Abstract
Objective
To evaluate patterns of elevations of isotypes of rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA) pre- and post-rheumatoid arthritis (RA) diagnosis.
Methods
Using the Department of Defense Serum Repository we identified 214 RA cases and 210 matched controls. A mean of 3 pre-RA and 1 post-RA diagnosis serum samples were tested for RF and ACPA immunoglobulin (Ig) A, IgG, and IgM. The timing and trajectories of elevations of autoantibodies were evaluated.
Results
Pre-RA autoantibody levels were elevated in cases vs. controls as follows: ACPA-IgG 17.9 years, RF-IgA 14.2 years, RF-IgM 7.2 years, ACPA-IgA 6.2 years and ACPA-IgM and RF-IgA both at 5.0 years (p<0.01, all comparisons). The autoantibodies as positive/negative showed similar relationships, with the median time of first positivity of ACPA-IgG at 1.9 years pre-RA, RF-IgA at 1.7 years, followed by the other isotypes. Only ACPA-IgA positivity significantly increased post-RA (19% 0–2 years pre-RA vs. 39% >2 years post-RA, p=0.04). All autoantibody levels demonstrated an early initial elevation, a period of stability, then an increase immediately Pre-RA diagnosis. A pre-RA endotype of early elevation of autoantibodies was associated with increased use of biologic therapy, and a higher prevalence of sicca symptoms and lung disease post-RA.
Conclusion
Differences in patterns of elevations of autoantibody isotypes have implications in understanding the pathophysiology of RA development. These include understanding what factors drive initial autoantibody elevations compared to what factors (including mucosal) drive later increases in autoantibodies and a transition to clinically-apparent RA, and how pre-RA endotypes may influence post-RA phenotypes.
Introduction
Serum autoantibodies including rheumatoid factor (RF), anti-citrullinated protein antibodies (ACPA) and other autoantibodies (e.g. antibodies to carbamylated proteins), as well as systemic inflammation, have been shown to be abnormal prior to the development of clinically-identifiable synovitis and a diagnosis of classified RA (1–11). Importantly, the discovery of this pre-RA diagnosis period has led to an improved understanding of the natural history and pathogenesis of RA, as well as provided rationale for strategies for prevention (12).
Elevations of serum autoantibodies prior to the onset of clinically-apparent synovitis also suggest autoantibodies may be initially generated outside of the joints and potentially related to mucosal processes (13). Findings supporting an important role for these processes in the early development of RA include elevations of serum RF and ACPA immunoglobulin A (IgA) isotypes pre-RA diagnosis (9, 14), and lung abnormalities in some individuals before they develop RA (15, 16). Furthermore, cross-sectional studies of subjects at-risk for future RA demonstrate serum ACPA and RF IgA elevations (17), IgA producing plasmablast expansion (18). In addition, epidemiologic studies link risk to RA with factors, such as exposure to tobacco smoke, that can drive mucosal inflammation (19).
The purpose of our current study was to evaluate serum RF and ACPA isotypes pre- and post-RA diagnosis using multiple serial samples from a new, large and well-characterized cohort of subjects with RA and matched controls obtained from the United States (US) military. Our findings highlight timing and trajectories of autoantibody isotype elevations in RA development, with a focus on the relationships between IgG and IgA isotypes.
Patients and Methods
Study subjects
A case-control study was performed using samples from the Department of Defense Serum Repository (DoDSR) from individuals pre and post-RA diagnosis, and controls. The DoDSR is part of a program to monitor the health of US military personnel, and samples are collected at enlistment, deployment and approximately every other year of service (20). For research purposes, the DoDSR can be used to identify individuals who develop incident RA with the availability of longitudinally collected pre-diagnosis data and samples.
For this project, candidate RA cases were initially identified based on documentation in the medical record of at least two International Classification of Disease (ICD) codes over time consistent with RA, and at least one rheumatologist encounter. Medical records for each candidate case were subsequently evaluated at Walter Reed National Military Medical Center (WRNMMC) by a rheumatologist and an experienced clinical rheumatology nurse with training in research methologies, and 346 RA cases were identified, all satisfying 1987 RA classification criteria (21) or receipt of an RA diagnosis from a rheumatologist. Notably, none of these 346 cases were included in an earlier DoDSR evaluation of 83 individuals with RA by our group (3, 5, 6, 22, 23)). Up to four DoDSR samples for each case were selected, including three samples from pre-RA diagnosis, and one sample from post-RA diagnosis. Up to four controls were identified per case, excluding individuals with a diagnosis of RA or inflammatory arthritis, and matched to cases based on age (at time of RA diagnosis for their matched cases), gender, race, region of enlistment, and duration of sample storage. When fewer samples were available from controls than cases, controls were matched by the overall timespan covered by the samples (i.e. oldest to newest) rather than a total number of serum samples.
For this analysis, we used a subset of 214 RA cases who had an RA diagnosis between 1995 and 2012, with 212 (99%) meeting 1987 RA classification criteria. The remaining two cases were diagnosed with RA by a board-certified rheumatologist. This subset of cases was selected because they had a clear date of RA diagnosis available from chart review, sufficient clinical information available to evaluate the clinical course of their RA post-diagnosis, and two or more pre-diagnosis serum samples as well as a post-diagnosis sample with adequate volume available for autoantibody testing. Notably, the 214 RA cases selected for analysis did not differ from the 132 remaining RA cases in terms of age at diagnosis, gender, race, smoking status, or ACPA positivity post-diagnosis (data not shown). We also used a subset of 210 matched controls. This was less than the 214 RA cases; however, 4 controls were removed from analysis because after initial selection and prior to autoantibody testing, they lacked sufficient information to exclude inflammatory arthritis.
Clinical data for each case was identified based on review of medical reports and ICD codes, and abstracted onto a standardized form by personnel blinded to autoantibody status. Data included demographics, date of RA diagnosis and symptom onset, comorbidities including ocular/oral dryness, lung disease, use of disease-modifying antirheumatic drugs (DMARDs), radiographic erosions, family history of RA (unavailable for controls), and smoking status (never vs. ever). Race/ethnicity was categorized as non-Hispanic white, black, and other, with the other category including Asian, Hispanic and Native American. Material for genetic studies was not available.
Autoantibody assays
Serum was tested in a blinded fashion at the University of Colorado in Exsera BioLabs. To provide a comparison with clinically-available ACPA assays, we tested serum for anti-cyclic citrullinated peptide 3.1 (CCP3.1) (IgG/A) (Inova Diagnostics, San Diego, CA USA). In addition, we tested RF-IgA, RF-IgG, and RF-IgM (Inova Diagnostics, San Diego, CA). ACPA isotypes IgA, IgG and IgM were measured using kits utilizing the same antigen plate as the commercial CCP3.1 assay but are only available in the isotype-specific assay for research (Inova Diagnostics, Inc., San Diego, CA). For each assay, serum was tested in duplicate and levels were determined using a standard curve. The final level for analyses determined from the mean of the two wells. For all isotype assays, the mean difference between wells was <15%.
Autoantibody positivity determination
We randomly selected 156 (~75%) of the 210 DoDSR controls and used their last available sample to set cut-offs for each antibody that were present in <2% of these controls. This also provided a subset of 54 (25%) controls to use to compare rates of positivity to cases. Of note, there were no significant differences between these 156 controls and the 214 cases in age, gender and race; furthermore, there were no significant differences between the sets of 156 and 54 controls in age, gender race, or levels of autoantibodies (see Supplemental Table 1). For the CCP3.1 assay, we used the positive cut-off level of >=20 units which is the manufacturer’s suggested cut-off that is also commonly used in general clinical practice.
Statistical analysis
For descriptive analyses, continuous data were analyzed using a t-test, and a chi-square test was used for categorical data.
For analyses of autoantibodies as continuous outcomes, levels were log (base 2) transformed, and values outside of the limit of detection for each assay were randomly imputed within a window around the limit to avoid truncation. To examine the timing of the isotypes, a mixed model was performed for each autoantibody isotype. Time was included as a continuous variable and modeled using cubic B-splines that were allowed to vary by group with internal knots placed at the tertiles and boundary knots placed at the extremes (24). The model included a random subject intercept and slope, which were assumed to have a multivariate normal distribution. Cholesky decomposition was used to constrain the covariance matrix of the random effects to be positive-definite. Isotype levels were compared at one-month intervals to identify the time at which levels differed significantly (p<0.05) between cases and controls. Comparisons were adjusted for multiple testing using a stepdown Holm-simulated method to control the family-wise type I error rate. Of note, a matched nested random effect was evaluated and did not change the results; as such, analyses of autoantibody levels do not include matched pair analyses.
Additionally, a proportional hazards frailty model was evaluated to compare differences in time to first positivity using dichotomous outcomes for each isotype within cases (25). This model took into account left censorship, and only included those cases who converted from negative to positive for the isotype of interest. Comparisons between survival curves were reported as hazard ratios (95% confidence intervals) and accounted for within-patient correlation, and Kaplan-Meier plots were generated. Contingency tables and Chi-squared testing were used to evaluate fluctuations in autoantibody positivity across the pre- and post-RA diagnosis periods.
Finally, estimated subject-specific autoantibody trajectories as levels from the mixed models were evaluated in an unsupervised random forest with 10,000 trees to estimate a proximity matrix. Partition around medoids (PAM) clustering was performed on the matrix and a silhouette plot was used to identify the number of clusters, which we defined as “endotypes” or subtypes of a condition with distinct pathophysiologic mechanisms based on biomarker patterns rather than clinically-observable phenotypes (e.g. gender) (26). The mixed effects model was applied to each isotype allowing for different B-spline between clusters and compared between cases and controls.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) or SPSS version 25 (IBM Corp., Armonk, NY, USA).
Ethical considerations
This study protocol was approved by the appropriate regulatory bodies at the DoDSR, WRNMMC as well as Colorado Multiple Institutional Review Board.
Results
Study subjects included 214 RA cases and 210 controls (Table 1). Overall, cases were similar to controls in age, gender and race, although cases had a non-signficant increase in ever smoking compared to controls (23% vs. 32%, p=0.06).
Table 1.
Subject Characteristics
| Characteristic | RA Cases n=214 |
Controls n=2101 |
p-value |
|---|---|---|---|
| Samples | |||
|
Number of samples tested, mean (range) Pre-RA diagnosis Post-RA diagnosis |
4 (3–4) 3 (2–3) 1 (1–1) |
3 (2–4) - - |
- |
| Time span of pre-RA diagnosis samples in years, mean (SD) [range] | −5.1 (5.7) [−22.8 to −0.1] |
- | - |
| Time span of post-RA diagnosis samples in years, mean (SD)[range] | 1.3 (0.9) [0.1 to 5.6] |
- | - |
| Time span, oldest to newest sample,in years, mean (SD) | 12.8 (5.6) | 12.3 (5.4) | 0.39 |
| Age at time of diagnosis, mean (SD) | 37 (7.9) | 37 (7.9) | 1.0 |
| Female, % | 48 | 48 | 1.0 |
| Non-Hispanic White, % | 58 | 55 | 0.93 |
| Ever Smoker, %2 | 322 | 232 | 0.06 |
| Autoantibody positivity post-RA diagnosis, n (%) | |||
| CCP3.1 | 162/214 (76) | 2/54 (4) | <0.01 |
| RF-IgA | 86/214 (40) | 1/54 (2) | <0.01 |
| RF-IgG | 35/215 (16) | 0/54 (0) | <0.01 |
| RF-IgM | 112/214 (52) | 2/54 (4) | <0.01 |
| ACPA-IgA | 56/214 (26) | 0/54 (0) | <0.01 |
| ACPA-IgG | 150/214 (70) | 1/54 (2) | <0.01 |
| ACPA-IgM | 52/214 (24) | 0/54 (0) | <0.01 |
| Any RF isotype positive | 129/214 (60) | 3/54 (6) | <0.01 |
| Any ACPA isotype positive | 151/214 (71) | 1/54 (2) | <0.01 |
| Any RF or ACPA isotype positive | 174/214 (81) | 4 (7) | <0.01 |
| No RF or ACPA isotype positive | 40/214 (19) | 50/54 (94) | <0.01 |
| Only RF isotype positive | 23/214 (11) | 3/54 (6) | <0.01 |
| Only ACPA isotype positive | 45/214 (21) | 1/54 (2) | <0.01 |
| Both ACPA and RF isotype positive | 106/214 (50) | 0/54 (0) | <0.01 |
| Follow-up time post-RA diagnosis by medical record review in years, mean (SD)3 | 6.9 (3.6)3 | 7.2 (4.4)3 | 0.54 |
| RA medications (Ever Used), % | |||
| Methotrexate | 87 | - | - |
| Anti-TNF inhibitor | 73 | - | - |
| Radiographic erosions, % | 45 | - | - |
| Self-reported first-degree relative with RA4, % | 16 | - | - |
A total of 210 individual controls were available for analyses. They were split into two groups of n=156 (~75%) and n=54 (~25%) and their last available sample was used to set cut-off levels for RF and ACPA isotypes in the n=156, and then compare prevalence rates of positivity of autoantibodies in the separate group (n=54). The demographic information presented here is for the entire group of n=210, while the autoantibody positivity rates are for the n=54 and using results from their last available sample. Additional comparisons between the n=156 and n=54 are presented in Supplemental Table 1.
Data was missing regarding ‘ever smoking’ in 5/214 (2%) of cases and 89/210 (42%) of controls.
For cases, this refers to the time post-RA diagnosis that charts were available for refview. For controls, it refers to the time available between the age they were at the time their matched case was diagnosed with RA and final chart information availible for review.
First-degree relative defined as parent, sibling or child with RA; this information was not available for controls.
Timing of autoantibody isotype elevations in cases compared to controls
In the mixed models, RF-IgA levels in cases diverged significantly from controls at 14.2 years prior to RA diagnosis (Figure 1A). This divergence was qualitatively earlier than the divergence for RF-IgG, which occurred at 5.0 years (Figure 1B). RF-IgM levels diverged between cases and controls at 7.2 years (data not shown).
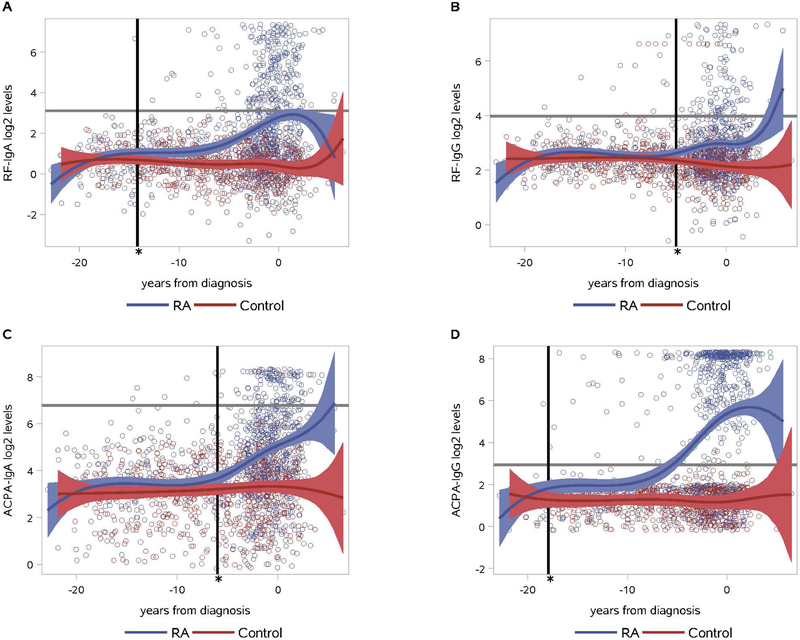
Figure 1. RF and ACPA IgA and IgG levels in RA cases and controls.
Using mixed model estimates, levels of RF-IgA diverged significantly between cases and controls at 14.2 years prior to RA diagnosis (mean (SE) log2 levels 1.02 (0.12) in cases vs. 0.69 (0.11) in controls, p<0.05), while for RF-IgG, a significant divergence between cases and controls occurred at 5.0 years pre-RA diagnosis (mean (SE) log2 levels 2.62 (0.08) in cases vs. 2.36 (0.07) in controls, p<0.05) (Figure 1A, 1B) While not shown, RF-IgM diverged from controls at 7.2 years. Levels of ACPA-IgG diverged between cases and controls at 17.9 years pre-RA diagnosis compared to a divergence of ACPA- IgA at 6.2 years (Figure 1C, 1D) While not shown, ACPA-IgM diverged from controls at 5.0 years. The horizontal black line in each figure represents the cut-off for positivity for that autoantibody isotype set in 156 controls.
For ACPA, IgG levels in cases diverged significantly from controls at 17.9 years prior to RA diagnosis. This divergence was qualitatively earlier than the divergence of 6.2 years that was observed for ACPA-IgA (Figure 1C and 1D). ACPA-IgM levels diverged between cases and controls at 5.0 years (data not shown).
Notably, the divergence times identified using the mixed models described above can only be compared in a qualitative fashion. Therefore, we additionally used analytic approaches that evaluated the differences between cases and controls on a subject level to quantitatively compare timing of divergence of autoantibody levels between cases and controls. In these analyses, the sequence of divergence was similar to that seen in the mixed models and are ordered as follows from earliest to latest separations from controls pre-RA diagnosis: ACPA-IgG, RF-IgA, RF-IgM, ACPA-IgA, ACPA-IgM and RF-IgG (p<0.01 for all comparisons; Supplemental Figure 1). There was no significant difference in the overall levels of autoantibodies based on smoking status (Supplemental Figure 2). Furthermore, there was no significant difference in the timing of autoantibody levels diverging from controls for any isotype based on smoking status (data not shown).
Survival models to evaluate the relative timing of autoantibody positivity prior to RA diagnosis
To complement the analyses of autoantibodies as levels, we performed survival analyses to evaluate the relative timing of earliest positivity of each autoantibody isotype, adjusting for age, gender and smoking. In these analyses, the time of first positivity for ACPA-IgG was earliest at a median time of 1.9 years prior to diagnosis, followed by the other autoantibodies closer to diagnosis (Table 2). The cumulative probability for a positive test by autoantibody isotype is shown in Supplemental Figures 3 and 4.
Table 2.
Timing of autoantibody isotype positivity for RA cases prior to RA diagnosis
| Autoantibody Isotype | Median years first positive1 (interquartile range) | Unadjusted hazard ratios (95% CI; p-value) | Adjusted hazard ratios (95% CI; p-value)2 |
|---|---|---|---|
| ACPA-IgG | 1.92 (1.35, 2.65) | reference | reference |
| RF-IgA | 1.74 (1.12, 2.57) | 1.72 (1.17, 2.53) p<0.01 | 1.71 (1.16, 2.53); p<0.01 |
| RF-IgM | 1.66 (0.83, 2.29) | 1.55 (1.10, 2.19); p<0.01 | 1.59 (1.13, 2.24); p=0.01 |
| ACPA-IgM | 1.59 (0.78, 2.1) | 1.934 (1.19, 3.14); p=0.01 | 1.974 (1.22, 3.21); p=0.01 |
| RF-IgG | 1.37 (0.57, 2.23) | 2.82 (1.51, 5.26); p<0.01 | 2.83 (1.52, 5.29); p<0.01 |
| ACPA-IgA | 1.35 (0.83, 2.56) | 2.81 (1.66, 4.75); p<0.01 | 2.83 (1.68, 4.77); p<0.01 |
Positivity for each autoantibody was determined by identifying a level that was positive in <2% of 156 controls.
Hazard ratios (HRs) are adjusted for age at RA diagnosis, gender, and smoking history. A HR >1 indicates that the duration of autoantibody positivity is shorter (i.e. closer to RA diagnosis) compared to the referent autoantibody isotype of ACPA-IgG.
Autoantibody fluctuations in levels and positivity pre- and post-RA diagnosis
To evaluate changes in autoantibody positivity in periods pre- and post-RA diagnosis, we analyzed positivity rates 0–2 years prior to RA diagnosis, 0–2 years immediately post-RA diagnosis and then >2 years post-RA diagnosis, using a single sample from each case per time interval. There were no significant changes across these time periods in prevalence of positivity for RF-IgA, M and G, or ACPA-IgM and G (Table 3). However, the prevalence of positivity of ACPA-IgA increased: 18.9% 0–2 years pre-RA, 23.4% 0–2 years post-RA and 38.5% >2 years post-RA (p=0.04) (Table 3). There were no significant differences between those who developed ACPA-IgA across these time periods in gender, age at diagnosis of RA, medication use or smoking history (data not shown).
Table 3.
Changes in autoantibody positivity in the peri-rheumatoid arthritis diagnosis period
| 0 to 2 years prior to RA diagnosis1 | 0–2 years post RA diagnosis1 | >2 years post RA diagnosis1 | p-value2 | |
|---|---|---|---|---|
| RF-IgA (% positive) | 41 | 39 | 5 | 0.83 |
| RF-IgM (% positive) | 53 | 52 | 55 | 0.93 |
| RF-IgG (% positive) | 19 | 16 | 18 | 0.71 |
| ACPA-IgA (% positive) | 19 | 23 | 39 | 0.04 |
| ACPA-IgM (% positive) | 25 | 25 | 23 | 0.97 |
| ACPA-IgG (% positive) | 67 | 68 | 80 | 0.30 |
| CCP3.1 (% positive) | 73 | 75 | 82 | 0.50 |
| Number of subjects per interval1 | 206 | 175 | 39 | - |
| Years of sample from time from diagnosis, mean (SD) | −0.7 (0.5) | 1.0 (0.5) | 2.6 (0.8) | - |
Each interval contains only one sample per subject; if more than one sample per subject was available per interval, the sample selected for analysis was the one closet to the middle of the interval; however, not all subjects have a sample in each time interval.
This p-value reflects contingency table Chi squared testing comparing the percentages of positivity across these 3 time intervals.
In addition, subject-specific plots identified that between-subject variability over time was similar between RF-IgA and RF-IgM (variance 0.001 for both)(Supplemental Figure 5). In contrast, there was greater between-subject variability for ACPA-IgA compared to ACPA-IgG (variance 0.03 vs. 0.001). Additional findings regarding the variability of autoantibody positivity in cases and controls where ‘reversion’ of autoantibody positivity was defined as positivity in one sample followed by negativity in the next sample are presented in Supplemental Table 2. In sum, these analyses showed that reversion was least common for ACPA-IgG (19%) and most common for RF-IgG (49%). Furthermore, a subset of cases were negative for a particular isotype Post-RA diagnosis yet had been positive pre-RA: the lowest rate of reversion to negative post-RA diagnosis was for ACPA-IgG (12%), and highest for RF-IgG (49%).
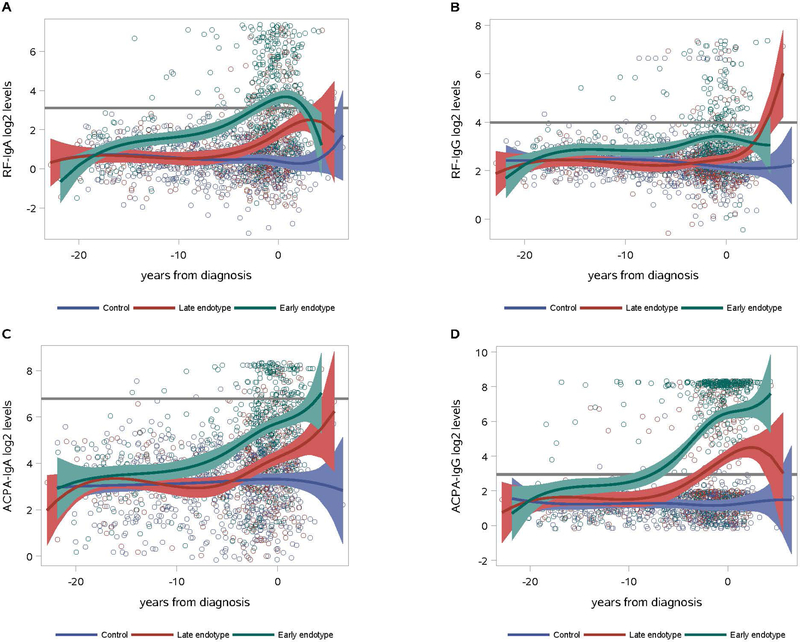
Endotype evaluation
Cluster analysis identified two endotypes based on subjects with similar trajectories of autoantibody elevations in the pre-RA period (Figure 2). An ‘Early Endotype’ was characterized by autoantibody levels rising early in the pre-RA period. In contrast, a ‘Late Endotype’ was characterized by autoantibody levels rising closer to RA diagnosis. There were no significant differences between the two endotypes for age at RA diagnosis, year of diagnosis, history of smoking, or prevalence of erosive disease assessed by review of radiologic reports from over the duration of follow-up post-RA (Table 4). However, the Early Endotype included more female subjects (55% vs. 39%, p=0.02), and also had longer duration of follow-up post-RA diagnosis (7.4 years vs. 6.3 years, p=0.03). Post-RA diagnosis, the Early Endotype also exhibited more frequent sicca symptoms (35% vs. 19%, p=0.04), lung disease (15% vs. 4%, p=0.01), and use of anti-TNF inhibitors (81% vs. 64%, p=0.01). The Early Endotype also had a higher mean number of RF and ACPA isotypes positive post-RA (2.9 vs. 1.6, p<0.01). Notably, while the timing of autoantibody elevations was different between the endotypes, the overall relationships between isotypes remained similar to those seen in other analyses, with ACPA-IgG elevated earliest.
Figure 2. Two endotypes identified among RA cases based on trajectories of autoantibody isotypes.
In the ‘early’ endotype (116 RA cases, green lines), RF and ACPA IgG and A autoantibodies began to increase from controls (blue lines) early in the pre-RA period (Figure 2A-D). RF-IgM and ACPA-IgM trajectories are not pictured but are within the range of IgG and A autoantibodies for each endotype. In contrast, in the ‘late’ endotype (98 cases, red lines) RF and ACPA IgG and A autoantibodies showed an increase from controls (blue lines) just proximal to the diagnosis of RA (Figure 2A-D, red lines).
Table 4.
Characteristics of two endotypes of RA cases with different autoantibody isotype patterns prior to RA diagnosis
| Characteristic | Early endotype (n = 116) | Late endotype (n = 98) | p-value |
|---|---|---|---|
| Duration of clinical follow-up available post-RA diagnosis, in years, mean (SD) | 7.4 (3.8) | 6.3 (3.20 | 0.03 |
| Age, mean years (SD) | 37.5 (8.5) | 36.2 (7.2) | 0.24 |
| Female Gender, % | 55 | 39 | 0.02 |
| Ever Smoke, % | 32 | 32 | 0.97 |
| Current smoker, % | 19 | 14 | 0.36 |
| X-ray Erosions, %1 | 47 | 41 | 0.33 |
| Lung Disease, % | 15 | 4 | 0.01 |
| Dry Eyes Mouth, % | 32 | 19 | 0.04 |
| Self-reported first-degree relative with RA, %2 | 18 | 14 | 0.29 |
| Body Mass Index | 0.75 | ||
| Normal, % | 21 | 22 | |
| Overweight, % | 47 | 46 | |
| Obese, % | 33 | 32 | |
| Medications, ever use % | |||
| Corticosteroids | 81 | 77 | 0.42 |
| Methotrexate | 91 | 83 | 0.06 |
| Leflunomide | 16 | 8 | 0.07 |
| Anti-TNF inhibitors | 81 | 64 | 0.01 |
| Rituximab | 7 | 8 | 0.73 |
| RA nodules | 8 | 7 | 0.86 |
| Mean # of Antibodies Positive post-diagnosis | 2.9 (1.7) | 1.6 (1.6) | <0.01 |
| Time of post-RA diagnosis serum sample for autoantibody testing, in years, mean (SD) | 1.4 (0.9) | 1.1 (0.9) | 0.05 |
Erosions were assessed by chart review of all available follow-up data from the RA cases; there was no significant difference in numbers of subjects who had radiographs performed, or timing of radiographs, between endotypes.
First-degree relative defined as parent, sibling or child with RA.
Discussion
In one of the largest cohorts studied to-date, we have identified that ACPA-IgG is elevated earlier pre-RA diagnosis than the other autoantibody isotypes, and there is an initial early elevation of autoantibody levels, followed by a period of stability, then an increase in levels immediately prior to a diagnosis of RA. We also identified that ACPA-IgA significantly increases in rates of positivity post-RA diagnosis, and that there appear to be two endotypes of autoantibody trajectories pre-RA diagnosis that are associated with differences in post-RA diagnosis phenotypes.
We had an a priori hypothesis for this project that ACPA-IgA would be elevated earlier pre-RA diagnosis than ACPA-IgG; however, this was not the observed outcome. Furthermore, in published studies, ACPA-IgG has been shown to be elevated pre-RA in more individuals than ACPA-IgA, similar to our findings herein (9). In aggregate, these findings suggest that ACPA-IgG indeed is elevated systemically earlier than ACPA-IgA pre-RA diagnosis. This finding may suggest that for ACPA, IgA processes are not the earliest drivers of autoimmunity.
However, several lines of evidence suggest that IgA and potentially mucosal responses are important in the evolution of RA, and in particular in pre-RA and transition to clinically-apparent RA. First, several cross-sectional studies of first-degree relatives (FDRs) of RA patients that have shown a higher prevalence of ACPA-IgA than ACPA-IgG (17, 27), although one study has shown higher rates of positivity for ACPA-IgG compared to ACPA-IgA among FDRs and non-FDR healthy controls (28). While mixed, these results may support that there is higher RF-IgA and ACPA-IgA levels due to ‘natural’ mucosal-related autoimmunity in individuals who do not develop RA - and perhaps in a T cell-independent fashion (29). In contrast, within individuals who progress to RA, certain factors may lead to more pathogenic immune responses, including early transition to IgG that may be facilitated through the presence of the shared epitope (4). Second, in the cases studied herein we may have missed earlier ACPA-IgA elevations related to the higher fluctuations of ACPA-IgA levels when compared to ACPA-IgG (Supplemental Figure 5). This varability may be in part due to a shorter circulating half-life of IgA vs. IgG (~5 days vs. ~21 days) that could make it difficult to capture persistent elevation/positivity of IgA until higher levels are sustained (30). Third, prior work from our group has demonstrated that ACPA-IgA is present in sputum, but may not simultaneously detected in the blood (31, 32). As such, our observation herein that ACPA-IgG was elevated in blood before ACPA-IgA could mean that IgG more efficiently transfers from a mucosal surface to the circulation and is therefore detected earlier (33). Fourth, for ACPA and RF, we measured total IgA. However, there are subtypes of IgA, including its secretory form, that may be more indicative of direct mucosal generation, although bone marrow or other sites of generation of IgA are also possible (34–36); future studies should evaluate these subtypes further. Finally, while IgA is the dominant antibody response at most mucosal sites, certain sites such as the cervico-vaginal tract can generate IgG locally (37), raising the possibility that even the early presence of serum IgG may be indicative of mucosal responses.
There are additional factors that suggest mucosal processes play an important role in RA development. In particular, RF and ACPA appear to be separate yet synergistic systems in RA, with distinct processes that drive the development of each autoantibody lineage (38–40). It is also known that these autoantibodies may be generated in the joints of individuals with established/Classified RA (41, 42) although it is not yet fully understood how and where RF and ACPA are generated pre-RA diagnosis when presumably there is no synovitis. However, earlier elevation of RF-IgA compared to RF-IgM and RF-IgG suggests that RF may initially be generated due to mucosal responses. In addition, the later elevations of ACPA-IgA compared to ACPA-IgG, and in particular the increased prevalence of positivity of ACPA-IgA across the pre- to post-RA diagnosis periods suggest that ACPA-IgA generation could represent mucosal processes that facilitate a transition to clinically-apparent synovitis. This latter concept is supported in other studies by the finding of circulating ACPA-IgA plasmablasts in early classified RA (43), as well as by findings in an animal model of inflammatory arthritis where a systemic autoimmune response is initiated through subcutaneous immunization yet there is a requirement for mucosal interactions with bacteria for the development of arthritis (44). Future studies can expand on these issues including by evaluating mucosal interventions that are applied after the initial development of RA-related autoimmunity and which could halt propagation of immunity and a transition to synovitis.
In our longitudinal analyses of samples we saw pre-RA diagnosis increases in levels of all autoantibody isotypes in the cases compared to the controls; for example, ACPA-IgG level diverged from controls at ~17 years prior to diagnosis (Figure 1). However, when treating the autoantibodies as positive/negative, the times of elevation were much closer to diagnosis (Table 2). This supports that autoantibody ‘positivity’ by a cut-off can be useful for predicting near-term onset of clinically-apparent RA. Indeed, positivity for ACPA and RF are being used in RA prevention trials that have the expectation of near-term development of clinically-apparent RA (12). However, when looking at the autoantibodies as levels, there was still a period of many years where the levels remained relatively low yet still higher than seen in controls. Importantly, for many cases, this level would be considered ‘negative’ using standard cut-off levels for RF or ACPA (Figure 1). Others have suggested that there are “two hits” in the evolution of RA, where there is an initial break in tolerance and early generation of autoantibodies, followed by a later expansion of autoimmunity and transition to clinically-apparent RA (5, 40, 45), and our findings herein strongly support this concept. The factors associated with the initial rise in autoantibodies and then later expansion closer to the onset of clinically-apparent RA RA need further evaluation.
Also of interest was our finding that there were flucutations of autoantibody positivity within cases pre- and post-RA diagnosis (Table 3, Supplemental Table 2). Fluctuations of autoantibody positivity post-RA diagnosis is not a novel finding, although less is known about the positive/negative states across the transition from pre- to post-RA (46). The findings post-diagnosis are somewhat limited in that the mean time of collection of post-RA samples was ~1.3 years and that we do not have serial post-RA diagnosis samples per individual. However, these result are consistent with the concept that there is continued evolution of autoantibodies across the span of RA development, although the factors that contribute to that (e.g. genetic, environmental, medication, etc.) need evaluation in the future.
There is emerging understanding that in individuals with established RA there can be a variety of ‘endotypes’ defined by a variety of features, including autoantibodies, that may have different clinical outcomes and response to therapy (47). Following this, among cases we uniquely identified that there were Early and Late Endotypes based on the temporal pattern of elevations of autoantibody isotype levels pre-RA diagnosis. This was an observational study and there were differences in the times of post-RA diagnosis follow-up between endotypes. As such, conclusions regarding the relationship between these endotypes and clinical outcomes are limited. However, the differences in TNF-inhibitor use and extra-articular disease yet similar erosive disease suggest there are underlying differences in disease pathogenesis between these endotypes. In addition, in prior work using a different and smaller (n=83) cohort of subjects we found an older age at RA diagnosis was significantly associated with a longer duration of autoantibody positivity pre-RA diagnosis (22). In this new cohort, we were unable to see a significant relationship between age and duration of autoantibody positivity pre-RA diagnosis. However, the mean age at RA diagnosis was non-significantly higher for the Early vs. Late Endotype (37.5 vs. 36.2) suggesting that there may be a similar effect of age on duration of autoantibody elevation pre-RA diagnosis. Additional genetic factors, inflammatory pathways, or other unmeasured variables that may also explain the differences between these endotypes will need further investigation (9, 27).
There are several additional caveats. This is one of the largest sample sets to date evaluated across the development of RA, however, we performed multiple analyses, many resulting in novel findings, and therefore welcome replication in additional studies. The military cohort may also represent a more severe form of RA than is seen in other populations given that the age at RA diagnosis was younger than other RA cohorts (~37 vs. 50–60 in other studies (48)), there was a high rate of RF and ACPA positivity, and a high percentage of individuals were treated with biologics. In addition, the percentage of males with RA (~50%) is higher than the typical ~30% seen in other RA cohorts (48). To explain this, our selection of cases who predominately met 1987 criteria may have led to the identification of more severe phenotypes. Furthermore, some of these issues may also be inherent to the US military that is typically young and predominately male, and a higher use of biologics may be attributed to access to health-care and a desire to maintain an individual’s active military status. However, given our analyses were adjusted for gender and age, it is likely that these findings are still generalizable to seropositive RA. Furthermore, 67% of samples were from the 0–5 year Pre-RA. Over 85% of autoantibody positive samples were in that 0–5 year window; as such, we believe that the analyses and findings regarding timing of elevations of autoantibodies are robust; however, this may limit our understanding of the behaviours of autoantibodies in earlier time periods. Furthermore, because of potential delays in individuals seeking medical attention for joint symptoms, the actual time of onset of clinically-apparent synovitis may also be earlier than the date of diagnosis, leading to a potential overestimation of the time between autoantibody elevation and onset of synovitis. In contrast, it is possible that individuals had clinically-apparent synovitis and RA prior to their recorded time-of-diagnois although herein the median time of symptoms attributed to RA was <6 months and as such we suspect this issue is not a major confounder. These latter points can be addressed in prospective studies with more frequent sample analyses as well as joint evaluations. The inherent properties of each of the autoantibody isotypes may also differ, and that could lead to differences in the ability to identify autoantibody elevations. For example, IgA affinity may be lower than IgG, and therefore serum levels may be more difficult to detect. However, the approaches that we have taken using the same assays and cut-off values for positivity, and the fact we observed a similar sequence of isotypes when examining both levels and dichotomous outcomes strengthens our conclusions on the timing of elevations of autoantibodies. Finally, for ACPA testing we used an antigen plate that is propriety and therefore the specific antigens are unknown; as such, the evolution of autoantibody isotypes and fine specificities pre- and post-RA diagnosis should be evaluated further in future studies (5, 28).
In conclusion, RF and ACPA have differences in isotype patterns across RA development. These findings have implications for understanding the pathophysiology of disease development.
Supplementary Material
Acknowledgments
Financial Support & Grants: This publication is supported by the Department of Defense Congressionally Directed Medical Research Program PR120839 (W81XWH-13-1-0408) and NIH/NCATS Colorado CTSA Grant Number UL1 TR001082. Contents are the authors’ sole responsibility and do not necessarily represent official DoD or NIH views. Dr. Kelmenson was also supported by the Rheumatology Research Foundation Scientist Development Award and previously by the NIH T32 AR07534. The authors thank the Department of the Defense and the members of US Armed Forces for the provision of the data and samples for this project.
Footnotes
Financial Conflicts of Interests: Inova Diagnostics, Inc. provided ACPA isotype kits for testing, and Dr. Kevin Deane has served as an advisory board member for Inova. Drs. Kevin Deane and V. Michael Holers have a patent with royalties for biomarker testing in rheumatoid arthritis.
Military Disclaimer: The identification of specific products or scientific instrumentation is considered an integral part of the scientific endeavor and does not constitute endorsement or implied endorsement on the part of the author, DoD, or any component agency. The views expressed in this article are those of the author and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
References
- 1.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003;48(10):2741–9. [DOI] [PubMed] [Google Scholar]
- 2.Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004;50(2):380–6. [DOI] [PubMed] [Google Scholar]
- 3.Deane KD, O’Donnell CI, Hueber W, Majka DS, Lazar AA, Derber LA, et al. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum 2010;62(11):3161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rakieh C, Nam JL, Hunt L, Hensor EM, Das S, Bissell LA, et al. Predicting the development of clinical arthritis in anti-CCP positive individuals with non-specific musculoskeletal symptoms: a prospective observational cohort study. Ann Rheum Dis 2015;74(9):1659–66. [DOI] [PubMed] [Google Scholar]
- 5.Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One 2012;7(5):e35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolfenbach JR, Deane KD, Derber LA, O’Donnell CI, Gilliland WR, Edison JD, et al. Autoimmunity to peptidyl arginine deiminase type 4 precedes clinical onset of rheumatoid arthritis. Arthritis Rheum 2010;62(9):2633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi J, van de Stadt LA, Levarht EW, Huizinga TW, Hamann D, van Schaardenburg D, et al. Anti-carbamylated protein (anti-CarP) antibodies precede the onset of rheumatoid arthritis. Ann Rheum Dis 2014;73(4):780–3. [DOI] [PubMed] [Google Scholar]
- 8.Gan RW, Trouw LA, Shi J, Toes RE, Huizinga TW, Demoruelle MK, et al. Anti-carbamylated protein antibodies are present prior to rheumatoid arthritis and are associated with its future diagnosis. The Journal of rheumatology 2015;42(4):572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kokkonen H, Mullazehi M, Berglin E, Hallmans G, Wadell G, Ronnelid J, et al. Antibodies of IgG, IgA and IgM isotypes against cyclic citrullinated peptide precede the development of rheumatoid arthritis. Arthritis research & therapy 2011;13(1):R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deane KD, Demoruelle MK, Kelmenson LB, Kuhn KA, Norris JM, Holers VM. Genetic and environmental risk factors for rheumatoid arthritis. Best Pract Res Clin Rheumatol 2017;31(1):3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catrina AI, Svensson CI, Malmstrom V, Schett G, Klareskog L. Mechanisms leading from systemic autoimmunity to joint-specific disease in rheumatoid arthritis. Nat Rev Rheumatol 2017;13(2):79–86. [DOI] [PubMed] [Google Scholar]
- 12.Deane KD, Striebich CC, Holers VM. Editorial: Prevention of Rheumatoid Arthritis: Now Is the Time, but How to Proceed? Arthritis & rheumatology (Hoboken, NJ) 2017;69(5):873–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holers VM, Demoruelle MK, Kuhn KA, Buckner JH, Robinson WH, Okamoto Y, et al. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat Rev Rheumatol 2018;14(9):542–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brink M, Hansson M, Mathsson-Alm L, Wijayatunga P, Verheul MK, Trouw LA, et al. Rheumatoid factor isotypes in relation to antibodies against citrullinated peptides and carbamylated proteins before the onset of rheumatoid arthritis. Arthritis research & therapy 2016;18:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer A, Solomon JJ, du Bois RM, Deane KD, Olson AL, Fernandez-Perez ER, et al. Lung disease with anti-CCP antibodies but not rheumatoid arthritis or connective tissue disease. Respir Med 2012;106(7):1040–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demoruelle MK, Weisman MH, Simonian PL, Lynch DA, Sachs PB, Pedraza IF, et al. Brief report: airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity? Arthritis Rheum 2012;64(6):1756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barra L, Scinocca M, Saunders S, Bhayana R, Rohekar S, Racape M, et al. Anti-citrullinated protein antibodies in unaffected first-degree relatives of rheumatoid arthritis patients. Arthritis Rheum 2013;65(6):1439–47. [DOI] [PubMed] [Google Scholar]
- 18.Kinslow JD, Blum LK, Deane KD, Demoruelle MK, Okamoto Y, Parish MC, et al. Elevated IgA Plasmablast Levels in Subjects at Risk of Developing Rheumatoid Arthritis. Arthritis & rheumatology (Hoboken, NJ) 2016;68(10):2372–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sparks JA, Karlson EW. The Roles of Cigarette Smoking and the Lung in the Transitions Between Phases of Preclinical Rheumatoid Arthritis. Curr Rheumatol Rep 2016;18(3):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perdue CL, Eick-Cost AA, Rubertone MV. A Brief Description of the Operation of the DoD Serum Repository. Military medicine 2015;180(10 Suppl):10–2. [DOI] [PubMed] [Google Scholar]
- 21.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31(3):315–24. [DOI] [PubMed] [Google Scholar]
- 22.Majka DS, Deane KD, Parrish LA, Lazar AA, Baron AE, Walker CW, et al. Duration of preclinical rheumatoid arthritis-related autoantibody positivity increases in subjects with older age at time of disease diagnosis. Ann Rheum Dis 2008;67(6):801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demoruelle MK, Parish MC, Derber LA, Kolfenbach JR, Hughes-Austin JM, Weisman MH, et al. Performance of anti-cyclic citrullinated Peptide assays differs in subjects at increased risk of rheumatoid arthritis and subjects with established disease. Arthritis Rheum 2013;65(9):2243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hastie TJTR, and Friedman JH. The Elements of Statistical Learning: Data Mining, Inference, and Prediction 1st ed. New York: Springer-Verlag; 2001. [Google Scholar]
- 25.Ripatti S, Palmgren J. Estimation of multivariate frailty models using penalized partial likelihood. Biometrics 2000;56(4):1016–22. [DOI] [PubMed] [Google Scholar]
- 26.Agache I, Akdis CA. Endotypes of allergic diseases and asthma: An important step in building blocks for the future of precision medicine. of Allergology international : official journal of the Japanese Society Allergology 2016;65(3):243–52. [DOI] [PubMed] [Google Scholar]
- 27.Arlestig L, Mullazehi M, Kokkonen H, Rocklov J, Ronnelid J, Dahlqvist SR. Antibodies against cyclic citrullinated peptides of IgG, IgA and IgM isotype and rheumatoid factor of IgM and IgA isotype are increased in unaffected members of multicase rheumatoid arthritis families from northern Sweden. Ann Rheum Dis 2012;71(6):825–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ioan-Facsinay A, Willemze A, Robinson DB, Peschken CA, Markland J, van der Woude D, et al. Marked differences in fine specificity and isotype usage of the anti-citrullinated protein antibody in health and disease. Arthritis Rheum 2008;58(10):3000–8. [DOI] [PubMed] [Google Scholar]
- 29.Bessa J, Bachmann MF. T cell-dependent and -independent IgA responses: role of TLR signalling. Immunological investigations 2010;39(4–5):407–28. [DOI] [PubMed] [Google Scholar]
- 30.Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol 2003;3(1):63–72. [DOI] [PubMed] [Google Scholar]
- 31.Willis VC, Demoruelle MK, Derber LA, Chartier-Logan CJ, Parish MC, Pedraza IF, et al. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum 2013;65(10):2545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demoruelle MK, Harrall KK, Ho L, Purmalek MM, Seto NL, Rothfuss HM, et al. Anti-Citrullinated Protein Antibodies Are Associated With Neutrophil Extracellular Traps in the Sputum in Relatives of Rheumatoid Arthritis Patients. Arthritis & rheumatology (Hoboken, NJ) 2017;69(6):1165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schroeder HW Jr., Cavacini L. Structure and function of immunoglobulins. The Journal of allergy and clinical immunology 2010;125(2 Suppl 2):S41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen IS, Baeten DLP, den Dunnen J. The inflammatory function of human IgA. Cell Mol Life Sci 2019;76(6):1041–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roos K, Martinsson K, Ziegelasch M, Sommarin Y, Svard A, Skogh T, et al. Circulating secretory IgA antibodies against cyclic citrullinated peptides in early rheumatoid arthritis associate with inflammatory activity and smoking. Arthritis research & therapy 2016;18(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Delft MAM, van der Woude D, Toes REM, Trouw LA. Secretory form of rheumatoid arthritis-associated autoantibodies in serum are mainly of the IgM isotype, suggesting a continuous reactivation of autoantibody responses at mucosal surfaces. Ann Rheum Dis 2019;78(1):146–8. [DOI] [PubMed] [Google Scholar]
- 37.Mestecky J, Moldoveanu Z, Russell MW. Immunologic uniqueness of the genital tract: challenge for vaccine development. Am J Reprod Immunol 2005;53(5):208–14. [DOI] [PubMed] [Google Scholar]
- 38.Lu DR, McDavid AN, Kongpachith S, Lingampalli N, Glanville J, Ju CH, et al. T Cell-Dependent Affinity Maturation and Innate Immune Pathways Differentially Drive Autoreactive B Cell Responses in Rheumatoid Arthritis. Arthritis & rheumatology (Hoboken, NJ) 2018;70(11):1732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ursum J, Bos WH, van de Stadt RJ, Dijkmans BA, van Schaardenburg D. Different properties of ACPA and IgM-RF derived from a large dataset: further evidence of two distinct autoantibody systems. Arthritis research & therapy 2009;11(3):R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sokolove J, Johnson DS, Lahey LJ, Wagner CA, Cheng D, Thiele GM, et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis & rheumatology (Hoboken, NJ) 2014;66(4):813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amara K, Steen J, Murray F, Morbach H, Fernandez-Rodriguez BM, Joshua V, et al. Monoclonal IgG antibodies generated from joint-derived B cells of RA patients have a strong bias toward citrullinated autoantigen recognition. J Exp Med 2013;210(3):445–55. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Humby F, Bombardieri M, Manzo A, Kelly S, Blades MC, Kirkham B, et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med 2009;6(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elliott SE, Kongpachith S, Lingampalli N, Adamska JZ, Cannon BJ, Mao R, et al. Affinity Maturation Drives Epitope Spreading and Generation of Proinflammatory Anti-Citrullinated Protein Antibodies in Rheumatoid Arthritis. Arthritis & rheumatology (Hoboken, NJ) 2018;70(12):1946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jubair WK, Hendrickson JD, Severs EL, Schulz HM, Adhikari S, Ir D, et al. Modulation of inflammatory arthritis by gut microbiota through mucosal inflammation and autoantibody generation. Arthritis & rheumatology (Hoboken, NJ) 2018. [DOI] [PMC free article] [PubMed]
- 45.Scherer HU, Huizinga TWJ, Kronke G, Schett G, Toes REM. The B cell response to citrullinated antigens in the development of rheumatoid arthritis. Nat Rev Rheumatol 2018;14(3):157–69. [DOI] [PubMed] [Google Scholar]
- 46.Barra L, Bykerk V, Pope JE, Haraoui BP, Hitchon CA, Thorne JC, et al. Anticitrullinated protein antibodies and rheumatoid factor fluctuate in early inflammatory arthritis and do not predict clinical outcomes. The Journal of rheumatology 2013;40(8):1259–67. [DOI] [PubMed] [Google Scholar]
- 47.Derksen VF, Ajeganova S, Trouw LA, van der Helm-van Mil AH, Hafstrom I, Huizinga TW, et al. Rheumatoid arthritis phenotype at presentation differs depending on the number of autoantibodies present. Ann Rheum Dis 2017;76(4):716–20. [DOI] [PubMed] [Google Scholar]
- 48.Gabriel SE, Crowson CS, O’Fallon WM. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955–1985. Arthritis Rheum 1999;42(3):415–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.