Abstract
Objectives:
Circulating endothelial cells (CECs), von Willebrand Factor (VWF) antigen, P-selectin and thrombomodulin are released from damaged endothelium, while decreases in circulating endothelial progenitor cells (CEPCs) have been associated with poor vascular outcomes. We examined these markers in the peripheral blood of juvenile dermatomyositis (JDM) patients and their correlations with disease assessments.
Methods:
Peripheral blood endothelial cells and biomarkers were assessed in 20 JDM patients, and matched healthy controls. CECs and CEPCs were quantitated by flow cytometry, while VWF antigen and activity, Factor VIII, P-selectin and thrombomodulin were measured in plate-based assays. Disease activity and damage, nailfold capillary (NFC) density, and brachial artery flow dilation were assessed. Serum cytokines/chemokines were measured by Luminex.
Results:
CECs, VWF antigen, Factor VIII, thrombomodulin, but not VWF activity, CEPCs or P-selectin, were elevated in the peripheral blood of JDM patients. CECs correlated with pulmonary activity (rs= 0.56). VWF antigen correlated with Patient/Parent Global, cutaneous and Extra-muscular Activity (rs= 0.47-0.59). CEPCs negatively correlated with muscle activity and physical function (rs= −0.52-−0.53). CEPCs correlated inversely with endocrine damage. VWF antigen and activity correlated with IL-10 and IP-10 (rs= 0.64-0.82),.
Conclusion:
Markers of endothelial injury are increased in JDM patients and correlate with extramuscular activity. CEPCs correlate inversely with muscle activity, suggesting a functional disturbance in repair mechanisms.
Keywords: juvenile dermatomyositis, myositis, endothelial function, circulating endothelial cells, endothelial progenitor cells, disease activity
Introduction.
The idiopathic inflammatory myopathies (IIM) are systemic autoimmune diseases characterized by chronic muscle inflammation (1). An immune attack on muscle capillary endothelium, infiltration of plasmacytoid dendritic cells with a resulting type I interferon (IFN) response, and upregulation of major histocompatibility complex (MHC) class I expression on the surface of myofibers appear to be central pathogenic events in adult and juvenile dermatomyositis (JDM) (2, 3).
Circulating endothelial cells (CECs) are biomarkers of endothelial function, which represent the detachment of mature cells from the endothelial monolayer following damage. CECs are rarely found in the peripheral blood of healthy individuals, but plasma levels of CECs are increased in vascular disease and are believed to reflect the degree of endothelial damage or stress (4). In a variety of inflammatory and autoimmune diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), systemic sclerosis, and antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis, patients with active disease have increased numbers of CECs, which correlate with disease activity (5–9). Circulating endothelial progenitor cells (CEPCs), which are part of the mononuclear cell component of the blood, are produced in the bone marrow and migrate into blood vessels, and have the ability to differentiate into mature endothelial cells, which results in the formation of new blood vessels (10). In response to vascular injury, the recruitment of CEPCs to sites of injury increases and bone marrow CEPC pools are depleted, resulting in a decrease in the number of CEPCs in the peripheral blood. In systemic rheumatic disease, CEPCs are often decreased in numbers and function. They have several important roles, including maintaining endothelial function following inflammatory stress, protecting against atherosclerosis, and stimulating angiogenesis (11).
CEPCs have been observed to be significantly less frequent in adult polymyositis (PM) patients compared with healthy controls (12). CEPCs also demonstrated a decreased capacity to differentiate into mature endothelial cells in adult DM/PM patients, and this impairment was associated with type I IFN and IL-18 serum activity (12). Recently, Xu et al. reported no difference in the number of CEPCs in JDM patients compared to healthy control children, and CEPCs did not correlate with JDM disease activity assessed by the Disease Activity Score (DAS) or with metabolic parameters (13).
The purpose of this study was to further evaluate CECs, CEPCs and other peripheral blood endothelial biomarkers in IIM patients compared to healthy controls, and to investigate the relationship between these endothelial markers with disease activity and damage measures in IIM.
Methods.
Subjects.
Twenty JDM patients fulfilled probable or definite Bohan and Peter criteria for IIM (14) and were enrolled in the National Institute of Environmental Health Sciences institutional review board-approved myositis natural history study (No. 94-E-0165). Patients provided written informed consent/assent according to the standards of the Declaration of Helsinki. Myositis-specific autoantibodies (MSAs) were identified in the sera of 20 patients using standard detection methods (15, 16). Median age at enrollment was 12.1 years, and the median time from diagnosis to enrollment was 23.2 months. JDM patient characteristics and their disease activity and damage measures (17) are shown in Table 1. Five patients underwent muscle biopsy to confirm a diagnosis of JDM; however, detailed muscle biopsy reports were available from only 2 patients. We also evaluated thigh and pelvis Magnetic Resonance Imaging (MRI), including Short Tau Inversion Recovery (STIR) muscle edema and T1 muscle atrophy and fatty infiltration, with scoring by one radiologist blinded to clinical status (18). Clinical laboratory studies were adjusted based on age-defined upper limits of normal.
Table 1:
Characteristics of 20 juvenile dermatomyositis patients in the present study.
| Characteristics | Median [IQR] or n (%) | |
|---|---|---|
| Age at evaluation, years | 12.1 [9.3-15.7] | |
| Gender | Female | 13 (65.0) |
| Race | Caucasian | 17 (85.0) |
| African American | 1 (5.0) | |
| Asian | 1 (5.0) | |
| Caucasian and Hispanic | 1 (4.5) | |
| Myositis specific autoantibodies | Anti-p155/140 (TIF1) | 7 (35.0) |
| Anti-MJ (NXP2) | 3 (15.0) | |
| Anti-MDA5 | 6 (30.0) | |
| Other MSAs (Jo-1, Mi-2, HMGCR)† | 3 (15.0) | |
| MSA negative | 1 (5.0) | |
| Disease Measures | Physician global activity score, 0-10 cm VAS | 2.7 [1.6-4.4] |
| Patient/Parent global activity score, 0-10 cm VAS | 4.2 [2.1-5.6] | |
| Physician global damage, 0-10 cm VAS | 1.9 [0.7-2.9] | |
| Physical Function | HAQ or CHAQ (0-3) | 0.4 [0.1-1.1] |
| CMAS (0-52) | 45.5 [37.3-49.0] | |
| Muscle strength | MMT8 (0-80) | 75.0 [68.5-78.8] |
| Muscle enzymes | CK (26 - 252 U/L) | 39.5 [29.0-66.0] |
| Aldolase (1-7 U/L) | 5.8 [4.7-5.8] | |
| AST (0 - 34 U/L) | 30.5 [23.3-35.8] | |
| LDH (105 - 226 U/L) | 204 [170-241] | |
| Myositis Disease Activity Assessment tool (MDAAT) VAS Organ System Scorest‡ | Cutaneous (0-10 cm VAS) | 2.6 [1.9-5.0] |
| Muscle (0-10 cm VAS) | 2.1 [0.6-3.4] | |
| Constitutional (0-10 cm VAS) | 1.0 [0.4-1.4] | |
| Pulmonary (0-10 cm VAS) | 0.6 [0-1.0] | |
| Skeletal (0-10 cm VAS) | 0 [0-0.3] | |
| Gastrointestinal (0-10 cm VAS) | 0 [0-0.5] | |
| Extra-muscular VAS score (0-60) | 4.6 [3.2-7.1] | |
| Disease Activity Score | Total (0-20) | 10 [8-12] |
| Muscle (0-11) | 5 [3-6] | |
| Cutaneous (0-9) | 6 [5-7] | |
| Myositis Damage Index | Total Extent of Damage score (0-38) | 5.0 [3.0-8.5] |
| Total Severity of Damage score (0-110) | 5.5 [3.3-8.0] | |
| Muscle Severity of Damage (0-10 cm VAS) | 1.5 [0.1-2.5] | |
| Skeletal Severity of Damage (0-10 cm VAS) | 1.5 [0.9-2.8] | |
| Endocrine Severity of Damage (0-10 cm VAS) | 0.6 [0-1.0] | |
| Pulmonary Severity of Damage (0-10 cm VAS) | 0 [0-0.9] | |
| Other Measures | MRI T1 atrophy and fatty muscle infiltration score (0-4) | 0 [0-0.5] |
| Brachial Artery Flow Mediated Dilation (% change) | 9.1 [8.0-14.8] | |
| Periungual nailfold capillary density (per mm) | 8.0 [6.8-10.0] | |
| Delay to diagnosis (months) | 4.0 [2.0-9.0] | |
| Laboratory | White blood cell count (3.4-9.6) (x103/mcL) | 6.9 [4.7-8.5] |
| Platelet count (161-380) (x103/mcL) | 300 [250-343] | |
| Erythrocyte sedimentation rate (ULN 42) (mm/hr) | 19.0 [6.0-44.0] | |
| Fasting serum insulin (mIU/L) | 15.1 [10.7-25.6] | |
| LDL (ULN 159) (mg/dl) | 118 [108-151] | |
| Total cholesterol (ULN 240) (mg/dl) | 202 [176-244] | |
| Medications | Daily oral prednisone dose (mg/day) | 15.0 [6.4-23.1] |
| Oral prednisone usage, n (%) | 18 (90.0) | |
| Intravenous methylprednisolone usage, n (%) | 16 (80.0) | |
| Methotrexate usage, n (%) | 20 (100) | |
| Intravenous immunoglobulin usage, n (%) | 12 (60.0) | |
| Other immunosuppressive usage§, n (%) | 9 (45.0) | |
| Hydroxychloroquine usage, n (%) | 15 (75.0) |
Other Myositis specific autoantibodies included 1 each patient with anti-Jo-1, anti-Mi-2, and anti-HMGCR.
Cardiovascular MDAAT and Gastrointestinal and Peripheral vascular severity of damage scores were all 0.
Other immunosuppressives include: azathioprine, mycophenolate mofetil, cyclosporine, cyclophosphamide, or anti-TNF therapy (Etanercept and Infliximab)
Abbreviation: AST, aspartate aminotransferase; [C]HAQ, (Childhood) Health Assessment Questionnaire; CK, creatine kinase; CMAS, childhood myositis assessment scale; HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase; LDH, lactate dehydrogenase; LDL, low-density lipoprotein; MDA5, melanoma differentiation-associated gene 5; MMT, manual muscle testing; MRI, magnetic resonance imaging; MSAs, myositis-specific autoantibodies; NXP2 nuclear matrix protein 2; STIR, short tau inversion recovery; TIF1, transcriptional intermediary factor 1; ULN, upper limit of normal; VAS, visual analogue scale.
A healthy control group (n=20) was recruited through the NIH Office of Patient Recruitment, consisting of 13 (65%) females with a median enrollment age of 11.7 years [Interquartile range (IQR) 9.7-15.9 years] and similar racial composition to the JDM patients. The controls had no evidence of autoimmune disease by history, physical examination, and laboratory testing as well as no infections, vaccines or use of anti-inflammatory medications within 2 months of enrollment.
Endothelial functional assessments:
JDM patients and healthy controls underwent evaluation of periungual nailfold capillaries (NFC) and brachial artery flow mediated dilation (FMD) (Table 1). NFC density was quantitated, blinded to patient clinical status, on the 4th digit of the right hand using a Nikon D810 digital camera (Nikon Inc., Melville, NY) with a 80 mm lens with ring light flash; mineral oil was applied to the periungual area for magnification, with a millimeter measuring tape used for reference in each photograph (19). Brachial artery FMD was assessed by a standard protocol using a high-resolution ultrasonography (12.5-MHz linear-array transducer, model ATL HDI 5000, Advanced Technology Laboratories, Signal Hill, CA), as previously described (20).
Laboratory methods.
All laboratory studies were performed by personnel blinded to patient characteristics and assessments. EDTA anti-coagulated peripheral blood was processed within 4 hours of collection. To detect CECs, whole blood lysis was performed using ammonium chloride as previously described (21) prior to staining for 30 minutes at 4°C with the following cocktail of antibodies (antibody concentration according to manufacturer’s recommendations): CD31-FITC (Becton Dickinson, San Jose, CA), CD146-PE, (P1H12 – Chemicon, Temecula, CA), CD45-APC (Becton Dickinson, San Jose, CA). 7-aminoactinomycin D Viability Dye (Beckman Coulter, Marseille, France) was added one minute prior to acquisition to discriminate live versus dead cells. 5 million cells were acquired per tube using a FACSCalibur (BD Biosciences, San Jose, CA). Live CECs were identified as 7AAD negative, CD45 negative, CD146 positive and CD31 positive cells.
CEPCs were quantitated by flow cytometry of mononuclear cells isolated from buffy coat cells from EDTA anti-coagulated peripheral blood, as previously described (22). Each tube of aliquoted cells was stained with phycoerythrin PE or fluorescein isothiocyanate-conjugated CD34 monoclonal antibody and PerCPCy5.5-conjugated CD45 monoclonal antibody (BD Biosciences, San Jose, CA). Two additional monoclonal antibodies for CEPC and endothelial cell markers were also added to each tube of cells, including biotin-conjugated KDR (VEGFR-2) (Sigma-Aldrich, St. Louis, MO), and PE-conjugated CD133 or activated protein C (APC)-conjugated CD133 (Miltenyi Biotec, Auburn, CA). Cell populations in subjects were also expressed as the number of circulating cells per volume of peripheral blood, based on the nucleated white blood cell count from the automated counter.
The von Willebrand factor (VWF) antigen assays were performed using Diagnostica Stago STA LIATEST VWF: antigen according to the manufacturer’s directions (Parsippany, NJ). VWF activity was measured in a Ristocetin cofactor assay as previously described (23). Factor VIII activity was measured in a one-stage aPTT assay using George King Biomedical factor VIII deficient plasma and automated aPTT from Diagnostica Stago (Parsippany, NJ). P-selectin levels and thrombomodulin levels were measured in ELISA assays (R&D Systems, Inc. Minneapolis, MN). Serum levels of 23 cytokines and chemokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, CXCL8 (IL-8), IL-10, IL-12, IL-13, IL-15, IL-17, TNF- α, IFN- γ, IFN- α, GM-CSF, macrophage inflammatory protein-1 α (MIP −1 α), macrophage inflammatory protein-1 β (MIP -1 β), IFN-induced-protein-10 (IP10), CCL11 (eotaxin), RANTES, Monocyte chemoattractant protein-1 (MCP-1), and IL-1 receptor antagonist (IL-1Ra)) were measured using a bead-based immunofluorescence assay (Luminex Inc. Austin, TX, USA) using multiplex cytokine reagents (Biosource International, Camarillo, CA, USA) as previously described (24). Sensitivity of the standards ranged from 1.95 to 32,000 pg/ml.
Statistical Analysis.
Analyses were performed using JMP for Windows, version 11.0.0 (SAS Institute Inc, Cary, NC, USA). Wilcoxon-rank sums tests were used to compare median values of endothelial markers between patients and healthy controls. Data were expressed as median [IQ.R]. Spearman’s rank correlations (Spearman’s rho or rs) assessed plausible relationships among the endothelial markers, and between endothelial markers and disease activity or damage measures, as well as among the endothelial functional assessments. This exploratory analysis defines possible association at the alpha = 0.05 level and significant association at the alpha = 0.01 level.
Results.
The median number of CECs was significantly higher in patients with JDM compared to healthy controls (median [IQR]: JDM: 0.85 [0.52-1.95] vs. healthy controls: 0.18 [0.11-0.33] cells/ml, Figure 1A). Levels of thrombomodulin (JDM: 7.0 [2.4-10.1] vs. healthy controls: 1.9 [0.68-3.5] ng/ml), VWF antigen (JDM: 142.0 [90.0-251.8] vs. healthy controls: 97.0 [81.0-131.0] IU/dL) and Factor VIII (JDM: 202 [152-254]; healthy controls: 137 [124-170] IU/dL) were also significantly higher in patients with JDM compared to healthy control subjects (Figure 1B–D). There were no significant differences in VWF activity (JDM: 154 [104-196]; healthy controls: 121 [97-150] %) , P-selectin levels (JDM: 31.0 [24.0-41.5]; healthy controls: 24.0 [24.0-38.0] ng/ml) or in the number of CEPCs (JDM: 35.0 [24.8-81.1]; healthy controls: 34.7 [29.0-92.3] cells/ml) between JDM patients and healthy control subjects (Figure 1E–F). There were no significant differences in endothelial cells or markers between patients who received intravenous methylprednisolone, intravenous immunoglobulin, hydroxychloroquine, or other immunosuppressive therapies compared to patients not receiving these medications. Corticosteroid dose did not correlate with any endothelial cells or markers. All patients studied received methotrexate, so effects of this medication could not be evaluated.
Figure 1:
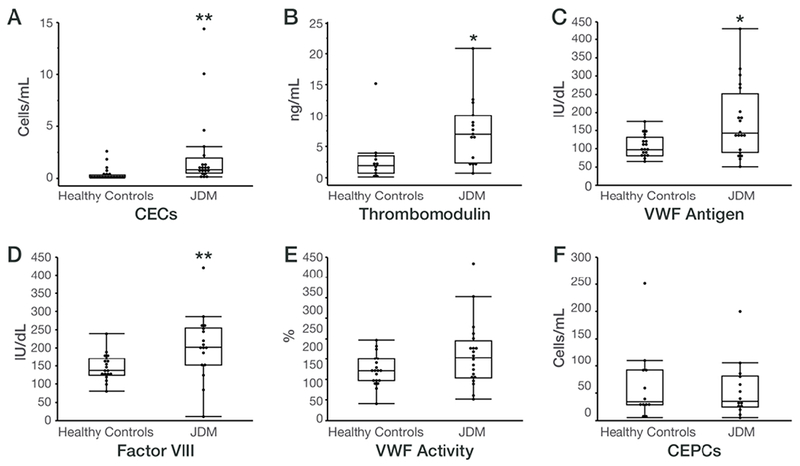
Endothelial cells and endothelial markers in juvenile dermatomyositis (JDM) patients and healthy control subjects.
Box and whisker plots are graphed with the median values, interquartile range (25%, 75%) within the boxes and the 5% and 95% also shown, in control subjects vs. JDM patients for the following endothelial cells and markers: (A) Circulating endothelial cells (CECs), (B) Thrombomodulin, (C) von Willebrand factor (VWF) antigen, (D) Factor VIII, (E) VWF activity, and (F) Circulating endothelial progenitor cells (CEPCs).
*P < 0.05, **P < 0.01 for JDM vs. controls.
We assessed correlations among endothelial markers, and found some endothelial cells and markers correlated with each other. VWF antigen, VWF activity, and Factor VIII all highly correlated with each other (rs= 0.82 - 0.90; P<0.001). There was no significant correlation of the number of CECs, CEPCs, P-selectin levels, or thrombomodulin levels with any of the other endothelial markers.
We assessed the relationship of endothelial cells and markers with myositis disease activity measures, and selected significant associations as shown in Figure 2. The number of CECs significantly correlated with the Myositis Disease Activity Assessment Tool (MDAAT) pulmonary visual analog scale (VAS) activity, which involved assessment of dyspnea, dysphonia, and interstitial lung disease, including pulmonary function testing (rs= 0.56, p=0.001, Figure 2A). The number of CEPCs correlated inversely with MDDAT muscle VAS activity, and physical function, as assessed by the (Childhood) Health Assessment Questionnaire ([C]HAQ) (rs= −0.52 - −0.53, p=0.054 – 0.055, Figures 2B–2C). VWF antigen correlated with Patient/Parent Global Activity and Extra-muscular VAS Activity, as well as with Cutaneous VAS activity (rs= 0.47–0.54, p=0.014- 0.046, Figure 2D–2F). P-selectin correlated with the serum levels of aldolase (rs=0.60, p=0.019).
Figure 2:
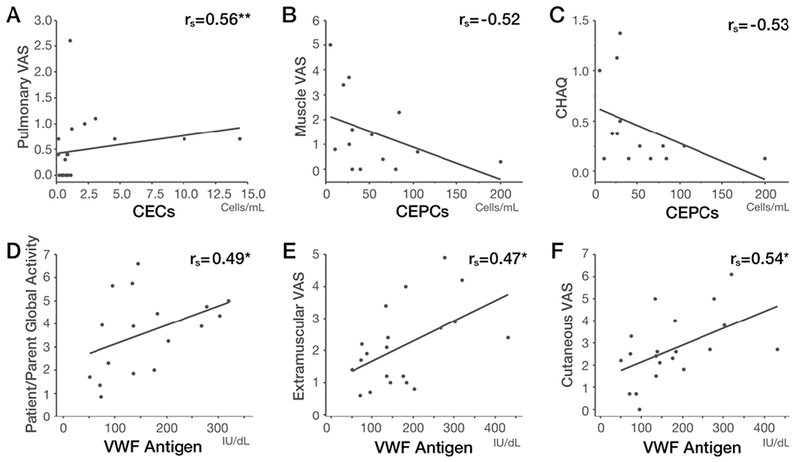
Correlations between endothelial cells/markers and disease activity measures in juvenile dermatomyositis patients.
Spearman rank correlations (rs) between (A) CECs and pulmonary VAS activity, (B) CEPCs and muscle VAS activity, (C) CEPCs and [C]HAQ, (D) VWF antigen and Patient/Parent Global Activity score, (E) VWF antigen and Extra-Muscular VAS activity, and (F) VWF antigen and cutaneous VAS activity.
*P < 0.05, **P < 0.01.
Abbreviations: CECs, circulating endothelial cells; CEPCs, circulating endothelial progenitor cells; [C]HAQ, (Childhood) Health Assessment Questionnaire; rs, Spearman’s rho; VAS, visual analog scale; VWF, von Willebrand factor.
MMT8, Childhood Myositis Assessment Scale (CMAS), Muscle DAS, Skin DAS, and STIR MRI muscle edema scores did not correlate with the number of endothelial cells (CECs, CEPCs) or with circulating levels of endothelial markers assessed. None of the endothelial markers correlated with NFC density.
In terms of laboratory data, erythrocyte sedimentation rate (ESR) correlated with VWF antigen and activity, and Factor VIII (rs= 0.63-0.82, P=0.0002- 0.017), and correlated inversely with P-selectin (rs= −0.56, p=0.048). White blood cell count (WBC) and platelet count did not correlate with endothelial cells or markers.
There was no correlation of Physician Global Damage or of Myositis Damage Index Extent or Severity of Damage scores with any of the endothelial cells or markers. Patients with a longer delay to diagnosis (>4 months) had higher number of CECs than patients with shorter delay (1.12 [0.73-3.83] vs. 0.56 [0.22-0.91] p<0.040). The number of CEPCs inversely correlated with endocrine damage severity in the Myositis Damage Index (rs= −0.56, p=0.039). MRI T1 muscle damage scores inversely correlated with CEPC cells (rs= −0.53, p=0.051). None of the other endothelial markers correlated with T1 MRI. Brachial artery FMD did not differ between JDM patients and healthy controls, and did not correlate with endothelial markers in JDM patients (data not shown). Fasting serum insulin positively correlated with VWF antigen and factor VIII level (rs= 0.50- 0.68, P=0.039 – 0.005). Total and low-density lipoprotein (LDL) cholesterol levels significantly inversely correlated with CEPCs (rs= −0.68 - −0.71, p=0.007-0.010).
Serum IL-10 (JDM: 37.1 [26.0-64.6], healthy controls: 19.8 [14.5-23.1] pg/ml), IP-10 (JDM: 100.7 [53.1-182.7], healthy controls: 18.4 [12.7-35.5] pg/ml), and MCP-1 (JDM: 918.4 [296.8-1303.0], healthy controls: 383.6 [257.5-558.1] pg/ml) were significantly increased in JDM patients compared with healthy controls (Figures 3A–C). These cytokines/chemokines correlated with disease activity measures. IL-10 correlated with PGA (rs=0.53, p=0.019), Patient/Parent Global Activity (rs=0.47, p=0.043), MDAAT Extramuscular activity (rs=0.52, p=0.023), serum ALT (rs=0.50, p=0.031), and Total DAS (rs=0.52, p=0.026). IP-10 correlated with MDDAT Muscle VAS (rs=0.52, p=0.021), MDAAT Extra-muscular activity (rs=0.52, p=0.021), Total DAS (rs=0.51, p=0.030), Patient/Parent Global Activity (rs=0.46, p=0.048), [C]HAQ (rs=0.49, p=0.048), and serum ALT (rs=0.47, p=0.041). MCP-1 correlated with Ttotal DAS (rs=0.48, p=0.043). Other serum cytokines/chemokines were not significantly different between JDM and controls, including levels of serum IFN-α (43.5 pg/ml vs.25.7 pg/ml, p=0.63). VWF antigen significantly correlated with IL-10 (rs= 0.81, p=0.0001, Figure 3D), and with eotaxin, IP-10, and MCP-1 (rs= 0.65 - 0.76, p=0.0006- 0.007), VWF activity significantly correlated with IL-10 (rs=0.82, p<0.0001), IP-10 (rs= 0.64, p=0.007, Figure 3E), eotaxin, and MCP-1 (rs= 0.66 - 0.70, p=0.002-0.003). CECs and CEPCs did not correlate with any cytokines/chemokines. Thrombomodulin correlated with IL-4 (rs = 0.52, p=0.046).
Figure 3:
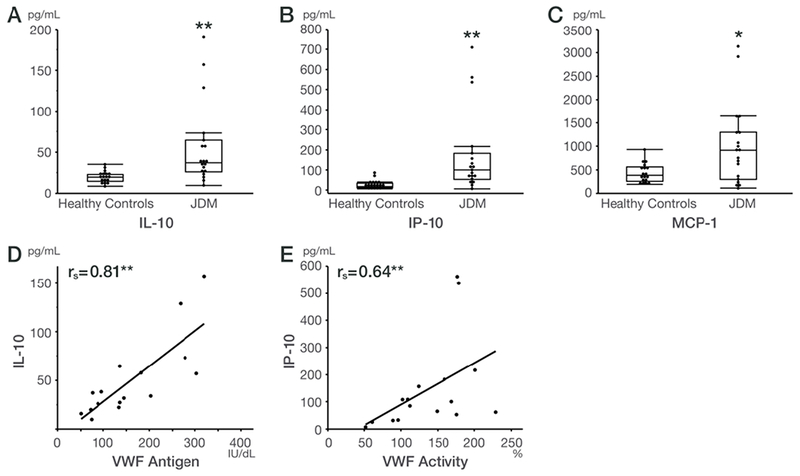
Cytokines/chemokines in juvenile dermatomyositis (JDM) patients and healthy control subjects and correlations between endothelial cells/markers and cytokines/chemokines.
Box and whisker plots are graphed with the median values, interquartile range (25%, 75%) within the boxes and the 5% and 95% also shown, in healthy control subjects vs. JDM patients for the following cytokines and chemokines: (A) IL-10, (B) IP10, and (C) MCP-1. *P < 0.05 and **P < 0.01 for JDM vs. controls.
Spearman’s rank correlations (rs) among endothelial cells/markers vs. cytokines and chemokines in JDM patients: (D) VWF antigen and IL-10, and (E) VWF activity and IP10.
**P < 0.01 for Spearman rank correlation coefficients.
Abbreviations: CEPCs, circulating endothelial progenitor cells; IL-10, Interleukin-10; IP-10, IFN-gamma-inducible protein 10; MCP-1, monocyte chemotactic protein-1; rs, Spearman’s rho; VWF, von Willebrand factor.
Discussion.
The findings of this study demonstrate that endothelial biomarkers are frequently altered in the peripheral blood of patients with JDM and are associated with myositis disease activity. The number of CECs, a marker of endothelial damage (4), levels of thrombomodulin, an angiogenic factor (25), and VWF antigenand Factor VIII, which are associated with endothelial dysfunction (26), are increased in JDM patients compared to healthy individuals. Increased numbers of CECs and increased levels of other endothelial activation markers have also been observed in patients with other inflammatory and systemic rheumatic diseases (5–9, 27, 28). Plasma VWF antigen has previously been reported to be elevated in the peripheral blood of active adult and JDM patients (29), and serum P-selectin levels, related to leucocyte recruitment at sites of vascular injury, was significantly increased in adult DM patients (30), although we did not see an elevation in our JDM population. We also observed a correlation of some endothelial markers with each other, including a strong relationship among VWF antigen, VWF activity, and Factor VIII, which are in the same activation pathway (31).
The number of CEPCs was not altered in JDM patients compared to healthy subjects in our study, nor in another report of JDM (13). We also did not examine the functional capacity of CEPCs to differentiate into mature cells. In contrast, in adult PM, CEPC numbers have been observed to be decreased (12), similar to patients with SLE (9, 11, 32), and to have decreased ability to differentiate into mature endothelial cells (12, 32, 33). This decrease in CEPCs numbers, maturation, and function correlates with Type I IFN and IL-18 serum activity (9, 12, 32, 33). The lack of decrease of CEPCs in most patients with myositis, despite a Type I IFN response, as evidenced by increases in serum Type I IFN-inducible cytokines and chemokines, may relate to deposition of CEPCs in affected muscle tissue (34), or to a combination of anti- and pro-angiogenic factors in the muscle tissue and periphery that may affect CEPC migration and detection (3, 35, 36).
Both CECs and VWF antigen are increased in JDM peripheral blood and both correlated with extra-muscular disease activity in our study, which mainly consisted of pulmonary and cutaneous features, but they did not correlate with measures of muscle activity or damage. Higher VWF levels have been previously associated with some adult DM symptoms, including weakness, fatigue, fever, and elevated muscle enzymes (37) and with disease flare in JDM (29). Plasma thrombomodulin levels were previously found to be higher in adult DM patients with interstitial lung disease (38). These reports suggest that endothelial markers may be associated with disease activity and vascular inflammation of DM, and our results also indicated that these endothelial markers correlated with extra-muscular disease activity of JDM, including in the skin.
In contrast, the number of CEPCs also inversely correlated with functional disability measured by the [C]HAQ, as well as with MDAAT muscle VAS. The inverse relationship of CEPCs with muscle function and disease activity suggests blood vessel regeneration may be diminished or there may be a functional disturbance in repair mechanisms during active disease. The correlation of CEPCs with measures of disease activity was not observed in the study by Xu et al (13), which may be related to differences in the subsets of CEPCs examined or to differences in disease duration or therapy. The lack of increased EPCs in the peripheral blood, but correlation of circulating endothelial activation markers with skin and extramuscular activity is consistent with findings in the muscle of JDM patients observed by Baumann et al. (40). In JDM muscle, endothelial cell activation is associated with early myogenesis, but an absence of increased endothelial progenitor cells suggests they are not contributing to the vascular repair process (40).
CEPCs also correlated inversely with endocrine and MRI muscle damage, which contrasted to childhood SLE in which CEPCs did not correlate with clinical damage (32). In JDM patients, we found CEPCs also inversely correlated with metabolic parameters, including serum lipids, but did not relate to brachial artery FMD, a functional measure of vascular damage. The number of CEPCs has been reported to inversely correlate with conventional cardiovascular risk factors, including total and LDL cholesterol. CEPCs correlate with coronary atherosclerosis, but and are inversely correlated with metabolic syndrome in patients with SLE (32, 41). These metabolic factors, by modulating the levels of oxidative stress, nitric oxide activity, or other physiologic processes, could directly influence the mobilization or half-life of CEPCs and lead to depletion of a presumed finite supply of CEPCs (42).
Type I IFNs have an important role in IIM pathogenesis and the severity of vasculopathic changes in affected tissues (3, 35). IL-10, IP-10 (CXCL10), and MCP1 (CCL2) are significantly increased in JDM patients compared with healthy controls, as previously reported (43, 44). VWF antigen and activity positively correlated with type I IFN cytokines/chemokines, which also have been reported to correlate with IIM extra-muscular disease activity (44, 45). Our data did not show a difference in other serum cytokines, between JDM and healthy controls, including IFN- α, probably due to a lack of sensitivity of the assay used. Type I IFN induces anti-angiogenic properties in CEPCs, through an IFN/IL-18 axis (12), consistent with several studies demonstrating toxic, anti-proliferative and anti-angiogenic effects of type I IFN towards endothelial cells (46). On the other hand, the role of the proangiogenic activity of myogenic progenitor cells appears to be driven by type I IFN, resulting in the stimulation of vessel remodeling and muscle recovery in JDM (47).
There are some limitations to this study. The number of JDM patients was relatively small. We could not evaluate these endothelial markers by myositis autoantibody status, because the number of patients was too small. Second, a single study visit precluded evaluation of the progression of vascular damage or changes in these biomarkers over time. Patients included this study were not new onset patients and received varying therapies; therefore, we could not evaluate the effects of treatment. Finally, endothelial activation markers could not be correlated with muscle biopsy microvascular changes, as only 2 patients in this study had a muscle biopsy available at the time of diagnosis with information on their biopsy features, and these were prevalent cases.
We conclude that markers of endothelial function, including CEC, VWF and thrombomodulin, were increased in JDM patients and correlate with extra-muscular disease activity. CEPCs were not increased in the peripheral blood of JDM patients, but CEPCs possibly correlate inversely with muscle activity measures, and with muscle and endocrine damage. These findings suggest a functional disturbance in repair mechanisms.
Acknowledgements:
We thank Drs. Mariana J. Kaplan and Sarfaraz A. Hasni for critical reading of the manuscript and Dr. J Philip McCoy for valuable discussions. We thank Dr. Lawrence Yao for MRI readings, Ms. Gloria Zalos for brachial artery flow mediated dilation, Dr. Gulnara Mamyrova for assistance with nailfold capillary density quantitation, and Dr. Richard Cannon for endothelial cell progenitor flow cytometry studies.
The source of support in the form of grants or industrial supports: This work was supported by in part by the Intramural Research Program of the National Institute of Environmental Health Sciences (project ES101074, Takayuki Kishi, Lisa Rider, and Frederick Miller). Takayuki Kishi was supported by a research fellowship of the Cure JM Foundation and by Tokyo Women’s Medical University.
Footnotes
Conflict of interest: None
References
- 1.Miller FW, Lamb JA, Schmidt J, Nagaraju K. Risk factors and disease mechanisms in myositis. Nat Rev Rheumatol 2018;14:255–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emslie-Smith AM, Engel AG. Microvascular changes in early and advanced dermatomyositis: a quantitative study. Ann Neurol 1990;27:343–56. [DOI] [PubMed] [Google Scholar]
- 3.Nagaraju K, Rider LG, Fan C, Chen YW, Mitsak M, Rawat R, et al. Endothelial cell activation and neovascularization are prominent in dermatomyositis. J Autoimmune Dis 2006; 3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burger D, Touyz RM. Cellular biomarkers of endothelial health: microparticles, endothelial progenitor cells, and circulating endothelial cells. J Am Soc Hypertens 2012;6:85–99. [DOI] [PubMed] [Google Scholar]
- 5.Del Papa N, Colombo G, Fracchiolla N, Moronetti LM, Ingegnoli F, Maglione W, et al. Circulating endothelial cells as a marker of ongoing vascular disease in systemic sclerosis. Arthritis Rheum 2004;50:1296–304. [DOI] [PubMed] [Google Scholar]
- 6.Foster W, Shantsila E, Carruthers D, Lip GY, Blann AD. Circulating endothelial cells and rheumatoid arthritis: relationship with plasma markers of endothelial damage/dysfunction. Rheumatology (Oxford). 2009;48:285–8. [DOI] [PubMed] [Google Scholar]
- 7.Clarke LA, Hong Y, Eleftheriou D, Shah V, Arrigoni F, Klein NJ, et al. Endothelial injury and repair in systemic vasculitis of the young. Arthritis Rheum 2010;62:1770–80. [DOI] [PubMed] [Google Scholar]
- 8.Clancy R, Marder G, Martin V, Belmont HM, Abramson SB, Buyon J. Circulating activated endothelial cells in systemic lupus erythematosus: further evidence for diffuse vasculopathy. Arthritis Rheum 2001;44:1203–8. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Carrio J, Prado C, de Paz B, Lopez P, Gomez J, Alperi-Lopez M, et al. Circulating endothelial cells and their progenitors in systemic lupus erythematosus and early rheumatoid arthritis patients. Rheumatology (Oxford). 2012;51:1775–84. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M, Malik AB, Rehman J. Endothelial progenitor cells and vascular repair. Curr Opin Hematol. 2014;21:224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westerweel PE, Verhaar MC. Endothelial progenitor cell dysfunction in rheumatic disease. Nat Rev Rheumatol 2009;5:332–40. [DOI] [PubMed] [Google Scholar]
- 12.Ekholm L, Kahlenberg JM, Barbasso Helmers S, Tjarnlund A, Yalavarthi S, Zhao W, et al. Dysfunction of endothelial progenitor cells is associated with the type I IFN pathway in patients with polymyositis and dermatomyositis. Rheumatology (Oxford). 2016;55:1987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu D, Kacha-Ochana A, Morgan GA, Huang CC, Pachman LM. Endothelial progenitor cell number is not decreased in 34 children with Juvenile Dermatomyositis: a pilot study. Pediatr Rheumatol Online J. 2017;15:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bohan A, Peter JB, Bowman RL, Pearson CM. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine. 1977;56:255–86. [DOI] [PubMed] [Google Scholar]
- 15.Targoff IN, Mamyrova G, Trieu EP, Perurena O, Koneru B, O’Hanlon TP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum 2006;54:3682–9. [DOI] [PubMed] [Google Scholar]
- 16.Arnett FC, Targoff IN, Mimori T, Goldstein R, Warner NB, Reveille JD. Interrelationship of major histocompatibility complex class II alleles and autoantibodies in four ethnic groups with various forms of myositis. Arthritis Rheum 1996;39:1507–18. [DOI] [PubMed] [Google Scholar]
- 17.Rider LG, Werth VP, Huber AM, Alexanderson H, Rao AP, Ruperto N, et al. Measures of adult and juvenile dermatomyositis, polymyositis, and inclusion body myositis: Physician and Patient/Parent Global Activity, Manual Muscle Testing (MMT), Health Assessment Questionnaire (HAQ)/Childhood Health Assessment Questionnaire (C-HAQ), Childhood Myositis Assessment Scale (CMAS), Myositis Disease Activity Assessment Tool (MDAAT), Disease Activity Score (DAS), Short Form 36 (SF-36), Child Health Questionnaire (CHQ), Physician Global Damage, Myositis Damage Index (MDI), Quantitative Muscle Testing (QMT), Myositis Functional Index-2 (FI-2), Myositis Activities Profile (MAP), Inclusion Body Myositis Functional Rating Scale (IBMFRS), Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), Cutaneous Assessment Tool (CAT), Dermatomyositis Skin Severity Index (DSSI), Skindex, and Dermatology Life Quality Index (DLQI). Arthritis Care Res(Hoboken). 2011;63 Suppl 11:S118–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao L, Yip AL, Shrader JA, Mesdaghinia S, Volochayev R, Jansen AV, et al. Magnetic resonance measurement of muscle T2, fat-corrected T2 and fat fraction in the assessment of idiopathic inflammatory myopathies. Rheumatology (Oxford). 2016;55:441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith RL, Sundberg J, Shamiyah E, Dyer A, Pachman LM. Skin involvement in juvenile dermatomyositis is associated with loss of end row nailfold capillary loops. J Rheumatol 2004;31:1644–9. [PubMed] [Google Scholar]
- 20.Vazquez E, Sethi AA, Freeman L, Zalos G, Chaudhry H, Haser E, et al. High-density lipoprotein cholesterol efflux, nitration of apolipoprotein A-I, and endothelial function in obese women. Am J Cardiol 2012;109:527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tembhare PR, Yuan CM, Venzon D, Braylan R, Korde N, Manasanch E, et al. Flow cytometric differentiation of abnormal and normal plasma cells in the bone marrow in patients with multiple myeloma and its precursor diseases. Leuk Res 2014;38:371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell TM, Paul JD, Hill JM, Thompson M, Benjamin M, Rodrigo M, et al. Granulocyte colony-stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease. Arterioscler Thromb Vas Biol 2005;25:296–301. [DOI] [PubMed] [Google Scholar]
- 23.Gralnick HR, Coller BS, Sultan Y. Studies of the human factor VIII/von Willebrand factor protein.III.Qualitative defects in von Willebrand’s disease. J Clin Invest 1975;56:814–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szodoray P, Alex P, Knowlton N, Centola M, Dozmorov I, Csipo I, et al. Idiopathic inflammatory myopathies, signified by distinctive peripheral cytokines, chemokines and the TNF family members B-cell activating factor and a proliferation inducing ligand. Rheumatology (Oxford). 2010;49:1867–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi CS, Shi GY, Chang YS, Han HS, Kuo CH, Liu C, et al. Evidence of human thrombomodulin domain as a novel angiogenic factor. Circulation. 2005;111:1627–36. [DOI] [PubMed] [Google Scholar]
- 26.Vischer UM. von Willebrand factor, endothelial dysfunction, and cardiovascular disease. J Thromb Haemost 2006;4:1186–93. [DOI] [PubMed] [Google Scholar]
- 27.Mannucci PM, Vanoli M, Forza I, Canciani MT, Scorza R. Von Willebrand factor cleaving protease (ADAMTS-13) in 123 patients with connective tissue diseases (systemic lupus erythematosus and systemic sclerosis). Haematologica. 2003;88:914–8. [PubMed] [Google Scholar]
- 28.Ho CY, Wong CK, Li EK, Tam LS, Lam CW. Elevated plasma concentrations of nitric oxide, soluble thrombomodulin and soluble vascular cell adhesion molecule-1 in patients with systemic lupus erythematosus. Rheumatology (Oxford). 2003;42:117–22. [DOI] [PubMed] [Google Scholar]
- 29.Guzman J, Petty RE, Malleson PN. Monitoring disease activity in juvenile dermatomyositis: the role of von Willebrand factor and muscle enzymes. J Rheumatol 1994;21:739–43. [PubMed] [Google Scholar]
- 30.Figarella-Branger D, Schleinitz N, Boutiere-Albanese B, Camoin L, Bardin N, Guis S, et al. Platelet-endothelial cell adhesion molecule-1 and CD146: soluble levels and in situ expression of cellular adhesion molecules implicated in the cohesion of endothelial cells in idiopathic inflammatory myopathies. J Rheumatol 2006;33:1623–30. [PubMed] [Google Scholar]
- 31.Terraube V, O’Donnell JS, Jenkins PV. Factor VIII and von Willebrand factor interaction: biological, clinical and therapeutic importance. Haemophilia. 2010;16:3–13. [DOI] [PubMed] [Google Scholar]
- 32.Mohan S, Barsalou J, Bradley TJ, Slorach C, Reynolds JA, Hasni S, et al. Endothelial progenitor cell phenotype and function are impaired in childhood-onset systemic lupus erythematosus. Arthritis Rheumatol 2015;67:2257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee PY, Li Y, Richards HB, Chan FS, Zhuang H, Narain S, et al. Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Arthritis Rheum 2007;56:3759–69. [DOI] [PubMed] [Google Scholar]
- 34.Hollemann D, Budka H, Loscher WN, Yanagida G, Fischer MB, Wanschitz JV. Endothelial and myogenic differentiation of hematopoietic progenitor cells in inflammatory myopathies. J Neuropathol Exp Neurol 2008;67:711–9. [DOI] [PubMed] [Google Scholar]
- 35.Fall N, Bove KE, Stringer K, Lovell DJ, Brunner HI, Weiss J, et al. Association between lack of angiogenic response in muscle tissue and high expression of angiostatic ELR-negative CXC chemokines in patients with juvenile dermatomyositis: possible link to vasculopathy. Arthritis Rheum 2005;52:3175–80. [DOI] [PubMed] [Google Scholar]
- 36.Lutz J, Huwiler KG, Fedczyna T, Lechman TS, Crawford S, Kinsella TR, et al. Increased plasma thrombospondin-1 (TSP-1) levels are associated with the TNF alpha-308A allele in children with juvenile dermatomyositis. Clin Immunol 2002;103:260–3. [DOI] [PubMed] [Google Scholar]
- 37.Komiya T, Negoro N, Kondo K, Miura K, Hirota Y, Yoshikawa J. Clinical significance of von Willebrand factor in patients with adult dermatomyositis. Clin Rheumatol 2005;24:352–7. [DOI] [PubMed] [Google Scholar]
- 38.Funauchi M, Shimadsu H, Tamaki C, Yamagata T, Nozaki Y, Sugiyama M, et al. Role of endothelial damage in the pathogenesis of interstitial pneumonitis in patients with polymyositis and dermatomyositis. J Rheumatol 2006;33:903–6. [PubMed] [Google Scholar]
- 39.Barth Z, Witczak BN, Flato B, Koller A, Sjaastad I, Sanner H. Microvascular Abnormalities Assessed by Nailfold Capillaroscopy In Juvenile Dermatomyositis After Medium to Long-Term Follow-Up. Arthritis Care Res (Hoboken). 2018;70:768–76. [DOI] [PubMed] [Google Scholar]
- 40.Baumann M, Gumpold C, Mueller-Felber W, Schoser B, Haberler C, Loescher WN, et al. Pattern of myogenesis and vascular repair in early and advanced lesions of juvenile dermatomyositis. Neuromuscul Disord 2018;28:973–85. [DOI] [PubMed] [Google Scholar]
- 41.Castejon R, Jimenez-Ortiz C, Rosado S, Tutor-Ureta P, Mellor-Pita S, Yebra-Bango M. Metabolic syndrome is associated with decreased circulating endothelial progenitor cells and increased arterial stiffness in systemic lupus erythematosus. Lupus. 2016;25:129–36. [DOI] [PubMed] [Google Scholar]
- 42.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003;348:593–600. [DOI] [PubMed] [Google Scholar]
- 43.De Paepe B, Creus KK, De Bleecker JL. Chemokine profile of different inflammatory myopathies reflects humoral versus cytotoxic immune responses. Ann N Y Acad Sci 2007;1109:441–53. [DOI] [PubMed] [Google Scholar]
- 44.Bilgic H, Ytterberg SR, Amin S, McNallan KT, Wilson JC, Koeuth T, et al. Interleukin-6 and type I interferon-regulated genes and chemokines mark disease activity in dermatomyositis. Arthritis Rheum 2009;60:3436–46. [DOI] [PubMed] [Google Scholar]
- 45.Walsh RJ, Kong SW, Yao Y, Jallal B, Kiener PA, Pinkus JL, et al. Type I interferon-inducible gene expression in blood is present and reflects disease activity in dermatomyositis and polymyositis. Arthritis Rheum 2007;56:3784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thacker SG, Berthier CC, Mattinzoli D, Rastaldi MP, Kretzler M, Kaplan MJ. The detrimental effects of IFN-alpha on vasculogenesis in lupus are mediated by repression of IL-1 pathways: potential role in atherogenesis and renal vascular rarefaction. J Immunol 2010;185:4457–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gitiaux C, Latroche C, Weiss-Gayet M, Rodero MP, Duffy D, Bader-Meunier B, et al. Myogenic Progenitor Cells Exhibit Type I Interferon-Driven Proangiogenic Properties and Molecular Signature During Juvenile Dermatomyositis. Arthritis Rheumatol 2018;70:134–45. [DOI] [PubMed] [Google Scholar]


