relatively more right frontal activity), and that negative frontal alpha asymmetry scores, in turn, are associated with increased child behavioral problems (H2a: mediation‐hypothesis). Furthermore, we expect that increased right frontal alpha asymmetry interacts with maternal childhood trauma in determining child behavioral problems (H2b: moderation‐hypothesis). For all hypotheses, child total behavior problems were examined, and when significant, contributions of externalizing and internalizing behavioral problems were examined separately.
2. METHODS
2.1. Procedure
The current study is part of a longitudinal study which follows mothers beginning in the early stages of pregnancy. Participants were recruited for the first wave of the study during routine obstetrical appointments at Hutzel Women's Hospital in Detroit, Michigan, USA. Physicians provided initial orientation to the study and interested, eligible women were introduced to the research team. Inclusion criteria for the mother were: maternal age between 18 and 40 years, English as a first language, singleton pregnancies, and no contraindication for magnetic resonance imaging (MRI). Children that later evidenced or experienced neurological abnormalities (e.g., cerebral palsy), severe illness (e.g., epilepsy), premature birth (<37 weeks of gestation), or low birth weight (<2,600 g) were subsequently excluded. Pregnant mothers underwent repeat assessments that included questionnaires, cognitive testing, biological samples, and fetal neurodevelopmental assessment by multimodal MRI. Subsequently, mothers were invited to participate in follow‐up visits when children were 7 months, 3 years, and 5 years old. Data collection is ongoing, with children presently aging into study windows.1 Details of the original sample are provided in the supplementary materials. Mothers received compensation for their time and travel expenses at the end of each lab visit and children received a small toy. The current study has been approved by the Human Investigation Committee of Wayne State University and all mothers signed an informed consent form before participation.
2.2. Participants
Data included in this study were drawn from all available cases that completed the age 5 study visit before May 2018, resulting in a sample of 67 women and their children. Seven children did not have EEG data available (three children were non‐compliant, and with four children there were technical issues with the EEG system). Five mothers did not fill out the childhood trauma questionnaire, and another eight mothers had non‐random missing values on the questionnaire. Finally, two children were excluded who did not have sufficient artifact‐free EEG‐data (>90 1‐s bins). This resulted in a total of 45 children (16 girls, 35.6%) with a mean age of 57.9 months (SD = 3.13) at the time of the EEG measurement. Their mothers were on average 30.0 years old (SD = 4.49) at the time their child participated at the 5‐year‐old lab visit. Characteristics of mothers and children included in the current study are provided in Table 1.
Table 1.
Participant characteristics
| Children | N | % | M | SD | Min | Max |
|---|---|---|---|---|---|---|
| Age at EEG‐measurement (months) | 45 | 57.9 | 3.13 | 54.2 | 70.6 | |
| Sex | ||||||
| Girl | 16 | 35.6 | ||||
| Boy | 29 | 64.4 | ||||
| Frontal alpha asymmetry | 45 | 0.005 | 0.004 | −0.1 | 0.01 | |
| Internalizing behavioral problems | 45 | 6.1 | 5.2 | 0 | 25 | |
| Externalizing behavioral problems | 45 | 10.3 | 8.4 | 0 | 33 | |
| Total behavioral problems | 45 | 25.9 | 19.6 | 0 | 87 | |
| Mothers | N | % | M | SD | Min | Max |
|---|---|---|---|---|---|---|
| Age at participation (years) | 45 | 30.0 | 4.49 | 23.2 | 39.4 | |
| Ethnicity | ||||||
| African American/Black | 35 | 77.8 | ||||
| Caucasian/White | 6 | 13.3 | ||||
| Asian | 1 | 2.2 | ||||
| Other | 3 | 6.7 | ||||
| Education | ||||||
| 10−12th grade | 9 | 20.0 | ||||
| High school degree/GED | 11 | 24.4 | ||||
| Partial college | 17 | 37.8 | ||||
| 2‐year college degree | 2 | 4.4 | ||||
| 4‐year college degree | 2 | 4.4 | ||||
| Graduate degree | 4 | 8.9 | ||||
| Childhood Trauma score (CTQ) | 45 | 36.3 | 12.6 | 2 | 74 |
2.3. Measurements
2.3.1. Maternal childhood trauma
The Childhood Trauma Questionnaire (CTQ) (Bernstein & Fink, 1998) was used to identify the extent of maternal childhood abuse and neglect history. The CTQ assesses five different dimensions, including emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect across 28 items. A 5‐point Likert scale is used for the responses which range from 1 (never true) to 5 (very often true). A higher total score indicates more severe childhood trauma. About 35.6% of women in our sample reported moderate‐to‐severe abuse on at least one of the five dimensions. The full distribution of trauma scores is listed in Table 2. The CTQ has provided evidence of validity and reliability (internal consistency of α = 91, Bernstein et al., 2003; Scher, Stein, Asmundson, McCreary, & Forde, 2001). In the current study, CTQ data were obtained from mothers at the child 3‐year old lab visit and observed internal consistency was α = 0.88. As previously mentioned, cases containing missing values (N = 8) were excluded because Little's MCAR test showed that missing values were not random, X 2(151, N = 45) = 222.80, p = <0.001. Shapiro–Wilk analysis showed that the remaining data were not normally distributed (p < 0.001), even when log‐transformed (p = 0.001). Therefore, we also conducted, when possible, non‐parametric tests.
Table 2.
Distribution of maternal childhood trauma
| None or minimal | Low to moderate | Moderate to severe | Severe to extreme | |
|---|---|---|---|---|
| Emotional abuse | 33 (73.3%) | 8 (17.8%) | 4 (8.9%) | – |
| Physical abuse | 36 (80.0%) | 5 (11.1%) | 3 (6.7%) | 1 (2.2%) |
| Sexual abuse | 33 (73.3%) | 2 (4.4%) | 4 (8.9%) | 6 (13.3%) |
| Emotional neglect | 30 (66.7%) | 9 (20%) | – | 6 (13.3%) |
| Physical neglect | 31 (68.9%) | 10 (22.2%) | 1 (2.2%) | 3 (6.7%) |
Based on the cut‐off scores provided in the CTQ manual by Bernstein and Fink (1998).
2.3.2. Child behavioral problems
The Child Behavior Checklist (CBCL) (Achenbach, 2001) is a standardized questionnaire that was completed by the child's mother during the 5‐year‐old lab‐visit to assess behavioral problems in the child. The CBCL consists of 99 behavior problem items using a 3‐point scale (0 = not true, 1 = somewhat or sometimes true, 2 = very true or often true). These 99 items are grouped in eight separate syndrome scale scores (Aggressive Behavior, Anxious/Depressed, Attention Problems, Rule‐breaking Behavior, Somatic Complaints, Social Problems, Thought Problems, and Withdrawn/Depressed). Furthermore, the CBCL provides two broad scales; Internalizing problems (combines Anxious/Depressed, Withdrawn/Depressed, and Somatic complaints) and Externalizing problems (combines Rule‐breaking and Aggressive behavior). Because there have been contrasting results whether and how FAA affects internalizing and externalizing behavior (Peltola et al., 2014) we used the total behavior problems score, as well as separate internalizing (36 items) and externalizing (24 items) problem behavior subscales in our analyses. Of note, the CBCL shows strong reliability and validity (Achenbach & Rescorla, 2001). For the current study, the internal consistency was α = 0.94. The data contained 0.2% missing values and Little's MCAR test showed that the missing values were at random, X 2(586, N = 45) = 167.52, p = 1.00, and were therefore substituted by the mean.
2.3.3. Pokémon‐themed eyes‐closed paradigm
Restful EEG was defined as a quiet, awake state with eyes‐closed and minimal movement for repeated 25‐s blocks of time. Using a restful EEG protocol developed by our group, quiescent behavior was reinforced by an animated Pokémon character that was presented on a computer screen at the conclusion at each 25‐s block. In brief, the child was instructed to place his/her chin on a custom‐built ‘incubator’ (See Figure 1) and to close eyes until signaled by the sound of a bell. The narrative behind the structured trials was that by remaining still with eyes closed they would cause a Pokémon egg to hatch. The bell signal was followed by a hatching sound and animated appearance of a new character, at which point the child received a sticker corresponding to the hatched Pokémon and placed it on a visit passport. The task consisted of eight 25‐s trials, preceded by one demonstration trial performed by a research assistant. During the game, video recording was obtained to document child behavior overlaid on stimuli presentation, for use in subsequent assessment of behavioral compliance.
Figure 1.
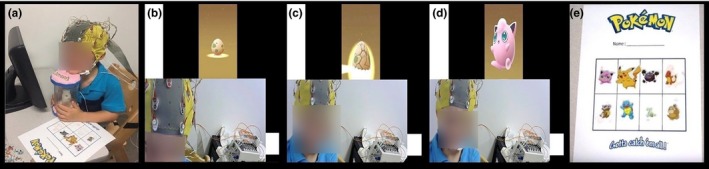
Children were informed to place their chin on the custom‐built Pokémon incubator and close their eyes (a). Once the children's eyes were closed, an egg appeared on the screen to cue the start of the trial. The children would stay still and seated with their eyes closed for 25 seconds during the trial (b). After the end of the allotted time, a bell rang via the computer program, along with the sound of an egg hatching to cue the children to open their eyes (c). The children would then see a Pokémon hatch out of the egg (d). A research‐assistant would provide the children the corresponding sticker of the hatched Pokémon that the children could put on their Pokémon passport (e). The children completed this process 7 more times until their passport was filled with a Pokémon from each trial (total of 8 trials). An informative video about our task is available on: https://www.youtube.com/watch?v=4JGlmUGoEyc
2.3.4. Covariates
Based on previous research (Choi et al., 2017; Min et al., 2013; Moog et al., 2018; Rijlaarsdam et al., 2014) on the association between maternal childhood trauma and child behavioral functioning and child brain function we considered several possible confounders.
Prenatal maternal stress
Studies have shown that prenatal maternal stress can affect brain functions, with changes in neuroendocrine regulation and behavior in offspring (Mulder et al., 2002; Van den Bergh et al., 2017). At the same time, it has been found that women with a history of childhood abuse exhibit hyperreactivity of the hypothalamic‐pituitary‐adrenal axis and autonomic nervous system (Heim et al., 2000). Because prenatal maternal stress is linked to both childhood trauma and the child's brain function and behavior, we included prenatal maternal stress as a covariate. To measure prenatal maternal stress, five different standardized questionnaires that measure internalizing problems and sources of stress were administered: Center for Epidemiological Studies Depression Scale (CES‐D; Radloff, 1977), the State Trait Anxiety Inventory (STAI‐T; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983), the Penn State Worry Questionnaire (PSWQ; Meyer, Miller, Metzger, & Borkovec, 1990), the Perceived Stress Scale (PSS; Cohen, Kamarck, & Mermelstein, 1994), and the Satisfaction with Life Scale (SWLS; Diener, Emmons, Larsen, & Griffin, 1985). Questionnaires were completed during pregnancy. To minimize the number of models run (to reduce the likelihood of Type I error), robustly account for shared variance in stress exposure, and reduce the need to control for multiple testing, we derived a single factor of maternal stress exposure using latent class analyses and confirmatory factor analyses that was used in subsequent analyses.
Maternal anxiety
Previous studies have shown relationships between maternal anxiety and parenting styles, as well as observable differences in dyadic interaction (Nicol‐Harper, Harvey, & Stein, 2007; Woodruff‐Borden, Morrow, Bourland, & Cambron, 2002). Due to the established relationship between parenting styles and child behavior (Aunola & Nurmi, 2005; Querido et al., 2002), we included maternal anxiety as a covariate. Maternal anxiety was measured using the total score of the State Trait Anxiety Inventory (STAI‐T; Spielberger et al., 1983). The STAI‐T is designed to measure trait anxiety using 20 items rated on a 4‐point intensity scale (1 = almost never, 4 = almost always) and was administered to the child's mom during the 5 year old lab visit. For this inventory, internal consistency has been calculated between 0.86 and 0.95 (Spielberger et al., 1983). The current data contained no missing values.
Maternal depression
Maternal depression is another psychosocial factor related to parenting and child outcomes. Several studies have noted associations between parental depressive symptoms and parenting styles (Albright & Tamis‐LeMonda, 2002; Lyons‐Ruth et al., 2000). Additionally, maternal depressive symptoms have been related to adverse child outcomes (Cummings & Davies, 1994; Gotlib & Lee, 1996). In order to examine maternal depression as a covariate, we used the Center for Epidemiological Studies Depression Scale (CES‐D; Radloff, 1977). The CES‐D consists of 20 items designed to measure the current level of depressive symptomatology, with emphasis on the affective component, depressed mood. During the 5‐year‐old lab visit, mothers were asked to indicate how often they experienced a particular behavior or feeling over the past two weeks with item responses ranging from 0 = rarely/none of the time to 3 = most/all of the time. High reliability and validity have been reported for the CES‐D, with Cronbach's alpha values between 0.90 and 0.94 (Cosco, Prina, Stubbs, & Wu, 2017; Yang, Jia, & Qin, 2015). The total score of the CES‐D was used as a covariate and the data contained no missing values.
Maternal intelligence
Due to the heritability of intelligence (Deary, Johnson, & Houlihan, 2009), and its relationship with behavioral problems (Pianta & Castaldi, 1989) and psychopathology (Dietz, Lavigne, Arend, & Rosenbaum, 1997), maternal intelligence was included as a covariate. The Wechsler Abbreviated Scale of Intelligence (second edition; WASI‐ⅠⅠ; Weschler, 2011) is a brief intelligence test intended for participants between 6 and 90 years old. A two‐subtest form that consisted of verbal and matrix reasoning was administered and resulted in a continuous variable with higher scores indicating higher intelligence. This abbreviated form has shown to be valid and reliable (Stano, 2004). The WASI was administered during pregnancy (N = 28), or, due to time constraints, when the child was 36 months old (N = 11). For six mothers (13.3%) the WASI was not administered.
Child trauma
Approximately one‐third of all individuals who are maltreated will later maltreat their own children (Kaufman & Zigler, 1987). To ensure that the child's behavioral problems were not due to a higher risk of being maltreated or other traumatizing experiences, we included childhood trauma of the child as a covariate. The Young Child PTSD Checklist (YCPC; Scheeringa, 2010) is a developmentally sensitive checklist used to assess traumatic events (including child abuse) and Post‐Traumatic Stress Disorder (PTSD) in young children. The YCPC includes a checklist for 12 traumatic events, 24 questions concerning PTSD symptoms, and 6 questions regarding functional impairment. The YCPC was completed by the child's mother during the age 5 study visit. Because our interest was solely the exposure of traumatic events, the 12‐item traumatic events checklist was used. A categorical variable was computed to distinguish children who did experience a traumatic event versus children who did not. The data contained one (2.2%) missing value.
Demographic factors
Child gestational age at delivery, birth weight, and sex were obtained from birth records. In addition, the child's age at the time of the EEG measurement, mother's age and educational level were attained when the mother filled out the questionnaires. Both gestational age and birth weight contained one (2.2%) missing value.
2.4. EEG data acquisition and processing
EEG was recorded using a 34‐electrode array with BioSemi ActiveTwo amplifiers and a sampling rate of 2048 Hz. During EEG set‐up, the child was occupied by an animation video. Electrodes were placed in accordance with the 10–20 system and the standard BioSemi reference (CMS‐DRL), and two additional electrodes on the left and right mastoids. Videos of the child during the task were examined to exclude trials where children opened their eyes or moved excessively (11.0%). The remaining EEG‐data were analyzed using EMSE software 5.6 (Cortech Solutions). A 60 Hz notch filter and a bandpass filter of 0.1–30 Hz were applied. From the filtered EEG data, we extracted data from the two frontal electrodes, F3 and F4. The remaining data were then extracted in one‐second bins using a hamming window with 50% overlap (cf. Allen, Coan, & Nazarian, 2004; Smith et al., 2017). Bins with a voltage change exceeding 100 µV/ms at any electrode were automatically excluded from further analysis. The remaining bins were processed through a Fourier Transform. As described above, cases with fewer than 90 bins of artifact‐free EEG‐data (N = 2) were removed from consideration, resulting in a total of 45 children. The average number of remaining bins included in the analyses was 304.44 (SD = 87.35).
Based on visual inspection of our data, we decided to use a broad alpha range of 5–11 Hz. This is slightly broader than the alpha frequency range recommended by Marshall, Bar‐Haim, and Fox (2002) of 6–9 Hz for children up to four years. This was chosen since our sample consisted mainly of African American children with low social economic backgrounds, while most pediatric EEG studies have highly educated Caucasian samples. Studies have shown variations in alpha frequency based on ethnicity (Ehlers & Phillips, 2003) and socioeconomic status (Harmony, Marosi, de León, Becker, & Fernández, 1990; Otero, Pliego‐Rivero, Fernández, & Ricardo, 2003). These factors could have led to higher individual variation in alpha peak frequency.
Most of our participants showed a clear alpha peak (73.3%), all within the 5–11 Hz window, see also Table S1. For participants with a clear alpha peak, alpha power was computed at their individual alpha peak at electrodes F3 and F4. For those participants that did not show a clear alpha peak, a fixed frequency of the mean (8 Hz) was used to extract peak power (cf. Smulders, Ten Oever, Donkers, Quaedflieg, & van de Ven, 2018). To compute FAA, we subtracted the log‐transformed peak power of F3 (left frontal electrode) from the log‐transformed peak power of F4 (right frontal electrode) and divided by the sum of log‐transformed F3 and F4, i.e.: F4log − F3log/(F4log + F3log). As power values tend to be positively skewed, they are typically log‐transformed (Allen et al., 2004). Furthermore, normalization by the sum of power provides an asymmetry measure which is independent of individual variability (Anokhin, Heath, & Myers, 2006). Because alpha power is inversely related to brain activity (Goldman et al., 2002; Laufs et al., 2003; Lindsley & Wicke, 1974; Pfurtscheller et al., 1996), higher alpha asymmetry scores indicate relative greater left frontal brain activity.
2.5. Statistical analysis
To test our first hypothesis, whether maternal childhood trauma is associated with child behavioral problems, we first conducted a multiple linear regression with maternal childhood trauma as the independent variable and child total behavioral problems as the dependent variable. In order to better understand observed effects, significant results were followed up by separating total behavior problems into internalizing and externalizing, and repeating analyses. For hypothesis 2a, to test the role of FAA as a significant mediator on behavioral problems, we followed the steps by Baron and Kenny (1986). Significant mediation requires that: (a) the independent variable is a significant predictor of the dependent variable, (b) the independent variable significantly predicts the mediator, (c) the mediator significantly predicts the dependent variable, controlling for the independent variable, and (d) the effect of the independent variable on the dependent variable, controlled for the mediator, should be zero. To investigate hypothesis 2b, whether FAA moderates the relationship of maternal childhood trauma on child behavioral problems, multiple regression analyses were conducted. First, maternal childhood trauma and frontal alpha asymmetry were entered as independent variables as well as an interaction between the two on total behavioral problems. Significant interactions were followed up with simple slope tests.
For all analyses described above, we selected the covariates based on forward‐selection regression analysis. The forward selection algorithm adds independent variables into the model as long as it significantly changes the value of explained variance (Efroymson, 1960). Missing values on the covariates were substituted by the mean in the regression analyses. Furthermore, the results were adjusted for multiple testing, using the false discovery rate (FDR; Benjamini & Hochberg, 1995). All statistical analyses were conducted in IBM SPSS Statistics (version 23). Diagnostic analysis was conducted to ensure that regression assumptions were met. Because the normality assumption was violated, Spearman rank correlations were conducted in addition, as a non‐parametric alternative.
2.5.1. Sensitivity analysis
Sensitivity analyses were conducted using three approaches. First, we considered the effect of the size of the bins. Previous studies have shown that variability within bins varies with bins size (Levy, 1987; Salinsky, Oken, & Morehead, 1991). We therefore reran analyses using two‐second bins instead of one‐second bins. Second, we looked for outliers using a 1.5 interquartile range. Outliers were winsorized to the closest non‐outlier value (Dixon, 1960). Analyses were performed with and without outlier correction to test the influence of this processing approach. Third, we examined the absence or presence of an alpha peak, see the supplementary materials for more details.
3. RESULTS
3.1. Descriptives
Prevalence of maternal childhood trauma did not differ by child sex, t(42.89) = 1.70, p = 0.097, d = 0.05, as determined by independent sample t tests. However, there was a significant difference on behavioral problems between boys (total behavioral problems M = 30.74, SD = 21.02, internalizing: M = 7.31, SD = 5.59, and externalizing, M = 12.65, SD = 9.06) and girls (total behavioral problems M = 17.09, SD = 13.37, internalizing: M = 4.01, SD = 3.61, and externalizing, M = 6.07, SD = 4.94), showing that boys have more total behavioral problems, as well as internalizing and externalizing behavioral problems than girls (total behavioral problems: t(43) = 2.34, p = 0.024, d = 0.08, internalizing: t(43) = 2.13, p = 0.039, d = 0.70 and externalizing, t(43) = 3.15, p = 0.003, d = 0.66). We focused on internalizing and externalizing as dimensions of interest; however, it should be noted that total behavioral problems includes more than internalizing and externalizing behavioral problems combined, it also comprises attentional, social, and thought problems. Inspection of the frontal alpha asymmetry scores revealed that only four of the 45 children (8.9%) had more relative right frontal activity and 41 children (91.1%) showed more relative left frontal activity. Their FAA scores ranged from −0.004 to 0.013 (M = 0.005, SD = 0.004). There was no significant difference in FAA between boys and girls, t(37.65) = −2.01, p = 0.052, d = 0.55 (boys, M:0.004, SD: 0.005, girls, M:0.006, SD: 0.002).
3.2. Maternal childhood trauma and child behavioral problems
Our first aim was to examine the relation between maternal childhood trauma and child behavioral problems. The unadjusted and adjusted for covariates findings are listed in Table 3. Here, we only report the results adjusted for covariates. Based on the forward‐selection algorithm (Efroymson, 1960), maternal depression was selected as a covariate for internalizing behavioral problems, and child sex for externalizing behavioral problems. Maternal childhood trauma was significantly associated with child total behavioral problems, β = 0.43, t(43) = 3.16, p = 0.008. Further analyses showed that this was the case for both internalizing, β = 0.32, t(42) = 2.156, p = 0.037, and externalizing behavioral problems, β = 0.34, t(42) = 2.47, p = 0.023, showing that more severe maternal trauma leads to more behavioral problems in the child. Because the scores of maternal childhood trauma and child behavioral problems were not normally distributed, even when log‐transformed, we also conducted Spearman rank correlations as a non‐parametric alternative. Results still displayed a significant association between maternal childhood trauma and internalizing, r = 0.32, p = 0.033, and externalizing behavioral problems, r = 0.34, p = 0.023, but not for total behavioral problems, r = 0.28, p = 0.064.
Table 3.
Beta coefficients from multiple linear regression analysis predicting child total, internalizing, and externalizing behavioral problems
| Variable | Total | Internalizing | Externalizing | ||
|---|---|---|---|---|---|
| Unadjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Partial model | |||||
| Maternal childhood trauma | 0.43** | 0.46** | 0.32*, a | 0.40** | 0.34*, b |
| Full model | |||||
| Maternal childhood trauma | 0.90** | 0.83** | 0.80** | 0.76b, ** | |
| Frontal asymmetry | 0.81 | 0.57 | 0.73 | 0.87b | |
| Frontal asymmetry x maternal childhood trauma | −1.22* | −0.94 | 1.04 | −1.12*, b | |
For all models, covariates were selected with a forward‐selection algorithm (Efroymson, 1960). In the partial model, none of the covariates significantly improved the fit of our model for total behavioral problems, therefore only the adjusted coefficients for internalizing and externalizing behavioral problems are reported. In the full model, only for externalizing behavioral problems did covariates significantly improve the fit of our model. The p‐values have been adjusted for multiple testing using the false discovery rate (Benjamini & Hochberg, 1995).
Adjusted for maternal depression.
Adjusted for child sex.
p = ≤0.05.
p = ≤0.01.
3.3. The role of frontal alpha asymmetry (FAA)
3.3.1. Mediation
For hypothesis 2a, we examined the role of child FAA as a mediator on the association between maternal childhood trauma and child behavioral problems. Linear regression showed that maternal childhood trauma is not correlated with FAA, β = 0.05, t(43) = 0.30, p = 0.768, nor is FAA correlated with child behavioral problems, β = −0.23, t(43) = −1.56, p = 0.252. According to the criteria for mediation by Baron and Kenny (1986), FAA cannot act as a mediating factor in the association between maternal childhood trauma and child behavioral problems, because FAA (mediator) is not related to maternal childhood trauma (independent variable) or child behavioral problems (dependent variable).
3.3.2. Moderation
To address hypothesis 2b we examined the role of child FAA as a moderator by including an interaction between FAA and maternal childhood trauma in our linear regression model. The unadjusted and adjusted for covariates findings are listed in Table 3. Here, we only report the results adjusted for covariates. Based on the forward‐selection algorithm (Efroymson, 1960), only child sex was selected as a covariate for externalizing behavioral problems. Results showed that there was a significant interaction between FAA and maternal childhood trauma on total behavioral problems, β = −1.22, t(41) = −2.59, p = 0.031. Subsequent analyses showed that there was a significant interaction between child FAA and maternal childhood trauma on externalizing behavioral problems, β = −1.12, t(40) = −2.32, p = 0.05, but not for internalizing behavioral problems, β = −0.94, t(41) = −1.98, p = 0.073. The significant interactions were followed up with a simple slope test (see also Figure 2). Only for children with low FAA scores (relative more right/less left frontal activity) was maternal childhood trauma related to higher child total behavioral problems, β = 1.26, t(41) = 4.29, p = <0.001, and externalizing behavioral problems, β = 0.45, t(40) = 3.51, p = 0.001 (low FAA: total behavioral problems: β = −0.004, t(41) = −0.013, p = 0.989, externalizing behavioral problems, β = −0.04, t(40) = −0.30, p = 0.763). These results indicate that children with relative more right/less left frontal activity scores show more total and externalizing behavioral problems when their mothers have been exposed to higher levels of childhood trauma than do children with more left/less right frontal activity. All three models showed a collective significant effect, total behavioral problems, F(3, 41) = 7.59, p < 0.001, R 2 = 35.7%, internalizing, F(3, 41) = 7.06, p = 0.001, R 2 = 34.0%, and externalizing, F(4, 40) = 5.39, p = 0.001, R 2 = 35.0.
Figure 2.
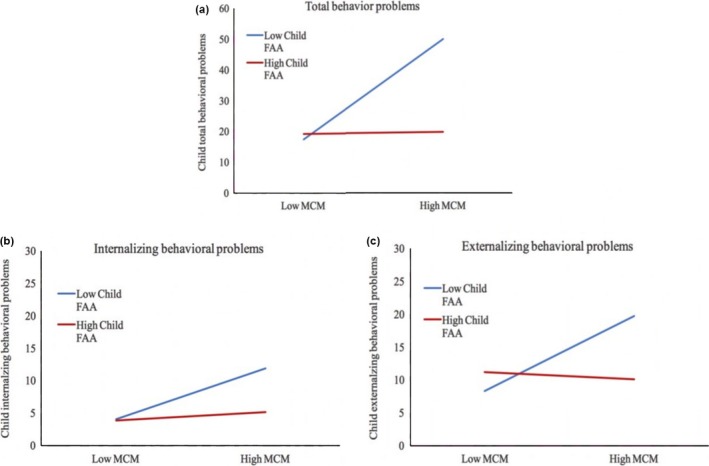
Interaction effect between maternal childhood maltreatment (MCM) and child frontal alpha assymetry (FAA, seperated by low, one standard deviation below the mean, and high, one standard deviation above the mean) on child total behavioral problems (p < .05) (a), internalzing behavioral problems (p > .05) (b) and externalzing behavioral problems (p < .05) (c)
3.4. Sensitivity analysis
Outlier analysis on FAA revealed one outlier. Although this score could still be a valid score, we wanted to examine the effect of this outlier and therefore repeated the multiple regression analysis with the outlier winsorized to the next lowest value. The significant interaction effect on total and externalizing behavioral problems was no longer significant (total: β = −1.09, t(41) = −2.30, p = 0.062, externalizing: β = −1.03, t(40) = −2.11, p = 0.062). The non‐significant interaction between maternal childhood trauma and child FAA on internalizing behavioral problems remained non‐significant, β = −0.83, t(41) = −1.73, p = 0.092. Finally, we ran multiple linear regressions with two‐second bins instead of one‐second bins, because it has been shown that variability within bins varies between different sizes of bins (Levy, 1987; Salinsky et al., 1991). The significant interaction effect on total and externalizing behavioral problems remained significant (total behavioral problems: β = −1.08, t(41) = −2.31, p = 0.039, externalizing behavioral problems: β = −0.96, t(40) = −2.03, p = 0.049). Furthermore, it became significant for internalizing behavioral problems, β = −1.11, t(41) = −2.40, p = 0.039.
4. DISCUSSION
The aim of this study was to investigate the effect of maternal childhood trauma on children's behavioral problems and identify the role of child frontal alpha asymmetry on this relationship. Results showed that childhood trauma of the mother were significantly associated with total, internalizing, and externalizing behavioral problems of her child. Furthermore, we examined the role of child FAA in this association. Results showed that FAA was not significantly associated with maternal childhood trauma or child behavioral problems, and therefore did not meet criteria for mediation. In contrast, FAA did moderate the relationship between maternal childhood trauma and total and externalizing behavioral problems, in support of our moderation hypothesis. More specifically, the results indicated that only children of mothers with trauma history that also show relative more right/less left frontal activity are at heightened risk for development of behavioral problems. Based on these results, we suggest that child FAA does not act as a mediator, but as a moderator and that FAA could be a potential susceptibility marker for child behavioral problems in children of mothers with a history of childhood trauma.
Our results partly replicate previous studies that found a relation between maternal childhood trauma and children's behavior (Choi et al., 2017; Collishaw et al., 2007; Dubowitz et al., 2001; Min et al., 2013; Miranda et al., 2011, 2013; Myhre et al., 2014; Rijlaarsdam et al., 2014; Roberts et al., 2004; Thompson, 2007). However, it should be noted that findings regarding the relationship between maternal childhood trauma and internalizing versus externalizing behavioral problems are mixed. One study reported an effect on internalizing behavior (Dubowitz et al., 2001), three studies found an effect on externalizing behavior (Miranda et al., 2011, 2013; Myhre et al., 2014), and five studies found an effect for both or total behavioral problems (Collishaw et al., 2007; Min et al., 2013; Rijlaarsdam et al., 2014; Roberts et al., 2004; Thompson, 2007; For a more extensive overview see Plant, Pawlby, Pariante, & Jones, 2017). These mixed results may result in part from specific approaches or populations studied, including child age and population demographics, and covariates included in the study. The present study adds to this extant literature by reporting that within our low‐resource, minority sample a significant effect of maternal childhood trauma on child total, internalizing and externalizing behavioral problems.
In addition to the relationship between maternal childhood trauma and child's behavior, we also examined the role of child FAA. In line with our moderation hypothesis, we found that child FAA moderates the relation between maternal childhood trauma and child total and externalizing behavioral problems. Interestingly, child FAA was only a significant moderator for total and externalizing behavioral problems, but not for internalizing behavioral problems. In addition, the significant interaction on total and externalizing behavioral problems became non‐significant when we winsorized the outlier, it could therefore be the case that one case drives the interaction. However, the case seemed valid and there was no reason for deleting the case, and the effects remained in the same direction without the outlier. In addition, the internalizing moderation did show a trend (p = 0.073), and when we changed the bin size for FAA computation, the internalizing moderation did reach significance, suggesting that this distinction should remain a target for future continued examination.
Having observed moderation but not mediation in these data contributes to our understanding of the origin of these differences. Prior research has yielded mixed results regarding whether FAA acts as moderator or mediator, or both (Coan & Allen, 2004). Our results are in line with prior studies that have shown that frontal asymmetry moderates the effects of stressful life events (Davidson & Fox, 1989; Goodman, Rietschel, Costanzo, & Hatfield, 2013; Lopez‐Duran et al., 2012; Quaedflieg, Meyer, Smulders, & Smeets, 2015), and modulates responses to stressors (Lewis et al., 2007; Tops et al., 2005). Our results suggest that frontal asymmetry acts solely as moderator, and not a mediator, between maternal childhood trauma and child behavioral problems, and explains up to 35% of the variance.
Our results show that frontal alpha asymmetry confers susceptibility to child behavioral problems when exposed to maternal childhood trauma, but it remains unclear what mechanisms are underlying this relationship. Firstly, it could act as a neural mechanism in and of itself. A study by Zotev et al. (2016) using fMRI, found that changes in FAA within subjects were related to changes in the activation of the amygdala and many regions associated with emotion regulation, indicating that children with higher right frontal alpha activity may be more reactive to stressful life events, including exposure to maternal childhood trauma. Furthermore, relative more right frontal activity has been associated with higher cortisol levels (Buss et al., 2003; Kalin, Larson, Shelton, & Davidson, 1998; Tops et al., 2005), although not in all studies (Lewis et al., 2007). More research is necessary to understand the underlying mechanisms and/or more complicated pathways of interaction, and explain why FAA ‘makes’ children more vulnerable to stressful life events.
Key findings in the literature suggest that other factors may contribute to negative outcomes observed in children of mothers with heightened stress and trauma backgrounds. For example, Deuschle et al. (2018) found that brain‐derived neurotrophic factor (BDNF) is positively related to maternal childhood trauma, suggesting this may be a possible mechanism in altered child neurological development. Furthermore, Thomas et al. (2018) suggested that the maternal prenatal HPA axis could be a mechanism by which stress from maternal adverse childhood experiences are transmitted across generations. However, in our study, maternal prenatal stress did not prove to be a significant covariate. Another potential mechanism could be epigenetics, as it was found that maternal adversity can lead to alterations in DNA methylation (Monk, Spicer, & Champagne, 2012; Roth, Lubin, Funk, & Sweatt, 2009; Scorza et al., 2019). Furthermore, trauma might also lead to mental health problems in mothers and/or poor parenting skills (Berlin et al., 2011; Lang et al., 2010; Rijlaarsdam et al., 2014; Thompson, 2007), although effects of maternal childhood trauma have already been found in newborns (Moog et al., 2018). These studies suggest that multiple pathways may put children at greater risk for emotional psychopathology, and future research may have to address these in aggregate to understand potential interactive and additive effects.
The findings of this study must be considered in light of certain limitations. First, there was a limited number of children with right frontal asymmetry in our study. Even though our sample consisted of women with a high risk for trauma, only four children displayed relatively more right frontal activity—an activation pattern related to negative environments and outcomes. Our limited number of children with right frontal activity might be explained by the age range of the children that participated in our study. There is not much known about how frontal asymmetry develops in children, but Marshall and Fox (2013) stated that hemispheric asymmetries occur as part of the typical development of EEG, and infants and young children show more left frontal asymmetry in early childhood and slight right frontal asymmetry in middle childhood. McLaughlin et al. (2011) reported that children exhibit more relative right activity between the second and fourth years of life, followed by an increase in relative left frontal activity between the fourth and eight years of life.
Another factor that may have contributed to fewer cases with more right frontal activity could be the paradigm used here to measure frontal asymmetry. To date, there is no consensus on a good measurement to obtain restful EEG in this age group due to frequent refusal to keep their eyes closed for long periods of time. In order to tackle this issue, we developed a Pokémon‐themed restful EEG task. Although it did succeed in letting children close their eyes and sit still, children might have also experienced the task as something positive, as it allowed them to catch Pokémon figures and receive stickers, and the task could therefore be biased by reward anticipation. Because studies have suggested that frontal asymmetry could be a state effect influenced by positive stimuli (Davidson & Fox, 1982) this alternative explanation cannot be ruled out. Furthermore, eye electrodes were not placed on children's faces during the restful EEG task. We worked to eliminate ocular artifacts in the data by visual inspection on both video data as well as EEG data, but there may be ocular artifacts that remained.
As with many studies in the field of developmental psychology, and certainly on the topic of trauma, results may be biased due to endogeneity (Duncan, Magnuson, & Ludwig, 2004). When studying childhood trauma, it is of course not ethical to run experiments and studies are therefore limited to observational data. Consequently, the reported associations could be due to self‐selection or unmeasured characteristics of the mother or child. Furthermore, the used questionnaires are self‐reports, and therefore common‐method variance could occur. We tried to limit these biases as much as possible by including many potential covariates, but it is possible that additional influences not characterized here are salient contributors.
Another bias might result from the sensitivity of the topic of this study. Analysis showed that the missing data of the trauma questionnaire was not random, and we therefore eliminated participants with missing data. It is however possible that mothers who experienced severe trauma in their childhood are less likely to answer certain questions about trauma than mothers who did not experience any trauma. It is therefore plausible that our remaining sample is an underrepresentation in terms of participants who experienced severe trauma. The same holds for our data on the children's trauma. Although filled out by their mothers, negative experiences might be withheld, especially if the mother was (part of) the cause of this negative experiences, for example, in the case of child abuse by the mother.
Lastly, the sample of this study is very specific, as the majority is African American with relatively low levels of education. Despite the fact that the women in our sample were at heightened risk for childhood trauma, the majority of the women reported no exposure to childhood trauma. This is also related to the violation of the normality assumption for the trauma questionnaire. Although we conducted Spearman rank correlations as a non‐parametric alternative that confirmed our findings, this analysis does not have the flexibility for inclusion of covariates and interactions. While the results of studies with large sample sizes (where the number of observations per variable is >10) often are not noticeably impacted by violations of the normality assumptions (Schmidt & Finan, 2017), it could increase the probability of Type I errors in smaller sample sizes. Although several sensitivity analyses were conducted that confirmed the robustness our results, further research with a larger sample size, including more women at risk for trauma, is recommended before these findings can be generalized to a broader population.
5. CLINICAL IMPLICATIONS
The results of this study, although requiring replication with a larger sample size, suggest that child frontal asymmetry is a potential susceptibility marker for child behavioral problems in response to maternal childhood trauma. Children with relative more left frontal activity appear to be more susceptible to the negative effects of maternal childhood trauma than children with relative more right frontal activity. Because FAA develops early in life and is a relativity stable trait (Coan & Allen, 2003; Davidson & Fox, 1989; Tomarken et al., 1992), it can be measured before behavioral problems arise. This poses the opportunity to target (preventive) interventions at those children most at risk to develop behavioral problems. It is believed that children who show a heightened susceptibility to adverse environments also have a heightened susceptibility to the beneficial effects of supportive environments (Belsky, Bakersmans‐Kranenburg, & Van Ijzendoorn, 2007) and could thus possibly benefit from interventions (for an overview of behavioral interventions in early childhood see Gleason, Goldson, Yogman, & Committee on Psychosocial Aspects of Child and Family Health, 2016). Furthermore, research has shown that FAA has a relatively low heritability (Anokhin et al., 2006; van Wijk et al., 2019), which suggests that it can be largely shaped by individual experience, rather than genetic factors. Because the frontal cortex is still developing during childhood (Casey, Giedd, & Thomas, 2000), it poses a lot of potential to intervene, to either prevent, or reverse behavioral problems in children whose mothers have been exposed to childhood trauma.
6. CONCLUSION
The time between when a girl experiences trauma during her childhood until her own child exhibits behavioral problems is long, and therefore complex in terms of the number of factors that can play a role. This is the first study to show that child frontal alpha asymmetry is one of these factors. Although frontal alpha asymmetry was not directly linked to behavioral problems, it did moderate the effect of maternal childhood trauma, showing that only children with relative more right/less left frontal activity are at risk for behavioral problems when their mothers have been exposed to trauma as a child. This study therefore identified frontal alpha asymmetry as a potential susceptibility marker for child behavioral problems. More generally, our results add to the understanding of why some children do develop behavioral problems whereas others do not, even though they might grow up in comparable stressful environments. This poses the opportunity to target interventions at those children who are most at risk to develop behavioral problems.
CONFLICT OF INTEREST
I hereby declare that the co‐authors have no conflicting interest.
DATA AVAILABILITY STATEMENT
The data and code used in this study will be made available via https://ndar.nih.gov/ and/or accessed upon direct request to M.E. Thomason.
Supporting information
ACKNOWLEDGMENTS
This project was supported by awards to M.E.T. from the National Institutes of Health, MH110793 and ES026022, and by a NARSAD Young Investigator Award. The authors thank Sarah Wilhoit, Abdulmohsen Ghuloum, Rosemary Joseph, Houda Ajrouche, Kowzar Hijazi, Zainab Altarjoman, Mohamed Hussain Mussa, Joshua Hammond, Sophia Neuenfeldt, Tamara Qawasmeh, and Jasmine Hect for their assistance in data acquisition and analyses. Special thanks to Dr. Rebecca Waller for construction of the stress factor score used in this study, and Dr. Mark Pflieger for his help on the EMSE software. The authors also thank participant families who generously shared their time.
van de Ven MCJ, van den Heuvel MI, Bhogal A, Lewis T, Thomason ME. Impact of maternal childhood trauma on child behavioral problems: The role of child frontal alpha asymmetry. Developmental Psychobiology. 2020;62:154–169. 10.1002/dev.21900
ENDNOTE
Data collection started early in 2012 and is ongoing. Here, we publish data from children that completed the age 5 visit before May 2018.
REFERENCES
- Achenbach, T. (2001). Child Behavior Checklist for Ages 1.5‐5 (CBCL/1.5‐5). Reporter, 10, 20.
- Achenbach, T. M. , & Rescorla, L. A. (2001). Manual for the ASEBA preschool forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Albright, M. B. , & Tamis‐LeMonda, C. S. (2002). Maternal depressive symptoms in relation to dimensions of parenting in low‐income mothers. Applied Developmental Science, 6(1), 24–34. 10.1207/S1532480XADS0601_03 [DOI] [Google Scholar]
- Allen, J. J. , Coan, J. A. , & Nazarian, M. (2004). Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology, 67(1–2), 183–218. 10.1016/j.biopsycho.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Anokhin, A. P. , Heath, A. C. , & Myers, E. (2006). Genetic and environmental influences on frontal EEG asymmetry: A twin study. Biological Psychology, 71(3), 289–295. 10.1016/j.biopsycho.2005.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aunola, K. , & Nurmi, J. E. (2005). The role of parenting styles in children's problem behavior. Child Development, 76(6), 1144–1159. 10.1111/j.1467-8624.2005.00840.x-i1 [DOI] [PubMed] [Google Scholar]
- Baron, R. M. , & Kenny, D. A. (1986). The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51(6), 1173–1182. 10.1037/0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- Baving, L. , Laucht, M. , & Schmidt, M. H. (2002). Frontal brain activation in anxious school children. Journal of Child Psychology and Psychiatry, 43(2), 265–274. 10.1111/1469-7610.00019 [DOI] [PubMed] [Google Scholar]
- Belsky, J. (2005). Differential susceptibility to rearing influence. Origins of the social mind: Evolutionary psychology and child development, 139–163.
- Belsky, J. , Bakermans‐Kranenburg, M. J. , & Van Ijzendoorn, M. H. (2007). For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science, 16(6), 300–304. [Google Scholar]
- Belsky, J. , & Pluess, M. (2009). Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin, 135(6), 885–908. 10.1037/a0017376 [DOI] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57(1), 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Berlin, L. J. , Appleyard, K. , & Dodge, K. A. (2011). Intergenerational continuity in child maltreatment: Mediating mechanisms and implications for prevention. Child Development, 82(1), 162–176. 10.1111/j.1467-8624.2010.01547.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein, D. P. , & Fink, L. (1998). Childhood trauma questionnaire: A retrospective self‐report. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Bernstein, D. P. , Stein, J. A. , Newcomb, M. D. , Walker, E. , Pogge, D. , Ahluvalia, T. , … Zule, W. (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect, 27(2), 169–190. 10.1016/S0145-2134(02)00541-0 [DOI] [PubMed] [Google Scholar]
- Buss, K. A. , Schumacher, J. R. M. , Dolski, I. , Kalin, N. H. , Goldsmith, H. H. , & Davidson, R. J. (2003). Right frontal brain activity, cortisol, and withdrawal behavior in 6‐month‐old infants. Behavioral Neuroscience, 117(1), 11–20. 10.1037/0735-7044.117.1.11 [DOI] [PubMed] [Google Scholar]
- Casey, B. J. , Giedd, J. N. , & Thomas, K. M. (2000). Structural and functional brain development and its relation to cognitive development. Biological Psychology, 54(1–3), 241–257. 10.1016/S0301-0511(00)00058-2 [DOI] [PubMed] [Google Scholar]
- Choi, K. W. , Sikkema, K. J. , Vythilingum, B. , Geerts, L. , Faure, S. C. , Watt, M. H. , … Stein, D. J. (2017). Maternal childhood trauma, postpartum depression, and infant outcomes: Avoidant affective processing as a potential mechanism. Journal of Affective Disorders, 211, 107–115. 10.1016/j.jad.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Cloitre, M. , Stolbach, B. C. , Herman, J. L. , Kolk, B. V. D. , Pynoos, R. , Wang, J. , & Petkova, E. (2009). A developmental approach to complex PTSD: Childhood and adult cumulative trauma as predictors of symptom complexity. Journal of Traumatic Stress, 22(5), 399–408. 10.1002/jts.20444 [DOI] [PubMed] [Google Scholar]
- Coan, J. A. , & Allen, J. J. (2003). State and trait of frontal EEG asymmetry in emotion. The Asymmetrical Brain, 566–615.
- Coan, J. A. , & Allen, J. J. (2004). Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology, 67(1–2), 7–50. 10.1016/j.biopsycho.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Cohen, S. , Kamarck, T. , & Mermelstein, R. (1994). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 386–396. [PubMed] [Google Scholar]
- Collishaw, S. , Dunn, J. , O'connor, T. G. , & Golding, J. (2007). Maternal childhood abuse and offspring adjustment over time. Development and Psychopathology, 19(2), 367–383. 10.1017/S0954579407070186 [DOI] [PubMed] [Google Scholar]
- Cosco, T. D. , Prina, M. , Stubbs, B. , & Wu, Y. T. (2017). Reliability and validity of the Center for Epidemiologic Studies Depression Scale in a population‐based cohort of middle‐aged US adults. Journal of Nursing Measurement, 25(3), 476–485. 10.1891/1061-3749.25.3.476 [DOI] [PubMed] [Google Scholar]
- Cummings, E. M. , & Davies, P. T. (1994). Maternal depression and child development. Journal of Child Psychology and Psychiatry, 35(1), 73–122. 10.1111/j.1469-7610.1994.tb01133.x [DOI] [PubMed] [Google Scholar]
- Davidson, R. J. , & Fox, N. A. (1982). Asymmetrical brain activity discriminates between positive and negative affective stimuli in human infants. Science, 218(4578), 1235–1237. [DOI] [PubMed] [Google Scholar]
- Davidson, R. J. , & Fox, N. A. (1989). Frontal brain asymmetry predicts infants' response to maternal separation. Journal of Abnormal Psychology, 98(2), 127–131. 10.1037/0021-843X.98.2.127 [DOI] [PubMed] [Google Scholar]
- Deary, I. J. , Johnson, W. , & Houlihan, L. M. (2009). Genetic foundations of human intelligence. Human Genetics, 126(1), 215–232. 10.1007/s00439-009-0655-4 [DOI] [PubMed] [Google Scholar]
- Deuschle, M. , Hendlmeier, F. , Witt, S. , Rietschel, M. , Gilles, M. , Sánchez‐Guijo, A. , … Hellweg, R. (2018). Cortisol, cortisone, and BDNF in amniotic fluid in the second trimester of pregnancy: Effect of early life and current maternal stress and socioeconomic status. Development and Psychopathology, 30(3), 1–10. 10.1017/S0954579418000147 [DOI] [PubMed] [Google Scholar]
- Diener, E. D. , Emmons, R. A. , Larsen, R. J. , & Griffin, S. (1985). The satisfaction with life scale. Journal of Personality Assessment, 49(1), 71–75. 10.1207/s15327752jpa4901_13 [DOI] [PubMed] [Google Scholar]
- Dietz, K. R. , Lavigne, J. V. , Arend, R. , & Rosenbaum, D. (1997). Relation between intelligence and psychopathology among preschoolers. Journal of Clinical Child Psychology, 26, 99–107. 10.1207/s15374424jccp2601_10 [DOI] [PubMed] [Google Scholar]
- Dixon, W. J. (1960). Simplified estimation from censored normal samples. The Annals of Mathematical Statistics, 31, 385–391. 10.1214/aoms/1177705900 [DOI] [Google Scholar]
- Dubowitz, H. , Black, M. M. , Kerr, M. A. , Hussey, J. M. , Morrel, T. M. , Everson, M. D. , & Starr, R. H. (2001). Type and timing of mothers' victimization: Effects on mothers and children. Pediatrics, 107(4), 728–735. 10.1542/peds.107.4.728 [DOI] [PubMed] [Google Scholar]
- Duncan, G. J. , Magnuson, K. A. , & Ludwig, J. (2004). The endogeneity problem in developmental studies. Research in Human Development, 1(1–2), 59–80. [DOI] [Google Scholar]
- Efroymson, M. A. (1960). Multiple regression analysis In Ralston A., & Wilf H. S. (Eds.), Mathematical methods for digital computers (pp. 191–203). New York, NY: Wiley. [Google Scholar]
- Ehlers, C. L. , & Phillips, E. (2003). EEG low‐voltage alpha and alpha power in African American young adults: Relation to family history of alcoholism. Alcoholism: Clinical and Experimental Research, 27(5), 765–772. 10.1097/01.ALC.0000065439.09492.67 [DOI] [PubMed] [Google Scholar]
- Ellis, B. J. , Boyce, W. T. , Belsky, J. , Bakermans‐Kranenburg, M. J. , & Van IJzendoorn, M. H. (2011). Differential susceptibility to the environment: An evolutionary–neurodevelopmental theory. Development and Psychopathology, 23(1), 7–28. 10.1017/S0954579410000611 [DOI] [PubMed] [Google Scholar]
- Etain, B. , Aas, M. , Andreassen, O. A. , Lorentzen, S. , Dieset, I. , Gard, S. , … Henry, C. (2013). Childhood trauma is associated with severe clinical characteristics of bipolar disorders. The Journal of Clinical Psychiatry, 74(10), 991–998. 10.4088/JCP.13m08353 [DOI] [PubMed] [Google Scholar]
- Fox, N. A. , & Davidson, R. J. (1986). Taste‐elicited changes in facial signs of emotion and the asymmetry of brain electrical activity in human newborns. Neuropsychologia, 24(3), 417–422. 10.1016/0028-3932(86)90028-X [DOI] [PubMed] [Google Scholar]
- Fox, N. A. , & Davidson, R. J. (1988). Patterns of brain electrical activity during facial signs of emotion in 10‐month‐old infants. Developmental Psychology, 24(2), 230–236. 10.1037/0012-1649.24.2.230 [DOI] [Google Scholar]
- Gabard‐Durnam, L. , Tierney, A. L. , Vogel‐Farley, V. , Tager‐Flusberg, H. , & Nelson, C. A. (2015). Alpha asymmetry in infants at risk for autism spectrum disorders. Journal of Autism and Developmental Disorders, 45(2), 473–480. 10.1007/s10803-013-1926-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason, M. M. , Goldson, E. , & Yogman, M. W. (2016). Addressing early childhood emotional and behavioral problems. Pediatrics, 138(6), e20163025 10.1542/peds.2016-3025 [DOI] [PubMed] [Google Scholar]
- Goldman, R. I. , Stern, J. M. , Engel, J. Jr. , & Cohen, M. S. (2002). Simultaneous EEG and fMRI of the alpha rhythm. NeuroReport, 13(18), 2487 10.1097/00001756-200212200-00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman, R. N. , Rietschel, J. C. , Lo, L. C. , Costanzo, M. E. , & Hatfield, B. D. (2013). Stress, emotion regulation and cognitive performance: The predictive contributions of trait and state relative frontal EEG alpha asymmetry. International Journal of Psychophysiology, 87(2), 115–123. 10.1016/j.ijpsycho.2012.09.008 [DOI] [PubMed] [Google Scholar]
- Gotlib, I. H. , Lee, C. M. , et al. (1996). Impact of parental depression on young children and infants In Mundt C., Goldstein M. J., Hahlweg K., & Fiedler P. (Eds.), Interpersonal factors in the origin and course of affective disorders (pp. 218–239). London, UK: Gaskell/Royal College of Psychiatrists. [Google Scholar]
- Hagemann, D. , Naumann, E. , Thayer, J. F. , & Bartussek, D. (2002). Does resting electroencephalograph asymmetry reflect a trait? An application of latent state‐trait theory. Journal of Personality and Social Psychology, 82(4), 619–641. 10.1037/0022-3514.82.4.619 [DOI] [PubMed] [Google Scholar]
- Hane, A. A. , & Fox, N. A. (2006). Ordinary variations in maternal caregiving influence human infants' stress reactivity. Psychological Science, 17(6), 550–556. 10.1111/j.1467-9280.2006.01742.x [DOI] [PubMed] [Google Scholar]
- Hane, A. A. , Henderson, H. A. , Reeb‐Sutherland, B. C. , & Fox, N. A. (2010). Ordinary variations in human maternal caregiving in infancy and biobehavioral development in early childhood: A follow‐up study. Developmental Psychobiology, 52(6), 558–567. 10.1002/dev.20461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon‐Jones, E. , & Allen, J. J. (1997). Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology, 106(1), 159–163. 10.1037/0021-843X.106.1.159 [DOI] [PubMed] [Google Scholar]
- Harmony, T. , Marosi, E. , de León, A. E. D. , Becker, J. , & Fernández, T. (1990). Effect of sex, psychosocial disadvantages and biological risk factors on EEG maturation. Electroencephalography and Clinical Neurophysiology, 75(6), 482–491. 10.1016/0013-4694(90)90135-7 [DOI] [PubMed] [Google Scholar]
- Heim, C. , & Nemeroff, C. B. (2001). The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biological Psychiatry, 49(12), 1023–1039. 10.1016/S0006-3223(01)01157-X [DOI] [PubMed] [Google Scholar]
- Heim, C. , Newport, D. J. , Heit, S. , Graham, Y. P. , Wilcox, M. , Bonsall, R. , … Nemeroff, C. B. (2000). Pituitary‐adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA, 284(5), 592–597. 10.1001/jama.284.5.592 [DOI] [PubMed] [Google Scholar]
- Hussey, J. M. , Chang, J. J. , & Kotch, J. B. (2006). Child maltreatment in the United States: Prevalence, risk factors, and adolescent health consequences. Pediatrics, 118(3), 933–942. 10.1542/peds.2005-2452 [DOI] [PubMed] [Google Scholar]
- Kalin, N. H. , Larson, C. , Shelton, S. E. , & Davidson, R. J. (1998). Asymmetric frontal brain activity, cortisol, and behavior associated with fearful temperament in rhesus monkeys. Behavioral Neuroscience, 112(2), 286–292. 10.1037/0735-7044.112.2.286 [DOI] [PubMed] [Google Scholar]
- Kaufman, J. , & Zigler, E. (1987). Do abused children become abusive parents? American Journal of Orthopsychiatry, 57(2), 186–192. 10.1111/j.1939-0025.1987.tb03528.x [DOI] [PubMed] [Google Scholar]
- Kemp, A. H. , Griffiths, K. , Felmingham, K. L. , Shankman, S. A. , Drinkenburg, W. , Arns, M. , … Bryant, R. A. (2010). Disorder specificity despite comorbidity: Resting EEG alpha asymmetry in major depressive disorder and post‐traumatic stress disorder. Biological Psychology, 85(2), 350–354. 10.1016/j.biopsycho.2010.08.001 [DOI] [PubMed] [Google Scholar]
- Kong, S. , & Bernstein, K. (2009). Childhood trauma as a predictor of eating psychopathology and its mediating variables in patients with eating disorders. Journal of Clinical Nursing, 18(13), 1897–1907. 10.1111/j.1365-2702.2008.02740.x [DOI] [PubMed] [Google Scholar]
- Lang, A. J. , Gartstein, M. A. , Rodgers, C. S. , & Lebeck, M. M. (2010). The impact of maternal childhood abuse on parenting and infant temperament. Journal of Child and Adolescent Psychiatric Nursing, 23(2), 100–110. 10.1111/j.1744-6171.2010.00229.x [DOI] [PubMed] [Google Scholar]
- Laufs, H. , Kleinschmidt, A. , Beyerle, A. , Eger, E. , Salek‐Haddadi, A. , Preibisch, C. , & Krakow, K. (2003). EEG‐correlated fMRI of human alpha activity. NeuroImage, 19(4), 1463–1476. 10.1016/S1053-8119(03)00286-6 [DOI] [PubMed] [Google Scholar]
- Levy, W. J. (1987). Effect of epoch length on power spectrum analysis of the EEG. Anesthesiology, 66(4), 489–495. 10.1097/00000542-198704000-00007 [DOI] [PubMed] [Google Scholar]
- Lewis, R. S. , Weekes, N. Y. , & Wang, T. H. (2007). The effect of a naturalistic stressor on frontal EEG asymmetry, stress, and health. Biological Psychology, 75(3), 239–247. 10.1016/j.biopsycho.2007.03.004 [DOI] [PubMed] [Google Scholar]
- Lindsley, D. B. , & Wicke, J. D. (1974). The electroencephalogram: Autonomous electrical activity in man and animals In Thompson R., & Patterson M. N. (Eds.), Bioelectric recording techniques (pp. 3–83). New York, NY: Academic Press. [Google Scholar]
- Lopez‐Duran, N. L. , Nusslock, R. , George, C. , & Kovacs, M. (2012). Frontal EEG asymmetry moderates the effects of stressful life events on internalizing symptoms in children at familial risk for depression. Psychophysiology, 49(4), 510–521. 10.1111/j.1469-8986.2011.01332.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons‐Ruth, K. , Wolfe, R. , & Lyubchik, A. (2000). Depression and the parenting of young children: Making the case for early preventive mental health services. Harvard Review of Psychiatry, 8(3), 148–153. 10.1080/hrp_8.3.148 [DOI] [PubMed] [Google Scholar]
- Malinosky‐Rummell, R. , & Hansen, D. J. (1993). Long‐term consequences of childhood physical abuse. Psychological Bulletin, 114(1), 68–79. 10.1037/0033-2909.114.1.68 [DOI] [PubMed] [Google Scholar]
- Marshall, P. J. , Bar‐Haim, Y. , & Fox, N. A. (2002). Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology, 113(8), 1199–1208. 10.1016/S1388-2457(02)00163-3 [DOI] [PubMed] [Google Scholar]
- Marshall, P. J. , & Fox, N. A. (2013). Infant EEG and ERP in relation to social and emotional development In De Haan M. (Ed.), Infant EEG and event‐related potentials (pp. 227–249). New York, NY: Psychology Press. [Google Scholar]
- Mathersul, D. , Williams, L. M. , Hopkinson, P. J. , & Kemp, A. H. (2008). Investigating models of affect: Relationships among EEG alpha asymmetry, depression, and anxiety. Emotion, 8(4), 560–572. 10.1037/a0012811 [DOI] [PubMed] [Google Scholar]
- McLaughlin, K. A. , Fox, N. A. , Zeanah, C. H. , & Nelson, C. A. (2011). Adverse rearing environments and neural development in children: The development of frontal electroencephalogram asymmetry. Biological Psychiatry, 70(11), 1008–1015. 10.1016/j.biopsych.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, T. J. , Miller, M. L. , Metzger, R. L. , & Borkovec, T. D. (1990). Development and validation of the penn state worry questionnaire. Behaviour Research and Therapy, 28(6), 487–495. 10.1016/0005-7967(90)90135-6 [DOI] [PubMed] [Google Scholar]
- Min, M. O. , Singer, L. T. , Minnes, S. , Kim, H. , & Short, E. (2013). Mediating links between maternal childhood trauma and preadolescent behavioral adjustment. Journal of Interpersonal Violence, 28(4), 831–851. 10.1177/0886260512455868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda, J. K. , de la Osa, N. , Granero, R. , & Ezpeleta, L. (2011). Maternal experiences of childhood abuse and intimate partner violence: Psychopathology and functional impairment in clinical children and adolescents. Child Abuse & Neglect, 35(9), 700–711. 10.1016/j.chiabu.2011.05.008 [DOI] [PubMed] [Google Scholar]
- Miranda, J. K. , de la Osa, N. , Granero, R. , & Ezpeleta, L. (2013). Maternal childhood abuse, intimate partner violence, and child psychopathology: The mediator role of mothers’ mental health. Violence against Women, 19(1), 50–68. 10.1177/1077801212475337 [DOI] [PubMed] [Google Scholar]
- Miskovic, V. , Schmidt, L. A. , Georgiades, K. , Boyle, M. , & MacMillan, H. L. (2009). Stability of resting frontal electroencephalogram (EEG) asymmetry and cardiac vagal tone in adolescent females exposed to child maltreatment. Developmental Psychobiology, 51(6), 474–487. 10.1002/dev.20387 [DOI] [PubMed] [Google Scholar]
- Monk, C. , Spicer, J. , & Champagne, F. A. (2012). Linking prenatal maternal adversity to developmental outcomes in infants: The role of epigenetic pathways. Development and Psychopathology, 24(4), 1361–1376. 10.1017/S0954579412000764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog, N. K. , Entringer, S. , Rasmussen, J. M. , Styner, M. , Gilmore, J. H. , Kathmann, N. , … Buss, C. (2018). Intergenerational effect of maternal exposure to childhood maltreatment on newborn brain anatomy. Biological Psychiatry, 83(2), 120–127. 10.1016/j.biopsych.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder, E. J. , De Medina, P. R. , Huizink, A. C. , Van den Bergh, B. R. , Buitelaar, J. K. , & Visser, G. H. (2002). Prenatal maternal stress: Effects on pregnancy and the (unborn) child. Early Human Development, 70(1–2), 3–14. 10.1016/S0378-3782(02)00075-0 [DOI] [PubMed] [Google Scholar]
- Myhre, M. C. , Dyb, G. A. , Wentzel‐Larsen, T. , Grøgaard, J. B. , & Thoresen, S. (2014). Maternal childhood abuse predicts externalizing behaviour in toddlers: A prospective cohort study. Scandinavian Journal of Public Health, 42(3), 263–269. 10.1177/1403494813510983 [DOI] [PubMed] [Google Scholar]
- Nicol‐Harper, R. , Harvey, A. G. , & Stein, A. (2007). Interactions between mothers and infants: Impact of maternal anxiety. Infant Behavior & Development, 30(1), 161–167. 10.1016/j.infbeh.2006.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero, G. A. , Pliego‐Rivero, F. B. , Fernández, T. , & Ricardo, J. E. E. G. (2003). EEG development in children with sociocultural disadvantages: A follow‐up study. Clinical Neurophysiology, 114(10), 1918–1925. 10.1016/S1388-2457(03)00173-1 [DOI] [PubMed] [Google Scholar]
- Peltola, M. J. , Bakermans‐Kranenburg, M. J. , Alink, L. R. , Huffmeijer, R. , Biro, S. , & van IJzendoorn, M. H. (2014). Resting frontal EEG asymmetry in children: Meta‐analyses of the effects of psychosocial risk factors and associations with internalizing and externalizing behavior. Developmental Psychobiology, 56(6), 1377–1389. 10.1002/dev.21223 [DOI] [PubMed] [Google Scholar]
- Pfurtscheller, G. , Stancak, A. Jr , & Neuper, C. (1996). Event‐related synchronization (ERS) in the alpha band—an electrophysiological correlate of cortical idling: A review. International Journal of Psychophysiology, 24(1–2), 39–46. 10.1016/S0167-8760(96)00066-9 [DOI] [PubMed] [Google Scholar]
- Pianta, R. C. , & Castaldi, J. (1989). Stability of internalizing symptoms from kindergarten to first grade and factors related to instability. Development and Psychopathology, 1(4), 305–316. 10.1017/S0954579400000493 [DOI] [Google Scholar]
- Plant, D. T. , Jones, F. W. , Pariante, C. M. , & Pawlby, S. (2017). Association between maternal childhood trauma and offspring childhood psychopathology: Mediation analysis from the ALSPAC cohort. The British Journal of Psychiatry, 211(3), 144–150. 10.1192/bjp.bp.117.198721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant, D. T. , Pawlby, S. , Pariante, C. M. , & Jones, F. W. (2017). When one childhood meets another–maternal childhood trauma and offspring child psychopathology: A systematic review. Clinical Child Psychology and Psychiatry, 23, 483–500. [DOI] [PubMed] [Google Scholar]
- Quaedflieg, C. W. E. M. , Meyer, T. , Smulders, F. T. Y. , & Smeets, T. (2015). The functional role of individual‐alpha based frontal asymmetry in stress responding. Biological Psychology, 104, 75–81. 10.1016/j.biopsycho.2014.11.014 [DOI] [PubMed] [Google Scholar]
- Querido, J. G. , Warner, T. D. , & Eyberg, S. M. (2002). Parenting styles and child behavior in African American families of preschool children. Journal of Clinical Child and Adolescent Psychology, 31(2), 272–277. 10.1207/S15374424JCCP3102_12 [DOI] [PubMed] [Google Scholar]
- Radloff, L. S. (1977). The CES‐D Scale: A self‐report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Read, J. , Os, J. V. , Morrison, A. P. , & Ross, C. A. (2005). Childhood trauma, psychosis and schizophrenia: A literature review with theoretical and clinical implications. Acta Psychiatrica Scandinavica, 112(5), 330–350. 10.1111/j.1600-0447.2005.00634.x [DOI] [PubMed] [Google Scholar]
- Rijlaarsdam, J. , Stevens, G. W. J. M. , Jansen, P. W. , Ringoot, A. P. , Jaddoe, V. W. V. , Hofman, A. , … Tiemeier, H. (2014). Maternal childhood maltreatment and offspring emotional and behavioral problems: Maternal and paternal mechanisms of risk transmission. Child Maltreatment, 19(2), 67–78. 10.1177/1077559514527639 [DOI] [PubMed] [Google Scholar]
- Roberts, A. L. , Lyall, K. , Rich‐Edwards, J. W. , Ascherio, A. , & Weisskopf, M. G. (2013). Association of maternal exposure to childhood abuse with elevated risk for autism in offspring. JAMA Psychiatry, 70(5), 508–515. 10.1001/jamapsychiatry.2013.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, R. , O'Connor, T. , Dunn, J. , & Golding, J. , & ALSPAC Study Team . (2004). The effects of child sexual abuse in later family life; mental health, parenting and adjustment of offspring. Child Abuse & Neglect, 28(5), 525–545. 10.1016/j.chiabu.2003.07.006 [DOI] [PubMed] [Google Scholar]
- Roth, T. L. , Lubin, F. D. , Funk, A. J. , & Sweatt, J. D. (2009). Lasting epigenetic influence of early‐life adversity on the BDNF gene. Biological Psychiatry, 65(9), 760–769. 10.1016/j.biopsych.2008.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinsky, M. C. , Oken, B. S. , & Morehead, L. (1991). Test‐retest reliability in EEG frequency analysis. Electroencephalography and Clinical Neurophysiology, 79(5), 382–392. 10.1016/0013-4694(91)90203-G [DOI] [PubMed] [Google Scholar]
- Sandberg, S. , Rutter, M. , Giles, S. , Owen, A. , Champion, L. , Nicholls, J. , … Drinnan, D. (1993). Assessment of psychosocial experiences in childhood: Methodological issues and some illustrative findings. Journal of Child Psychology and Psychiatry, 34(6), 879–897. 10.1111/j.1469-7610.1993.tb01096.x [DOI] [PubMed] [Google Scholar]
- Santesso, L. D. , Dana, L. R. , Schmidt, L. A. , & Segalowitz, S. J. (2006). Frontal electroencephalogram activation asymmetry, emotional intelligence, and externalizing behaviors in 10‐year‐old children. Child Psychiatry and Human Development, 36(3), 311–328. 10.1007/s10578-005-0005-2 [DOI] [PubMed] [Google Scholar]
- Scheeringa, M. S. (2010). Young child PTSD checklist. New Orleans, LA: Tulane University. [Google Scholar]
- Scher, C. D. , Stein, M. B. , Asmundson, G. J. , McCreary, D. R. , & Forde, D. R. (2001). The childhood trauma questionnaire in a community sample: Psychometric properties and normative data. Journal of Traumatic Stress, 14(4), 843–857. 10.1023/A:1013058625719 [DOI] [PubMed] [Google Scholar]
- Schmidt, A. F. , & Finan, C. (2017). Linear regression and the normality assumption. Journal of Clinical Epidemiology, 98, 146–151. 10.1016/j.jclinepi.2017.12.006 [DOI] [PubMed] [Google Scholar]
- Scorza, P. , Duarte, C. S. , Hipwell, A. E. , Posner, J. , Ortin, A. , Canino, G. , … Program Collaborators for Environmental influences on Child Health Outcomes . (2019). Research review: Intergenerational transmission of disadvantage: Epigenetics and parents' childhoods as the first exposure. Journal of Child Psychology and Psychiatry, 60(2), 119–132. 10.1111/jcpp.12877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman, E. K. , & Weingartner, H. (1986). Hemispheric lateralization of functions related to emotion. Brain and Cognition, 5(3), 322–353. 10.1016/0278-2626(86)90035-7 [DOI] [PubMed] [Google Scholar]
- Simeon, D. , Guralnik, O. , Schmeidler, J. , Sirof, B. , & Knutelska, M. (2001). The role of childhood interpersonal trauma in depersonalization disorder. American Journal of Psychiatry, 158(7), 1027–1033. 10.1176/appi.ajp.158.7.1027 [DOI] [PubMed] [Google Scholar]
- Smith, E. E. , Reznik, S. J. , Stewart, J. L. , & Allen, J. J. (2017). Assessing and conceptualizing frontal EEG asymmetry: An updated primer on recording, processing, analyzing, and interpreting frontal alpha asymmetry. International Journal of Psychophysiology, 111, 98–114. 10.1016/j.ijpsycho.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smulders, F. T. , Ten Oever, S. , Donkers, F. C. , Quaedflieg, C. W. , & van de Ven, V. (2018). Single‐trial log transformation is optimal in frequency analysis of resting EEG alpha. European Journal of Neuroscience, 48, 2585–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger, C. D. , Gorsuch, R. L. , Lushene, R. , Vagg, P. R. , & Jacobs, G. A. (1983). Manual for the state‐trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Stano, J. F. (2004). Wechsler abbreviated scale of intelligence. Rehabilitation Counseling Bulletin, 48(1), 56. [Google Scholar]
- Tang, A. , Miskovic, V. , Lahat, A. , Tanaka, M. , MacMillan, H. , Van Lieshout, R. J. , & Schmidt, L. A. (2018). Trajectories of resting frontal brain activity and psychopathology in female adolescents exposed to child maltreatment. Developmental Psychobiology, 60(1), 67–77. 10.1002/dev.21585 [DOI] [PubMed] [Google Scholar]
- Teicher, M. H. , Andersen, S. L. , Polcari, A. , Anderson, C. M. , Navalta, C. P. , & Kim, D. M. (2003). The neurobiological consequences of early stress and childhood maltreatment. Neuroscience & Biobehavioral Reviews, 27(1), 33–44. 10.1016/S0149-7634(03)00007-1 [DOI] [PubMed] [Google Scholar]
- Thomas, J. C. , Letourneau, N. , Campbell, T. S. , & Giesbrecht, G. F., & Apron Study Team . (2018). Social buffering of the maternal and infant HPA axes: Mediation and moderation in the intergenerational transmission of adverse childhood experiences. Development and Psychopathology, 30(3), 921–939. 10.1017/S0954579418000512 [DOI] [PubMed] [Google Scholar]
- Thompson, R. (2007). Mothers' violence victimization and child behavior problems: Examining the link. American Journal of Orthopsychiatry, 77(2), 306–315. 10.1037/0002-9432.77.2.306 [DOI] [PubMed] [Google Scholar]
- Tomarken, A. J. , Davidson, R. J. , Wheeler, R. E. , & Kinney, L. (1992). Psychometric properties of resting anterior EEG asymmetry: Temporal stability and internal consistency. Psychophysiology, 29(5), 576–592. 10.1111/j.1469-8986.1992.tb02034.x [DOI] [PubMed] [Google Scholar]
- Tops, M. , Wijers, A. A. , van Staveren, A. S. , Bruin, K. J. , Den Boer, J. A. , Meijman, T. F. , & Korf, J. (2005). Acute cortisol administration modulates EEG alpha asymmetry in volunteers: Relevance to depression. Biological Psychology, 69(2), 181–193. 10.1016/j.biopsycho.2004.07.005 [DOI] [PubMed] [Google Scholar]
- Van den Bergh, B. R. H. , van den Heuvel, M. I. , Lahti, M. , Braeken, M. , de Rooij, S. R. , Entringer, S. , … Schwab, M. (2017). Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neuroscience & Biobehavioral Reviews, 1–39. 10.1016/j.neubiorev.2017.07.003 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- van Wijk, I. C. , Huffmeijer, R. , Bosdriesz, J. R. , Bakermans‐Kranenburg, M. J. , Kolijn, L. , van IJzendoorn, M. H. , … van den Bulk, B. G. (2019). Behavioral genetics of temperament and frontal asymmetry in early childhood. Journal of Experimental Child Psychology, 179, 348–361. 10.1016/j.jecp.2018.11.015 [DOI] [PubMed] [Google Scholar]
- Weschler, D. (2011). Weschler Abbreviated Scale of Intelligence – Second Edition (WASI‐II). San Antonio, TX: NCS Pearson. [Google Scholar]
- Woodruff‐Borden, J. , Morrow, C. , Bourland, S. , & Cambron, S. (2002). The behavior of anxious parents: Examining mechanisms of transmission of anxiety from parent to child. Journal of Clinical Child and Adolescent Psychology, 31(3), 364–374. 10.1207/S15374424JCCP3103_08 [DOI] [PubMed] [Google Scholar]
- World Health Organization . (2016). Child maltreatment. Retrieved from http://www.who.int/news-room/fact-sheets/detail/child-maltreatment
- Yang, L. , Jia, C. X. , & Qin, P. (2015). Reliability and validity of the Center for Epidemiologic Studies Depression Scale (CES‐D) among suicide attempters and comparison residents in rural China. BMC Psychiatry, 15(76), 1–8. 10.1186/s12888-015-0458-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev, V. , Yuan, H. , Misaki, M. , Phillips, R. , Young, K. D. , Feldner, M. T. , & Bodurka, J. (2016). Correlation between amygdala BOLD activity and frontal EEG asymmetry during real‐time fMRI neurofeedback training in patients with depression. NeuroImage: Clinical, 11, 224–238. 10.1016/j.nicl.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and code used in this study will be made available via https://ndar.nih.gov/ and/or accessed upon direct request to M.E. Thomason.


