Abstract
Objective:
To examine five-year outcomes of treatment of meniscal tear in osteoarthritis.
Methods:
We examined 5-year follow-up data from the MeTeOR (Meniscal Tear in Osteoarthritis Research) Trial of physical therapy (PT) vs. arthroscopic partial meniscectomy (APM). We performed primary intention-to-treat (ITT) and secondary as-treated analyses. The primary outcome was the Knee Osteoarthritis and Injury Outcome Score (KOOS) Pain scale; total knee replacement (TKR) was a secondary outcome. We used piecewise linear mixed models to describe change in KOOS Pain. We calculated 5-year cumulative TKR incidence and used a Cox model to estimate hazard ratios (HR) for TKR.
Results:
351 participants were randomized. In the ITT analysis, KOOS Pain scores were ~46 (0–100, 100 worst) at baseline in both arms. Pain scores improved substantially in both arms over the first three months, continued to improve through 24 months (to ~18 in each arm) and were stable from 24 to 60 months. Results of the as-treated analyses of KOOS Pain were similar. Twenty-five participants (7.1% (95% CI: 4.4%, 9.8%)) underwent TKR over five years. In the ITT model, the HR for TKR was 2.0 (95% CI: 0.8, 4.9) for subjects randomized to APM, compared to those randomized to PT. The as-treated HR for TKR was 4.9 (95% CI: 1.1, 20.9) for subjects ultimately treated with APM, compared to those treated nonoperatively.
Conclusion:
Pain improved considerably in both arms over 60 months. While ITT analysis revealed no statistically significant differences in TKR use, greater TKR utilization in those receiving APM merits further study.
Introduction
Knee pain in the setting of meniscal tear and damage to cartilage, bone, and other intra-articular knee structures is common in middle-aged and older adults. Patients with this constellation of findings are typically treated non-operatively with medications, physical therapy (PT), and corticosteroid injections. When these measures fail, patients are often offered a surgical option, arthroscopic partial meniscectomy (APM).
Several randomized controlled trials have reported on the short-term (1–2 year) outcomes of non-operative and surgical treatments for meniscal tear in this patient population.(1–8) These trials demonstrated clinically important improvements in pain and function from baseline to 1–2 years of follow-up in both the non-operative and surgical treatment arms. In intention-to-treat (ITT) analyses, one trial showed an advantage of APM over PT,(6, 7) while several others did not reveal clinically important differences in the outcomes of these two strategies.(1–5) In some trials,(1–3) investigators also performed as-treated analyses, in which participants who were randomized to PT but then elected to have APM were analyzed within the APM group. These as-treated analyses suggest a benefit from crossing over to APM among those not responding to PT.(1–3) In summary, the literature suggests that, irrespective of treatment, middle-aged and older patients presenting with knee pain, osteoarthritis (OA) changes, and meniscal tear are likely, on average, to improve considerably over a 1–2-year period. These short-term trials point to non-operative therapy as a sensible initial treatment strategy. APM appears to be effective in those who do not respond to initial PT.(9)
Relatively little is known about the effects of treatment on outcomes beyond 1–2 years in patients with meniscal tear and OA changes. To address this gap in the literature, we used data from the five-year follow-up of MeTeOR (Meniscal Tear in Osteoarthritis Research) Trial participants to evaluate the effect of treatment on pain, functional status, and total knee replacement (TKR) use over five years of follow-up.
Patients and Methods
Design:
This study was a longitudinal five-year follow-up of participants in the MeTeOR Trial, a multicenter randomized controlled trial.
Sample:
Participants were at least 45 years of age and had knee pain of at least one month’s duration, ascribed by their treating physicians to meniscal tear. Additional inclusion criteria included MRI evidence of meniscal tear and either one or more cartilage defects on MRI or an osteophyte or joint space narrowing on radiograph. We excluded individuals with greater than 50% joint space narrowing (a criterion often used to define Kellgren-Lawrence (KL) grade 4 on radiographs, as the precise definition of KL 4 has not been established).(10) Full details are reported elsewhere.(1, 11)
Interventions:
Participants were randomized 1:1 to either a standardized, strengthening-based twelve-week PT regimen or APM followed by the PT regimen. Participating surgeons performed APM in a standardized fashion, with resection of the tear to achieve a stable edge and no osteochondral drilling.
Groups for this analysis:
We adopted an ITT approach for the primary analysis, in which all subjects are analyzed in the group to which they were assigned at randomization, irrespective of the treatment(s) they actually received. Because some subjects randomized to PT crossed over and had APM over the follow-up period, we also performed an as-treated analysis in which we categorized participants into 3 groups: randomized to and received APM; randomized to PT without crossover; and randomized to PT with crossover to APM. Ten subjects were randomized to but did not receive APM; this group was too small to examine meaningfully and was excluded from the as-treated analysis.
Outcome measures:
Participants completed questionnaires just prior to randomization, at 3- and 6-months post-randomization, and every 6 months thereafter through 60 months of follow-up. The primary outcome for this analysis was the Knee Osteoarthritis and Injury Outcome Scale (KOOS) Pain Scale,(12, 13) which was assessed in each follow-up questionnaire. The KOOS Pain Scale consists of nine items that ask about the severity of pain related to a range of activities. We transformed the score to a 0–100 scale, with 100 representing the most pain and 0 representing no pain. We used KOOS Pain rather than the WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) Pain Scale(14) as the primary outcome because the WOMAC Pain Scale asks about pain with largely sedentary activities, while the KOOS Pain Scale contains the WOMAC Pain items and adds four more items on knee pain with bending, straightening, twisting, or pivoting, and on knee pain frequency. A secondary outcome was the (WOMAC) Functional Status scale,(14) which is also scaled 0–100 with 100 representing worst functional status.
We evaluated the occurrence of TKR in the index knee in all participants as an additional outcome. Participants were asked about TKR in the questionnaires, and we examined medical records of each subject to verify the TKR. Since the procedures could have occurred at a different hospital from the one at which the subject was enrolled into MeTeOR, we used data from both self-report and medical record review. If the date of the TKR procedure was self-reported by the participant in the questionnaire with only the month and year of procedure, we assigned the first day of the reported month as the TKR date. In subjects receiving TKR of the index knee, any data collected following the date of TKR was not included in our analyses.
Baseline covariates:
We collected data on several baseline factors including age, sex, body mass index (BMI), KL grade, musculoskeletal comorbidity index(15) (painful sites other than the index knee), five-item mental health index (MHI-5),(16) and baseline measures of the primary and secondary outcome metrics.
Statistical analyses:
We conducted the statistical analyses in two parts: evaluation of pain and function and evaluation of TKR rates. Our principal analyses used an ITT approach and secondary analyses used an as-treated approach.
Pain and Function:
We used descriptive statistics and a piecewise linear mixed model to describe the trend in KOOS Pain and WOMAC Function outcomes over time. Piecewise linear models, or spline models, describe non-linear trends well when the mean change in response varies over some duration.(17) Points at which the slope changes are referred to as knots. We evaluated models with knots at 3, 6, 12, 24, 36, and 48 months and chose the final model based on the goodness of fit assessed by the Bayesian Information Criterion (BIC).(17) These models were adjusted for baseline age, BMI, sex, musculoskeletal comorbidity index, and mental health index. We included covariate-by-time interactions for treatment group, for baseline KL grade, and for treatment group and KL grade. These interactions reveal whether the trend in outcomes over time differed by level of each covariate.
We utilized pattern mixture models (PMM) to investigate the potential effect of dropout on longitudinal estimates of self-reported outcomes. Pattern mixture modeling is a technique to account for potentially informative dropouts; the models stratify the population by the pattern of dropout and then separately model each group.(18) The final estimate is a weighted average of these patterns. To do this, we stratified the population into three mutually exclusive groups: subjects undergoing TKR prior to 60 months, study completers (defined as participants who completed either the 48-, 54-, or 60-month questionnaire), and study non-completers (those who did not complete questionnaires at 48, 54, or 60 months and who did not have TKR). This analysis assesses whether the trend in outcome over time is different for different dropout groups (suggesting that dropout may be not at random) and evaluates whether these potentially not-at-random dropouts bias the overall estimate of longitudinal pain and function trajectory.
While we incorporated pre-TKR KOOS Pain scores in the longitudinal model, we did not follow these subjects post-TKR. To address the concern that subjects dropping out of the study to undergo TKR may have worse pain and not be missing at random, we performed a sensitivity analysis to examine the effect of TKR dropout on KOOS Pain score across the two randomized groups. First, we performed multiple imputation under a missing at random assumption, using baseline covariates associated with outcome and observed KOOS Pain values. Next, we assumed that subjects dropping out to undergo TKR would have worse KOOS Pain values than predicted by the multiple imputation model (i.e. a not-at-random dropout mechanism.) We increased imputed KOOS Pain for TKA dropouts by either 33% or 50% at all time points; we chose these increases in pain score based on the results of the PMM suggesting that TKR dropouts had worse pain and function and because they are clinically plausible. We then fit a mixed effects model on the imputed dataset with knots at 3, 12, and 24 months to estimate the effect of treatment groups on KOOS Pain scores.
Total Knee Replacement:
We calculated the 5-year cumulative incidence of TKR, overall and stratified by the two randomized groups. In a secondary, as-treated analysis we stratified by the three treatment groups (randomized to and received APM; randomized to and received PT; randomized to PT and crossed over to APM). We fit Cox proportional hazard models to evaluate the association between group (randomized group in the primary analysis and received APM vs. didn’t receive APM in the as-treated analysis) and time to TKR, adjusting for baseline covariates including KL grade. We examined associations between model residuals and time to confirm the proportional hazards assumption.
Results
Sample:
Three hundred and fifty-one participants were randomized: 174 to APM and 177 to PT. These subjects were included in the ITT analyses. In the as-treated analyses, we excluded the 10 randomized to APM who never underwent surgery. The as-treated groups included 164 subjects randomized to and receiving APM, 109 randomized to PT who did not cross over to APM, and 68 who were randomized to PT and crossed over to APM. 79% of cross-overs occurred in the first six months following randomization, 12% between six and 12 months, and 9% after 12 months.
Baseline features:
Participants in the two randomized groups were similar in age (mean 57.2 – 58.6 in each group), sex (~43% male in each group), BMI (mean ~30 in each group), and KL grade (~1/3 of subjects in each group had KL 3 radiographs). The KOOS Pain score at baseline was 46.4 – 47.4 in each group (Table 1). The baseline characteristics of the as-treated groups are shown in Appendix Table 1.
Table 1:
Descriptive statistics of randomized groups
| Randomized to PT | Randomized to APM | |||
|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | |
| Age | 177 | 57.2 (6.7) | 174 | 58.6 (7.9) |
| BMI | 168 | 30.2 (6.1) | 164 | 30.2 (6.2) |
| KOOS Pain | 175 | 47.4 (16.2) | 172 | 46.4 (16.1) |
| WOMAC Function | 176 | 37.6 (18.2) | 173 | 37.5 (18.2) |
| MHI-5 Index | 176 | 73.6 (14.2) | 171 | 74.8 (12.8) |
| Sex | N (%) | N (%) | ||
| Male | 75 (42) | 75 (43) | ||
| Female | 102 (58) | 99 (57) | ||
| Race | ||||
| White | 145 (82) | 151 (87) | ||
| Black | 19 (11) | 15 (9) | ||
| Hispanic | 5 (3) | 2 (1) | ||
| Other or missing | 8 (5) | 6 (3) | ||
| Index Knee | ||||
| Left | 106 (60) | 96 (55) | ||
| Right | 71 (40) | 78 (45) | ||
| KL Grade | ||||
| [0] Normal | 14 (8) | 14 (8) | ||
| [1] Questionable osteophyte | 43 (24) | 33 (19) | ||
| [2] Definite osteophyte | 59 (33) | 69 (40) | ||
| [3] < 50% joint space narrowing | 61 (34) | 58 (33) | ||
SD: standard deviation; APM: arthroscopic partial meniscectomy; PT: physical therapy
Subject retention:
Among the 351 participants in the primary analytic cohort four participants died (1%), 25 had TKR (7%), 65 (19%) withdrew participation over five years, and 18 (5%) were lost to follow-up. The percentage of participants completing at least 9 of the 12 questionnaires was 66% in both the APM and PT groups. Detailed data on questionnaire completion by timepoint are presented in Appendix Table 2.
KOOS Pain:
The unadjusted data on KOOS Pain over the 60-month follow-up period in the two randomized groups are shown in Figure 1. We evaluated piecewise linear mixed models with knots at 3, 6, 12, 24, 36, and 48 months. The best fit based on BIC(17) was the model with knots at 3, 12, and 24 months (Figure 2), defining four distinct pain slopes: baseline to 3 months, 3 to 12 months, 12 to 24 months, and 24 to 60 months.
Figure 1:
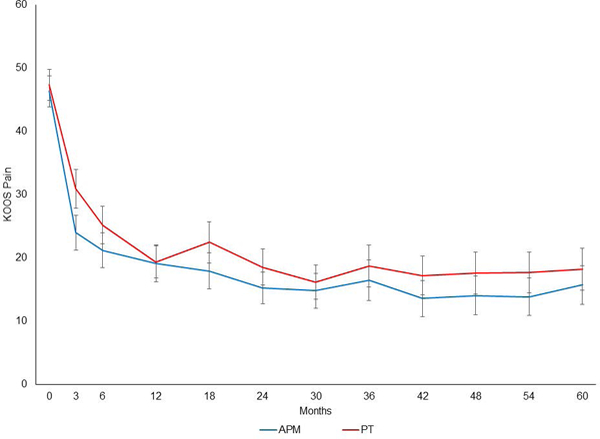
Crude KOOS Pain scores with 95% CI for subjects randomized to APM (blue) and randomized to PT (red).
Figure 2:
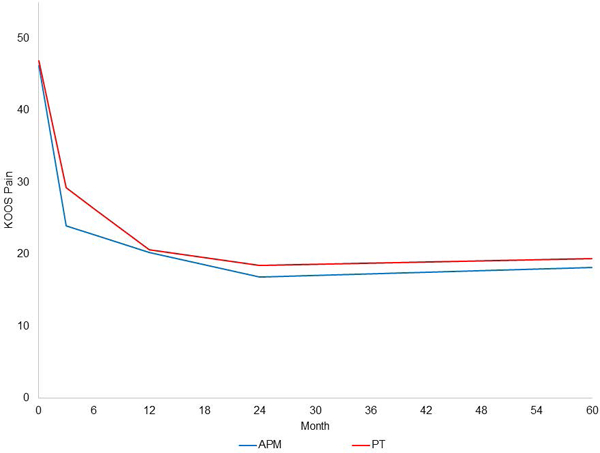
Piecewise linear mixed model of KOOS Pain scores in subjects randomized to APM (blue) and randomized to PT (red). Slopes are estimated in four time periods: 0–3 months, 3–12 months, 12–24, and 24–60 months.
The slope for the first 3 months for those randomized to APM was −7.4 points/month, while the slope for those randomized to PT was −5.90 points/month. The slope for the period from 3 to 12 months was −0.41 in the APM group and −0.96 in the PT group. For the period of 12 to 24 months, the slopes were −0.29 and −0.18 in the APM and PT groups, respectively, while the slopes from 24 to 60 months were 0.038 and 0.027 in the APM and PT groups, respectively. The interaction between randomization group and time on KOOS Pain was statistically significant, due largely to less robust improvement in the PT group in months 0–3. The effect of randomized group on pain did not change significantly when adjusted for age, BMI, gender, KL grade, musculoskeletal index, and mental health index.
The crude, unadjusted data on KOOS Pain in the as-treated analysis are shown in Appendix Figure 1 and the piecewise linear model with knots at 3, 12 and 24 months in the as-treated groups is shown in Appendix Figure 2. These as-treated analyses highlight the less rapid improvement in pain from baseline to three months in the group that crossed over from PT to APM. By 12 months, scores in all three of the as-treated groups were similar.
The PMM separately modeled each dropout pattern and indicated that participants ultimately undergoing TKR started with the highest KOOS Pain scores and experienced the slowest improvement in pain over the first 3 months post-randomization. The TKR group improved from 50 to 41 points, on average; the non-completers improved from 48 to 28, on average; and the study completers improved from 46 to 22, on average (Figure 3). The overall combined estimate using the PMM approach (Figure 3, light dotted black line) is similar to the combined estimate obtained using the piecewise linear mixed model (Figure 3, darker dashed black line). The similarity in these estimates reflects that only 7% underwent TKR.
Figure 3:
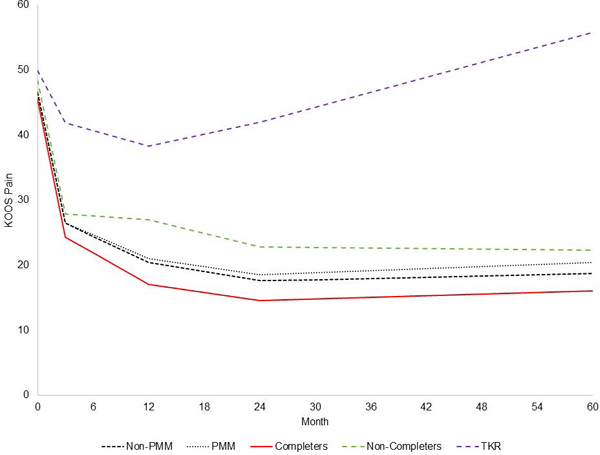
KOOS Pain scores are estimated for three groups: those who had TKR, those who dropped out of the study for reasons other than TKR (non-completers); and those who completed the study (completers). The pattern mixture model (PMM) shows the weighted average of the TKR, non-completers, and completer groups. The Non-PMM trace shows the results of the piecewise linear mixed model. The figure demonstrates that the PMM and non-PMM traces are very similar (since only 7% of subjects had TKR). The non-completer and TKR lines are dashed to indicate that the slopes are generated from data obtained before members of these groups left the cohort.
In the sensitivity analysis, we imputed KOOS Pain scores for subjects who dropped out and then increased KOOS Pain by 33% and 50% for those who dropped out due to TKR (Appendix Figures 3 and 4). The effect of treatment group on KOOS Pain over time in these models changed little compared with the original piecewise mixed model that used data without imputation (Figure 2).
WOMAC Function:
Analyses of WOMAC Function score were largely similar to those of KOOS Pain, with substantial improvements in the first year in both randomized groups. Improvements achieved in the first year were largely maintained in the two randomized groups over the five-year follow-up (Appendix Figure 5).
Total knee replacement:
Twenty-five participants (7.1%, 95% CI: 4.4, 9.8) underwent TKR of the index knee over the course of follow-up. From an ITT perspective, 9.2% (95% CI: 4.9, 13.5) of those randomized to APM underwent TKR as compared with 5.1% (95% CI: 1.9, 8.3) of those randomized to PT (Figure 4a). The ITT Cox regression model included KL grade and randomized group. TKR occurred more often in those randomized to APM than in those randomized to PT (HR 2.0, 95% CI: 0.84, 4.9). The model also showed that subjects with KL 3 radiographs had greater risk of TKR than those with KL 0–2 radiographs (HR 3.0, 95% CI: 1.3, 6.9). As the confidence intervals suggest, the TKR risk in this ITT model did not reach statistical significance (p=0.11).
Figures 4a-b:
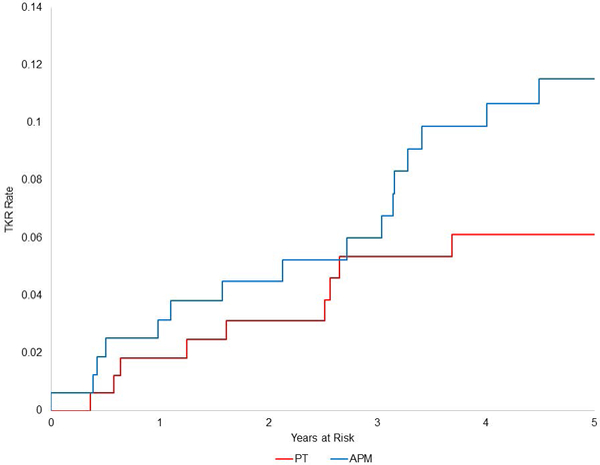
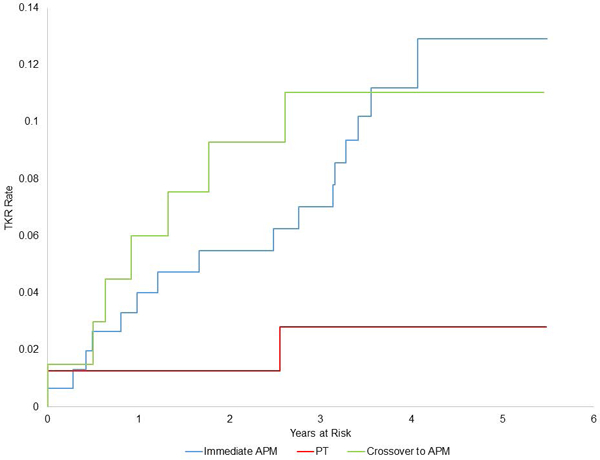
Kaplan Meier plots of time to TKR in two intention to treat groups (4a): randomized to APM (blue) and randomized to PT (red); and in three as-treated groups (4b): subjects randomized to and receiving APM (blue), randomized to PT and not crossing over (red), and randomized to PT and crossover over to APM (green).
From an as-treated perspective, TKRs were performed in 1.8% (95% CI: 0, 4.4) of subjects treated with PT alone, 9.8% (95% CI: 5.2, 14.3) of those treated with APM alone, and 10.3% (95% CI: 3.1, 17.5) of those that crossed over from PT to APM (Figure 4b). In the as-treated analysis, which adjusted for KL radiographic grade, the hazard of TKR was greater in those treated with APM (either immediate or crossover) than in those treated with only PT (HR 4.9, 95% CI: 1.1, 20.9).
Discussion
We found that participants in the MeTeOR Trial experienced considerable pain relief in the first year, which was maintained over five years. These generally favorable results were observed in subjects randomized to APM and in those randomized to nonoperative therapy. TKR rates were generally low (7.1% in the whole cohort), and greater among those who were randomized to APM. As-treated analyses also documented favorable five-year outcomes irrespective of treatment received, with greater TKR risk in those treated with APM.
Our findings are consistent with those of Herrlin et al., who conducted a smaller single-center trial of APM with PT vs. PT alone.(3) These authors followed subjects to five years and observed considerable improvement in pain and function in both the APM and PT groups, with the improvement sustained over five years. Herrlin et al. observed, as we did, that those randomized to PT who crossed over to APM had five-year outcomes similar to those randomized to APM. These findings are also consistent with cohort data that show favorable long-term pain outcomes of APM.(19, 20) There are relatively few studies of outcomes of non-operative therapy beyond 1–2 years.
The less robust reduction in pain over the first 3 months in the PT arm of the ITT analysis reflects that some subjects randomized to PT crossed over to APM due to the persistence of pain in the first several months. By 12 months, pain levels of subjects who crossed over to APM were similar to levels of those who had immediate APM.
The greater likelihood of TKR in those who ultimately received APM merits comment. The as-treated analysis suggested a five-fold increased TKR risk among those exposed to APM over the follow-up period, as compared to those treated nonoperatively. The ITT analysis of the five-year TKR risk in the two randomized groups showed a 2-fold increased hazard of TKR among those randomized to APM, though this hazard ratio was not statistically significant. Our analyses of TKR use were adjusted for baseline KL grade, suggesting that the findings are not due to greater pre-APM radiographic severity. The group that crossed over had severe symptoms at baseline and then failed PT; these factors may underly the decision to ultimately undergo TKR. APM may be associated with greater progression of OA and increased risk of TKR on that basis. Our analyses of 18-month progression in cartilage damage documented by MRI support this hypothesis.(21) Finally, subjects who undergo APM become more familiar and comfortable with the process of undergoing surgical therapy and may be more inclined to select TKR. These potential explanations require further examination.
We acknowledge several limitations. Participants enrolled in MeTeOR may not be representative of the larger population of persons with degenerative meniscal tear, as just 26% of eligible participants agreed to participate in the MeTeOR trial.(1) Thirty percent of participants in MeTeOR crossed over from PT to APM, and drop-out was substantial over five years, potentially disturbing the matching of baseline characteristics across randomized groups. Because of these post-randomization events, we performed both an ITT and an as-treated analysis and addressed potential selection bias with adjustment for potential confounders. We acknowledge that unmeasured variables could create residual confounding. While losses to follow-up occurred at a similar rate across the randomized groups, the rate of dropout due to TKR differed. We used pattern mixture models and multiple imputation to account for potentially informative dropouts due to TKR. Finally, we acknowledge that our pre-TKR pain scores may have been gathered up to six months before the TKR and may have underestimated actual pain levels at the time of surgery.
In summary, the MeTeOR cohort experienced, on average, substantial symptom relief in the first year following treatment, which was maintained over five years, with a relatively low cumulative incidence of TKR (7.1%). These findings provide reassurance that both strategies -- early treatment with APM and PT with the opportunity for delayed APM -- are associated with generally favorable outcomes in middle-aged and older persons presenting with knee pain, osteoarthritic changes, and degenerative meniscal tear. While we did not observe statistically significant differences in TKR use in the ITT analysis, the greater TKR utilization observed in those receiving APM merits further study.
Supplementary Material
Crude KOOS Pain scores with 95% CI for subjects randomized to and receiving APM (blue), randomized to PT and not crossing over (red), and randomized to PT and crossover over to APM (green).
Piecewise linear mixed model of KOOS Pain scores in subjects randomized to and receiving APM (blue), randomized to PT and not crossing over (red), and randomized to PT and crossover over to APM (green). Slopes are estimated in four time periods: 0–3 months, 3–12 months, 12–24 months, and 24–60 months.
Piecewise mixed linear regression model in which KOOS Pain scores were increased 33% for subjects who dropped out due to TKR. Groups include subjects randomized to APM (blue) and randomized to PT (red).
Piecewise mixed linear regression model in which KOOS Pain scores were increased 50% for subjects who dropped out due to TKR. Groups include subjects randomized to APM (blue) and randomized to PT (red).
Piecewise linear mixed model of KOOS Function scores in subjects randomized to APM (blue) and randomized to PT (red). Slopes are estimated in four time periods: 0–3 months, 3–12 months, 12–24 months, and 24–60 months.
Acknowledgement:
We appreciate the data management support of Joseph Palmisano and the staff at the Boston University Data Coordinating Center, and we thank the participants enrolled in the MeTeOR Trial.
Support: NIH/NIAMS R01 AR05557, K24 AR057827, P30 AR072577, K23AR066133; Rheumatology Research Foundation
Footnotes
Disclosures: Dr. Jeffrey Katz is a deputy editor at JBJS and receives research funding from Samumed and Flexion Therapeutics. Swastina Shrestha has no disclosures to report. Dr. Elena Losina is a deputy editor of JBJS and is a consultant to Regeneron and receives research funding from Samumed and Genentech. Dr. Morgan Jones had received consulting fees from Samumed, publishing income from JBJS, and is on the editorial board of the Orthopedic Journal of Sports Medicine. Dr. Robert Marx is the Deputy Editor of Sports Medicine and the Associate Editor of Evidence Based Orthopedics for the Journal of Bone and Joint Surgery, receives royalties from books published by Springer and Demos Health, and receives equity compensation for Seat on Science Advisory Board for Mend. Dr. Lisa Mandl is an associate editor at the Annals of Internal Medicine and receives royalties from Up-to-Date. Dr. Bruce Levy is a consultant for Arthrex, and Smith and Nephew, he receives research support from Arthrex, Stryker and Bioment, and he receives royalties from Arthrex. He is the deputy editor or sports medicine for CORR. Dr. Lindsey MacFarlane receives research support from Samumed. Dr. Kurt Spindler is a consultant for the NFL, Samumed, and Flexion Therapeutics, on the advisory board for Cytori, and received research support from the DJO. Genevieve S Silva has no disclosures to report. Dr. Jamie Collins is a consultant to BICL.
References
- 1.Katz JN, Brophy RH, Chaisson CE, de Chaves L, Cole BJ, Dahm DL, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. The New England journal of medicine. 2013;368(18):1675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrlin S, Hallander M, Wange P, Weidenhielm L, Werner S. Arthroscopic or conservative treatment of degenerative medial meniscal tears: a prospective randomised trial. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2007;15(4):393–401. [DOI] [PubMed] [Google Scholar]
- 3.Herrlin SV, Wange PO, Lapidus G, Hallander M, Werner S, Weidenhielm L. Is arthroscopic surgery beneficial in treating non-traumatic, degenerative medial meniscal tears? A five year follow-up. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2013;21(2):358–64. [DOI] [PubMed] [Google Scholar]
- 4.Kise NJ, Risberg MA, Stensrud S, Ranstam J, Engebretsen L, Roos EM. Exercise therapy versus arthroscopic partial meniscectomy for degenerative meniscal tear in middle aged patients: randomised controlled trial with two year follow-up. BMJ (Clinical research ed). 2016;354:i3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yim JH, Seon JK, Song EK, Choi JI, Kim MC, Lee KB, et al. A comparative study of meniscectomy and nonoperative treatment for degenerative horizontal tears of the medial meniscus. The American journal of sports medicine. 2013;41(7):1565–70. [DOI] [PubMed] [Google Scholar]
- 6.Gauffin H, Sonesson S, Meunier A, Magnusson H, Kvist J. Knee Arthroscopic Surgery in Middle-Aged Patients With Meniscal Symptoms: A 3-Year Follow-up of a Prospective, Randomized Study. The American journal of sports medicine. 2017;45(9):2077–84. [DOI] [PubMed] [Google Scholar]
- 7.Gauffin H, Tagesson S, Meunier A, Magnusson H, Kvist J. Knee arthroscopic surgery is beneficial to middle-aged patients with meniscal symptoms: a prospective, randomised, single-blinded study. Osteoarthritis and cartilage. 2014;22(11):1808–16. [DOI] [PubMed] [Google Scholar]
- 8.van de Graaf VA, Noorduyn JCA, Willigenburg NW, Butter IK, de Gast A, Mol BW, et al. Effect of Early Surgery vs Physical Therapy on Knee Function Among Patients With Nonobstructive Meniscal Tears: The ESCAPE Randomized Clinical Trial. Jama. 2018;320(13):1328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz JN, Wright J, Spindler KP, Mandl LA, Safran-Norton C, Reinke EK, et al. Predictors and Outcomes of Crossover to Surgery from Physical Therapy for Meniscal Tear and Osteoarthritis: A Randomized Trial Comparing Physical Therapy and Surgery. J Bone Joint Surg Am. 2016;98(22):1890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spector TD, Cooper C. Radiographic assessment of osteoarthritis in population studies: whither Kellgren and Lawrence? Osteoarthritis and cartilage. 1993;1(4):203–6. [DOI] [PubMed] [Google Scholar]
- 11.Katz JN, Chaisson CE, Cole B, Guermazi A, Hunter DJ, Jones M, et al. The MeTeOR trial (Meniscal Tear in Osteoarthritis Research): rationale and design features. Contemporary clinical trials. 2012;33(6):1189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roos EM, Roos HP, Ekdahl C, Lohmander LS. Knee injury and Osteoarthritis Outcome Score (KOOS)--validation of a Swedish version. Scandinavian journal of medicine & science in sports. 1998;8(6):439–48. [DOI] [PubMed] [Google Scholar]
- 13.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)--development of a self-administered outcome measure. The Journal of orthopaedic and sports physical therapy. 1998;28(2):88–96. [DOI] [PubMed] [Google Scholar]
- 14.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. The Journal of rheumatology. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 15.Katz JN, Wright EA, Baron JA, Losina E. Development and validation of an index of musculoskeletal functional limitations. BMC musculoskeletal disorders. 2009;10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berwick DM, Murphy JM, Goldman PA, Ware JE Jr., Barsky AJ, Weinstein MC. Performance of a five-item mental health screening test. Medical care. 1991;29(2):169–76. [DOI] [PubMed] [Google Scholar]
- 17.Fitzmaurice GM. Applied longitudinal analysis. Hoboken, N.J: Wiley; 2011. [Google Scholar]
- 18.RJ L A class of pattern-mixture models for normal incomplete data. Biometrika. 1994;81(3):471–83. [Google Scholar]
- 19.Higuchi H, Kimura M, Shirakura K, Terauchi M, Takagishi K. Factors affecting long-term results after arthroscopic partial meniscectomy. Clin Orthop Relat Res. 2000(377):161–8. [DOI] [PubMed] [Google Scholar]
- 20.Schimmer RC, Brulhart KB, Duff C, Glinz W. Arthroscopic partial meniscectomy: a 12-year follow-up and two-step evaluation of the long-term course. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 1998;14(2):136–42. [DOI] [PubMed] [Google Scholar]
- 21.Collins JE, Losina E, Marx RG, Guermazi A, Jarraya M, Jones MH, et al. Early MRI-based Changes in Patients with Meniscal Tear and Osteoarthritis. Arthritis Care Res. 2018;Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crude KOOS Pain scores with 95% CI for subjects randomized to and receiving APM (blue), randomized to PT and not crossing over (red), and randomized to PT and crossover over to APM (green).
Piecewise linear mixed model of KOOS Pain scores in subjects randomized to and receiving APM (blue), randomized to PT and not crossing over (red), and randomized to PT and crossover over to APM (green). Slopes are estimated in four time periods: 0–3 months, 3–12 months, 12–24 months, and 24–60 months.
Piecewise mixed linear regression model in which KOOS Pain scores were increased 33% for subjects who dropped out due to TKR. Groups include subjects randomized to APM (blue) and randomized to PT (red).
Piecewise mixed linear regression model in which KOOS Pain scores were increased 50% for subjects who dropped out due to TKR. Groups include subjects randomized to APM (blue) and randomized to PT (red).
Piecewise linear mixed model of KOOS Function scores in subjects randomized to APM (blue) and randomized to PT (red). Slopes are estimated in four time periods: 0–3 months, 3–12 months, 12–24 months, and 24–60 months.


