Abstract
Objectives
Sociodemographic disparities in the incidence and mortality of HPV-associated conditions have been well-documented in the pre-HPV vaccine era. It is still unknown if the introduction of routine vaccination has been effective in reducing these pre-vaccine era inequalities. The purpose of this review was to determine the utilization of sociodemographic variables to assess for disparities in population-level HPV vaccine impact research and to evaluate the current evidence for disparities in the reduction of HPV-associated conditions post-vaccine introduction in the United States.
Study Design
A systematic review of the literature from January 2007 through March 2018 was carried out to identify studies evaluating the impact HPV vaccines have had on the rates of HPV infection, genital warts, and cervical dysplasia (cervical intraepithelial neoplasia grades 1+) in the US. An in-depth review was then performed to synthesize these data and to assess the way prior studies have reported and evaluated for potential disparities in the vaccine’s impact within various racial, ethnic, and/or socioeconomic subgroups of the population.
Methods
Vaccine impact studies measure the change in the population-level burden of disease pre- versus post-licensure of the vaccine. We systematically searched Pubmed/Medline and Embase, combining search terms related to the HPV vaccine, sentinel surveillance, and HPV-associated conditions. Eligible studies were those with population-level, post-vaccine introduction data that were conducted in the United States. Finally, a cited reference search was conducted for all included articles using Web of Science platform that accesses three major citation indexes: Science Citation Index, Social Sciences Citation Index, and Arts and Humanities Citation Index. This allowed us to not only screen the articles that were cited by our final collection of studies, but also the articles that used our selected studies as one of their references. The study protocol is registered in PROSPERO (#CRD42018107579).
Results
Overall, 23 of the 4,139 references retrieved assessed the population-level impact of HPV vaccines between 1/1/2007 – 3/29/2018. Among these, 13 (57%) reported sociodemographic data. Only two articles reported stratified results by sociodemographic factors, thereby allowing assessment for potential disparate impact. One of these studies described differences in the impact of the vaccine by race, ethnicity, and income.
Conclusion
Though approximately half of the studies that assessed the impact of the HPV vaccine measured sociodemographic characteristics, few presented results in a way that allowed for the identification of potential differences in impact between the relevant subgroups of the population. Determining to what extent, if any, vaccines are reducing known sociodemographic disparities is an important public health priority and an essential step to developing immunization strategies that are beneficial for all.
Keywords: HPV, Vaccine, Impact, Health disparities
Introduction
Human papillomavirus (HPV) is the most common sexually transmitted infection in the United States (US).1, 2 HPV causes virtually all cases of cervical cancer. Each year in the US, more than 12,000 new cases of cervical cancer are diagnosed, and over 4,000 women die of the disease.3 The clinical burden of HPV extends beyond cervical cancer. Anogenital warts and cervical dysplasia are two more common clinical manifestations of genital HPV infection and pose a substantial economic burden to patients and the healthcare system.4
Three highly efficacious vaccines have been licensed in the US since 2006 that protect against the most common oncogenic HPV types.5–7 It has been estimated that more than half of all high-grade cervical dysplasia and > 90% of anogenital warts could be prevented with the HPV vaccines.8, 9 The real-world benefits of HPV vaccines, however, may not mirror the degree of benefit estimated from pre-licensure efficacy trials. Vaccine impact studies measure the population-level performance of the vaccine post-licensure.10 Monitoring for changes in rates of HPV-associated conditions over time is important to determine whether the vaccine is achieving its fullest potential and reducing the population-level burden of HPV-related conditions.
Sociodemographic disparities in HPV-associated conditions during the pre-vaccine era have been well documented. Individuals of racial and ethnic minority groups, and individuals of lower socioeconomic status (SES), are more likely to be infected with HPV and to die from an HPV-associated cancer.3,11 Eliminating these health disparities requires strategies and public health efforts that are informed by high-quality data.12 Vaccine impact studies primarily examine the effect of uptake of the vaccine on the overall burden of vaccine-preventable diseases in that population. However, focusing solely on the correlation between vaccine uptake and reduction of overall burden of disease may fail to account for the multifaceted determinants of impact. Factors unrelated to uptake of a vaccine may also affect its real-world impact. For example, the introduction of a vaccine in one subgroup of the population may alter the dynamics of transmission of disease to other subgroups and may worsen the disparities in health outcomes. Thus, after implementation of a new vaccine in the population, it is important to monitor not only the trends of disease in the overall population, but also the trends within relevant subgroups of the population to assess its effect on disparities in health outcomes.
Healthcare systems and academic research often collect demographic data, yet these data are not always utilized to optimally assess for disparities in impact.13–15 This systematic review, therefore, aims to evaluate the extent to which sociodemographic characteristics are being examined in population-based HPV vaccine research and to evaluate the data for potential disparities in the impact of the vaccine.
Methods
Information sources
We conducted a systematic review according to PRISMA guidelines.16 Pubmed, Medline, and Embase were searched using a combination of Medical Subject Heading terms and keywords related to the vaccine (e.g.”HPV vaccine”, or “Gardasil”), study type (e.g. “impact”, or “sentinel surveillance”), and outcomes (e.g. “HPV infection”, “genital warts”, or “cervical intraepithelial neoplasia”). See supplemental tables S1, S2, and S3 for the complete search strategy.
Eligibility criteria
The HPV vaccine was first introduced in the United States in 2006. Therefore, this search was limited to articles published after January 1, 2007. To be eligible, studies had to have been published and peer-reviewed. Data had to have been reported, at the population-level, as trends of disease over time after the vaccine was introduced. The impact of HPV vaccination on the rate of cancer is unlikely to be observable during this study period due to the lag between incident infection and diagnosis of cancer.17–19 Consequently, eligible studies were those that reported the HPV vaccine’s impact as it pertains to the reduction in the rates of earlier HPV-related outcomes: HPV infection, genital warts, and cervical dysplasia. Cervical dysplasia was defined as cervical intraepithelial neoplasia (CIN) grades 1, 2, or 3 or adenocarcinoma in situ (AIS). This review focused on the racial, ethnic, and socioeconomic disparities in the impact of an HPV vaccine in the US. Therefore, studies from outside the US were excluded. Eligibility criteria were applied sequentially in a review of titles, abstracts, and remaining full papers. Finally, a cited reference search was conducted for all included articles using Web of Science platform that accesses three major citation indexes: Science Citation Index, Social Sciences Citation Index, and Arts and Humanities Citation Index. This allowed us to not only screen the articles that were cited by our final collection of studies, but also the articles that used our selected studies as one of their references.
Data Collection and Analysis
Characteristics of eligible articles were extracted using a standardized form including geographic region, size of the study population, outcome(s) measured, a summary of results, and statistical methods for analyzing sociodemographic variables if these variables were collected. Findings of studies that modeled for interactions or stratified the trends of disease by race, ethnicity or SES were synthesized separately in a second standardized form, which included a summary of the stratified data and whether a disparity was observed. The quality of the studies that modeled for interactions or stratified their outcome-trends by sociodemographic characteristics were separately assessed by two authors (L.A.S. and C.R.O) using a modified version of the PRISMA criteria that addressed three main areas: selection bias, information bias, and external validity.20
This review is registered in Prospero (number CRD42018107579), an international database of ongoing systematic reviews in healthcare. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or in the writing of the manuscript. The corresponding author had full access to all the data in this review and had final responsibility for the decision to submit for publication.
Results
Our search criteria yielded 4,139 potentially eligible articles, 23 of which met inclusion criteria and were reviewed in-full (Figure 1).21–43 Of these, 11 described the impact of HPV vaccine on the trends of HPV infection,21–31 5 on the trends of genital warts,32–36 and 7 on the trends of cervical dysplasia.37–43 Among those that evaluated for impact on cervical dysplasia, 1 assessed all grades of cervical dysplasia (CIN 1 or greater),36 4 assessed only high-grade lesions (CIN 2 or greater),37–39, 41 1 assessed for abnormalities pap smear results (high grade squamous epithelial lesions) in addition to CIN 2+,40 and 1 assessed for impact on only CIN 3 and AIS.42
Figure 1. Flowchart of study selection.
Search results were screened for eligibility by title, abstract, and finally by in-depth review of full papers.
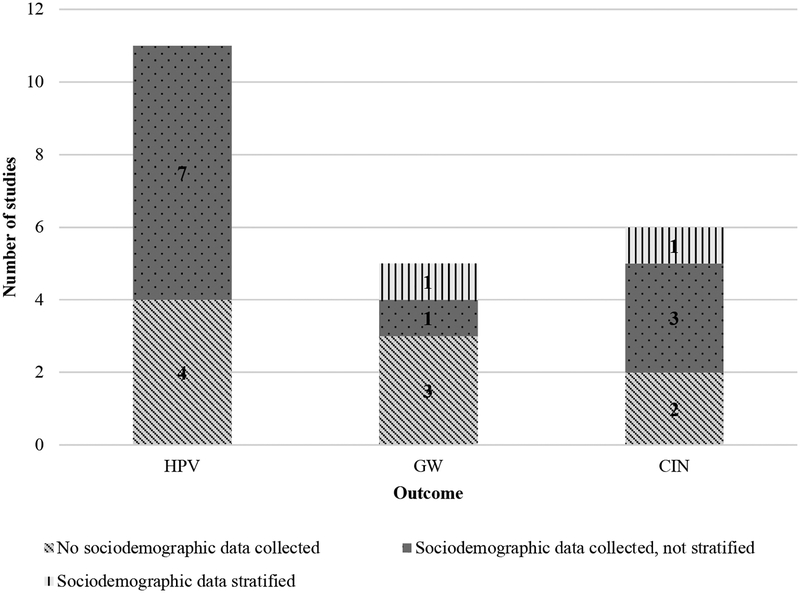
Of the 23 articles included in this review, 43% (n=10) did not mention sociodemographic variables (Table 1).24, 25, 27, 32–34, 36, 37, 41, 42 The remaining 57% (n=13) reported some information on sociodemographic variables (13 included race/ethnicity and 4 included SES measures).20,28,34,37 Of the 13 articles that mentioned race/ethnicity, ten described how this information was determined (six by self-report,21, 23, 28–31 and four by investigators).35, 38–40 Beyond describing the sample population, seven studies that collected and reported sociodemographic data assessed whether there was an association between these sociodemographic variables and the outcome of interest (Figure 2). Most of those (5/7; 71%) did so by using a multivariable statistical model that included the sociodemographic variable as an adjustment factor. In the studies that only adjusted for sociodemographic characteristics the differences between the unadjusted and the adjusted results were not substantially different. Only two articles performed subgroup analyses to specifically assess whether the effect of vaccine introduction on the rates of disease differed across the various sociodemographic groups (i.e., they either evaluated interactions in the multivariable model or they performed stratified analyses.)
Table 1. Characteristics of all articles meeting inclusion criteria.
Eligible studies were read in full, relevant characteristics were extracted, and results were summarized.
| Reference | Location Study Period | Study Population Sample Size | Outcome | Findings | Race, Ethnicity, or SES Data Reported | Statistical Analysis of Demographic Data |
|---|---|---|---|---|---|---|
| Kahn Pediatrics 2012 | Ohio 2006–2010 |
Women aged 13–26 with evidence of sexual activity N=793 |
Cervicovaginal HPV infection |
Overall: VT HPV prevalence rate decreased (31⋅7%–13⋅4%, p=0⋅0001) Vaccinated: VT HPV decreased (31⋅8%–9⋅ 9%, p=0⋅0001), NVT HPV increased (60⋅7%–75⋅9%, p =0⋅0001) Unvaccinated: VT HPV decreased (30⋅2%–15⋅4%, p=0⋅0001) |
Yes | Description of the overall rates of disease by demographic variables. Assessed for overall trends in outcome over time but did not stratify or model with demographic variables. |
| MSMR 2013 | USA 2000–2012 |
All active component service members who served during the study period N=304,021 |
HPV infection |
Overall: incidence rate reached a low of 159⋅1 in 2012 Men: incidence rates declined, from 173.6 in 2000 to 135⋅9 in 2010 Women: rates rose to a peak of 481⋅2 in 2007 but declined thereafter by 42% to 279⋅5 in 2012, fall associated with dramatic declines in the rates for women in the youngest age groups after peaking in 2007 [p-values not provided] |
Yes | Description of the overall rates of disease by demographic variables. Assessed for overall trends in outcome over time but did not stratify or model with demographic variables. |
| Markowitz JID 2013 | USA 2003–2010 |
Women aged 14–59 N=8,403 |
HPV infection |
Age 14–19 years: among women, VT HPV prevalence decreased from 11⋅5% (95% CI: 9⋅2–14⋅4) in 2003–2006 to 5⋅1% (95% CI: 3⋅8–6⋅6) in 2007–2010, a decline of 56% (95% CI: 38–69) [p-values not given] Other age groups: for both men and women the prevalence did not differ significantly between the two time periods (p>0⋅05) |
Yes | Vaccine coverage stratified by race/ethnicity. Assessed for overall trends in HPV infections over time. Overall trends were adjusted for race/ethnicity. Did not model for interactions or stratify trends with demographic variables. |
| Wilson OJPHI 2014 | USA 2004–2013 |
Women of all ages N=735,437 |
HR HPV infection |
Overall: decrease from 2004 to 2013, unadjusted positivity rate of 27⋅2% Women aged 14–19 years: highest rates occurred Higher age groups: showed lesser decline than did younger age groups following vaccine introduction Age 20–24 and 25–29 years: men and women showed a significantly different downward disease trend between pre⋅ and post-vaccine periods (−0⋅1% to −1⋅5%, and +0⋅4% to −1⋅5%, respectively) All other age groups: decrease in rates of change, a slower rate of decline over time [p-values not provided] |
No | N/A |
| Dickson EI 2015 | New Hampshire, Iowa, California 2004–2011 |
Women aged 21–65 N=220,914 |
HPV infection |
Overall: rates decreased, while the number of HPV tests increased Younger women: greater decrease in infection rates than in older women Older women: rates were consistent [p-values not provided] |
No | N/A |
| Dunne JID 2015 | Oregon, Washington 2007, 2012–2013 |
Women aged 20–29 N=8,309 |
VT HPV infection |
Overall: prevalence decreased from 10⋅6% in 2007 to 6⋅2% in 2012–2013 (p<0⋅001) <19 years: among vaccinated (≥1 dose) in 2012–2013, prevalence significantly lower (APR: 0⋅1; 95% CI: 0⋅1–0⋅3) than among unvaccinated [p-values not provided] |
Yes | Descriptive characteristics of the overall study population by race/ethnicity. Assessed for overall trends in HPV infections over time. Overall trends were modeled and adjusted for race/ethnicity. Did not stratify trends or model interactions in trends using demographic variables. |
| Berenson JID 2016 | USA 2009–2012 |
Women aged 18–59 N=1,952 |
VT HPV infection |
Age 18–26 years: prevalence decreased from 15⋅4% in 2009–2010 to 8⋅5% in 2011–2012 (PR: 0⋅51; 95% CI: 0⋅28–0⋅92) All ages: HR VT HPV decreased from 13⋅1% to 6⋅5% (0.46; 0⋅25–0⋅86) [p-values not provided] |
No | N/A |
| Markowitz Pediatrics 2016 | USA 2003–2012 |
Women aged 14–34 N=2,099 |
VT HPV infection |
Age 14–19 years: prevalence declined from 11⋅5% to 4⋅3% (APR: 0⋅36, 95% CI: 0⋅21–0⋅61) Aged 20–24 years: decrease from 18⋅5% to 12⋅1% (APR: 0⋅66, 95% CI: 0⋅47–0⋅93) Older age groups: no decrease seen Aged 14–24 years: prevalence in sexually active women lower in vaccinated (≥1 dose) compared with unvaccinated females: 2⋅1% vs 16⋅9% (APR: 0⋅11, 95% CI: 0⋅05–0⋅24) [p-values not provided] |
Yes | Assessed for overall trends in HPV infections over time. Overall trends were adjusted for race/ethnicity. Did not model interactions or stratify trends with demographic variables. |
| Tarney OG 2016 | USA 2003–2012 |
Women aged 18–29 N=2,309 |
HPV 16/18 infection |
Vaccinated (≥1 dose): prevalence of VT HPV declined (p=0⋅003): 10⋅1% (95% CI: 7⋅1–13⋅8%) in the pre-vaccine era to 4⋅ 2% (95% CI: 3⋅3–10⋅ 9%) in post-vaccine, no change in prevalence of NVT HPV Unvaccinated: no change in prevalence of VT HPV among from the pre-vaccine era 10⋅1% (95% CI: 7⋅1–13⋅8%) to 8⋅ 8% (95% CI: 5⋅6–12⋅9%) in the post-vaccine era (p=0⋅4) |
Yes | Descriptive demographics of the study population. Assessed for overall trends in outcome over time Did not stratify or model with demographic variables. |
| Berenson OG 2017 | USA 2009–2014 |
Women aged 18–59 N=2,244 |
Vaginal HPV Infection |
Age 18–59 years: overall decrease in prevalence of VT HPV from 2009–2010 to 2013–2014, only significant in age 18–26 years Vaccinated age 18–26 years: prevalence declined from 2009–2010 (39%) to 2013–2014 (2⋅ 0%; PR: 0⋅51, 95% CI: 0⋅18–1⋅46) Unvaccinated age 18–26 years: decrease from 19⋅5% in 2009–2010 to 9⋅7% in 2013–2014 (PR: 0⋅44, 95% CI: 0⋅22–0⋅91) Unvaccinated age 26+ years: no change observed [p-values not provided] |
Yes | Assessed for overall trends in HPV infections over time. Overall trends were adjusted for race/ethnicity. Did not model interactions or stratify trends with demographic variables. |
| Oliver JID 2017 | USA 2003–2014 |
Women aged 14–34 N=6,686 |
VT HPV infection |
Age 14–19 years: prevalence decreased from 11⋅5% (95% CI: 9⋅1%–14⋅4%) in 2003–2006 to 3⋅3% (95% CI: 1⋅9%–5⋅8%) in 2011–2014 Age 20–24 years: prevalence decreased from 18⋅ 5% (95% CI: 14⋅ 9%–22⋅ 8%) in 2003–2006 to 7⋅2% (95% CI: 4⋅7%–11⋅1%) in 2011⋅2014 Age 14–24 years: From 2003–2006 to 2011–2014, prevalence in sexually active women decreased 89% in vaccinated and 34% in unvaccinated Trends of non⋅vaccine type HPV infections: decreases were noted among 14- to 19-year-olds in 2011–2014, compared with 2003–2006, but these were attenuated when adjusted for race/ethnicity, poverty index, and number of lifetime partners.[p-values not provided] |
Yes | Vaccine coverage stratified by race/ethnicity. Assessed for overall trends in HPV infections over time. Overall trends were adjusted for race/ethnicity and poverty index. Did not model interactions or stratify trends with demographic variables. |
| Bauer AJPH 2012 | California 2007–2010 |
Men and women of all ages N=8,053,738 |
Genital warts |
Age <21 years: women: 35% decrease from 0⋅94% to 0⋅61% (p<0⋅001), men: 19% decrease Age 21–25 years: women: 10% decrease, men: 11% decrease Older age groups: incidence stabilized or increased [p-values not provided] |
No | N/A |
| Flagg AJPH 2013 | USA 2003–2010 |
Men and women aged 10–39 N=13,000,000 |
Anogenital warts |
Age 15–19 years: (women and men) prevalence increased in 2003–2006, then declined (p<0⋅ 05) in 2007–2010 Age 20–24 years: increase in men and women in 2003–2007, remained stable through 2009, and declined in 2010 among women Age 25–39 years: increased through 2009 (men and women) but not in 2010 for women Ages 15–39 years: among men prevalence for each 5-year age group increased in 2003–2009, no changes observed for 2010 [p-values not provided for age groups 20–39 years] |
No | N/A |
| Nsouli-Maktabi MSMR 2013 | USA 2000–2012 |
All individuals serving in the active component of the U.S. Armed Forces N>1,400,000 |
Genital warts |
HPV4 vaccine-eligible age range: incidence rates declined among females from 2007 to 2010 Age 25+ years: for women annual rates of GW diagnoses remained low and stable from 2000 through 2010 Men of all age groups annual rates of GW diagnoses remained low and stable from 2000 through 2010 [p-values not provided] |
No | N/A |
| Perkins STD 2015 | Massachusetts 2004–2013 |
Men and women aged 16–26 N=45,787 |
Genital warts |
Women: rates decreased from 3⋅5% to 1⋅5% (p<0⋅05) Men: rates decreased from 3⋅6% to 2⋅9% (p<0⋅05), began to decrease after the introduction of female vaccination and continued to decrease after male vaccination was introduced |
Yes | Trends in outcome over time stratified by demographics |
| Flagg AJPH 2018 | USA 2006–2014 |
Men and women aged 15–39 N=35,000,000 |
Anogenital warts |
Women Age 15–19 years: prevalence decreased in 2008–2014 (APC: −14⋅1%; p<0⋅001) Age 20–24 years: decrease in 2009–2014 (APC: − 12⋅9%; p<0⋅001) Age 25–29 years: decrease from 2009–2014 (APC: −6⋅0%; p=0⋅001) Men Age 20–24 years: significant declines seen (APC: −6⋅5%; p=0⋅005) All other sex and age groups: prevalence increased or remained stable |
No | N/A |
| Benard JAMA Oncol 2017 |
New Mexico Period not reported |
Women aged 15–29 N=20,639 |
CIN1, CIN2+ |
Age 15–19 years: reductions in incidence significant for all grades of CIN: From 3468⋅ 3 to 1590⋅6 for CIN1 (APC: −9⋅0, 95% CI: −12⋅0 to ⋅5⋅8, p<0⋅001), from 896⋅4 to 414⋅9 for CIN2 (APC: −10⋅5, 95% CI: −18⋅8 to −1⋅2, p=0⋅03), from 240⋅2 to 0 for CIN3 (APC: −41⋅3, 95% CI: −65⋅7 to 0⋅3, p=0⋅05) Age 20–24 years: reductions in the CIN2 incidence significant from 1027⋅ 7 to 627⋅1 (APC: −6⋅ 3, 95% CI: −10⋅9 to −1⋅4, p=0⋅02) |
No | N/A |
| Niccolai CEBP 2013 | Connecticut 2008–2011 |
Women aged 21–39 N=8,435 |
CIN2+ |
Overall: annual rate per 100,000 declined by 36 from 2008–2011 (p=0⋅002) Age 25–39 years: no significant declines [p-values not provided] |
Yes | Trends in outcome over time stratified by demographics |
| Hariri Cancer 2015 | California, Connecticut, New York, Oregon 2008 2012 |
Women aged ≥18 N=9,119 |
CIN2+ |
Age 18–20 years: from 2008 to 2012, the incidence per 100,000 women significantly decreased [p-values not provided] Age 21–29-years: decrease in Connecticut (from 762 to 589), decrease in New York (from 770 to 465; p<0⋅001) Ages 30–39 years: rates did not change [p-values not provided] |
Yes | Description of the overall rates of disease by demographic variables. Assessed for overall trends in outcome over time but did not stratify or model with demographic variables. |
| Hariri Vaccine 2015 | California, Connecticut, New York, Oregon 2008–2012 |
Women aged ≥18 N=4,678 |
CIN2+ |
Vaccinated (≥1 dose): from 2008 to 2012, prevalence of HPV 16/18 in CIN2+ lesions decreased from 53⋅6% to 28⋅4% (p<0⋅001) Unvaccinated: no significant decrease (57⋅1% vs 52⋅5%, p=0⋅08) Unknown vaccination status: no significant decrease (55⋅0% vs 50⋅5%, p=0⋅71) |
Yes | Descriptive demographics of the immunized population. Assessed for overall trends in outcome over time but did not stratify or model with demographic variables. |
| Flagg AJPH 2016 | USA 2007–2014 |
Women aged 15–39 N=9,000,000 |
HSIL and CIN2+ |
Age 15–19 years: prevalence of HSIL and CIN2+ decreased, APC during 2007⋅2014 for HSIL and CIN2+ was −8⋅3% and −14⋅4%, respectively (p<0⋅001 for both) Age 20–24 years: prevalence of HSIL and CIN2+ also decreased significantly [p-values not provided] Aged 25–39 years: no decreases seen [p-values not provided] |
No | N/A |
| Niccolai CID 2017 | Connecticut 2008–2015 |
Women aged 21–39 N=15,218 |
CIN2+ |
Age 21 years: significant decline in CIN2+ seen in 2010 Aged 22–26 years: significant decline in 2011–2012 Aged 21–26 years: in 2008–2015, rates declined by 30%–74%, with greater declines observed in younger women Birth cohorts between 1985–1994: all experienced significant declines ranging from 25% to 82% [p-values not provided] |
No | N/A |
| Watson PM 2017 | Michigan 2009–2012 | Women aged 15–85 N=21,770 |
CIN III/AIS |
Overall rates Kentucky reported highest rate (69⋅ 8), followed by Michigan (55⋅4), Louisiana (42⋅ 3), and Los Angeles (19⋅2) White women had the highest rates (41⋅6), followed by black women (37⋅ 5). Michigan trends Age 15–19 years: rates declined by 37% each year Age 20–24 years: rates declined by 14% each year Age 25–29 years: rates declined by 7% each year [p-values not provided] |
Yes | Description of the overall rates of disease by demographic variablesvariables. Assessed for overall trends in disease over time but did not stratify or model trends with demographic variables. |
Abbreviations: PR=prevalence ratio, CI=confidence interval, VT=vaccine type (16, 18, 6, 11), NVT = non-vaccine type, HR HPV= high risk (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68), LR HPV= low risk (types 6, 11, 40, 42, 43, 44, 53, 54, 61, 72, 73, 81), APC=annual percent change, APR=adjusted prevalence ratio, CIN1=low grade cervical intraepithelial neoplasia, CIN2+=high grade cervical intraepithelial neoplasia, HSIL= high grade squamous intraepithelial lesion, AIS=cervical adenocarcinoma in situ
Figure 2. Sociodemographic data collection by included studies.
23 studies were analyzed in-depth and were assessed for how they reported and analyzed race/ethnicity/SES data. Most of eligible studies examined HPV infection, none of which stratified time-trends by sociodemographic variables.
Abbreviations: GW=genital warts, CIN=cervical intraepithelial neoplasia
Only two articles performed subgroup analysis to specifically examine if the effect of vaccine introduction on the rates of disease differed across the various sociodemographic groups (i.e., performed a formal evaluation of interactions or stratified analyses.)35, 38 Perkins and colleagues evaluated for changes in diagnoses of genital warts post-vaccine introduction. This study observed a steady increase in rates of genital warts in both males and females during the pre-vaccine period, followed by sharp declines in the post-vaccine period with no statistically significant difference between the various racial and ethnic subgroups (Table 2). Niccolai and colleagues stratified the rates of cervical dysplasia over time by sociodemographic variables. They observed that the counties with a lower proportion of racial and ethnic minority group residents (<5% Black or <5% Hispanic) experienced a significant decline in the incidence of cervical dysplasia over time. However, this decline was not observed in the counties with a higher proportion of minority groups residents (≥20% Black or ≥20% Hispanic) (Table 2). A similar disparity was seen in income level; where significant declines in the incidence of cervical dysplasia were only observed in the wealthier communities. The overall quality of both studies was medium, with a low risk of selection bias, a medium risk of information bias, and a medium external validity score (Table S4).
Table 2. Characteristics and findings of articles that stratify data by race/ethnicity and SES.
Two studies examined their time-trend data by race, ethnicity, and SES were analyzed in-depth by two authors. Results regarding rates over time within these groups were summarized.
| Reference | Location Study period |
Study population Sample size |
Outcome | Disparity observed | Findings | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Perkins STD 2015 | Massachusetts 2004–2013 |
Men and women aged 16–26 N=45,787 |
Genital warts | No | Cases per 100 patients per period (males and females) | ||||||
| 2004–2006 | 2007–2010 | 2011–2013 | |||||||||
| F | M | F | M | F | M | ||||||
| Total | 5·1 | 4·6 | 5·1 | 5·3 | 2·4 | 3·5 | |||||
| White | 6·9 | 8·3 | 6·2 | 7·5 | 3·5 | 6·5 | |||||
| Black | 4·8 | 3·2 | 5·0 | 4·2 | 1·7 | 2·7 | |||||
| Hispanic | 4·8 | 5·0 | 6·5 | 6·1 | 1·0 | 4·0 | |||||
| Asian | 3·4 | 5·4 | 3·2 | 3·6 | 1·3 | 1·8 | |||||
| Other | 4·3 | 3·5 | 3·5 | 6·4 | 1·3 | 3·8 | |||||
| APC(%) | +9·1* | +7·7 | −9·8* | −0·4 | −22·1* | −13·5* | |||||
| Niccolai CEBP 2013 | Connecticut 2008–2011 |
Women aged 21–39 N=8,146 |
CIN2+ | Yes | Cases per 100,000 women per year | ||||||
| 2008 | 2011 | Δ (95% CI) | p-value | ||||||||
| Total | 512 | 476 | −36 (−66 to −5) | 0·002 | |||||||
| <5% Black | 497 | 455 | −42 (−86−2) | 0·006 | |||||||
| <5% Hispanic | 499 | 451 | −48 (−100−4) | 0·002 | |||||||
| <5% in poverty | 510 | 451 | −58 (−106 to −11) | 0·003 | |||||||
| ≥20%Black | 558 | 512 | −45 (−113−23) | 0·283 | |||||||
| ≥20% Hispanic | 525 | 507 | −17 (−74−58) | 0·477 | |||||||
| ≥20% in poverty | 550 | 501 | −49 (−125−27) | 0·267 | |||||||
p<0.05
Discussion
Numerous studies have demonstrated that during the pre-vaccine era, the burden of HPV-associated conditions was disproportionally high among individuals of low-income and of racial and ethnic minority groups.44–46 Although HPV vaccines have the potential to reduce disparities in the incidence of HPV-related conditions, the complex epidemiology of this sexually transmitted infection may also lead to a scenario in which health disparities are worsened after the vaccine is introduced.47 For example, several studies have shown that sexual mixing patterns differ considerably between the various racial, ethnic, and SES subgroups in the US. Black Americans, for example, are more likely than Whites to have their first sexual encounter before 15 years of age and also are more likely to have more than one HPV infection during adolescence.48 Thus, even if high immunization coverage is achieved in all groups, if the first dose of HPV vaccine is usually given to adolescents after 15 years of age, the vaccine may have less of an impact in Black than in White adolescents, and disparities may increase.
Earlier systematic reviews have compiled evidence on the impact of HPV vaccine on HPV-associated conditions (HPV infections, anogenital warts, and cervical cancer).20, 49,17, 47, 50 However, none specifically reviewed the evidence for sociodemographic disparities in the impact of the vaccine on these outcomes. This is the first systematic review that examines potential disparities in the impact of the vaccine on the most common HPV-associated diseases. This study identified 23 peer-reviewed HPV vaccine impact studies from the US, all of which described robust reductions in the burden of HPV-associated conditions post-vaccine introduction. However, few of these studies examined for changes in trends within various racial, ethnic, or SES subgroups, leaving the question of persistent disparities largely unanswered.
This review found that nearly half of the published articles did not include any sociodemographic data in their reports. It is important to acknowledge that collecting high-quality data on race, ethnicity or SES can be challenging. How individuals identify themselves is a complex matter and can even change over time. Socioeconomic status, in particular, can be defined in multiple ways, including but not limited to, percent below the federal poverty line, annual income, or type of insurance. It is also worth noting that the effect of SES on health outcomes is not dichotomous (i.e., not just above or below an arbitrary income level), rather it is a gradient that can have varying magnitudes of effect at any given SES.51 How investigators define these important variables may substantially change how the results are interpreted. Therefore, in future research on HPV vaccine, priority should be placed on deliberate, precise, and systematic collection of sociodemographic data – including data about race, ethnicity, and SES.
While collecting high-quality sociodemographic data is important, it is equally important to analyze these data in a way that allows for an assessment of whether the magnitude of the vaccine’s impact differs across specific subgroups of the population. This review found that among the few studies that reported race/ethnicity or SES data, most utilized these variables either to present baseline characteristics of study populations or as an adjustment factor in a multivariable statistical model without presenting the association. Including sociodemographic variables as an adjustment factor in a statistical model without modeling an interaction can be useful if these factors are confounders, controlling for which permits the investigators to isolate the association between the timing of the introduction of the vaccine and the incidence of disease over time. To answer questions about disparities in the vaccine’s impact – such as whether women of low-income on average derive less benefit from the immunization program than do women of high-income – investigators need to assess how the joint association of the factors (i.e., timing of the introduction of the vaccine and income) jointly modify the incidence of disease over time. These questions can be assessed better by performing analyses of subgroups either by using stratified analyses or by incorporating interaction terms in the statistical models.52 However, very few studies in this review either stratified their analyses by sociodemographic characteristics or performed a formal evaluation of interaction.
This review identified two important vaccine impact studies, one of genital warts and another of cervical dysplasia that performed these stratified analyses. Perkins and colleagues found marked declines in trends of genital warts diagnoses and found that although the initial incidence was disparate between various racial and ethnic groups, there was no disparity seen in the rates of decline over time.35 Though these results are promising for equal impact, the study population was relatively homogeneous and thus may have had limited power to detect small differences between groups. This review also identified one article by Niccolai and colleagues that reported on the disparities in trends of cervical dysplasia over time. This study demonstrated that declines were lower in populations with higher proportions of residents living in poverty and of racial/ethnic minorities compared to those of higher income and a smaller proportion of minority women.38 It is unclear why these differences were seen in rates of cervical dysplasia but were not seen in genital warts. According to the most recent reports, HPV immunization rates among minority, and low SES individuals are not significantly lower than among white individuals of high SES.48 Some studies indicate that racial/ethnic and socioeconomic differences in HPV type distribution, unequal screening rates, or unequal linkage to care may be influencing the disparate outcomes, though consensus has not been established.16, 49–51The epidemiology of HPV-related conditions is complex. Various population-level factors (e.g., the prevalence of high-risk HPV types, sexual mixing patterns in the community, inequity in implementation of healthcare interventions) and individual-level factors (e.g., sexual behaviors, access to screening and treatment options) can directly influence an individual’s risk of developing an HPV-related condition. In a longitudinal study across several states in the US, Jemal and colleagues show that in areas where there are higher numbers of individuals living below the poverty line, the rate of sexual activity is often higher, the utilization of cervical cancer screening programs is often lower, and as a consequence, the rates of cervical cancer and precancers are higher.45 Future work in this area is needed to deepen our understanding of these differences.
Vaccines are one of the key preventative strategies that are used to promote population health and reduce health disparities. Often, racial/ethnic minorities and low-income individuals bear the heaviest burden of infectious diseases and these health disparities could, in theory, be alleviated by robust vaccination programs. However, questions remain about the extent to which HPV vaccines are realizing their full potential and eliminating these disparities. Our results support the need for a more systematic approach to both the collection and the analysis of health disparities in research on HPV vaccine. It is important that future work not only assess the trends in HPV-associated conditions, but also that they consider the effects of race, ethnicity, and SES on these health outcomes.
Supplementary Material
Highlights:
Few HPV vaccine impact studies assess potential sociodemographic disparities
Only 2 of 23 studies identified reported stratified results by these variables
One study saw greater impact in low-proportion minority and low-income populations
Determining if vaccines reduce known sociodemographic disparities is critical
Funding:
This work was supported, in part, from grants from the American Cancer Society (Oliveira), the Robert E. Leet and Clara Guthrie Patterson Trust (Oliveira), National Institutes of Health grant numbers R01AI123204 (Niccolai), K07CA230234 (Sheth), CTSA grant numbers KL2 TR001862 (Sheth, Shapiro), UL1TR000142 (Shapiro) from the National Center for Advancing Translational Science (NCATS) at the National Institutes of Health and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical approval: Not required as no human or animal subjects were used in this literature review study.
Declarations of Interest: Dr. Niccolai has served as a Scientific Advisor for Merck. Dr. Sheth receives Gardasil 9 from Merck at no cost for research.
References
- 1.Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MC, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013; 40:187–93. [DOI] [PubMed] [Google Scholar]
- 2.McQuillan G, Kruszon-Moran D, Markowitz LE, Unger ER, Paulose-Ram R. Prevalence of HPV in Adults Aged 18–69: United States, 2011–2014. NCHS Data Brief. 2017:1–8. [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017; 67:7–30. [DOI] [PubMed] [Google Scholar]
- 4.Frederiksen ME, Njor S, Lynge E, Rebolj M. Psychological effects of diagnosis and treatment of cervical intraepithelial neoplasia: a systematic review. Sex Transm Infect. 2015; 91:248–56. [DOI] [PubMed] [Google Scholar]
- 5.Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009; 374:301–14. [DOI] [PubMed] [Google Scholar]
- 6.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007; 356:1928–43. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, Zhang J, Xia N, Zhao Q. Expanded strain coverage for a highly successful public health tool: Prophylactic 9-valent human papillomavirus vaccine. Hum Vaccin Immunother. 2017; 13:2280–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garland SM, Steben M, Sings HL, James M, Lu S, Railkar R, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009; 199:805–14. [DOI] [PubMed] [Google Scholar]
- 9.Joura EA, Ault KA, Bosch FX, Brown D, Cuzick J, Ferris D, et al. Attribution of 12 high-risk human papillomavirus genotypes to infection and cervical disease. Cancer Epidemiol Biomarkers Prev. 2014; 23:1997–2008. [DOI] [PubMed] [Google Scholar]
- 10.Hanquet G, Valenciano M, Simondon F, Moren A. Vaccine effects and impact of vaccination programmes in post-licensure studies. Vaccine. 2013; 31:5634–42. [DOI] [PubMed] [Google Scholar]
- 11.Singh GK, Jemal A. Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950–2014: Over Six Decades of Changing Patterns and Widening Inequalities. J Environ Public Health. 2017; 2017:2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vickers SM, Fouad MN. An overview of EMPaCT and fundamental issues affecting minority participation in cancer clinical trials: enhancing minority participation in clinical trials (EMPaCT): laying the groundwork for improving minority clinical trial accrual. Cancer. 2014; 120 Suppl 7:1087–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mastroianni AC, Faden R, Federman D. Women and health research: a report from the Institute of Medicine. Kennedy Inst Ethics J. 1994; 4:55–62. [DOI] [PubMed] [Google Scholar]
- 14.Bonham VL, Umeh NI, Cunningham BA, Abdallah KE, Sellers SL, Cooper LA. Primary Care Physicians’ Collection, Comfort, and Use of Race and Ethnicity in Clinical Practice in the United States. Health Equity. 2017; 1:118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma IW, Khan NA, Kang A, Zalunardo N, Palepu A. Systematic review identified suboptimal reporting and use of race/ethnicity in general medical journals. J Clin Epidemiol. 2007; 60:572–8. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musselwhite LW, Oliveira CM, Kwaramba T, de Paula Pantano N, Smith JS, Fregnani JH, et al. Racial/Ethnic Disparities in Cervical Cancer Screening and Outcomes. Acta Cytol. 2016; 60:518–26. [DOI] [PubMed] [Google Scholar]
- 18.Bakir AH, Skarzynski M. Health Disparities in the Immunoprevention of Human Papillomavirus Infection and Associated Malignancies. Front Public Health. 2015; 3:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeudin P, Liveright E, Del Carmen MG, Perkins RB. Race, ethnicity, and income factors impacting human papillomavirus vaccination rates. Clin Ther. 2014; 36:24–37. [DOI] [PubMed] [Google Scholar]
- 20.Drolet M, Bénard É, Boily MC, Ali H, Baandrup L, Bauer H, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2015; 15:565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahn JA, Brown DR, Ding L, Widdice LE, Shew ML, Glynn S, et al. Vaccine-type human papillomavirus and evidence of herd protection after vaccine introduction. Pediatrics. 2012; 130:e249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Center AFHS. Sexually transmitted infections, active component, U.S. Armed Forces, 2000–2012. MSMR. 2013; 20:5–10. [PubMed] [Google Scholar]
- 23.Markowitz LE, Hariri S, Lin C, Dunne EF, Steinau M, McQuillan G, et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis. 2013; 208:385–93. [DOI] [PubMed] [Google Scholar]
- 24.Wilson AR, Welch RJ, Hashibe M, Greenwood J, Jackson B, She RC. Surveillance of human papilloma virus using reference laboratory data for the purpose of evaluating vaccine impact. Online J Public Health Inform. 2014; 6:e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickson EL, Vogel RI, Luo X, Downs LS. Recent trends in type-specific HPV infection rates in the United States. Epidemiol Infect. 2015; 143:1042–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunne EF, Naleway A, Smith N, Crane B, Weinmann S, Braxton J, et al. Reduction in Human Papillomavirus Vaccine Type Prevalence Among Young Women Screened for Cervical Cancer in an Integrated US Healthcare Delivery System in 2007 and 2012–2013. J Infect Dis. 2015; 212:1970–5. [DOI] [PubMed] [Google Scholar]
- 27.Berenson AB, Laz TH, Rahman M. Reduction in Vaccine-Type Human Papillomavirus Prevalence Among Women in the United States, 2009–2012. J Infect Dis. 2016; 214:1961–4. [DOI] [PubMed] [Google Scholar]
- 28.Markowitz LE, Liu G, Hariri S, Steinau M, Dunne EF, Unger ER. Prevalence of HPV After Introduction of the Vaccination Program in the United States. Pediatrics. 2016; 137:e20151968. [DOI] [PubMed] [Google Scholar]
- 29.Tarney CM, Klaric J, Beltran T, Pagan M, Han J. Prevalence of Human Papillomavirus in Self-Collected Cervicovaginal Swabs in Young Women in the United States Between 2003 and 2012. Obstet Gynecol. 2016; 128:1241–7. [DOI] [PubMed] [Google Scholar]
- 30.Berenson AB, Hirth JM, Chang M. Change in Human Papillomavirus Prevalence Among U.S. Women Aged 18–59 Years, 2009–2014. Obstet Gynecol. 2017; 130:693–701. [DOI] [PubMed] [Google Scholar]
- 31.Oliver SE, Unger ER, Lewis R, McDaniel D, Gargano JW, Steinau M, et al. Prevalence of Human Papillomavirus Among Females After Vaccine Introduction-National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis. 2017; 216:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bauer HM, Wright G, Chow J. Evidence of human papillomavirus vaccine effectiveness in reducing genital warts: an analysis of California public family planning administrative claims data, 2007–2010. Am J Public Health. 2012; 102:833–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flagg EW, Schwartz R, Weinstock H. Prevalence of anogenital warts among participants in private health plans in the United States, 2003–2010: potential impact of human papillomavirus vaccination. Am J Public Health. 2013; 103:1428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nsouli-Maktabi H, Ludwig SL, Yerubandi UD, Gaydos JC. Incidence of genital warts among U.S. service members before and after the introduction of the quadrivalent human papillomavirus vaccine. MSMR. 2013; 20:17–20. [PubMed] [Google Scholar]
- 35.Perkins RB, Legler A, Hanchate A. Trends in Male and Female Genital Warts Among Adolescents in a Safety-Net Health Care System 2004–2013: Correlation With Introduction of Female and Male Human Papillomavirus Vaccination. Sex Transm Dis. 2015; 42:665–8. [DOI] [PubMed] [Google Scholar]
- 36.Flagg EW, Torrone EA. Declines in Anogenital Warts Among Age Groups Most Likely to Be Impacted by Human Papillomavirus Vaccination, United States, 2006–2014. Am J Public Health. 2018; 108:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benard VB, Castle PE, Jenison SA, Hunt WC, Kim JJ, Cuzick J, et al. Population-Based Incidence Rates of Cervical Intraepithelial Neoplasia in the Human Papillomavirus Vaccine Era. JAMA Oncol. 2017; 3:833–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niccolai LM, Julian PJ, Meek JI, McBride V, Hadler JL, Sosa LE. Declining rates of high-grade cervical lesions in young women in Connecticut, 2008–2011. Cancer Epidemiol Biomarkers Prev. 2013; 22:1446–50. [DOI] [PubMed] [Google Scholar]
- 39.Hariri S, Johnson ML, Bennett NM, Bauer HM, Park IU, Schafer S, et al. Population-based trends in high-grade cervical lesions in the early human papillomavirus vaccine era in the United States. Cancer. 2015; 121:2775–81. [DOI] [PubMed] [Google Scholar]
- 40.Hariri S, Bennett NM, Niccolai LM, Schafer S, Park IU, Bloch KC, et al. Reduction in HPV 16/18-associated high grade cervical lesions following HPV vaccine introduction in the United States - 2008–2012. Vaccine. 2015; 33:1608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flagg EW, Torrone EA, Weinstock H. Ecological Association of Human Papillomavirus Vaccination with Cervical Dysplasia Prevalence in the United States, 2007–2014. Am J Public Health. 2016; 106:2211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niccolai LM, Meek JI, Brackney M, Hadler JL, Sosa LE, Weinberger DM. Declines in Human Papillomavirus (HPV)-Associated High-Grade Cervical Lesions After Introduction of HPV Vaccines in Connecticut, United States, 2008–2015. Clin Infect Dis. 2017; 65:884–9. [DOI] [PubMed] [Google Scholar]
- 43.Watson M, Soman A, Flagg EW, Unger E, Deapen D, Chen VW, et al. Surveillance of high-grade cervical cancer precursors (CIN III/AIS) in four population-based cancer registries, United States, 2009–2012. Prev Med. 2017; 103:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kahn JA, Lan D, Kahn RS. Sociodemographic factors associated with high-risk human papillomavirus infection. Obstet Gynecol. 2007; 110:87–95. [DOI] [PubMed] [Google Scholar]
- 45.Jemal A, Simard EP, Dorell C, Noone AM, Markowitz LE, Kohler B, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013; 105:175–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Virnig BA, Baxter NN, Habermann EB, Feldman RD, Bradley CJ. A matter of race: early-versus late-stage cancer diagnosis. Health Aff (Millwood). 2009; 28:160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brisson M, Drolet M, Malagón T. Inequalities in Human Papillomavirus (HPV)-associated cancers: implications for the success of HPV vaccination. J Natl Cancer Inst. 2013; 105:158–61. [DOI] [PubMed] [Google Scholar]
- 48.Biello KB, Niccolai L, Kershaw TS, Lin H, Ickovics J. Residential racial segregation and racial differences in sexual behaviours: an 11-year longitudinal study of sexual risk of adolescents transitioning to adulthood. J Epidemiol Community Health. 2013; 67:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garland SM, Kjaer SK, Muñoz N, Block SL, Brown DR, DiNubile MJ, et al. Impact and Effectiveness of the Quadrivalent Human Papillomavirus Vaccine: A Systematic Review of 10 Years of Real-world Experience. Clin Infect Dis. 2016; 63:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Menzies RI, Singleton RJ. Vaccine preventable diseases and vaccination policy for indigenous populations. Pediatr Clin North Am. 2009; 56:1263–83. [DOI] [PubMed] [Google Scholar]
- 51.Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, et al. Socioeconomic status and health. The challenge of the gradient. Am Psychol. 1994; 49:15–24. [DOI] [PubMed] [Google Scholar]
- 52.Warren RC, Hahn RA, Bristow L, Yu ES. The use of race and ethnicity in public health surveillance. Public Health Rep. 1994; 109:4–6. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.