Abstract
There are no pharmacological interventions to prevent the development of epilepsy, although many promising compounds have been identified in the animal laboratory. Clinical trials to validate their effectiveness, however, would currently be prohibitively expensive due to the large subject population and duration of follow-up necessary. There is, therefore, the need to identify biomarkers of epileptogenesis that could identify patients at high risk for epilepsy following a potential epileptogenic insult to enrich the subject population, as well as biomarkers that could determine the effectiveness of therapeutic intervention without the need to wait for seizures to occur. Putative biomarkers under investigation for epileptogenesis and its treatment include genetic, molecular, cellular, imaging, and electrophysiological measures that might reliably predict the development or progression of an epileptic condition, the effects of antiepileptogenic treatment, or cure after surgery. To be clinically useful for most purposes, ideal biomarkers should be noninvasive, and it is anticipated that a profile of multiple biomarkers will likely be required. Ongoing animal research involves a number of experimental models of epileptogenesis, with traumatic brain injury, offering the best potential for translational clinical investigations. Collaborative and multicenter research efforts by multidisciplinary teams of basic and clinical neuroscientists with access to robust, well-defined animal models, extensive patient populations, standardized protocols, and cutting-edge analytical methodologies are likely to be most successful. Such biomarker research should also provide insights into fundamental neuronal mechanisms of epileptogenesis suggesting novel targets for antiepileptogenic treatments.
Keywords: antiepileptogenesis, area under the curve, diagnosis, electroencephalogram, epileptogenesis, magnetic resonance imaging, microRNA, receiver operating characteristics
Introduction
Despite decades of productive translational research on fundamental neuronal mechanisms underlying epileptogenicity, and the introduction of over 20 new antiseizure drugs in the past 30 years, many of which were designed to correct specific pathophysiological substrates of seizure generation, there remain no pharmacological treatments to prevent epilepsy. The reasons for this are twofold: (a) Mechanisms of epileptogenesis are not the same as mechanisms of ictogenesis; therefore, drugs designed specifically to stop seizures would not be expected to also prevent the development of epilepsy. (b) Even if compounds were identified as potentially antiepileptogenic, and there are several, clinical trials to validate their effectiveness would be prohibitively expensive. Such a trial would require a subject population with a known epileptogenic insult, such as traumatic brain injury (TBI). Even with severe TBI, the incidence of later posttraumatic epilepsy (PTE) is 15 to 20%, making a very large subject population necessary to achieve statistically significant results. Furthermore, PTE most commonly begins one to two years following TBI and may occur ten years or more later, indicating that a trial to determine the efficacy of an antiepileptogenic agent would need to last, at least, several years.
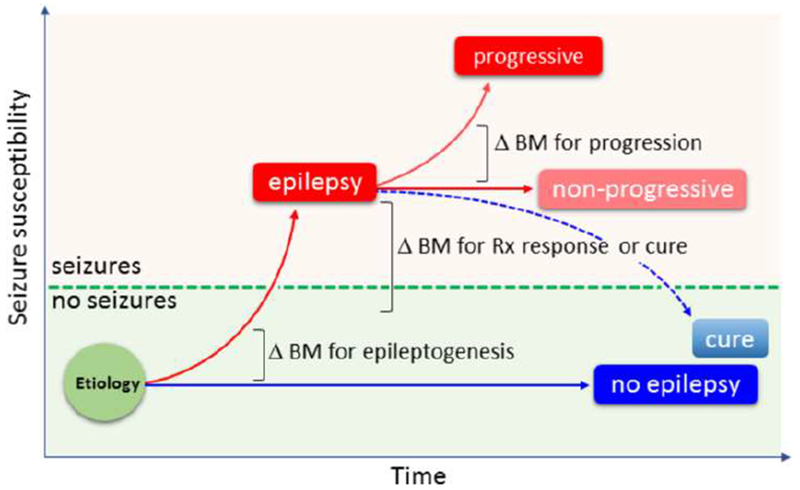
Research to develop prospective antiepileptogenic agents to prevent epilepsy is a high priority, and identification of biomarkers of epileptogenesis is viewed as the most practical solution to the problem of designing economically feasible clinical trials (Engel Jr. et al., 2013; Pitkänen and Engel Jr., 2014). This review will be specifically concerned with biomarkers of epileptogenesis and their relevance for studies to develop antiepileptogenic treatments. Epileptogenesis refers to the development and extension of tissue capable of generating spontaneous seizures, resulting in (a) the development of an epileptic condition and/or (b) progression of the epilepsy after it is established (Pitkänen and Engel Jr., 2014). Like any progressive pathology (e.g., Alzheimer’s disease), the evolution of epileptogenesis is a “moving target”. Antiepileptogenesis is a process that counteracts the effects of epileptogenesis, including prevention, seizure modification, and cure (Pitkänen and Engel Jr., 2014). Definitions of relevant terms appear in Table 1.
Table 1.
Concepts and definitions.
|
Epileptogenesis: the development and extension of tissue capable of generating spontaneous seizures, resulting in a) development of an epileptic condition and/or b) progression of the epilepsy after it is established. |
|
Ictogenesis: a propensity to generate epileptic seizures, including initiation and evolution of the epileptic seizures (also referred to as epileptogenicity). |
| Disease or Syndrome Modification: consists of antiepileptogenesis and comorbidity modification |
|
Antiepileptogenesis: a process that counteracts the effects of epileptogenesis, including prevention, seizure modification, and cure. Antiepileptogenesis can also prevent or reduce the progression of epilepsy after it has already been established. |
| • Prevention: Complete prevention aborts the development of epilepsy. Partial prevention can delay the development of epilepsy or reduce its severity. |
| • Cure: The complete and permanent reversal of epilepsy, such that no seizures occur after treatment withdrawal. |
|
Comorbidity Modification: treatment that alleviates or reverses the symptomatic development or progression of epilepsy-related comorbidities, such as anxiety, depression, somato-motor impairment, or cognitive decline. |
| Antiepileptogenic Treatment: can be given prior to or after epilepsy onset. When an antiepileptogenic treatment is given prior to epilepsy onset it prevents or delays the development of epilepsy. If seizures occur, they may be fewer in frequency, shorter, or of milder severity. When such a treatment is given after the diagnosis of epilepsy, it can alleviate seizure severity, prevent, or reduce the progression of epilepsy, or change the seizures from drug-resistant to drug-sensitive. |
Adapted from Pitkänen and Engel Jr. (2014) with permission.
Reliable biomarkers of epileptogenesis would permit selection of subjects with a very high likelihood of developing epilepsy due to genetic or structural causes, in order to enrich the subject population and reduce it to a workable number. Biomarkers would also be useful for tracking the treatment effect of the antiepileptogenic intervention (Pitkänen et al., 2018).
Biomarker – definition and categories
In 2015, the FDA-NIH Joint Leadership Council developed the BEST [BEST (Biomarkers, EndpointS, and other Tools) Resource; http://www.biostatsolutions.com/wp-content/uploads/2016/11/Bookshelf_NBK326791.pdf] to improve accuracy in the understanding and use of biomarker terminology in biomedical research, clinical practice, and medical product development.
A biomarker is a characteristic that is measured as an indicator of normal biologic processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions. Biomarkers may have molecular, histologic, radiographic, and physiologic characteristics. The BEST Glossary of biomarker categories includes: (a) susceptibility/risk biomarkers, (b) diagnostic biomarkers, (c) monitoring biomarkers, (d) prognostic biomarkers, (e) predictive biomarkers, (f) pharmacodynamic/response biomarkers, and (g) safety biomarkers. Biomarkers are developed through the process of analytical validation, clinical validation, and the demonstration of clinical utility (Frisoni et al., 2017). Epileptogenesis biomarkers can be categorized accordingly (Table 2).
Table 2.
Molecular, imaging, and electrophysiologic prognostic/diagnostic biomarkers for epileptogenesis, severe drug-refractory epilepsy and pharmacological and surgical therapy response.
| Study design | Modality | Tissue | Analysis platform | Biomarker | AUC | Reference |
|---|---|---|---|---|---|---|
| Prognostic & Diagnostic biomarker for epileptogenesis | ||||||
| Rats which develop epilepsy vs. rats which do not develop epilepsy after electrical stimulation - induced SE | Molecular | Plasma | ELISA | Increased total HMGB1 | 1.00* | Walker et al. (2017) |
| Children with benign childhood epilepsies vs. controls | Molecular | Hair | ECLIA | Increased hair cortisol within 24 h after the 1st seizure | 0.817 | Stavropoulos et al. (2017) |
| Rats with or without epilepsy after hyperthermia-induced SE at P11 | Imaging | Brain tissue - amygdala, thalamus | T2 MRI | T2 relaxation time ⇩ in basolateral amygdala T2 relaxation time ⇩ in medial amygdala T2 relaxation time ⇩ in medial thalamus |
0.910 0.820 0.780 |
Choy et al. (2014) |
| Rats with or without increased seizure susceptibility in the PTZ test after TBI | Imaging | Brain tissue - amygdala, hippocampus, thalamus | T2-w DTI MRI | T1σ in S1 cortex T1σ in Prh cortex T2 in thalamus T1σ in HC | 0.881 0.929 0.893 0.857 |
Pitkänen and Immonen (2014) |
| P21 rats which develop epilepsy vs. P21 rats which do not develop epilepsy after systemic pilocarpine -induced SE | Imaging | Septal pole of the hippocampus | MRS | Increased hippocampal mIns/tCr on day 72 post-SE | 0.830 | Pascente et al. (2016) |
| Patients with vs. without epilepsy after TBI | Imaging | Cerebral cortex | Gadolinium-MRI | Area of gadolinium leakage around cortical lesion after TBI | 0.850 | Data by A Friedman in Pitkänen et al. (2016) |
| Rats with epilepsy vs. no epilepsy after lateral fluid-percussion induced TBI | Electrophysiology | Brain | Sleep-EEG | Shortening of the duration of sleep spindles occurring at transition from N3 to REM | 0.907 | Andrade et al. (2016) |
| HFOs differentiate rats which develop epilepsy after intrahippocampal kainite injection or lateral fluid-percussion injury from those that will not | Electrophysiology | Brain | EEG | Presence of HFOs during the first 2 post-injury weeks | no data | Bragin et al. (2004, 2017) |
| Diagnostic biomarker for epileptogenesis or Prognostic biomarker for development epilepsy in rodents with brain injury |
Mice with or without epilepsy after i.c.v. injection of albumin, TGF-β, or IL-6 Rats with or without epilepsy after photothrombotic stroke Rats with or without epilepsy after bilateral hippocampal electrical stimulation - induced SE |
Brain | Electrophysiology Theta dynamics in EEG on days 2-4 |
Absolute slope value of dynamic change in theta band Distance from the mean control group |
0.910 0.997 |
Milikovsky et al. (2017) |
| Patients with acute anterior circulation ischemic stroke developing epilepsy vs. not developing epilepsy | Electrophysiology | Brain | EEG | Background asymmetry Interictal epileptiform activity |
0.810 | Bentes et al. (2018) |
| Three models | Electrophysiology | Brain | EEG | Decrease in non-linear dynamics dimension in EEG/ECoG | ≥0.886 in different models | Rizzi et al. (2019) |
| Prognostic/Diagnostic biomarkers for progression of epilepsy (drug-refractoriness) or epileptogenicity | ||||||
| Patients with high (median ≥4 sz/month) vs. low (<4) seizure frequency undergoing temporal lobectomy with amygdalohippocampectomy due to intractable TLE | Molecular | Lateral temporal cortex | Affymetrix GeneChip Human Gene 1.0 ST Array | Relative down-regulation of 35 genes and up-regulation of 5 genes in ≥4 sz/month group | ≥0.904* * logistic regression |
McCallum et al. (2016) |
| Drug-sensitive vs. drug-refractory patients | Molecular | Plasma | ELISA | Total HBMG1 | 0.990 | Walker et al. (2017) |
| Patients with benign vs. refractory mTLE | Imaging | Brain tissue - temporal lobe grey and white matter | DTI MRI | ⇧ ipsilateral MD ⇩ ipsilateral FA ⇩ ipsilateral HC vol ipsilateral HS FA+HS |
0.670 0.770 0.670 0.660 0.768 |
Labate et al. (2015) |
| Diagnostic biomarker for localization of seizure onset zone (tissue epileptogenicity) |
Intrahippocampal kainate model of TLE and lateral FPI model of PTE in rat Seizure onset zone vs. other brain areas in humans evaluated for epilepsy surgery |
Brain | Electrophysiology iHC or iECEEG scalp EEG |
occurrence of HFOs stereotypical HFOs with waveform similarity Spikes x HFO |
no data |
Bragin et al. (1999) Liu et al. (2018) Roehri et al. (2018) |
| Predictive biomarker for antiepileptogenic treatment effect | ||||||
| Post-SE rats treated with vehicle vs. anakinra+BoxA+ifenprodil polytherapy | Molecular | Plasma | ELISA | Prevention in the increase in total HMGB1 | ≥0.900* | Walker et al. (2017) |
| Predictive biomarker for cure after epilepsy surgery | ||||||
| Patients undergoing temporal lobectomy with amygdalohippocampectomy due to intractable TLE with seizure-free vs. non-seizure-free outcome | Molecular | Lateral temporal cortex | Affymetrix GeneChip Human Gene 1.0 ST Array | Relative down-regulation of ZNF852 CDCP2 PRRT1 FLJ41170 and 7 RNA probes in sz-free subjects |
0.958* 0.941* 0.942* 0.908* * logistic regression |
Gallek et al. (2016) |
Abbreviations: AUC, area under the curve; DTI, diffusion tensor imaging; ECLIA, electrochemiluminecence assay; EEG, electroencephalogram; ELISA, enzyme linked immune assay; HFO, high-frequency oscillation; HMGB1, high-mobility group box 1 protein; i.c.v., intracerebroventricular; i.p., intraperitoneal; MRI, magnetic resonance imaging; mTLE, medial temporal lobe epilepsy; P, postnatal day; PTE, post-traumatic epilepsy; PTZ, pentylenetetrazol; SE, status epilepticus; T2, a time constant for the decay of transverse magnetization arising from natural interactions at the atomic or molecular levels; TBI, traumatic brain injury; TLE, temporal lobe epilepsy
Clinical utility of epileptogenic biomarkers has four major needs: (a) Identification of patients who need treatment after potential epileptogenic insults, (b) verification of potential anti-epileptogenic treatments, (c) identification of progressive epilepsies that need more aggressive treatments, such as surgery, and (d) diagnosis of cure or prevention.
Biomarkers for epileptogenesis and antiepileptogenesis – the need for a multimodal approach
At the molecular and cellular levels, epileptogenesis after a structural cause refers to a process in which an initial brain-damaging insult triggers a cascade of molecular and cellular changes that eventually lead to the occurrence of unprovoked seizures. Cellular alterations include neurodegeneration, neurogenesis, axonal sprouting, axonal and myelin injury, dendritic remodeling, various types of gliosis, inflammatory cell invasion, blood-brain-barrier damage, angiogenesis, alterations in extracellular matrix composition, possible aggregation of materials (e.g., iron and calcium), and acquired channelopathies (Pitkänen and Lukasiuk, 2011, 2009). These pathologies generate a “cellular and molecular ecosystem” which eventually triggers seizures, and can be modulated by treatments. The epileptogenic tissue milieu expresses and secretes molecules that can be measured as biomarkers in different epilepsy indications, including therapy trials, using various approaches such as blood/cerebrospinal fluid (CSF) analysis, brain tissue analysis (e.g., cortical or hippocampal tissue), and indirectly by imaging, or electrophysiology. Importantly, at each stage, the epileptogenic process and biomarker discovery can be modulated by the genetic make-up, microbiota, and exposome.
At the network level, epileptogenesis refers to cell loss and neurogenesis, resulting in axonal sprouting and synaptic reorganization, which not only alter local circuit and more widespread excitability, but also predispose to enhance synchronization of pathological neuronal discharges such as burst firing. The subsequent development of a localized epileptogenic region and a distributed epileptogenic network can be measured by recording specific electrophysiological patterns (Engel et al., 2018; Frauscher et al., 2017) such as interictal spikes, high-frequency oscillations (HFOs) or sleep spindles, as well as by structural and functional magnetic resonance imaging, and newer technologies, including optogenetics, and DREADDs (designer receptor exclusively activated by designer drugs).
Considering the complexity of the epileptogenic process, it is anticipated that no single biomarker will be a robust measure of any of the functions necessary to design a cost-effective clinical trial of an antiepileptogenic agent, but, rather, a profile of biomarkers will eventually be necessary to increase sensitivity and specificity.
The optimal biomarker
Optimal biomarkers should be specific for epilepsy and for a given epilepsy indication. It should be translatable so that biomarker(s) used for therapy development in the animal laboratory can be applied in clinical antiepileptogenesis trials. This means that, for most clinical purposes, the optimal biomarker must be noninvasive. Therefore, molecular and cellular biomarkers determined from analysis of brain tissue in experimental animals would need to inform noninvasive measures using imaging techniques such as positron emission tomography (PET). Electrophysiological biomarkers, such as HFOs, that are recorded directly from the brain in experimental animals may eventually be visible with scalp EEG (Cuello-Oderiz et al., 2017), or by alternative techniques such as magnetoencephalography (MEG) (Velmurugan et al., 2018). Sleep-EEG could also provide a translatable modality for therapy development and monitoring of treatment effect (Andrade et al., 2017). Biomarkers should also have a long expression time window and stability. The analysis platform should be accessible, rapid, reproducible, and economically feasible.
Epileptogenesis biomarkers - State of the art in 2019
Diagnosis of epileptogenesis.
Biomarkers for epileptogenesis can be “prognostic” that indicate likelihood of epilepsy after a brain insult or “diagnostic” that identify the presence of ongoing epileptogenesis at a given time point. We previously estimated that by increasing the likelihood of developing epilepsy from 20% to 50% in a study population would reduce the cost of the powered human antiepileptogenesis study from 18 M$ to 3.6 M$ (Engel et al., 2013a). There are great hopes that this goal will be achieved by identification of sensitive and specific (a) susceptibility/risk biomarkers for a given epilepsy syndrome (e.g., genetic biomarkers with strong association with epileptogenesis), (b) prognostic biomarkers which anticipate the risk of epilepsy, for example, at 2-year timepoint after TBI or (c) diagnostic biomarkers which would reveal ongoing epileptogenesis even without knowledge of accurate timing of the potential preceding brain insult. Table 2 lists the studies which have reported prognostic and/or diagnostic biomarkers for epileptogenesis.
Genetics -
A few genetic markers have been linked to increased risk of structural epileptogenesis such as mutations in CD-40-1C/T or Rs671 genes for post-stroke epilepsy (for review, see (Pitkänen et al., 2016b, 2016a). However, the study populations have been small and the studies wait to be replicated before these genetic markers would be ready for patient stratification into antiepileptogenesis studies, possibly jointly with the expression of other prognostic/diagnostic biomarkers in a given subject.
Molecular analysis -
So far, two molecular biomarkers have shown promise in epileptogenesis diagnosis. Walker et al. (2017) reported that increased total plasma high-mobility group box 1 protein (HMGB1) levels differentiated animals which will develop epilepsy after unilateral hippocampal electrical stimulation induced status epilepticus from those that will not. An interesting study of Stavropoulos et al. (2017) reported that 6-12 y old children with benign childhood epilepsy syndromes had a 1.5-fold increase in cortisol levels in a 3-cm scalp hair sample when assessed within 24 h after the first seizure as compared to controls, reporting on preceding chronic stress for approximately 3 months. This observation is interesting as it takes weeks for the cortisol to accumulate in hair (Russell et al., 2012; Stalder and Kirschbaum, 2012; Wester and Van Rossum, 2015), and thus, suggests increased hair cortisol levels and hypothalamic-pituitary-adrenal axis dysfunction even before the occurrence of the 1st seizure.
Imaging -
Four imaging studies have reported imaging parameters as prognostic & diagnostic biomarkers for epileptogenesis for various structural epilepsies. Choy et al. (2014) found that reduced T2 relaxation time in the basolateral or medial amygdala or in the medial thalamus identified rats with or without later epilepsy induced by hyperthermic seizures at postnatal day (P) 11. Pitkänen and Immonen (2014) reported that increased diffusion trace T1σ) in the somatosensory and perirhinal cortices and hippocampus and increased T2 in the thalamus indicated rats with increased seizure susceptibility after pentylenetetrazol administration after lateral fluid-percussion induced TBI. More recently, Pascente et al. (2016) showed that increased hippocampal myo-inositol/total creatine ratio diagnosed P21 animals which were going to develop epilepsy after lithium-pilocarpine -induced SE from those that will not. Finally, human data from Friedman’s laboratory showed that the area of the gadolinium leakage around the cortical lesion rather than the area of the cortical lesion itself indicated the presence of PTE (see Pitkänen et al., 2016a).
Electrophysiology -
Six studies have identified potential epileptogenesis biomarkers in EEG. Andrade et al. (2017) found that shortening of sleep spindles at N3-REM transition indicated animals with PTE after lateral fluid-percussion TBI. Bragin et al. (2004) found that HFOs appeared days after intrahippocampal kainic acid induced SE in rats, only in animals that later developed epilepsy. Furthermore, the duration of time before HFO appearance correlated with the delay to first seizure. Bragin et al. (2016) also found that perilesional HFOs and repetitive HFOs on EEG spikes were present in animals which later developed epilepsy in the lateral FPI model of TBI. Milikovsky et al. (2017) revealed that a dynamic change in the theta band presented a diagnostic biomarker for epileptogenesis in several structural epilepsy models. Rizzi et al. (2019) demonstrated that a decrease in non-linear dynamics dimensions in the EEG/electrocorticogram differentiated epileptogenic from non-epileptogenic rats in three SE model of epileptogenesis. A clinical study by Bentes et al. (2018) showed that background EEG asymmetry differentiated patients with acute anterior circulation ischemic stroke who developed epilepsy from those who did not.
Identification of progressive epilepsies that need more aggressive treatments, such as surgery.
Two clinical studies have compared patient populations with drug-responsive and drug-refractory epilepsy to identify biomarkers for drug-refractoriness (Table 2). Walker et al. (2017) reported that elevated total plasma HBMG1 levels differentiated drug-refractory patients from drug-sensitive patients. Labate et al. (2015) found that increased ipsilateral medial diffusivity, decreased fractional anisotropy, and reduced hippocampal volume associated with refractory medial TLE.
Another two studies have proposed biomarkers for epileptogenicity of the brain tissue. McCallum et al. (2016) analyzed transcriptomics signatures of the brain tissue available from epilepsy surgery. They reported a relative downregulation of 35 genes and upregulation of 5 genes in patients with ≥4 seizures per month as compared to those with ≥4 seizures per month. As in animals, aspects of pHFOs are considered to be an indicator of epileptogenic brain tissue in humans (Bragin et al., 1999; Liu et al., 2018; Roehri et al., 2017).
Development of anti-epileptogenic treatments.
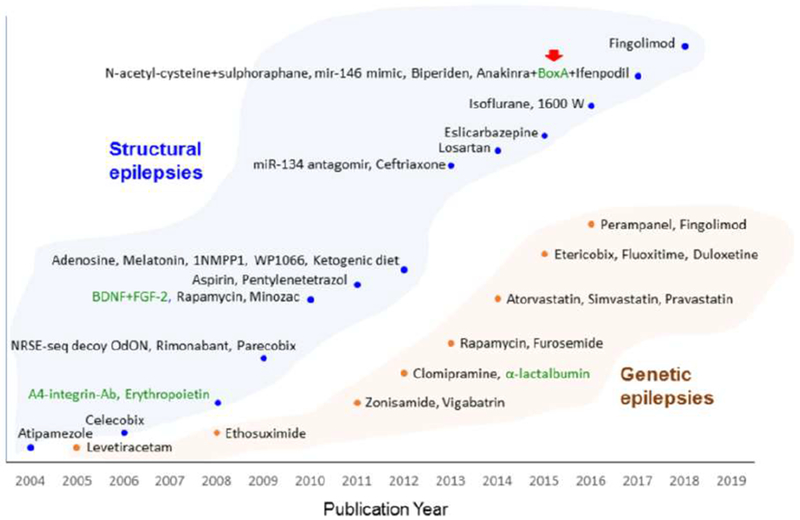
The development of small molecule as well as biological treatments to combat epileptogenesis is in great need of biomarkers which (a) predict therapy response, (b) monitor treatment effect, (c) report on pharmacodynamics, and (d) safety. Despite of over 40 treatment approaches (Fig. 2), so far, only one study has been published of epilepsy biomarkers related to drug therapy. Walker et al. (2017) reported that the total prevention of the increase in the level of total HBMG1 in plasma indicated a favorable therapy response to anakinra-BoxA-ifenprodil polytherapy after SE-induced epileptogenesis in rats.
Figure 2. Proof-of-concept trials of antiepileptogenic treatment and discovery of predictive biomarkers for therapy response.
Treatments that have been demonstrated to have a disease-modifying effect on epileptogenesis in animal models of structural (blue dots) and genetic (brown dots) epilepsies are plotted according to their publication year [for references, see Pitkänen et al. (2014); Russo and Citraro (2018)]. Compounds include mostly small molecules and only a few biologicals (green text) have been tested, so far. Red arrow indicates the only therapy trial that applied predictive biomarkers to demonstrate treatment effect. Walker et al. (2017) showed that a decrease in total plasma HMBG1 levels predicted therapy response to anakinra+BoxA+ifenpodil polytherapy in a status epilepticus model of epileptogenesis in rat.
Epilepsy surgery outcome - Cure.
Surgery is the only therapy that can cure epilepsy. Using molecular profiling, Gallek et al. (2016) reported a relative down-regulation of ZNF852, CDCP2, PRRT1, FLJ41170 and 7 RNA probes in subjects who became seizure free as compared to non-seizure free subjects following anterior temporal lobectomy for intractable temporal lobe epilepsy (TLE).
How to facilitate the progress in epileptognenesis biomarker discovery and validation
Despite many promising biomarker discovery studies, there are no epileptogenesis-related biomarkers in clinical use. As Table 2 summarizes, biomarkers investigated in the laboratory are not investigated in the clinic. The only exceptions, so far, are plasma HMBG1 levels and presence of pHFOs in EEG recordings. These proof-of-concept studies suggest that translation can be possible both in plasma proteins as well as in electrophysiological signals. Still, neither one is validated in large cohorts and target populations need to be specified. Also, the analysis platforms need to be developed that qualify for rapid affordable analysis and fill the standards for clinical use (Pitkänen et al., 2018).
Model selection.
Preclinical studies to identify reliable biomarkers of epileptogenesis and ictogenesis are best performed with an animal model in which the timing of a potentially epileptogenic insult to a normal brain is known, in order to follow the course of the epileptogenic process from insult to the appearance of epilepsy. An animal model must also have translational potential. A good candidate clinical condition that fits these criteria for modeling is TBI, and several approaches are available to mimic various forms of TBI that lead to PTE in humans. Animal models showing post-TBI spontaneous seizures investigated in the TBI field include lateral fluid-percussion injury modeling a mixed type (both focal and diffuse) injury, controlled cortical impact (focal contusion), and weight-drop model (diffuse injury)[for review, see Pitkänen et al. (2017)]. In addition, highly epileptogenic cortical iron injection mimicking post-TBI hemorrhage (Willmore et al., 1978), cortical undercut model in mice and rats mimicking stab wound TBI (Graber and Prince, 2006; Ping and Jin, 2016), or intracerebral metals mimicking gun-shot injuries (Kendirli et al., 2014) have been described and used in drug discovery but not yet in biomarker discovery.
The most studied animal models of epileptogenesis, and therefore the best understood, are those that model hippocampal sclerosis, which is induced in the laboratory by various forms of experimental status epilepticus (Aronica et al., 2017). Unfortunately, neither the timing, nor the nature, of the epileptogenic insults responsible for hippocampal sclerosis in humans is known so translational studies are not possible. Like PTE after TBI, epilepsy following status epilepticus is variable. Intracranial hemorrhage and stroke can be reproduced in the animal laboratory (Kelly, 2017; Klahr et al., 2014) and are a common cause of epilepsy in humans. Animal models of epilepsy following these conditions have not been as well established, however, as the animal model for TBI.
Finally, tuberous sclerosis has been suggested as a particularly appropriate condition to be modeled because 80% of children born with this disease will develop epilepsy within the first two years of life, which automatically constrains the subject population and the duration of a clinical trial. Animal models of tuberous sclerosis exist, and research on this condition is proceeding (Jeoang and Wong, 2017; Davis et al., 2019). It is not clear, however, to what extent there is a process of epileptogenesis after birth that can be interrupted by an antiepileptogenic intervention, as opposed to an epileptogenic process that occurred in utero, in which the appearance of epileptic seizures one or two years after birth reflects brain maturation that eventually is capable of supporting ictogenesis.
Validation.
Theoretically, it may not be necessary to know the exact timing of an epileptogenic insult in order to validate the effectiveness of an antiepileptogenic intervention. For instance, there is no reason to assume that the epileptogenic process in every situation is completed, and therefore ceases, as soon as the first epileptic seizure occurs. For many patients, the first ictal event of chronic epilepsy is an indication that the epileptogenic process is continuing and will result in more frequent and more severe seizures. Animal models, therefore, could be developed utilizing a variety of epileptogenic interventions in order to study the effectiveness of a potential antiepileptogenic intervention introduced after the first seizure occurs. Genetic epilepsies begin at various times after birth, and at least some of these could represent an epileptogenic process over time, as opposed to an effect of brain maturation. Indeed, there is evidence, in a rat model of absence epilepsy, that treatment with anti-absence medications before seizures appear prevents their later occurrence [Figure 2, Russo and Citraro (2018)]. Genetic biomarkers indicating the risk of developing a genetic epilepsy provide the opportunity for antiepileptogenic interventions, and these conditions could also be modeled in the animal laboratory.
Statistical power and big data.
Many more investigations have been published in recent years of putative molecular and cellular (Diamond et al., 2015; Gorter et al., 2014; Hegde and Lowenstein, 2014; Holtman et al., 2013; Loeb, 2011; Löscher and Brandt, 2010), electrophysiological (Gabriel and Rowe, 2014; Huneau et al., 2013; Staba et al., 2014; Staba and Bragin, 2011; Worrell and Gotman, 2011), and imaging (Engel et al., 2013b; Gomes and Shinnar, 2011; Lin et al., 2007, 2005; Obenaus, 2013; Savic and Engel, 2014) epilepsy biomarkers that could indicate epileptogenesis; however, a primary obstacle has been the fact that both animal and clinical studies have invariably been underpowered. Furthermore, the lack of standardized approaches has prevented serious comparative and translational analyses among published studies.
Collaborative and multicenter research efforts, composed of multidisciplinary teams of basic and clinical neuroscientists with access to robust, well-defined animal models, extensive patient populations, standardized protocols, and cutting-edge analytic methodologies, including big data and artificial intelligence approaches, offer the best opportunity to realize the goal of identifying reliable epileptogenic biomarkers. Adequate predictive power will likely require a combination of biochemical, electrophysiologic, and neuroimaging biomarkers, measured at different post-injury time points, to diagnose epileptogenesis, with high sensitivity and specificity, independent of the severity of brain damage. Several large collaborative efforts have been funded by the European Commission and the U.S. National Institutes of Health in recent years, and an increasing number of reports on potential epileptogenic biomarkers are appearing in the literature. Such studies will likely also provide insights into the fundamental neuronal mechanisms of these processes and inform basic research into novel targets for antiepileptogenic interventions.
Figure 1. Epileptogenesis biomarkers.
As epileptogenic process is progressive, the sensitivity and specificity of a given biomarker is tied to the timing of its measurement (x-axis). The same time point can present different stages of progression in different epilepsy syndromes. For example, in status epilepticus -induced epileptogenesis models, a 3-wk time point is often “late epileptogenesis” whereas in ischemic stroke and traumatic brain injury models it is “early epileptogenesis”. Such staging does not, however, exclude the possibility that the same biomarker could be expressed in all models at the same time point despite it would be reporting of the different stage of epileptogenesis in a given model. For example, in most of the structural epileptogenesis models one could expect to see expression of cell death markers during the first post-insult week. As summarized in Table 2, proof-of-concept studies have identified preclinical and clinical prognostic & diagnostic biomarkers for epileptogenesis, diagnosis of drug-refractory epilepsy, diagnosis of tissue epiletogenicity, prediction of drug-therapy response and prediction of epilepsy surgery outcome.
Highlights.
There are no pharmacological interventions to prevent the development of epilepsy, in large part because clinical trials to validate their efficacy would be prohibitively expensive.
There’s a great need to identify biomarkers of epileptogenesis in order to facilitate clinical trials of antiepileptogenic interventions, by enriching the subject population, and documenting efficacy without the need to wait for seizures to occur.
Several animal models of human epilepsy are appropriate for translational investigations to identify putative genetic, molecular, cellular, imaging, and electrophysiological biomarkers that can predict the development or progression of epilepsy.
Large collaborative multicenter research efforts by multidisciplinary teams of basic and clinical neuroscientists with access to well-defined animal models, extensive patient populations, standardized protocols, and cutting-edge analytical methodologies are currently underway.
Acknowledgments
Original research reported by the authors was supported by grants NS002808, NS015654, NS033310, NS042372, NS080181, NS100064, R01NS065877 (JE), and U54NS100064 (EpiBioS4Rx JE, AP) from the National Institutes of Health, grants 272249 and 273909 (AP) from the Medical Research Council of the Academy of Finland, and grant 602102 (EPITARGET JE, AP) from the European Union.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andrade P, Nissinen J, Pitkänen A, 2017. Generalized Seizures after Experimental Traumatic Brain Injury Occur at the Transition from Slow-Wave to Rapid Eye Movement Sleep. J. Neurotrauma 34 10.1089/neu.2016.4675 [DOI] [PubMed] [Google Scholar]
- Aronica E, Muhlebner A, van Vliet E, Gorter J, 2017. Characterization of pathology, in: Pitkänen A, Buckmaster P, Galanopoulou A, Moshe SL (Eds.), Models of Sezures and Epilepsy. London, pp. 139–160. [Google Scholar]
- Bentes C, Martins H, Peralta AR, Morgado C, Casimiro C, Franco AC, Fonseca AC, Geraldes R, Canhão P, Pinho e Melo T, Paiva T, Ferro JM, 2018. Early EEG predicts poststroke epilepsy. Epilepsia Open 3, 203–212. 10.1002/epi4.12103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEST (Biomarkers, EndpointS, and other Tools) Resource [WWW Document], n.d. URL https://www.ncbi.nlm.nih.gov/books/NBK326791/
- Bragin A, Engel J, Wilson CL, Fried I, Mathern GW, 1999. Hippocampal and entorhinal cortex high-frequency oscillations (100--500 Hz) in human epileptic brain and in kainic acid--treated rats with chronic seizures. Epilepsia 40, 127–37. [DOI] [PubMed] [Google Scholar]
- Bragin A, Li L, Almajano J, Alvarado-Rojas C, Reid AY, Staba RJ, Engel J, 2016. Pathologic electrographic changes after experimental traumatic brain injury. Epilepsia 57, 735–45. 10.1111/epi.13359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Almajano J, Mody I, Engel J, 2004. High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia 45, 1017–23. 10.1111/j.0013-9580.2004.17004.x [DOI] [PubMed] [Google Scholar]
- Choy M, Dubé CM, Patterson K, Barnes SR, Maras P, Blood AB, Hasso AN, Obenaus A, Baram TZ, 2014. A novel, noninvasive, predictive epilepsy biomarker with clinical potential. J. Neurosci 34, 8672–84. 10.1523/JNEUROSCI.4806-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello-Oderiz C, von Ellenrieder N, Dubeau F, Gotman J, 2017. Influence of the location and type of epileptogenic lesion on scalp interictal epileptiform discharges and high-frequency oscillations. Epilepsia 58, 2153–2163. 10.1111/epi.13922 [DOI] [PubMed] [Google Scholar]
- Davis PE, Kapur K, Filip-Dhima R, Trowbridge SK, Little E, Wilson A, Leuchter A, Bebin EM, Krueger D, Northrup H, Wu JY, Sahin M, peters JM Increased electroencephalography connectivity precedes epileptic spasm onset in infants with tuberous sclerosis complex. Epilepsia. 2019. July 12 10.1111/epi.16284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ML, Ritter AC, Failla MD, Boles JA, Conley YP, Kochanek PM, Wagner AK, 2015. IL-1β associations with posttraumatic epilepsy development: A genetics and biomarker cohort study. Epilepsia 56, 991–1001. 10.1111/epi.13100 [DOI] [PubMed] [Google Scholar]
- Engel J, Bragin A, Staba R, 2018. Nonictal EEG biomarkers for diagnosis and treatment. Epilepsia open 3, 120–126. 10.1002/epi4.12233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Pitkänen A, Loeb JA, Dudek FE, Bertram EH, Cole AJ, Moshé SL, Wiebe S, Jensen FE, Mody I, Nehlig A, Vezzani A, 2013a. Epilepsy biomarkers. Epilepsia 54 Suppl 4, 61–9. 10.1111/epi.12299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Thompson PM, Stern JM, Staba RJ, Bragin A, Mody I, 2013b. Connectomics and epilepsy. Curr. Opin. Neurol 26, 186–94. 10.1097/WCO.0b013e32835ee5b8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J Jr., Pitkanen A, Loeb JA, Dudek FE, Bertram EH III, Cole AJ, Moshé SL, Wiebe S, Jensen FE, Mody I, Nehlig A, Vezzani A, 2013. Epilepsy biomarkers. Epilepsia 54 10.1111/epi.12299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauscher B, Bartolomei F, Kobayashi K, Cimbalnik J, van’t Klooster MA, Rampp S, Otsubo H, Höller Y, Wu JY, Asano E, Engel J, Kahane P, Jacobs J, Gotman J, 2017. High-frequency oscillations: The state of clinical research. Epilepsia 58, 1316–1329. 10.1111/epi.13829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoni GB, Boccardi M, Barkhof F, Blennow K, Cappa S, Chiotis K, Démonet J-F, Garibotto V, Giannakopoulos P, Gietl A, Hansson O, Herholz K, Jack CR, Nobili F, Nordberg A, Snyder HM, Ten Kate M, Varrone A, Albanese E, Becker S, Bossuyt P, Carrillo MC, Cerami C, Dubois B, Gallo V, Giacobini E, Gold G, Hurst S, Lönneborg A, Lovblad K-O, Mattsson N, Molinuevo J-L, Monsch AU, Mosimann U, Padovani A, Picco A, Porteri C, Ratib O, Saint-Aubert L, Scerri C, Scheltens P, Schott JM, Sonni I, Teipel S, Vineis P, Visser PJ, Yasui Y, Winblad B, 2017. Strategic roadmap for an early diagnosis of Alzheimer’s disease based on biomarkers. Lancet. Neurol 16, 661–676. 10.1016/S1474-4422(17)30159-X [DOI] [PubMed] [Google Scholar]
- Gabriel WM, Rowe AS, 2014. Long-term comparison of GOS-E scores in patients treated with phenytoin or levetiracetam for posttraumatic seizure prophylaxis after traumatic brain injury. Ann. Pharmacother 48,1440–4. 10.1177/1060028014549013 [DOI] [PubMed] [Google Scholar]
- Gallek MJ, Skoch J, Ansay T, Behbahani M, Mount D, Manziello A, Witte M, Bernas M, Labiner DM, Weinand ME, 2016. Cortical gene expression: prognostic value for seizure outcome following temporal lobectomy and amygdalohippocampectomy. Neurogenetics 17, 211–218. 10.1007/s10048-016-0484-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes WA, Shinnar S, 2011. Prospects for imaging-related biomarkers of human epileptogenesis: a critical review. Biomark. Med 5, 599–606. 10.2217/bmm.11.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter JA, Iyer A, White I, Colzi A, van Vliet EA, Sisodiya S, Aronica E, 2014. Hippocampal subregion-specific microRNA expression during epileptogenesis in experimental temporal lobe epilepsy. Neurobiol. Dis 62, 508–20. 10.1016/j.nbd.2013.10.026 [DOI] [PubMed] [Google Scholar]
- Graber K, Prince DA, 2006. Chronic partial cortical isolation, in: Pitkäonen A, PA S, SL M (Eds.), Models of Seizures and Epilepsy. Elsevier, Amsterdam, pp. 465–476. [Google Scholar]
- Hegde M, Lowenstein DH, 2014. The search for circulating epilepsy biomarkers. Biomark. Med 8, 413–27. 10.2217/bmm.13.142 [DOI] [PubMed] [Google Scholar]
- Holtman L, van Vliet EA, Aronica E, Wouters D, Wadman WJ, Gorter JA, 2013. Blood plasma inflammation markers during epileptogenesis in post-status epilepticus rat model for temporal lobe epilepsy. Epilepsia 54, 589–95. 10.1111/epi.12112 [DOI] [PubMed] [Google Scholar]
- Huneau C, Benquet P, Dieuset G, Biraben A, Martin B, Wendling F, 2013. Shape features of epileptic spikes are a marker of epileptogenesis in mice. Epilepsia 54, 2219–27. 10.1111/epi.12406 [DOI] [PubMed] [Google Scholar]
- Jeoang A, Wong M, 2017. Tuberous sclerosis and other mTORopathies, in: Pitkänen A, Buckmaster PS, Galanopoulou AS, Moshe SL (Eds.), Animal Modles of Seizures and Epilepsy. Elsevier, London, pp. 797–812. [Google Scholar]
- Kelly KM, 2017. Poststroke epilepsy, in: Pitkänen A, Buckmaster PS, Galanopoulou AS, Moshe SL (Eds.), Animal Models of Seizures and Epilepsy. Elsevier, London, pp. 727–742. [Google Scholar]
- Kendirli MT, Rose DT, Bertram EH, 2014. A model of posttraumatic epilepsy after penetrating brain injuries: effect of lesion size and metal fragments. Epilepsia 55, 1969–77. 10.1111/epi.12854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahr AC, Dickson CT, Colbourne F, 2014. Seizure Activity Occurs in the Collagenase but not the Blood Infusion Model of Striatal Hemorrhagic Stroke in Rats. Transl. Stroke Res 6, 29–38. 10.1007/s12975-014-0361-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labate A, Cherubini A, Tripepi G, Mumoli L, Ferlazzo E, Aguglia U, Quattrone A, Gambardella A, 2015. White matter abnormalities differentiate severe from benign temporal lobe epilepsy. Epilepsia 56, 1109–16. 10.1111/epi.13027 [DOI] [PubMed] [Google Scholar]
- Lin JJ, Salamon N, Dutton RA, Lee AD, Geaga JA, Hayashi KM, Toga AW, Engel J, Thompson PM, 2005. Three-dimensional preoperative maps of hippocampal atrophy predict surgical outcomes in temporal lobe epilepsy. Neurology 65, 1094–7. 10.1212/01.wnl.0000179003.95838.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JJ, Salamon N, Lee AD, Dutton RA, Geaga JA, Hayashi KM, Luders E, Toga AW, Engel J, Thompson PM, 2007. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cereb. Cortex 17, 2007–18. 10.1093/cercor/bh1109 [DOI] [PubMed] [Google Scholar]
- Liu S, Gurses C, Sha Z, Quach MM, Sencer A, Bebek N, Curry DJ, Prabhu S, Tummala S, Henry TR, Ince NF, 2018. Stereotyped high-frequency oscillations discriminate seizure onset zones and critical functional cortex in focal epilepsy. Brain, 10.1093/brain/awx374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb JA, 2011. Identifying targets for preventing epilepsy using systems biology. Neurosci. Lett 497, 205–12. 10.1016/j.neulet.2011.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löscher W, Brandt C, 2010. Prevention or modification of epileptogenesis after brain insults: experimental approaches and translational research. Pharmacol. Rev 62, 668–700. 10.1124/pr.110.003046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum AP, Gallek MJ, Ramey W, Manziello A, Witte MH, Bernas MJ, Labiner DM, Weinand ME, 2016. Cortical gene expression correlates of temporal lobe epileptogenicity. Pathophysiology 23, 181–190. 10.1016/j.pathophys.2016.05.006 [DOI] [PubMed] [Google Scholar]
- Milikovsky DZ, Weissberg I, Kamintsky L, Lippmann K, Schefenbauer O, Frigerio F, Rizzi M, Sheintuch L, Zelig D, Ofer J, Vezzani A, Friedman A, 2017. Electrocorticographic Dynamics as a Novel Biomarker in Five Models of Epileptogenesis. J. Neurosci 37, 4450–4461. 10.1523/JNEUROSCI.2446-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obenaus A, 2013. Neuroimaging biomarkers for epilepsy: advances and relevance to glial cells. Neurochem. Int 63, 712–8. 10.1016/j.neuint.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascente R, Frigerio F, Rizzi M, Porcu L, Boido M, Davids J, Zaben M, Tolomeo D, Filibian M, Gray WP, Vezzani A, Ravizza T, 2016. Cognitive deficits and brain myo-lnositol are early biomarkers of epileptogenesis in a rat model of epilepsy. Neurobiol. Dis 93, 146–155. 10.1016/j.nbd.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Ping X, Jin X, 2016. Chronic posttraumatic epilepsy following neocortical undercut lesion in mice. PLoS One 11, 1–12. 10.1371/journal.pone.0158231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, Ekolle Ndode-Ekane X, Lapinlampi N, Puhakka N, 2018. Epilepsy biomarkers – Toward etiology and pathology specificity. Neurobiol. Dis 10.1016/j.nbd.2018.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, Engel J Jr., 2014. Past and Present Definitions of Epileptogenesis and Its Biomarkers. Neurotherapeutics 11 10.1007/s13311-014-0257-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, Huusko N, Ndode-Ekane XE, Kyyriäinen J, Lipponen A, Lipsanen A, Sierra A, Bolkvadze T, 2014. Gender issues in antiepileptogenic treatments. Neurobiol. Dis 72, 224–232. 10.1016/j.nbd.2014.05.037 [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Immonen R, 2014. Epilepsy Related to Traumatic Brain Injury. Neurotherapeutics. 10.1007/s13311-014-0260-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, Kyyriäinen J, Andrade P, Pasanen L, Ndode-Ekane X, 2017. Epilepsy after traumatic brain injury, in: Pitkänen A, Buckmaster PS, Galanopoulou AS, Moshé SL (Eds.), Animal Models of Seizures and Eplilepsy. Elsevier, Amsterdam, p. in press. [Google Scholar]
- Pitkänen A, Löscher W, Vezzani A, Becker AJ, Simonato M, Lukasiuk K, Gröhn O, Bankstahl JP, Friedman A, Aronica E, Gorter JA, Ravizza T, Sisodiya SM, Kokaia M, Beck H, 2016a. Advances in the development of biomarkers for epilepsy. Lancet Neurol. 15 10.1016/S1474-4422(16)00112-5 [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Lukasiuk K, 2011. Mechanisms of epileptogenesis and potential treatment targets. Lancet. Neurol 10, 173–86. 10.1016/S1474-4422(10)70310-0 [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Lukasiuk K, 2009. Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav. 14 10.1016/j.yebeh.2008.09.023 [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Roivainen R, Lukasiuk K, 2016b. Development of epilepsy after ischaemic stroke. Lancet Neurol. 15 10.1016/S1474-4422(15)00248-3 [DOI] [PubMed] [Google Scholar]
- Rizzi M, Brandt C, Weissberg I, Milikovsky DZ, Pauletti A, Terrone G, Salamone A, Frigerio F, Löscher W, Friedman A, Vezzani A, 2019. Changes of dimension of EEG/ECoG nonlinear dynamics predict epileptogenesis and therapy outcomes. Neurobiol. Dis 124, 373–378. 10.1016/j.nbd.2018.12.014 [DOI] [PubMed] [Google Scholar]
- Roehri N, Pizzo F, Lagarde S, Lambert I, Nica A, McGonigal A, Giusiano B, Bartolomei F, Bénar C-G, 2017. High-frequency oscillations are not better biomarkers of epileptogenic tissues than spikes. Ann. Neurol, 10.1002/ana.25124 [DOI] [PubMed] [Google Scholar]
- Russell E, Koren G, Rieder M, Van Uum S, 2012. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology 37, 589–601. 10.1016/j.psyneuen.2011.09.009 [DOI] [PubMed] [Google Scholar]
- Russo E, Citraro R, 2018. Pharmacology of epileptogenesis and related comorbidities in the WAG/Rij rat model of genetic absence epilepsy. J. Neurosci. Methods 310, 54–62. 10.1016/j.jneumeth.2018.05.020 [DOI] [PubMed] [Google Scholar]
- Savic I, Engel J, 2014. Structural and functional correlates of epileptogenesis - does gender matter? Neurobiol. Dis 70, 69–73. 10.1016/j.nbd.2014.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staba RJ, Bragin A, 2011. High-frequency oscillations and other electrophysiological biomarkers of epilepsy: underlying mechanisms. Biomark. Med 5, 545–56. 10.2217/bmm.11.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staba RJ, Stead M, Worrell GA, 2014. Electrophysiological biomarkers of epilepsy. Neurotherapeutics 11, 334–46. 10.1007/s13311-014-0259-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, 2012. Analysis of cortisol in hair - State of the art and future directions. Brain. Behav. Immun 26, 1019–1029. 10.1016/j.bbi.2012.02.002 [DOI] [PubMed] [Google Scholar]
- Stavropoulos I, Pervanidou P, Gnardellis C, Loli N, Theodorou V, Mantzou A, Soukou F, Sinani O, Chrousos GP, 2017. Increased hair cortisol and antecedent somatic complaints in children with a first epileptic seizure. Epilepsy Behav. 68, 146–152. 10.1016/j.yebeh.2016.12.015 [DOI] [PubMed] [Google Scholar]
- Velmurugan J, Nagarajan SS, Mariyappa N, Ravi SG, Thennarasu K, Mundlamuri RC, Raghavendra K, Bharath RD, Saini J, Arivazhagan A, Rajan J, Mahadevan A, Rao MB, Satishchandra P, Sinha S, 2018. Magnetoencephalographic imaging of ictal high-frequency oscillations (80-200 Hz) in pharmacologically resistant focal epilepsy. Epilepsia 59,190–202. 10.1111/epi.13940 [DOI] [PubMed] [Google Scholar]
- Walker LE, Frigerio F, Ravizza T, Ricci E, Tse K, Jenkins RE, Sills GJ, Jorgensen A, Porcu L, Thippeswamy T, Alapirtti T, Peltola J, Brodie MJ, Park BK, Marson AG, Antoine DJ, Vezzani A, Pirmohamed M, 2017. Molecular isoforms of high-mobility group box 1 are mechanistic biomarkers for epilepsy. J. Clin. Invest 127, 2118–2132. 10.1172/JCI92001 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wester VL, Van Rossum EFC, 2015. Clinical applications of cortisol measurements in hair. Eur. J. Endocrinol 173, M1–M10. 10.1530/EJE-15-0313 [DOI] [PubMed] [Google Scholar]
- Willmore LJ, Sypert GW, Munson JV, Hurd RW, 1978. Chronic focal epileptiform discharges induced by injection of iron into rat and cat cortex. Science 200, 1501–3. [DOI] [PubMed] [Google Scholar]
- Worrell G, Gotman J, 2011. High-frequency oscillations and other electrophysiological biomarkers of epilepsy: clinical studies. Biomark. Med 5, 557–66. 10.2217/bmm.11.74 [DOI] [PMC free article] [PubMed] [Google Scholar]