Abstract
Kidney disease affects ~10% of the population worldwide, resulting in millions of deaths each year. Mechanistically, oxidative stress is a major driver of various kidney diseases, and promotes the progression from acute to chronic injury, as well as renal cancer development. NRF2, the master regulator of redox balance, has been shown to protect against kidney disease through its negation of reactive oxygen species (ROS). However, many kidney diseases exhibit high levels of ROS as a result of decreased NRF2 protein levels and transcriptional activity. Many studies have tested the strategy of using NRF2 inducing compounds to alleviate ROS to prevent or slow down the progression of kidney diseases. Oppositely, in specific subsets of renal cancer, NRF2 is constitutively activated and contributes to tumor burden and overall poor prognosis; therefore, there has been a recent interest in studies investigating the benefits of NRF2 inhibition. In this review, we summarize recent literature investigating the role of NRF2 and oxidative stress in various kidney diseases, and how pharmacological modification of NRF2 signaling could play a protective role.
Keywords: NRF2, KEAP1, oxidative stress, kidney disease, renal cell carcinoma, hypertension
Introduction
Perturbance of redox homeostasis leaves the kidneys susceptible to numerous diseases. Healthy, functioning kidneys are responsible for a variety of processes including blood filtration, hormone production, blood pressure regulation, and fluid balance; most notably, the kidneys filter out byproducts of food, medications, and toxicants that are then excreted in urine. However, when the kidneys are damaged, these respective processes are compromised; and when left untreated, pathogenesis is rapid and, in many cases, irreversible. At the later stages of kidney disease, patients will have to undergo costly and painful treatment options, such as dialysis and even organ transplantation. While each kidney disease has its own unique pathogenic characteristics, disturbance of redox homeostasis consistently plays a key role in disease onset and progression (Okamura and Pennathur 2015).
Impaired redox homeostasis is characterized by an increase in oxidative stress, which is usually indicative of increased production of reactive oxygen species (ROS) exceeding the cell’s antioxidant capacity. While some basal ROS exist and are required for cell signaling, increased ROS are typically damaging to the cell (Schieber and Chandel 2014). Many stressors, including radiation, chemotherapies, and environmental exposures, all increase ROS levels in the cell. Formation of promiscuous free radicals can cause oxidative damage to many cellular components. In the kidney, this has been observed in the form of DNA damage and increased cell death (Siddarth et al. 2018). Importantly, increased oxidative stress levels potentiate a variety of pathologies in the kidneys, including acute kidney injury, chronic kidney disease, polycystic kidney disease, diabetic nephropathy, hypertension, glomerulonephritis, hydronephrosis, and renal cancer. Due to its normal physiological functions, the kidney is rich in oxidative reactions; this leaves the kidney more susceptible to damage caused by oxidative stress (Anders 1980). Thus, maintenance of redox homeostasis via combatting enhanced ROS levels is essential for proper kidney function.
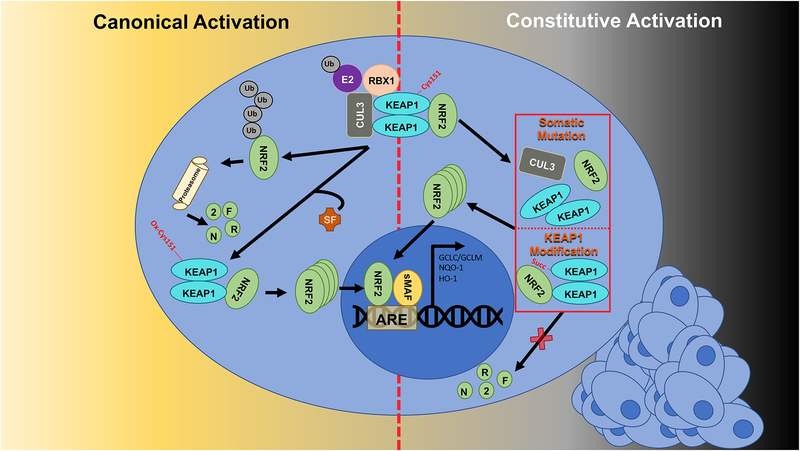
Kidney cells contain an endogenous cellular defense system designed to combat the effects of oxidative stress. A key regulator of this system is the transcription factor nuclear-factor (erythroid-derived 2)-like 2 (NRF2). NRF2 is ubiquitously expressed in all cell types, and during increased cellular stress, translocates into the nucleus, heterodimerizes with small musculoaponeurotic fibrosarcoma (sMAF) proteins, and binds to antioxidant response elements (AREs) to initiate transcription of target genes encoding proteins associated with redox regulation, xenobiotic efflux, protein homeostasis, iron metabolism, prevention of apoptosis, and DNA repair, all of which are upregulated to restore homeostasis (Dodson et al. 2019). Under basal conditions, NRF2 levels are kept low by its binding to Kelch-like ECH associated protein 1 (KEAP1), a substrate adaptor protein of a Cullin 3-Ring box 1 (CUL3-RBX1) E3 ubiquitin ligase complex. NRF2 contains two motifs (ETGE and DLG) that each bind to a homodimer of KEAP1; upon binding, the NRF2-KEAP1 interaction stabilizes the complex allowing for ubiquitylation, and ultimately proteasomal degradation, of NRF2 (Itoh et al. 1999).
During increased oxidative stress, important cysteine residues in KEAP1 (i.e. Cys151) become oxidized; this oxidation event prevents the binding of the DLG motif of NRF2 to KEAP1, and thus NRF2 can no longer be ubiquitylated and degraded (Zhang and Hannink 2003, Tong et al. 2006). However, since NRF2 remains bound to KEAP1 via its ETGE motif, and new NRF2 is continually being translated, NRF2 protein accumulates and translocates into the nucleus to activate transcription. Thus, in response to pro-oxidants, NRF2-based transcription is activated in order for its downstream target genes to be upregulated and neutralize ROS. Accordingly, many studies have shown that activation of NRF2 prior to disease onset is effective in conferring chemoprevention and maintaining overall health. Similarly, activation of the NRF2-KEAP1 signaling pathway prior to, or early in, disease onset may play a critical role in the prevention and treatment of various oxidative stress-related kidney diseases (Guerrero-Hue et al. 2017). However, it was recently discovered that NRF2 is constitutively activated in certain types of cancer, including renal cell carcinomas. There are several mechanisms by which NRF2 becomes constitutively active in renal cancers, including post-translational modifications to KEAP1, as well as somatic gain-of-function mutations in NRF2 or loss-of-function mutations in KEAP1 and CUL3 (Figure 1). Due to the cytoprotective effects of NRF2 upregulation, constitutive activation of NRF2 yields cancer cells resistant to therapies, and ultimately increases the morbidity of these patients (Wang et al. 2008). Therefore, there is a significant need to develop inhibitors of NRF2 to treat cancer. Overall, the role of NRF2 in kidney disease is wide ranging, with the need to maintain normal redox homeostasis being critical for healthy kidney function.
Figure 1: The NRF2 signaling pathway.
Under normal conditions, NRF2 is bound by KEAP1 as part of a CUL3-RBX1 complex that facilitates its ubiquitylation and proteasomal degradation. Electrophiles (i.e. sulforaphane [SF]) oxidize key cysteine residues in KEAP1 that causes a conformational change in the NRF2-KEAP1 complex that prevents ubiquitylation of NRF2. Thus, newly synthesized NRF2 accumulates and transcription of its target genes begins (left side). In renal cancers, somatic mutations in KEAP1, CUL3, or NRF2, or post-translational modifications to KEAP1 prevent ubiquitylation of NRF2 and result in constitutive activation of the NRF2 cascade. In this case, NRF2 is constantly transcribing its target genes, thus protecting the cancer cell (right side).
I. Kidney Disease and Redox Balance
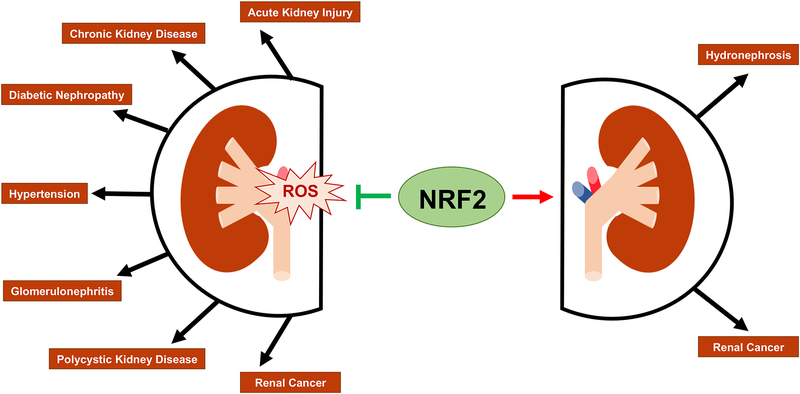
Due to the integral role of various kidney cell types in maintaining kidney function, oxidative damage to specific cell populations can lead to different diseases, varying in severity. In addition, initial injury to the kidney can be exacerbated over time and contribute to disease progression, leading to a worse prognosis. As mentioned above, recent literature has shown that NRF2 plays a vital role in alleviating the damage caused by oxidative stress in the kidneys; however, hyperactivation of NRF2 leads to resistance to therapies and poor prognosis in cancer patients. Below, we outline several kidney diseases related to oxidative stress and the role of NRF2 in each (Figure 2).
Figure 2: The role of NRF2 in kidney disease.
Accumulation of Reactive Oxygen Species (ROS) is linked to a variety of kidney diseases. NRF2 negates the effects of ROS, and therefore can prevent or delay the onset of these diseases. However, high levels of NRF2 have also been reported to promote hydronephrosis and renal cancers.
a. Acute Kidney Injury
Acute kidney injury (AKI) refers to an abrupt disruption of normal kidney function, which is typically characterized by an increase in creatinine levels and a decrease in urine output. There are three distinct phases of AKI, each increasing in severity: (1) prerenal is the most common phase, and is associated with damage caused by medications and pathological complications such as hypercalcemia, (2) the intrinsic renal phase results from infections, as well as other stresses (i.e. ischemic injury), and (3) postrenal is the phase linked to obstructions (i.e. kidney stones) and cancer (Rahman et al. 2012). Upregulation of NRF2 has been shown to combat the damage seen in both prerenal and intrinsic renal AKI. Specifically, activation of NRF2 has been shown to neutralize oxidative stress in T lymphocytes and thus prevent AKI (Noel et al. 2015). Furthermore, other studies have shown that environmental nephrotoxicity caused by arsenic (measured via increased apoptosis and DNA damage in rats) was negated by supplementation with the NRF2 inducer sulforaphane (Thangapandiyan et al. 2019). Similarly, nephrotoxicity caused by cisplatin was more severe in NRF2-deficient (Nrf2−/−) mice compared to wild type mice (Liu et al. 2009, Aleksunes et al. 2010). Thus, these studies indicate that NRF2 plays a critical role in preventing AKI via combatting oxidative stress (Nezu et al. 2017). Not only is AKI itself a health issue for patients, but it also presents a problem for drug discovery and development, as many new drug candidates undergo cessation due to drug-induced AKI (Shelton et al. 2013). Therefore, enhancing our understanding of the role of NRF2 in preventing AKI serves a valuable purpose in developing therapeutics to prevent kidney damage in patients, as well as limit the number of drugs withdrawn from clinical trials.
b. Chronic Kidney Disease
If left untreated, AKI can progress to chronic kidney disease (CKD) (Basile et al. 2016). Similar to AKI, CKD is characterized by destruction of the kidneys, just over a more prolonged period of time; however, in this context, irreversibility and total loss of function are eminent. Other pathologies, such as type II diabetes and hypertension can also contribute to the onset of CKD due to uncontrolled oxidative stress and increased inflammation. Metabolomics data gathered from 2155 participants, showed that CKD patients had decreased 5-methoxytryptophan (5-MTP); and, supplementation of 5-MTP in mice with ischemia/reperfusion injury activated the NRF2 signaling pathway and stopped renal interstitial fibrosis (Chen et al. 2019). Also in CKD patients, an accumulation of indoxyl sulfate has been observed, which induced nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (the master regulator of interleukins and the inflammatory response), and conversely decreased NRF2 transcriptional activity (Bolati et al. 2013). The inverse relationship between NF-κB and NRF2 has been attributed to several factors: (1) competition for binding with the transcriptional coactivator CREB-binding protein (CBP), (2) upregulation of target genes of NRF2 (NADPH quinone dehydrogenase 1 [NQO1], heme oxygenase 1 [HO-1])) can affect NF-κB-based transcription, and (3) NRF2 inducing compounds (bardoxolone methyl, sulforaphane) suppress NF-κB signaling (Wardyn et al. 2015, Dodson et al. 2019). Thus, in CKD patients with chronic inflammation, NRF2 target gene levels could remain low due to hyperactivation of NF-κB. Interestingly, treatment with resveratrol, a known NRF2 inducer, in CKD patients showed no difference in inflammatory or antioxidant biomarkers (Saldanha et al. 2016). On the other hand, treatment with dh404 (a known bardoxolone analog) was able to restore NRF2 activity and protect against CKD (Aminzadeh et al. 2014). CKD patients that received bardoxolone methyl were observed to have an increased estimated glomerular filtration rate (eGFR) and less severe adverse renal effects; however, this compound failed clinical trials due to an increased incidence of heart failure (Chin et al. 2018). Ultimately, this suggests that increasing NRF2 levels in CKD patients could preserve kidney function and prevent the onset of end-stage renal disease (ESRD) and total organ failure; however, off-target effects, as well as appropriately timed utilization and treatment regimens, need to be carefully considered. Thus, activation of NRF2 remains a promising therapeutic target for the treatment of CKD (Ruiz et al. 2013).
c. Autosomal Dominant Polycystic Kidney Disease
Unlike many other kidney diseases, autosomal dominant polycystic kidney disease (ADPKD) is a hereditary disease caused by mutations in polycystin-1 and polycystin-2 (PKD1 and PKD2) (Andries et al. 2018). However, despite having a genetic cause of onset, the effects of ADPKD are exacerbated by high oxidative stress levels. Unlike normal kidney cysts, ADPKD involves aberrant chronic cyst development that ultimately leads to enlargement of the kidneys and subsequent loss of function. It has been shown that resveratrol could delay ADPDK via attenuation of NF-κB (Wu et al. 2016); however, other studies suggest this phenomenon is due to the pro-antioxidant and anti-inflammatory target genes of NRF2, that in turn decrease cystogenesis and delay disease progression (Moradi and Vaziri 2016). Therefore, while evidence suggests that NRF2 plays a role in mediating ADPKD, the link between the two needs further elucidation.
d. Diabetic Nephropathy
A common result of untreated diabetes in patients is diabetic nephropathy, also known as diabetic kidney disease (DKD). DKD is characterized by a swelling of the mesangial cells that results in lowered eGFR and an ultimate halt in filtration. Hence, DKD is the leading cause of ESRD. A major driver of DKD is NADPH oxidase 4 (NOX4), which through production of hydrogen peroxide increases oxidative stress levels in the kidneys (Sedeek et al. 2010). Our group has shown that Nrf2−/− mice have higher levels of ROS and renal damage compared to wild type controls in a streptozotocin-induced diabetic nephropathy model (Jiang et al. 2010). Additionally, we showed that natural products isolated from food common in the diet (i.e. broccoli, cinnamon) could activate NRF2 and alleviate renal damage caused by diabetes (Zheng et al. 2011). Further studies indicated that diabetic mice that overexpress thrombomodulin domain 1 had improved renal function as a result of decreased NF-κB and inflammasome formation, as well as a simultaneous increase in NRF2 levels, resulting in decreased oxidative stress and apoptosis (Yang et al. 2014). Therefore, the interplay between inflammation and oxidative stress plays a key role in driving DKD, with activation of NRF2 preventing and reducing the onset of disease.
e. Hypertension
A major contributing factor to CKD is hypertension. The renin-angiotensin system (RAS) controls blood pressure in the kidneys, and when activated increases blood pressure (Sowers 2002). Activation of the RAS leads to the production of angiotensin II (ANG II), which in turn leads to increased aldosterone production; aldosterone signals the body to retain water, thus increasing blood pressure (Yim and Yoo 2008). Importantly, RAS activity was associated with increased ROS in the brains of salt-induced hypertensive rats (Su et al. 2017). Oxidative stress itself also leads to upregulation of the RAS, thus creating a positive feedback loop between increased ROS, RAS function, and hypertension (Touyz 2004). Treatment with ANG II has also been linked to decreased glutathione levels and suppression of NRF2 target genes as a result of enhancement of the ARE negative regulator: activating transcription factor 3 (ATF3) (Kang et al. 2011). Upregulation of NRF2 with curcumin was shown to reduce oxidative stress levels and alleviate the effects of glomerular hypertension (increased glomerular capillary pressure leading to increased blood pressure) in nephrectomized rats (Tapia et al. 2012). Similar to CKD and DKD, hypertension can be attributed to high levels of oxidative stress, but when NRF2 is activated prior to onset of disease, the pathological effects of hypertension can be mitigated. Opposingly, activation of the RAS in NRF2-deficient mice improved fetal and maternal survival in preeclamptic mice. Studies have demonstrated that ROS was essential for maintaining placental angiogenesis, and that hyperactivation of NRF2 (via KEAP1 knockdown) negated the function of necessary cytokines, indicating that NRF2 enhances preeclampsia (Nezu et al. 2017). Therefore, the role of NRF2 in regulating the interplay between the RAS, oxidative stress, and proper kidney function is context dependent.
f. Glomerulonephritis
Glomeruli, responsible for the filtration of blood, become inflamed during glomerulonephritis (GN), leaving them incapable of proper function and leading to increased proteinuria, ultimately contributing to AKI and CKD. Recently, it was established that activation of extracellular regulated kinase (ERK) signaling was linked to increased GN in mesangial cells; however, exogenous antioxidant treatment reduced glomerular injury (Budisavljevic et al. 2003). Oxidative stress is a known driver of the mitogen-activated protein kinases (MAPK) pathway as it can activate epidermal growth factor receptor (EGFR) and subsequently ERK signaling (Zhang et al. 2016); this could explain how oxidative stress drives GN. Additionally, it was shown that female HO-1−/− mice developed severe GN, associated with high levels of oxidative stress and chronic inflammation as a result of iron toxicity (Poss and Tonegawa 1997). Meanwhile, induction of NRF2 activity by treatment with green tea polyphenol (−)- epigallocatechin-3-gallate (EGCG) in mice seven days post nephritis development showed less severe GN due to increased transcription of the glutamate-cysteine ligase catalytic (GCLC) and glutamate-cysteine ligase modifier (GCLM) subunits of glutamate-cysteine ligase (Ye et al. 2015). Thus, production of glutathione lowers the oxidative stress that drives GN, and ultimately prevents the onset of CKD.
g. Hydronephrosis
Swelling of the kidneys due to lack of urine drainage, known as hydronephrosis, helps drive AKI and CKD. While a link between the onset of hydronephrosis and oxidative stress is yet to be established, it has been shown that hydronephrosis leads to increased oxidative stress and mitochondrial damage (Cao et al. 2015). Evidence indicated that in KEAP1-deficient (Keap1−/−) mice, NRF2 hyperactivation drives bilateral hydronephrosis as indicated by more severe swelling of the bladder, dilation of the collecting ducts, and damaged glomeruli as compared to wild type mice (Suzuki et al. 2017). Additionally, other reports also indicated that Keap1−/− mice developed hydronephrosis as evidenced by the enlarged kidney size and lack of medullary tissue (Noel et al. 2016). Therefore, these animal models suggest that KEAP1 deficiency, and thus persistently high expression of NRF2, may contribute to the onset of hydronephrosis.
h. Renal Cancer
Treatment of renal cancers, like many other cancer types, may require focusing on the dual role of NRF2 in cancer progression: i.e. utilizing NRF2 induction for chemoprevention prior to malignant transformation, as compared to NRF2 inhibition for chemotherapy post tumor initiation (Tao et al. 2018). NRF2 levels decrease with age, yielding cells more susceptible to damage from oxidative stress, a common feature of age-related diseases including cancer (Schmidlin et al. 2019). The resulting accumulation of oxidative damage can result in malignant transformation, and ultimately result in tumor development. In this context, induction of NRF2 prior to insult is an important chemoprevention tactic, as its target genes protect the cell and repair the initial damage caused by ROS. However, in renal cancers, modifications to the KEAP1-NRF2-CUL3 complex allow for constitutive activation of NRF2 that aid in the proliferation and survival of the tumor (Kitamura and Motohashi 2018). Post-translational modifications to KEAP1 also plays an important role in renal cancer progression. Specifically, in hereditary leiomyomatosis and renal cell cancer (HLRCC), mutations in fumarate hydratase (FH) lead to an accumulation of fumarate, which eventually results in the succination of cysteine residues in KEAP1 (Trpkov et al. 2016), which prevents ubiquitylation/degradation of NRF2, and results in uncontrolled upregulation of NRF2 target genes (Ooi et al. 2011). Additionally, somatic gain-of-function mutations in NRF2 and loss-of function mutations in CUL3 cause constitutive activation of NRF2 in HLRCC (Ooi et al. 2013). In clear-cell renal cell carcinoma, whole genome sequencing revealed mutations in KEAP1, NRF2, and CUL3 as well (Sato et al. 2013). Hence, oxidative stress levels are kept to a minimum in these tumors. In addition, due to the cytoprotective nature of these target genes, renal cell carcinomas with hyperactivated NRF2 could become resistant to therapies, resulting in a poorer prognosis. While induction prior to malignant transformation is beneficial, high NRF2 levels in a cancer cell could lead to a more aggressive tumor phenotype, indicating a need for NRF2 inhibition. Thus, NRF2 levels present a challenging target for the prevention and treatment of cancer, as well as other metabolic diseases; hence, caution should be taken in the application of pharmacological modulators of the NRF2-KEAP1 signaling pathway to treat kidney disease, as the manner in which NRF2 functions in kidney disease is time and context-dependent.
II. Pharmacological Modulation of NRF2 in Kidney Disease
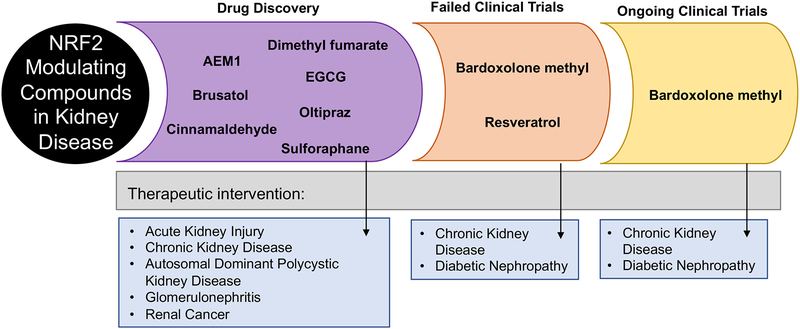
In order to prevent the onset of several of the diseases discussed above, induction of NRF2 prior to or immediately after insult is necessary. In contrast, inhibition of NRF2 in renal cancers is imperative to prevent tumor progression. Below, we outline the current state of pharmacological modulators of NRF2 and their potential role in treating kidney diseases (Figure 3).
Figure 3: Pharmacological modulation of NRF2 and its effect on kidney diseases.
Several NRF2 modulating compounds have been shown to protect against kidney diseases; however, most have either not entered or failed clinical trials. Currently, some clinical trials are ongoing testing NRF2 modulating compounds in the treatment of chronic kidney disease and diabetic nephropathy.
a. Inducers
Several inducers of NRF2 have been characterized and studied regarding the prevention and treatment of a number of kidney diseases (Atilano-Roque et al. 2016). Currently, clinical trials are ongoing for bardoxolone methyl for the amelioration of CKD (Chin et al. 2018). It has been suggested bardoxolone methyl may be more effective in early stages of kidney disease and may have failed clinical trials due to the severity of CDK and off target effects (Zhang 2013). Oltipraz, another oxidative stress causing NRF2 inducer, showed promise in protecting kidney cells from cisplatin exposure (Atilano-Roque et al. 2016); however, oltipraz is non-specific and can modify any cysteine-containing proteins and thus have many off-target effects (Bhattacharyya et al. 2010). Following successful clinical trials, dimethyl fumarate (DMF) has been approved for use in humans for treating multiple sclerosis (Bomprezzi 2015). In the context of the kidney, DMF has been shown to reduce renal cyst formation, but had little efficacy in reducing renal dysfunction and proteinuria (Oey et al. 2018). Several other inducers of NRF2 have been studied in the context of the kidney in animal models, including sulforaphane and cinnamaldehyde, yet none have been used in clinical trials for the treatment of kidney diseases. While pharmacological induction of NRF2 has been shown to mitigate and negate the effects of oxidative stress in several kidney disease models, there are still no FDA approved NRF2 inducers that can be used in the treatment of kidney disease in humans. Therefore, further research is needed to increase the efficacy, specificity, and amount of NRF2 inducers that make it from bench to bedside.
b. Inhibitors
Opposingly, while many inducers of NRF2 are available, inhibition of NRF2 is much more difficult. Inhibition of NRF2 is a potential individual or adjuvant therapeutic against cancers that rely on NRF2 hyperactivation for proliferation and survival. Target genes of NRF2 have been linked to each of the Hallmarks of Cancer, indicating that NRF2 plays a key role in cancer progression and maintenance (Rojo de la Vega et al. 2018). While genetic knockdown of NRF2 or pharmacological inhibition of NRF2 was shown to sensitize cancer cells to established chemotherapies and decrease tumor burden, the effects of NRF2 inhibition in the context of renal cancers has yet to be established. In 2011, our group developed the first known NRF2 inhibitor: brusatol; brusatol was able to decrease NRF2 expression levels, ultimately increasing the efficacy of chemotherapy in lung cancer models (Ren et al. 2011, Tao et al. 2014). However, further investigations into brusatol revealed it targeted general mRNA translation, and thus was not a specific NRF2 inhibitor (Harder et al. 2017). Recently, it was suggested that AEM1 inhibits NRF2 transcriptional activity without targeting general protein synthesis (Bollong et al. 2015). In these studies, AEM1 was able to increase chemosensitivity by inhibiting HO-1 and other NRF2 target genes in cancer cells; yet, the exact mechanisms by which AEM1 targets the NRF2 pathway need further elucidation. To date, no known NRF2 inhibitor has entered clinical trials, despite evidence showing the effectiveness of NRF2 inhibition in enhancing the efficacy of chemotherapy and in reducing tumor progression. Hence, there is a need for the development of specific NRF2 inhibitors, especially in the context of cancers or other metabolic diseases where NRF2 hyperactivation contributes to disease progression.
Conclusion
NRF2 is vital to healthy kidney function. The ability of NRF2 to negate oxidative stress makes modulation of this pathway crucial in preventing the onset of many oxidative stress-associated kidney diseases. For example, AKI, CKD, DKD, ADPKD, hypertension, and GN, are all driven by high oxidative stress levels with recent studies demonstrating that NRF2 can prevent each of these diseases by combatting the damage caused by ROS. Meanwhile, high levels of NRF2 have been shown to cause hydronephrosis and enhance the aggressiveness of renal cancers; in this context, inhibition of NRF2 would blunt disease progression and enhance the efficacy of chemotherapies. This dual role of NRF2 signaling in kidney disease presents a unique challenge in the targeting of this pathway to alleviate disease burden. This, coupled with the off-target effects of many of the current pharmacological modulators of NRF2, is part of the reason that none have been approved for use in humans for the treatment of kidney disease. Future work in the NRF2 and kidney disease fields should thus focus on the role of NRF2 in each specific type of kidney disease, the development of specific NRF2 modulating compounds with less off-target toxicities, and the clinical application of NRF2 modulators in ameliorating kidney pathologies in a temporal- and context-dependent manner. In addition, specific downstream target genes associated with the protective aspects of NRF2 activation, and their subsequent effects on kidney diseases need to be further elucidated. Overall, as demonstrated in recent literature, there are many potential therapeutic benefits of NRF2 modulation in kidney disease. However, there is a continued need for more detailed mechanistic investigations into the NRF2 signaling cascade and its function, which will in turn facilitate the development of more specific modulators of NRF2, thus improving the efficacy of current and future therapeutic strategies so that we can successfully translate NRF2 biology into a clinical setting to improve human health.
Acknowledgements
The lab of Dr. Zhang is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (DK109555 [D.D.Z.]), and the National Institute of Environmental Health Sciences (ES026845 [D.D.Z.], ES004940 [D.D.Z.], ES006694 [under center]).
Footnotes
Conflicts of Interests
The authors have no conflicts of interests to declare.
References
- Aleksunes LM, Goedken MJ, Rockwell CE, Thomale J, Manautou JE and Klaassen CD (2010) Transcriptional regulation of renal cytoprotective genes by Nrf2 and its potential use as a therapeutic target to mitigate cisplatin-induced nephrotoxicity. J Pharmacol Exp Ther 335(1): 2–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminzadeh MA, Reisman SA, Vaziri ND, Khazaeli M, Yuan J and Meyer CJ (2014) The synthetic triterpenoid RTA dh404 (CDDO-dhTFEA) restores Nrf2 activity and attenuates oxidative stress, inflammation, and fibrosis in rats with chronic kidney disease. Xenobiotica 44(6): 570–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders MW (1980) Metabolism of drugs by the kidney. Kidney Int 18(5): 636–647 [DOI] [PubMed] [Google Scholar]
- Andries A, Daenen K, Jouret F, Bammens B, Mekahli D and Van Schepdael A (2018) Oxidative stress in autosomal dominant polycystic kidney disease: player and/or early predictor for disease progression? Pediatr Nephrol [DOI] [PubMed] [Google Scholar]
- Atilano-Roque A, Wen X, Aleksunes LM and Joy MS (2016) Nrf2 activators as potential modulators of injury in human kidney cells. Toxicol Rep 3: 153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile DP, Bonventre JV, Mehta R, Nangaku M, Unwin R, Rosner MH, Kellum JA, Ronco C and Group AXW (2016) Progression after AKI: Understanding Maladaptive Repair Processes to Predict and Identify Therapeutic Treatments. J Am Soc Nephrol 27(3): 687–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Zhou H, Seiner DR and Gates KS (2010) Inactivation of protein tyrosine phosphatases by oltipraz and other cancer chemopreventive 1,2-dithiole-3-thiones. Bioorg Med Chem 18(16): 5945–5949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolati D, Shimizu H, Yisireyili M, Nishijima F and Niwa T (2013) Indoxyl sulfate, a uremic toxin, downregulates renal expression of Nrf2 through activation of NF-kappaB. BMC Nephrol 14: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollong MJ, Yun H, Sherwood L, Woods AK, Lairson LL and Schultz PG (2015) A Small Molecule Inhibits Deregulated NRF2 Transcriptional Activity in Cancer. ACS Chem Biol 10(10): 2193–2198 [DOI] [PubMed] [Google Scholar]
- Bomprezzi R (2015) Dimethyl fumarate in the treatment of relapsing-remitting multiple sclerosis: an overview. Ther Adv Neurol Disord 8(1): 20–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budisavljevic MN, Hodge L, Barber K, Fulmer JR, Durazo-Arvizu RA, Self SE, Kuhlmann M, Raymond JR and Greene EL (2003) Oxidative stress in the pathogenesis of experimental mesangial proliferative glomerulonephritis. Am J Physiol Renal Physiol 285(6): F1138–1148 [DOI] [PubMed] [Google Scholar]
- Cao Z, Yu W, Li W, Cheng F, Rao T, Yao X, Zhang X and Larre S (2015) Oxidative Damage and Mitochondrial Injuries Are Induced by Various Irrigation Pressures in Rabbit Models of Mild and Severe Hydronephrosis. PLoS One 10(6): e0127143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DQ, Cao G, Chen H, Argyopoulos CP, Yu H, Su W, Chen L, Samuels DC, Zhuang S, Bayliss GP, Zhao S, Yu XY, Vaziri ND, Wang M, Liu D, Mao JR, Ma SX, Zhao J, Zhang Y, Shang YQ, Kang H, Ye F, Cheng XH, Li XR, Zhang L, Meng MX, Guo Y and Zhao YY (2019) Identification of serum metabolites associating with chronic kidney disease progression and anti-fibrotic effect of 5-methoxytryptophan. Nat Commun 10(1): 1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin MP, Bakris GL, Block GA, Chertow GM, Goldsberry A, Inker LA, Heerspink HJL, O’Grady M, Pergola PE, Wanner C, Warnock DG and Meyer CJ (2018) Bardoxolone Methyl Improves Kidney Function in Patients with Chronic Kidney Disease Stage 4 and Type 2 Diabetes: Post-Hoc Analyses from Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes Study. Am J Nephrol 47(1): 40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson M, de la Vega MR, Cholanians AB, Schmidlin CJ, Chapman E and Zhang DD (2019) Modulating NRF2 in Disease: Timing Is Everything. Annu Rev Pharmacol Toxicol 59: 555–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Hue M, Farre-Alins V, Palomino-Antolin A, Parada E, Rubio-Navarro A, Egido J, Egea J and Moreno JA (2017) Targeting Nrf2 in Protection Against Renal Disease. Curr Med Chem 24(33): 3583–3605 [DOI] [PubMed] [Google Scholar]
- Harder B, Tian W, La Clair JJ, Tan AC, Ooi A, Chapman E and Zhang DD (2017) Brusatol overcomes chemoresistance through inhibition of protein translation. Mol Carcinog 56(5): 1493–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD and Yamamoto M (1999) Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13(1): 76–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Huang Z, Lin Y, Zhang Z, Fang D and Zhang DD (2010) The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes 59(4): 850–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SJ, You A and Kwak MK (2011) Suppression of Nrf2 signaling by angiotensin II in murine renal epithelial cells. Arch Pharm Res 34(5): 829–836 [DOI] [PubMed] [Google Scholar]
- Kitamura H and Motohashi H (2018) NRF2 addiction in cancer cells. Cancer Sci 109(4): 900–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Grigoryev DN, Crow MT, Haas M, Yamamoto M, Reddy SP and Rabb H (2009) Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int 76(3): 277–285 [DOI] [PubMed] [Google Scholar]
- Moradi H and Vaziri ND (2016) Effect of resveratrol on progression of polycystic kidney disease: a case of cautious optimism. Nephrol Dial Transplant 31(11): 1755–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezu M, Souma T, Yu L, Sekine H, Takahashi N, Wei AZ, Ito S, Fukamizu A, Zsengeller ZK, Nakamura T, Hozawa A, Karumanchi SA, Suzuki N and Yamamoto M (2017) Nrf2 inactivation enhances placental angiogenesis in a preeclampsia mouse model and improves maternal and fetal outcomes. Sci Signal 10(479) [DOI] [PubMed] [Google Scholar]
- Nezu M, Suzuki N and Yamamoto M (2017) Targeting the KEAP1-NRF2 System to Prevent Kidney Disease Progression. Am J Nephrol 45(6): 473–483 [DOI] [PubMed] [Google Scholar]
- Noel S, Arend LJ, Bandapalle S, Reddy SP and Rabb H (2016) Kidney epithelium specific deletion of kelch-like ECH-associated protein 1 (Keap1) causes hydronephrosis in mice. BMC Nephrol 17(1): 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel S, Martina MN, Bandapalle S, Racusen LC, Potteti HR, Hamad AR, Reddy SP and Rabb H (2015) T Lymphocyte-Specific Activation of Nrf2 Protects from AKI. J Am Soc Nephrol 26(12): 2989–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oey O, Rao P, Luciuk M, Mannix C, Rogers NM, Sagar P, Wong A and Rangan G (2018) Effect of dimethyl fumarate on renal disease progression in a genetic ortholog of nephronophthisis. Exp Biol Med (Maywood) 243(5): 428–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura DM and Pennathur S (2015) The balance of powers: Redox regulation of fibrogenic pathways in kidney injury. Redox Biol 6: 495–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi A, Dykema K, Ansari A, Petillo D, Snider J, Kahnoski R, Anema J, Craig D, Carpten J, Teh BT and Furge KA (2013) CUL3 and NRF2 mutations confer an NRF2 activation phenotype in a sporadic form of papillary renal cell carcinoma. Cancer Res 73(7): 2044–2051 [DOI] [PubMed] [Google Scholar]
- Ooi A, Wong JC, Petillo D, Roossien D, Perrier-Trudova V, Whitten D, Min BW, Tan MH, Zhang Z, Yang XJ, Zhou M, Gardie B, Molinie V, Richard S, Tan PH, Teh BT and Furge KA (2011) An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma. Cancer Cell 20(4): 511–523 [DOI] [PubMed] [Google Scholar]
- Poss KD and Tonegawa S (1997) Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci U S A 94(20): 10919–10924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M, Shad F and Smith MC (2012) Acute kidney injury: a guide to diagnosis and management. Am Fam Physician 86(7): 631–639 [PubMed] [Google Scholar]
- Ren D, Villeneuve NF, Jiang T, Wu T, Lau A, Toppin HA and Zhang DD (2011) Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci U S A 108(4): 1433–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo de la Vega M, Chapman E and Zhang DD (2018) NRF2 and the Hallmarks of Cancer. Cancer Cell 34(1): 21–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz S, Pergola PE, Zager RA and Vaziri ND (2013) Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int 83(6): 1029–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha JF, Leal VO, Rizzetto F, Grimmer GH, Ribeiro-Alves M, Daleprane JB, Carraro-Eduardo JC and Mafra D (2016) Effects of Resveratrol Supplementation in Nrf2 and NF-kappaB Expressions in Nondialyzed Chronic Kidney Disease Patients: A Randomized, Double-Blind, Placebo-Controlled, Crossover Clinical Trial. J Ren Nutr 26(6): 401–406 [DOI] [PubMed] [Google Scholar]
- Sato Y, Yoshizato T, Shiraishi Y, Maekawa S, Okuno Y, Kamura T, Shimamura T, Sato-Otsubo A, Nagae G, Suzuki H, Nagata Y, Yoshida K, Kon A, Suzuki Y, Chiba K, Tanaka H, Niida A, Fujimoto A, Tsunoda T, Morikawa T, Maeda D, Kume H, Sugano S, Fukayama M, Aburatani H, Sanada M, Miyano S, Homma Y and Ogawa S (2013) Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet 45(8): 860–867 [DOI] [PubMed] [Google Scholar]
- Schieber M and Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24(10): R453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidlin CJ, Dodson MB, Madhavan L and Zhang DD (2019) Redox regulation by NRF2 in aging and disease. Free Radic Biol Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM and Hebert RL (2010) Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 299(6): F1348–1358 [DOI] [PubMed] [Google Scholar]
- Shelton LM, Park BK and Copple IM (2013) Role of Nrf2 in protection against acute kidney injury. Kidney Int 84(6): 1090–1095 [DOI] [PubMed] [Google Scholar]
- Siddarth M, Chawla D, Raizada A, Wadhwa N, Banerjee BD and Sikka M (2018) Lead-induced DNA damage and cell apoptosis in human renal proximal tubular epithelial cell: Attenuation via N-acetyl cysteine and tannic acid. J Biochem Mol Toxicol 32(3): e22038. [DOI] [PubMed] [Google Scholar]
- Sowers JR (2002) Hypertension, angiotensin II, and oxidative stress. N Engl J Med 346(25): 1999–2001 [DOI] [PubMed] [Google Scholar]
- Su Q, Huo CJ, Li HB, Liu KL, Li X, Yang Q, Song XA, Chen WS, Cui W, Zhu GQ, Shi XL, Liu JJ and Kang YM (2017) Renin-angiotensin system acting on reactive oxygen species in paraventricular nucleus induces sympathetic activation via AT1R/PKCgamma/Rac1 pathway in salt-induced hypertension. Sci Rep 7: 43107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Seki S, Hiramoto K, Naganuma E, Kobayashi EH, Yamaoka A, Baird L, Takahashi N, Sato H and Yamamoto M (2017) Hyperactivation of Nrf2 in early tubular development induces nephrogenic diabetes insipidus. Nat Commun 8: 14577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S, Rojo de la Vega M, Chapman E, Ooi A and Zhang DD (2018) The effects of NRF2 modulation on the initiation and progression of chemically and genetically induced lung cancer. Mol Carcinog 57(2): 182–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S, Wang S, Moghaddam SJ, Ooi A, Chapman E, Wong PK and Zhang DD (2014) Oncogenic KRAS confers chemoresistance by upregulating NRF2. Cancer Res 74(24): 7430–7441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia E, Soto V, Ortiz-Vega KM, Zarco-Marquez G, Molina-Jijon E, Cristobal-Garcia M, Santamaria J, Garcia-Nino WR, Correa F, Zazueta C and Pedraza-Chaverri J (2012) Curcumin induces Nrf2 nuclear translocation and prevents glomerular hypertension, hyperfiltration, oxidant stress, and the decrease in antioxidant enzymes in 5/6 nephrectomized rats. Oxid Med Cell Longev 2012: 269039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangapandiyan S, Ramesh M, Miltonprabu S, Hema T, Jothi GB and Nandhini V (2019) Sulforaphane potentially attenuates arsenic-induced nephrotoxicity via the PI3K/Akt/Nrf2 pathway in albino Wistar rats. Environ Sci Pollut Res Int [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tong KI, Kobayashi A, Katsuoka F and Yamamoto M (2006) Two-site substrate recognition model for the Keap1-Nrf2 system: a hinge and latch mechanism. Biol Chem 387(10–11): 1311–1320 [DOI] [PubMed] [Google Scholar]
- Touyz RM (2004) Reactive oxygen species and angiotensin II signaling in vascular cells -- implications in cardiovascular disease. Braz J Med Biol Res 37(8): 1263–1273 [DOI] [PubMed] [Google Scholar]
- Trpkov K, Hes O, Agaimy A, Bonert M, Martinek P, Magi-Galluzzi C, Kristiansen G, Luders C, Nesi G, Comperat E, Sibony M, Berney DM, Mehra R, Brimo F, Hartmann A, Husain A, Frizzell N, Hills K, Maclean F, Srinivasan B and Gill AJ (2016) Fumarate Hydratase-deficient Renal Cell Carcinoma Is Strongly Correlated With Fumarate Hydratase Mutation and Hereditary Leiomyomatosis and Renal Cell Carcinoma Syndrome. Am J Surg Pathol 40(7): 865–875 [DOI] [PubMed] [Google Scholar]
- Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT, Wong PK and Zhang DD (2008) Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis 29(6): 1235–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardyn JD, Ponsford AH and Sanderson CM (2015) Dissecting molecular cross-talk between Nrf2 and NF-kappaB response pathways. Biochem Soc Trans 43(4): 621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Gu J, Mei S, Xu D, Jing Y, Yao Q, Chen M, Yang M, Chen S, Yang B, Qi N, Hu H, Wuthrich RP and Mei C (2016) Resveratrol delays polycystic kidney disease progression through attenuation of nuclear factor kappaB-induced inflammation. Nephrol Dial Transplant 31(11): 1826–1834 [DOI] [PubMed] [Google Scholar]
- Yang SM, Ka SM, Wu HL, Yeh YC, Kuo CH, Hua KF, Shi GY, Hung YJ, Hsiao FC, Yang SS, Shieh YS, Lin SH, Wei CW, Lee JS, Yang CY and Chen A (2014) Thrombomodulin domain 1 ameliorates diabetic nephropathy in mice via anti-NF-kappaB/NLRP3 inflammasome-mediated inflammation, enhancement of NRF2 antioxidant activity and inhibition of apoptosis. Diabetologia 57(2): 424–434 [DOI] [PubMed] [Google Scholar]
- Ye T, Zhen J, Du Y, Zhou JK, Peng A, Vaziri ND, Mohan C, Xu Y and Zhou XJ (2015) Green tea polyphenol (−)-epigallocatechin-3-gallate restores Nrf2 activity and ameliorates crescentic glomerulonephritis. PLoS One 10(3): e0119543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim HE and Yoo KH (2008) Renin-Angiotensin system - considerations for hypertension and kidney. Electrolyte Blood Press 6(1): 42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DD (2013) Bardoxolone brings Nrf2-based therapies to light. Antioxid Redox Signal 19(5): 517–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DD and Hannink M (2003) Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol 23(22): 8137–8151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y and Dong W (2016) ROS and ROS-Mediated Cellular Signaling. Oxid Med Cell Longev 2016: 4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Whitman SA, Wu W, Wondrak GT, Wong PK, Fang D and Zhang DD (2011) Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes 60(11): 3055–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]