Abstract
Objective:
While chronic inflammation is a well-established risk factor for malignancy, studies evaluating the relationship between allergic inflammation and cancer have revealed conflicting results. Here, we aimed to assess the association between allergic inflammation in the lung (asthma), skin (eczema) or esophagus (eosinophilic esophagitis; EoE) and cancer at the organ site.
Design:
We conducted a systematic review of the literature to identify observational studies (case-control, cohort, and cross-sectional) evaluating the association between asthma and lung cancer, eczema and skin cancer, or EoE and esophageal cancer. Random-effects meta-analysis was performed to define pooled estimates of effects.
Data sources:
PubMED, EMBASE, and Web of Science
Eligibility criteria for selection:
Included studies evaluated the incidence of cancer.
Results:
Thirty-two studies met the inclusion criteria, 27 in the lung, 4 in the skin, and 1 in the esophagus. Metanalysis of the 3 studies with prospective data collection of asthma diagnosis revealed a positive association with incident lung cancer (OR 1.27, 95% CI 1.09–1.44); however, this result was not consistently supported by the larger dataset of retrospective studies (OR 1.37, 95%CI 0.90–1.83). Overall, studies in the lung displayed significant heterogeneity (I2 98%, p<0.0001), but no significant effect-modification on the association between asthma and lung cancer was identified for the variables of sex, smoking, or study design. Meta-analysis could not be applied to the 4 papers reviewed in the skin, but 3 suggested an association between eczema and non-melanoma skin cancer, while the remaining study failed to identify an association between melanoma and eczema. A single study meeting inclusion criteria showed no association between EoE and esophageal malignancy.
Conclusions:
The current data cannot exclude the possibility of an association between atopy and malignancy the lung, skin and esophagus. The relationship between allergy and cancer should be explored further in prospective studies that any association identified between these conditions has the potential for significant public health implications
Systematic review registration:
PROSPERO registration CRD42018092447
INTRODUCTION
Allergic disease is increasing in incidence world-wide and may manifest in different organs, including the lung, skin, and gastrointestinal tract1,2. Asthma, eczema, and eosinophilic esophagitis (EoE) cause chronic allergic inflammation of the lungs, skin, and esophagus, respectively. Allergic inflammation differs from autoimmune and infectious inflammation in that it tends to be driven by a T-helper 2 predominant inflammatory response (with interleukin 4, 5, 13) and an eosinophil and mast cell predominance. While clinicians strive to identify and mitigate antigen exposures, and provide therapeutic interventions where necessary, the efficacy of such approaches are variable. As a result, atopic patients often experience long-standing inflammation. These challenges underscore the importance of understanding the natural history of allergic diseases and how they may influence tissue biology.
Chronic inflammation is a well-established risk factor for malignancy across tissue types. Mechanisms through which a persistent inflammatory microenvironment facilitates tumorigenesis include increased cell turnover, free radical production, diminished DNA repair capacity and stromal activation3. In the gastrointestinal tract alone, ulcerative colitis has been associated with colon cancer, pancreatitis with pancreatic cancer, and gastroesophageal reflux disease with esophageal adenocarcinoma4,5. Despite this paradigm of chronic inflammation as a tumor promoter, various studies have demonstrated a negative association between allergic inflammation and cancer risk, with enhanced immunosurveillance and activation of cellular protective mechanisms (e.g. apoptosis, senescence) suggested as proposed mechanisms through which malignant transformation is limited in atopic tissues6,7. Additionally, allergic inflammation may be eosinophil-predominant, compared to other forms of chronic inflammation which are either mixed or neutrophil-predominant. The risk of allergic inflammation on cancer risk is not well established.
To assess the relationship between atopy and incidence of cancer specifically in the tissue in which allergic inflammation occurs, we performed a systematic review of literature analyzing history of allergic conditions affecting the lung (asthma), skin (eczema) or esophagus (eosinophilic esophagitis; EoE) and incidence of malignancy in those organs. While few data exist regarding the risk of skin cancer and esophageal cancer in patients with eczema and EoE, respectively, there is a breadth of literature regarding the association between asthma and lung cancer. We, therefore, performed meta-analysis of relevant publications, overall and also individually in retrospective and prospective studies, to synthesize the evidence for a relationship between asthma and lung cancer.
METHODS
Design
We conducted a systematic review of the literature to assess whether organ specific atopy is associated with malignancy (PROSPERO registration CRD42018092447). The data have been reported according to the PRISMA guidelines for systematic review and meta-analyses (Supplemental Figure S1).
Literature Search
PubMED, EMBASE, and Web of Science databases were systematically searched with the assistance of a library scientist using key words and MeSH terms for each organ (e.g. eosinophilic esophagitis AND esophageal cancer). Included studies were English language, case-control, cohort, and cross-sectional design studies. The final literature search was performed on 26 January 2018.
Study Selection
Primary exposures for each key question were the allergic conditions asthma, atopic dermatitis, and EoE. Primary outcomes were incident lung cancer, skin cancer, and esophageal cancer, respectively. Diagnoses were accepted as presented in the original studies. Studies focused on populations at elevated cancer risk from non-tobacco toxin exposure (e.g. asbestos, coal) were excluded. Studies that failed to separate the allergic condition exposure in question from other comorbidities were excluded (e.g. co-association of asthma and hay fever with lung cancer). Studies that evaluated only cancer mortality (without separating cancer incidence) were excluded.
Two authors (ABM, KW) independently reviewed all abstracts to determine whether the study met study inclusion criteria. Differences were resolved by discussion and any disagreement was resolved by consulting additional reviewers (ESD, ETJ), who made the final decision. Full texts of all studies selected were obtained for data extraction.
Data Extraction
Two authors (ABM, KW) independently extracted data from full text articles. Details of the studies were documented in a standardized table, including the following data elements: first author, year, study design, sample size(s), measure obtained (e.g. odds ratio, hazard ration, relative risk), effect estimates, and potential for bias, including possible confounding factors.
Risk of Bias Assessment
Quality assessment of all eligible articles was done by two authors (ABM, KW), with any differences discussed and resolved with a third author (MD). Study quality was assessed using the NIH National Heart, Lung, and Blood Institute Quality Assessment Tools (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). Case control and cohort modules were utilized where appropriate.
Statistical Analyses
For comparisons where two or more studies reported a similar outcome and an odds ratio could be calculated from each, we estimated a pooled odds ratio using random-effects meta-analysis, according to the inverse-variance weighted method8. For studies that only reported a standardized incidence ratio (SIR), we used the SIR to impute numbers of subjects with and without the outcome in question from a comparison group of equal size, allowing combination with other studies in a pooled odds ratio. The main analyses pooled adjusted odds ratios from each study, when these were provided. We also performed sensitivity analyses pooling the raw numbers from each study. The meta-analysis only examined odds ratios, either reported or calculated, but did not pool odds ratios with other measures (such as hazard or risk ratio). Primary analyses considered only those study estimates that accounted for latency of lung cancer diagnosis (excluding asthma diagnoses reported within 1–5 years of cancer diagnosis), to reduce protopathic bias due to misattribution of early pulmonary symptoms, A sensitivity analysis included all estimates extracted irrespective of whether the cancer diagnosis was made within 1–5 years of the diagnosis of the allergic disease. We assessed between-study heterogeneity visually using forest plots and quantitatively with the I-squared (I2) statistic, which is the percentage of total variance from between-study rather than within-study variance9. For each pooled effect, we estimated 95% confidence and predictive intervals, representing the confidence interval for the mean of the random-effects distribution and the range within which 95% of future study effects are predicted to fall, respectively10. To explore causes of heterogeneity, we performed subgroup analyses according to study characteristics including sex, smoking status, study design, risk of bias, and year of publication, when there were at least two studies in each subgroup. Specifically, we separated studies with data collected prospectively versus retrospectively when this affected the summary estimate. For asthma, where there were >10 studies assessing asthma in relation to lung cancer, funnel plots were examined visually for asymmetry suggestive of small-study effects (such as publication bias), supplemented with quantification by the Egger test11. If publication bias was detected, the “trim-and-fill” method was used to determine a random-effects summary estimate adjusted for publication bias12. All analyses were performed in STATA 14.2 (StataCorp, College Station, TX), with a significance threshold of a 2-sided p<0.05.
RESULTS
Literature Search Results
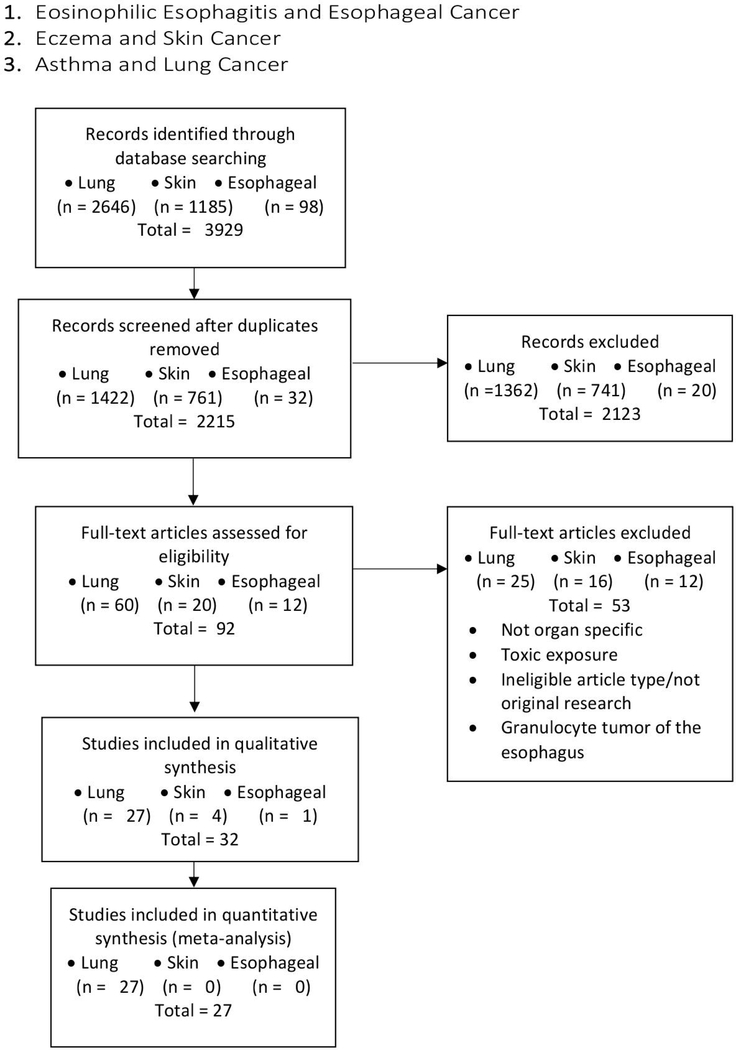
Our initial literature search identified 3929 records. After excluding duplicates and studies that did not meet the defined inclusion criteria, 92 articles were subjected to full-text review, identifying 32 relevant studies: 27 in the lung, 4 in the skin, and 1 in the esophagus (Figure 1; Table 1–2).
Figure 1:
Flow chart of searches performed.
Table 1:
Characteristics of published studies on asthma and lung cancer
| Author | Year | Study Type | Sex (M, F) | Smoking (S, NS, B, U) |
Covariates | Latency Considered (Yes/No) |
Total | Quality assessment |
|---|---|---|---|---|---|---|---|---|
| Alavanja et al | 1992 | Population-Based Case Control | F | NS | age, education, marital status, drivers status, health care finance registration, smoking history | Yes | 2020 | Fair |
| Boffetta et al | 2002 | Prospective population-based cohort | M, F | U | duration of follow-up, calendar year at entry, age at entry, other diagnoses at index hospitalization, dept of index hospitalization, # of hospitalizations during 1st year of follow up, presence of emphysema, chronic bronchitis, histological type of lung cancer | Yes | 92986/std. pop. | Fair |
| Brenner et al | 2001 | Population-Based Case Control | M, F | B | active smoking and socioeconomic status | Yes | 2651 | Good |
| Brownson et al | 2000 | Population-Based Case Control | F | B | pack-years of smoking | Yes | 1376 | Good |
| Colak et al | 2015 | Prospective Cohort | M,F | B | age; sex; BMI; allergy; familial predisposition for asthma; childhood asthma, hay fever, or eczema; use of asthma medication; occupational exposure to dust and/or fumes; daily exposure to passive smoking; leisure time physical activity; education; annual household income; and cumulative tobacco consumption, alcohol consumption, systolic and diastolic blood pressure, total cholesterol, LDL, HDL, triglycerides, use of cholesterol-lowering medication, and presence of diabetes | No | 94079 | Poor |
| Denholm et al | 2014 | large international case–control consortium | M,F | B | sex, age, center, ever-employed in a high-risk occupation, education, smoking status, cigarette pack-years, and time since quitting smoking. | Yes | 27684 | Good |
| El-Zein et al (and Ramanakumar et al) | 2010 | Population-Based Case Control | M | B | smoking, asbestos, ancestry, silica- all included in regression models | Yes | 1267 | Fair |
| El-Zein et al | 2014 | Case Control | M,F | B | adjusted for age, sex (except in sex-specific analyses), education, respondent status, ethnocultural origin, fruit and vegetable consumption, and smoking (represented by the comprehensive smoking index). lifetime occupational history; and history of 11 selected medical conditions among which were asthma, allergic eczema and hay fever. | Yes | 2655 | Good |
| Gabriel et al | 1972 | Case control | M | U | allergy: hay fever, eczema, urticaria, food reactions, smoking status | No | 300 | Poor |
| Gonzales-Perez et al | 2006 | Cohort study with nested case control | M,F | B | age, sex, calendar year, BMI, alcohol, smoking, prior comorbidities (cardiovascular disease, diabetes, osteoarthritis/rheumatoid arthritis), health services utilization, use of aspirin, NSAID, paracetamol | Yes | 19658 | Good |
| Gorlova et al | 2006 | Case control | M,F | NS | age, gender, ethnicity, income, years of education | Yes | 522 | Good |
| Huang et al (and Jian et al) | 2015 | Population based cohort | M,F | U | age, gender, low income, lung diseases, comorbidities, urbanization and geographic area | No | 15219024 | Poor |
| Ji et al | 2009 | Longitudinal cohort | M,F | 5-year age (person-years calculated from the last hospitalization admission for asthma until diagnosis of cancer, death emigration of closing date), gender, period (5-year group), socioeconomic class, residential area | Yes | 140425/std pop | Poor | |
| Koshiol et al (total) | 2009 | Population-based case control | M,F | B | smoking, other previous lung diseases, and study design variables | Yes | 3523 | Good |
| Liang et al | 2009 | Case Control | F | NS | age, marital status, years of schooling, ethnicity, 5 years ago BMI, passive smoking exposure, coal fuel use, exposure to coal smoke and cooking fumes, history of mental trauma in last 20 years, lifetime exercise habits, family cancer history in 1st-degree relatives | Yes | 505 | Fair |
| Lim et al | 2011 | Case Control | F | B | age, history of cancer in first-degree relative, fruit and vegetable consumption, country of origin, dialect group, housing type, number of years in school, environmental tobacco exposure at home, environmental tobacco exposure at work and study | No | 1808 | Fair |
| Littman et al | 2004 | Prospective Cohort | M,F | S | sex, and exposure cohort (female; male, in smoker cohort; male, in asbestos-exposed worker cohort), study arm (intervention or placebo), education, BMI, years smoked and years smoked squared, average number of cigarettes smoked per day and average number of cigarettes smoked per day squared, and all other lung diseases, and stratified by smoking status (former or current). | No | 17698 | Poor |
| Mayne et al | 1999 | Population based case-control | M,F | NS | years of schooling, cigarettes per day, lifetime passive smoke exposure, frequency of consumption of fresh fruit and vegetables, whole milk and supplemental vitamin E | Yes | 874 | Good |
| Osann et al | 2000 | Case control | F | B | age, education, smoking | No | 302 | Fair |
| Pirie et al | 2016 | Cohort | F | NS | age, oral contraceptive use, region, deprivation quintile, height | Yes | 1.2 million | Good |
| Samet et al | 1986 | Case control | M,F | B | tobacco use, residence history, occupational history, diet, passive exposure to tobacco smoke and certain occupational agents, history of respiratory diseases, personal history of physician-diagnosed chronic bronchitis, emphysema, asthma, tuberculosis, and other chest illnesses | No | 1314 | Poor |
| Vena et al | 1985 | Case control | M,F | B | age, smoking, lifetime history of asthma, hay fever, hives, eczema, | Yes | 5225 | Good |
| Vesterinen et al | 1993 | Cohort | M,F | U | age, sex | Yes | 77952/std pop | Fair |
| Wang et al | 2009 | Population based control | F | B | age, employment, total dish-year, intake of yellow/orange/dark green veggies, intake of multivitamins | Yes | 504 | Fair |
| Wang et al | 2006 | Case control | M,F | B | age, education, BMI, family history of cancer (1st degree), smoking status, alcohol consumption | No | 4467 | Fair |
| Wu et al | 1995 | Case control | F | NS | Adjusted for age, area, ethnicity or adjusted for age, area, ethnicity, education, exposure to tobacco during early life | No | 1665 | Fair |
| Wu et al | 1988 | Case Control | F | B | Pack-years smoking, years since smoking stopped, depth of inhalation, adenocarcinoma | No | 672 | Poor |
Table 2:
Characteristics of published studies on eczema and skin cancer
| Author | Year | Study Type |
Control | Case | Total | Cancer Type | Covariates | Latency Considered (Yes/No) |
Sex (M, F) |
Odds Ratio |
Confidence Interval |
Quality Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cheng et al | 2015 | Case control | BCC: 251 SCC: 432 | BCC: 375 SCC: 254 | 1312 | Early onset BCC and invasive SCC | age, sex, smoking status, corticosteriod use, immunosuppressant drug use, organ transplantation, ultraviolet radiation therapy, number of lifetime painful sunburns, skin reaction to first hour of summer sun, solar elastosis, actinic keratosis, family history of keratinocyte cancer,recreation outdoors time, mole/freckles history, alcohol consumed per month, coffee/tea consumption, tanning lamp use, history of UV radiation therapy, history of radiation therapy | yes | M,F | BCC: 1.52 SCC: 1.83 | BCC: 0.77–3.01 SCC: 0.97–3.45 | Fair |
| Dyer et al | 2012 | Cohort | 1079 | 52 | 1131 | BCC | sex, age, education, BCCs/SCCs in prior 5 years, family history of skin cancer, history of eczema, smoking status (current/former), sunburn history, Use of 5-FU, ACE/ARB, history of warts, sun sensitivity, latitude of residence history, total occupational decades outdoors in sun history, vacation history, total recreational months, ethnicity of grandparents, days spent outside, sunscreen use | yes | M,F | 1.38 | 0.95–2.03 | Fair |
| El-Zien | 2010 | Case control | 512 | 94 | 606 | Melanoma | ancestry, sports/outdoor activities | yes | M | 0.64 | 0.2–2.2 | Fair |
| Olesen et al (adults) | 2005 | Cohort | 2014 | 16 | 2030 | All keratinocyte cancers | N/A | yes | M,F | 2.4 | 1.4–3.9 | Poor |
Asthma and incidence of lung cancer
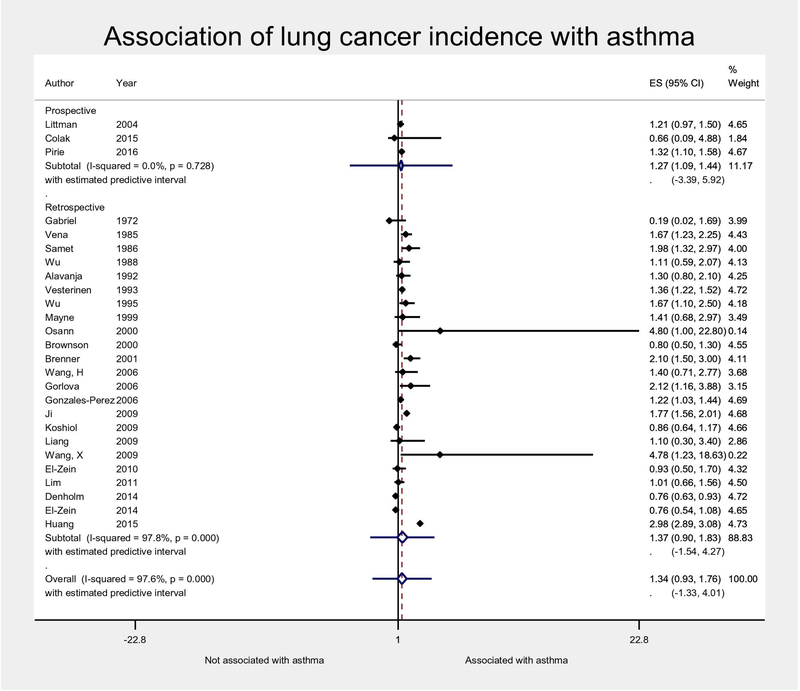
Twenty-seven studies of 939,248 unique asthmatic subjects examined the association of asthma with lung cancer incidence, and were pooled using random-effects meta-analysis (Table 1). The majority of the studies (22) were case-control studies, and only 3 studies collected data prospectively13–15. Asthma was associated with increased odds of incident lung cancer, but this was not statistically significant overall (OR 1.34, 95%CI 0.93–1.76, Figure 2) or in the subgroup of studies with retrospective data collection of asthma diagnosis (OR 1.37, 95%CI 0.90–1.83). The pooled estimate from the 3 prospective studies was significant due to less heterogeneity resulting in narrower confidence intervals, though with similar magnitude (OR 1.27, 95% CI 1.09–1.44). Heterogeneity resulted in a 95% predictive interval spanning the null in all analyses however. Indeed, 15 of the 26 studies had confidence intervals either spanning the null13,14,16–26,26 or with a significant inverse association27. When restricting the analysis to studies that reported adjusted odds ratios (AORs), the magnitude of the association attenuated slightly and results were less heterogeneous (OR 1.19, 95% CI 0.99–1.38, I2 47%, Supplemental Figure S2). The associations were very similar for a variety of sensitivity analyses, including analyses pooling raw numbers of patients rather than aORs (OR 1.38, 95% CI 1.09–1.74, Supplemental Figure S3) as well as analyses emphasizing non-latency-adjusted estimates along with either pooling AORs (OR 1.20, 95% CI 1.04–1.35) or pooling raw numbers of participants (OR 1.40, 95% CI 1.10–1.77) (Supplemental Figures S4, S5). In fact, accounting for latency did not significantly affect overall estimates (p=0.91, Supplemental Figure S4).
Figure 2:
Random-effects meta-analysis of association of lung cancer incidence and asthma, with 95% confidence interval (diamond) and estimated predictive interval (lines extending on either side of diamond). Forest plot is stratified by study design. Adjusted odds ratios were used if provided by original study, and otherwise calculated from raw numbers of the original study (and therefore crude, unadjusted). Estimates that accounted for latency between asthma and lung cancer diagnosis were selected when available. ES, estimate (here, adjusted odds ratio); CI, confidence interval.
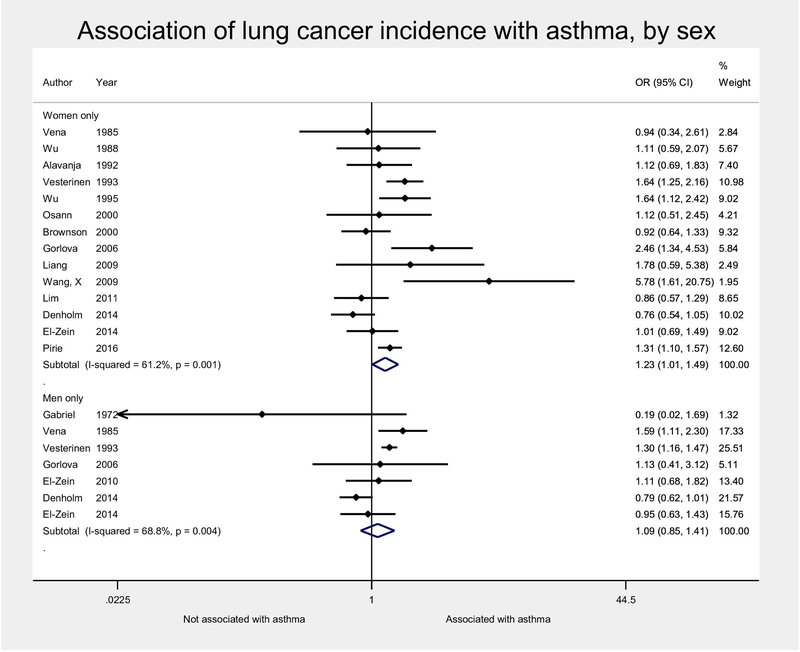
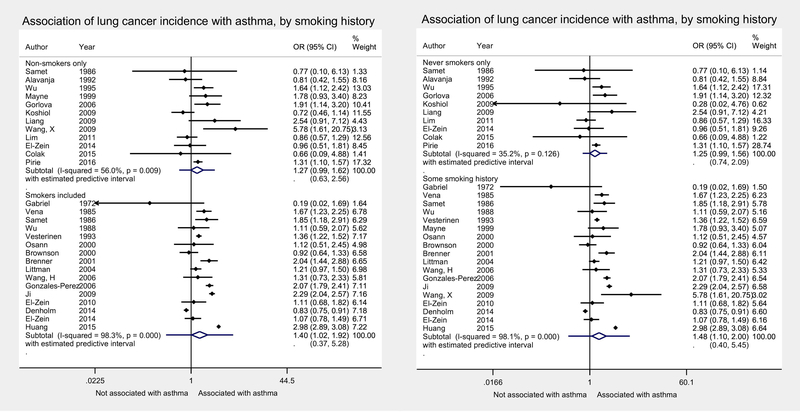
In subgroup analyses by patient characteristics, we emphasized meta-analytic estimates derived from raw numbers rather than adjusted odds ratios, since the reported aORs often adjusted for these factors already, by design. No significant effect-modification was found for the effects of sex or smoking on the association between asthma and lung cancer in the main analyses (Figures 3, 4, with sensitivity analyses using pooled aORs in Supplemental Figures S6, S7). For sex, this was true whether stratifying by studies that reported estimates by subgroups (Figure 3), or evaluating the effect of the percentage of male subjects in each study by metaregression (p=0.46). There were a greater number of studies including just women, which led to a very small, but statistically significant association between asthma and lung cancer in pooled estimates for females only, which was not significant in subgroups of only males (Figure 3). The association between asthma and lung cancer was not significantly different by smoking status either, whether stratifying by current smoking status (Figure 4a) or history of ever smoking (Figure 4b), or by metaregression of percentage of never-smokers (p= 0.62). Due to the larger number of smokers, as well as greater cancer incidence in this population, the association between asthma and lung cancer was still observed in the smoking subgroup, but did not meet statistical significance in the subgroup of non-smokers (Figure 4).
Figure 3:
Random-effects meta-analysis of association of lung cancer incidence and asthma, stratified by sex. The association of asthma with lung cancer was not significantly different by sex (p=0.46). Meta-analytic estimates calculated from raw numbers, given that most adjusted odds ratios already adjusted for sex. Estimates accounting for latency were used when provided. OR, odds ratio; CI, confidence interval.
Figure 4:
Random-effects meta-analysis of association of lung cancer incidence and asthma, stratified by inclusion of any current smokers (A) or any ever-smokers (B). Meta-analytic estimates calculated from raw numbers, given that most adjusted odds ratios already adjusted for smoking. OR, odds ratio; CI, confidence interval.
The magnitude of the odds ratios was consistent across other sensitivity analyses of study characteristics as well. An analysis removing the four studies where control group numbers were imputed from SIRs 14,28–30 reduced the overall power of the analysis without any change in the magnitude of the effect size (OR 1.32, 95% CI 0.85–1.79). Meta-regression did not reveal any statistically significant effect modification by study design (retrospectively vs prospectively collected data, p= 0.68) or year of publication (p=0.65). Of the 26 studies included in any analysis, 10 were at low risk of bias, 9 at medium, and 7 at high risk of bias (Supplemental Table 1). There was a trend toward lower pooled aORs with decreasing risk of bias, but this was not statistically significant (p=0.10). A sensitivity analysis excluding those studies at high risk of bias somewhat attenuated the magnitude of the estimate, and with less heterogeneity, but with some loss in the precision of the estimate given the reduced sample (OR 1.17, 95% CI 0.99–1.36, Supplemental Figure S8). There was statistically significant funnel plot asymmetry for the entire group of studies in the main analysis (Egger’s test p<0.001, Supplemental Figure S9), but (atypically) with smaller studies showing smaller magnitudes of an association between asthma and lung cancer. This suggests the possibility that publication bias or other small-study effects could have biased the results toward the null. The pooled random-effects odds ratio adjusted for publication bias by the trim-and-fill method was 1.39 (95% CI 1.09–1.77, vs OR 1.34 for main effect, above), suggesting the contribution of small study effects was minor.
Eczema and incidence of skin cancer
In the skin, we identified 4 studies evaluating the relationship between eczema and cancer (Table 2). Three of these studies31–33 reported a positive association between eczema and skin cancer. Olesen et al. identified 16 skin cancers in a cohort study of 2030 adults from Denmark in which the expected incidence was 6.6 (SMR 2.4, 95% CI 1.4–3.9)31. Cancers in this cohort were equally distributed between both males and females as well as squamous cell carcinomas (SCC) and basal cell carcinomas (BCC). Increased risk for skin cancer was statistically significant with follow up of 5–9 years, but failed to show statistical significance for cancers diagnosed in the population with >10 years of follow up. In an additional study, Dyer et al. reported a non-statistically significant positive association between history of eczema alone and BCC risk (HRR 1.38, 95% CI; 0.95–2.03; p=0.095)32. However, history of eczema was identified as an independent risk factor for developing BCC upon incorporation into a multivariate model adjusting for factors including age, 5-year history of skin cancer, and family history of skin cancer (HRR 1.52, 95% CI 1.01–2.09; p=0.037)32. A positive association between eczema and either SCC (OR 1.83, 95% CI 0.97–3.45) or early-onset BCC (OR 1.52, 95% CI 0.77–3.01) was identified by Cheng et al33; however, these associations did not reach statistical significance. Upon stratification by sex, men with a history of eczema displayed an increased risk of SCC (OR 3.5, 95% CI 1.33–11.15). By contrast, in women, neither a history of eczema nor allergy was associated with SCC (eczema: OR 0.95, 95% CI 0.35–2.57; allergy: OR 0.59, 95% CI 0.29–1.19). Both men (OR 2.02, 95% CI 0.61–6.72) and women (OR 1.59, 95% CI 0.66–3.80) exhibited a positive, but non-statistically significant association between history of eczema and BCC. Finally, in a cohort consisting of 94 Canadian men, El-Zein found no significant association between history of eczema and melanoma (OR 0.64, 95% CI 0.2–2.2)18. Two out of the three studies reporting a positive association between eczema and skin cancer were judged to be at medium risk of bias and one, Olesen et al., was found to be at a high risk of bias due to failure to evaluate the effect of time or possible confounders, including sun-exposure. Conversely, El-Zein et al. was found to be at medium risk of bias due to failure to assess latency effects.
EoE and risk of esophageal cancer
Our literature search of PubMed, WOS, and Embase revealed one paper that met our inclusion criteria in the esophagus, Syed et al. There were two case series, Lipka et al and Straumann et al, which followed EoE patients for an average of 13.6 and 7.2 years respectively34,35. Lipka et al. was a single-center, retrospective case series, reporting no identification of esophageal malignancy via endoscopy in 13 EoE patients followed up over a 13.6-year mean period (range 5–24 years). Additionally, while 50% of EoE patients exhibited Barrett’s esophagus, the premalignant precursor to esophageal adenocarcinoma, dysplasia was not detected upon histological review. Straumann et al. prospectively followed 30 adults with EoE for 11.5 years and found that none of these patients developed malignancy.
The study by Syed et al. was a cross-sectional population-based study utilizing an administrative database36 with over 340 hospitals and 22 major health care systems participating. With over 27 million patients, 0.02% had a diagnosis of EoE. None of these EoE patients had documentation of a diagnosis code for co-morbid esophageal cancer. A limitation of this study was that only 5 years of data were available for these patients, which may not be a long enough time of exposure for malignant transformation.
DISCUSSION
The current study provides a comprehensive evaluation of data regarding asthma, eczema, and EoE, and their association with cancer risk in the affected organ. In contrast to the skin and esophagus, where few studies have been conducted to assess associations between allergy and organ-specific malignancy, numerous case-control and cohort studies have evaluated the association between asthma and cancer in the lung. Meta-analysis of these data revealed an increase in lung cancer risk in asthma patients that was significant when examining 3 studies with prospective collection of asthma diagnosis, but that was not consistently supported when evaluating either retrospective studies alone or the collective dataset. While at these differences with regard to data significance may be a result of decreased heterogeneity in the limited dataset provided by the prospective studies, these findings support the need additional studies with prospective collection of allergy diagnoses to further investigate the relationship between atopy and cancer. Notably, the positive association between asthma and lung cancer risk remained consistent upon sub-analyses for effects of sex and smoking status. Geographic location and distribution of histologic lung cancer subtypes represent two potential sources of heterogeneity among these studies, and there were no studies with eosinophilic or neutrophilic asthma specifically assessed. A limitation of the body of evidence on atopic disease and organ-specific cancer is the potential for bias. In those studies using surveys to ascertain past medical histories from cases and controls,13–16,18–23,25–27,37–43 there is a potential for recall bias. Additionally, the temporality of atopic disease state and cancer is difficult to ascertain. Similarly, depending on duration of atopy and treatment efficacy, risk of cancer could differ. Risk of cancer in the setting of inflammatory diseases with cancer predisposition, including pancreatitis, ulcerative colitis and gastroesophageal reflux disease are dependent on disease duration. While not all studies evaluated for duration of illness prior to onset of malignancy, we did make note of this in our systematic review. In the case of asthma, other meta-analyses have shown that increased risk of lung cancer diagnosis in the 2 years following the diagnosis of asthma, and no increase in the population with >10 years of asthma44. This may signify ascertainment bias, where lung cancer cases are identified due to increased health care surveillance at the time of asthma diagnosis. Additionally, eosinophilic asthma has been recently identified as an important subtype with different treatment algorithms. The studies we identified did not stratify by peripheral eosinophil count or eosinophilic asthma status, and it would be interesting to determine this risk in this purely allergic subpopulation.
In evaluating the association between eczema and skin cancer, our literature review identified only 4 studies meeting our inclusion criteria, each reporting different combinations of skin cancer subtypes which precluded meta-analysis. Three of these studies identified increased skin cancer risk in eczema patients (two of which were not statistically significant) while the fourth failed to define any association between eczema and melanoma. In the study by Cheng et al., the positive association between eczema and skin cancer was present in both early-onset basal and invasive squamous cell carcinoma while sub-type specific effects were not explored in the two other studies identifying increased cancer risk in eczema patients33. The study by El-Zein et al. found no evidence of an association between eczema and melanoma, however, given that other skin cancer types were not evaluated, the potential remains that eczema may differentially influence specific histologic subtypes of skin cancer18. Of these 4 studies, only that performed by Olesen et al. included a primary diagnosis of eczema as an inclusion criterion. While this may alleviate concerns of recall bias, it also raises the potential for detection bias, as patients with eczema have an increased likelihood of undergoing clinical skin examinations during which cancer and pre-cancerous lesions may be detected. Importantly, as latency exclusion was not utilized in any of the 4 studies that met inclusion criteria in the skin, there exists the potential for misdiagnosis of eczema occurring close to the time cancer diagnosis. Failure to control for severity of atopic inflammation represented in these 4 studies represents an additional source of heterogeneity. While it is possible that control of atopic dermatitis inflammation could reduce subsequent cancer risk, as is the case for inflammatory conditions such as ulcerative colitis45, careful consideration must be given with regard to the agents used to alleviate allergic responses as links between several therapies and increased malignancy rates have been identified. For example, calcenurin inhibitors which are used topically to treat eczema have been associated with increased cancer risk in various organs, including the skin46–48. Additionally, the FDA has raised concerns regarding increased cancer rates in patients treated the anti-IgE antibody omalizumab. Although several studies failed to validate such concerns49,50, further investigations are warranted.
While the relationship between EoE and esophageal cancer is only beginning to be explored, such investigations may be particularly valuable for physicians who remain hesitant to treat asymptomatic EoE patients given the high burden of therapy for treatment of EoE51,52 and the fact that it is widely believed that EoE does not contribute to increased risk of malignant transformation. Supporting this notion, Lipka and Straumann et al. both failed to detect esophageal malignancy in small (n=30 and 13 respectively) EoE patient cohorts34,35. The large cross-sectional population-based study by Syed et al. revealed that in 5,370 EoE patients evaluated, not a single case of esophageal malignancy was documented over a 5-year period36. Limitations for these studies include inability to examine sex-specific effects, follow-up periods that are short with regard to cancer development, which occurs over decades, and lack of reporting on the histopathologic subtype of esophageal malignancy being detected. While malignancy was not detected in EoE patients in the three studies described herein, eosinophils have been detected in esophageal cancer lesions53,54, and patients with esophageal cancer often present with symptoms that are prevalent in EoE, including dysphagia55. Given that EoE has only been characterized as distinct disease entity in the past two decades, it is possible that undiagnosed EoE cases may have influenced carcinogenesis in the esophagus. Several studies have reported esophageal granular cell tumors in EoE patients56,57; however, such reports were excluded from the current analysis as these lesions are generally benign and of neuroendocrine origin. Nevertheless, the relationship between EoE and granular cell tumors would merit future assessment to determine if there is a true association or if it is incidental.
The strengths of the current study included its rigorous systematic review methods conforming to current guidelines, and its large sample size with nearly 1 million cases of lung cancer evaluated, allowing for evaluation of both smoking and sex differences within the studies. There were limitations in our ability to fully understand allergic inflammation and cancer risk in the skin and esophagus due to lack of studies in this area. There is a great need to further understand the consequences of long-term eosinophilic inflammation in the esophagus. EoE diagnosis is often delayed58 and treatment response rates are low, therefore many patients experience ongoing inflammation despite attempts at therapy59. Recently developed in vitro and ex vivo tissue engineering strategies, such as esophageal organoids, offer the unique ability to culture differentiated esophageal epithelia with eosinophils for prolonged periods60,61. These experiments may provide further better understanding of the mucosal stress responses and the evolution of carcinogenesis in the esophagus.
As allergic disorders increase in incidence, it is important that we consider the long-term effects of allergic inflammation. The current study indicates some weak associations between allergic inflammation (e.g. asthma and eczema) and carcinogenesis within the organ in which they occur; however, heterogeneity and potential for confounding in the available literature preclude firm conclusions on the presence of such associations. This review highlights the need for future prospective cohort studies, with careful consideration for duration of allergic disease, type of inflammation, medication usage, success of treatment and disease activity control, and well-defined outcomes related to malignancy to elucidate the clinical impact of the complex interplay between chronic inflammation and carcinogenesis. Such studies will provide population-based data that will inform our understanding of how allergic inflammation influences carcinogenesis and may have critical implications for cancer prevention and therapy.
Supplementary Material
Acknowledgments
Grant Support
This study was supported by the following NIH Grants: K01DK103953 (KAW), K08DK106444 and R03DK118310 01A1 (ABM), T32 DK007634 (MD), CEGIR (U54 AI117804) (ABM, ESD) is part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including APFED, CURED, and EFC.
Footnotes
Conflict of interest: The authors have nothing to declare
REFERENCES
- 1.Pawankar R Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organ J 2014;7:12–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prescott SL, Pawankar R, Allen KJ, et al. A global survey of changing patterns of food allergy burden in children. World Allergy Organ J 2013;6:21–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol 2004;287:G7–17. [DOI] [PubMed] [Google Scholar]
- 5.Shaheen N, Ransohoff DF. Gastroesophageal reflux, barrett esophagus, and esophageal cancer: scientific review. JAMA 2002;287:1972–1981. [DOI] [PubMed] [Google Scholar]
- 6.Profet M. The function of allergy: immunological defense against toxins. Q Rev Biol 1991;66:23–62. [DOI] [PubMed] [Google Scholar]
- 7.Sherman PW, Holland E, Sherman JS. Allergies: their role in cancer prevention. Q Rev Biol 2008;83:339–362. [DOI] [PubMed] [Google Scholar]
- 8.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549–d549. [DOI] [PubMed] [Google Scholar]
- 11.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duval S, Tweedie R. A Nonparametric “Trim and Fill” Method of Accounting for Publication Bias in Meta-Analysis. Journal of the American Statistical Association 2000;95:89. [Google Scholar]
- 13.Çolak Y, Afzal S, Nordestgaard BG, et al. Characteristics and Prognosis of Never-Smokers and Smokers with Asthma in the Copenhagen General Population Study. A Prospective Cohort Study. Am. J. Respir. Crit. Care Med 2015;192:172–181. [DOI] [PubMed] [Google Scholar]
- 14.Littman AJ, Thornquist MD, White E, et al. Prior lung disease and risk of lung cancer in a large prospective study. Cancer Causes Control 2004;15:819–827. [DOI] [PubMed] [Google Scholar]
- 15.Pirie K, Peto R, Green J, et al. Lung cancer in never smokers in the UK Million Women Study. Int. J. Cancer 2016;139:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabriel R, Dudley BM, Alexander WD. Lung cancer and allergy. Br J Clin Pract 1972;26:202–204. [PubMed] [Google Scholar]
- 17.El-Zein M, Parent M-E, Siemiatycki J, et al. History of allergic diseases and lung cancer risk. Ann. Allergy Asthma Immunol 2014;112:230–236. [DOI] [PubMed] [Google Scholar]
- 18.El-Zein M, Parent M-E, Kâ K, et al. History of asthma or eczema and cancer risk among men: a population-based case-control study in Montreal, Quebec, Canada. Ann. Allergy Asthma Immunol 2010;104:378–384. [DOI] [PubMed] [Google Scholar]
- 19.Koshiol J, Rotunno M, Consonni D, et al. Chronic obstructive pulmonary disease and altered risk of lung cancer in a population-based case-control study. Vij N, ed. PLoS ONE 2009;4:e7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brownson RC, Alavanja MC. Previous lung disease and lung cancer risk among women (United States). Cancer Causes Control 2000;11:853–858. [DOI] [PubMed] [Google Scholar]
- 21.Alavanja MC, Brownson RC, Boice JD, et al. Preexisting lung disease and lung cancer among nonsmoking women. Am. J. Epidemiol 1992;136:623–632. [DOI] [PubMed] [Google Scholar]
- 22.Wu AH, Yu MC, Thomas DC, et al. Personal and family history of lung disease as risk factors for adenocarcinoma of the lung. Cancer Res. 1988;48:7279–7284. [PubMed] [Google Scholar]
- 23.Mayne ST, Buenconsejo J, Janerich DT. Previous lung disease and risk of lung cancer among men and women nonsmokers. Am. J. Epidemiol 1999;149:13–20. [DOI] [PubMed] [Google Scholar]
- 24.Osann KE, Lowery JT, Schell MJ. Small cell lung cancer in women: risk associated with smoking, prior respiratory disease, and occupation. Lung Cancer 2000;28:1–10. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Rothenbacher D, Löw M, et al. Atopic diseases, immunoglobulin E and risk of cancer of the prostate, breast, lung and colorectum. Int. J. Cancer 2006;119:695–701. [DOI] [PubMed] [Google Scholar]
- 26.Liang H, Guan P, Yin Z, et al. Risk of lung cancer following nonmalignant respiratory conditions among nonsmoking women living in Shenyang, Northeast China. J Womens Health (Larchmt) 2009;18:1989–1995. [DOI] [PubMed] [Google Scholar]
- 27.Denholm R, Schüz J, Straif K, et al. Is previous respiratory disease a risk factor for lung cancer? Am. J. Respir. Crit. Care Med 2014;190:549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vesterinen E, Pukkala E, Timonen T, et al. Cancer incidence among 78,000 asthmatic patients. International Journal of Epidemiology 1993;22:976–982. [DOI] [PubMed] [Google Scholar]
- 29.Ji J, Shu X, Li X, et al. Cancer risk in hospitalised asthma patients. Br. J. Cancer 2009;100:829–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boffetta P, Ye W, Boman G, et al. Lung cancer risk in a population-based cohort of patients hospitalized for asthma in Sweden. Eur. Respir. J 2002;19:127–133. [DOI] [PubMed] [Google Scholar]
- 31.Olesen AB, Engholm G, Storm HH, et al. The risk of cancer among patients previously hospitalized for atopic dermatitis. J. Invest. Dermatol 2005;125:445–449. [DOI] [PubMed] [Google Scholar]
- 32.Dyer RK, Weinstock MA, Cohen TSD, et al. Predictors of basal cell carcinoma in high-risk patients in the VATTC (VA Topical Tretinoin Chemoprevention) trial. J. Invest. Dermatol 2012;132:2544–2551. [DOI] [PubMed] [Google Scholar]
- 33.Cheng J, Zens MS, Duell E, et al. History of allergy and atopic dermatitis in relation to squamous cell and Basal cell carcinoma of the skin. Cancer Epidemiol. Biomarkers Prev 2015;24:749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipka S, Keshishian J, Boyce HW, et al. The natural history of steroid-naïve eosinophilic esophagitis in adults treated with endoscopic dilation and proton pump inhibitor therapy over a mean duration of nearly 14 years. Gastrointest. Endosc 2014;80:592–598. [DOI] [PubMed] [Google Scholar]
- 35.Straumann A, Spichtin H-P, Grize L, et al. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology 2003;125:1660–1669. [DOI] [PubMed] [Google Scholar]
- 36.Syed A, Maradey-Romero C, Fass R. The relationship between eosinophilic esophagitis and esophageal cancer. Dis. Esophagus 2017;30:1–5. [DOI] [PubMed] [Google Scholar]
- 37.Vena JE, Bona JR, Byers TE, et al. Allergy-related diseases and cancer: an inverse association. Am. J. Epidemiol 1985;122:66–74. [DOI] [PubMed] [Google Scholar]
- 38.Samet JM, Humble CG, Pathak DR. Personal and family history of respiratory disease and lung cancer risk. Am. Rev. Respir. Dis 1986;134:466–470. [DOI] [PubMed] [Google Scholar]
- 39.Gorlova OY, Zhang Y, Schabath MB, et al. Never smokers and lung cancer risk: a case-control study of epidemiological factors. Int. J. Cancer 2006;118:1798–1804. [DOI] [PubMed] [Google Scholar]
- 40.Wang X-R, Yu ITS, Chiu YL, et al. Previous pulmonary disease and family cancer history increase the risk of lung cancer among Hong Kong women. Cancer Causes Control 2009;20:757–763. [DOI] [PubMed] [Google Scholar]
- 41.Sherrill JD, Kc K, Wu D, et al. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol 2014;7:718–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim W-Y, Chen Y, Ali SM, et al. Polymorphisms in inflammatory pathway genes, host factors and lung cancer risk in Chinese female never-smokers. Carcinogenesis 2011;32:522–529. [DOI] [PubMed] [Google Scholar]
- 43.Brenner AV, Wang Z, Kleinerman RA, et al. Previous pulmonary diseases and risk of lung cancer in Gansu Province, China. International Journal of Epidemiology 2001;30:118–124. [DOI] [PubMed] [Google Scholar]
- 44.Rosenberger A, Bickeböller H, McCormack V, et al. Asthma and lung cancer risk: a systematic investigation by the International Lung Cancer Consortium. Carcinogenesis 2012;33:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin. Gastroenterol. Hepatol 2012;10:639–645. [DOI] [PubMed] [Google Scholar]
- 46.Castellsague J, Kuiper JG, Pottegård A, et al. A cohort study on the risk of lymphoma and skin cancer in users of topical tacrolimus, pimecrolimus, and corticosteroids (Joint European Longitudinal Lymphoma and Skin Cancer Evaluation - JOELLE study). Clin Epidemiol 2018;10:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hui RL, Lide W, Chan J, et al. Association between exposure to topical tacrolimus or pimecrolimus and cancers. Ann Pharmacother 2009;43:1956–1963. [DOI] [PubMed] [Google Scholar]
- 48.Arellano FM, Arana A, Wentworth CE, et al. Lymphoma among patients with atopic dermatitis and/or treated with topical immunosuppressants in the United Kingdom. J. Allergy Clin. Immunol 2009;123:1111–6– 116.e1–13. [DOI] [PubMed] [Google Scholar]
- 49.Johnston A, Smith C, Zheng C, et al. Influence of prolonged treatment with omalizumab on the development of solid epithelial cancer in patients with atopic asthma and chronic idiopathic urticaria: A systematic review and meta-analysis. Clin. Exp. Allergy 2019;27:66. [DOI] [PubMed] [Google Scholar]
- 50.Burns SO, Plagnol V, Gutierrez BM, et al. Immunodeficiency and disseminated mycobacterial infection associated with homozygous nonsense mutation of IKKβ. J. Allergy Clin. Immunol 2014;134:215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jensen ET, Kappelman MD, Martin CF, et al. Health-care utilization, costs, and the burden of disease related to eosinophilic esophagitis in the United States. Am. J. Gastroenterol 2015;110:626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muir A, Moore H, Spergel J. To treat or not to treat: The minimally symptomatic EoE patient. Ann. Allergy Asthma Immunol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishibashi S, Ohashi Y, Suzuki T, et al. Tumor-associated tissue eosinophilia in human esophageal squamous cell carcinoma. Anticancer Res. 2006;26:1419–1424. [PubMed] [Google Scholar]
- 54.Ohashi Y, Ishibashi S, Suzuki T, et al. Significance of tumor associated tissue eosinophilia and other inflammatory cell infiltrate in early esophageal squamous cell carcinoma. Anticancer Res. 2000;20:3025–3030. [PubMed] [Google Scholar]
- 55.Rustgi A, El-Serag HB. Esophageal carcinoma. N. Engl. J. Med 2015;372:1472–1473. [DOI] [PubMed] [Google Scholar]
- 56.Nojkov B, Amin M, Ghaith G, et al. A Statistically Significant Association Between Esophageal Granular Cell Tumors and Eosinophilic Esophagitis: A 16-year Analysis at Two Large Hospitals of 167,434 EGDs. Digestive Diseases and Sciences 2017;62:3517–3524. [DOI] [PubMed] [Google Scholar]
- 57.Lucendo AJ, De Rezende L, Martín-Plaza J, et al. Esophageal granular cell tumor and eosinophilic esophagitis: two interesting entities identified in the same patient. Case Rep Gastroenterol 2008;2:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 2013;145:1230–6.e1–2. [DOI] [PubMed] [Google Scholar]
- 59.Dellon ES. Management of refractory eosinophilic oesophagitis. Nat Rev Gastroenterol Hepatol 2017;14:479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kasagi Y, Chandramouleeswaran PM, Whelan KA, et al. The Esophageal Organoid System Reveals Functional Interplay Between Notch and Cytokines in Reactive Epithelial Changes. Cell Mol Gastroenterol Hepatol 2018;5:333–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whelan KA, Muir AB, Nakagawa H. Esophageal 3D Culture Systems as Modeling Tools in Esophageal Epithelial Pathobiology and Personalized Medicine. Cell Mol Gastroenterol Hepatol 2018;5:461–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.